Abstract
In this study we evaluated whether the activation of endogenous angiotensin-converting enzyme (ACE) 2 would improve the cardiovascular autonomic dysfunction of diabetic rats. Ten days after type 1 diabetes induction (Streptozotocin, STZ, 50mg/kg i.v.), the rats were orally treated with 1-[(2-dimethylamino)ethylamino]-4-(hydroxymethyl)-7-[(4-methylphenyl) sulfonyl oxy]-9H-xanthene-9-one (XNT), a newly discovered ACE2 activator (1mg/kg/day), or saline (equivalent volume) during 30 days. Autonomic cardiovascular parameters were evaluated in unanesthetized animals and an isolated heart preparation was used to analyze the cardiac function. Diabetes induced a significant decrease in the baroreflex bradycardia sensibility, as well as in the chemoreflex chronotropic response and parasympathetic tone. The XNT treatment improved these parameters by ~76% (0.82±0.09 vs. 1.44±0.17ΔPI/ΔmmHg), ~85% (−57±9 vs. −105±10 Δbpm) and ~205% (22±2 vs. 66±12 Δbpm), respectively. Also, XNT administration enhanced the bradycardia induced by the chemoreflex activation by ~74% in non-diabetic animals (−98±16 vs. −170±9 Δbpm). No significant changes were observed in the mean arterial pressure, baroreflex tachycardia sensibility, chemoreflex pressor response and sympathetic tone among any of the groups. Furthermore, chronic XNT treatment ameliorated the cardiac function of diabetic animals. However, the coronary vasoconstriction observed in diabetic rats was unchanged by ACE2 activation. These findings indicate that XNT protects against the autonomic and cardiac dysfunction induced by diabetes. Thus, our results evidenced the viability and effectiveness of oral administration of an ACE2 activator for the treatment of the cardiovascular autonomic dysfunction caused by diabetes.
Keywords: Baroreflex, Chemoreflex, ACE2 activation
Introduction
The current Diabetes Atlas predicts a global prevalence of diabetes by 7.8% (438 million people) in 2030 (IDF, 2011). One of the most serious, prevalent and poorly understood complications of the diabetes is the diabetic autonomic neuropathy (DAN), which is widely spread in the body and causes important impact in the quality of live and survival of diabetic patients (Kennedy et al., 1995; Edwards et al., 2008; Tesfaye et al., 2010). Cardiovascular autonomic neuropathy (CAN) is the most studied and clinically important form of DAN. The CAN is classically related to parasympathetic and subsequent sympathetic dysfunction (Bennett et al., 1975; Pop-Busui, 2010), which may result in silent myocardial ischemia, prolonged QT interval, arrhythmias and sudden death (Hume et al., 1986; Nathan, 1993; Giunti et al., 2007; Ieda et al., 2008; Schonauer et al., 2008). More importantly, epidemiological studies show that CAN increases the risk of cardiovascular mortality and cardiovascular events in individuals with diabetes (Maser et al., 2003; Freeman, 2005; Giunti et al., 2007).
The cardiovascular reflexes, acting through the autonomic modulation of the heart, lungs and vessels, are responsible for the maintenance of blood pressure (BP) and blood-gas composition. While the baroreflex modulates the beat-to-beat control of heart rate (HR) to correct fluctuations of the BP (Kirchheim, 1976), the chemoreflex regulates the respiration, the HR and BP to maintain the blood-gas composition (Wright, 1936; Gonzalez et al., 1994). It has been shown that diabetic population presents an impairment of the cardiovascular reflexes and the earliest clinical indicator of CAN in these individuals is a decrease in the HR variability (Bernardi et al., 1997; Frattola et al., 1997; Tantucci et al., 1997; Lefrandt et al., 1999; Mancini et al., 1999; Chessa et al., 2002).
The renin-angiotensin system (RAS) is an important player in the neural cardiovascular control, as evidenced by its ability to modulate the baroreflex and chemoreflex activities (Allen, 1998; Paton & Kasparov, 1999; Phillips & de Oliveira, 2008; Allen et al., 2009). While Angiotensin (Ang) II reduces the sensibility of the baroreflex, Ang-(1-7) via Mas receptor increases the baroreflex sensitivity (Campagnole-Santos et al., 1992; Oliveira et al., 1996; Britto et al., 1997; Phillips & Sumners, 1998; Averill & Diz, 2000; Chaves et al., 2000; Diz et al., 2008a; de Moura et al., 2010). In accordance with these data, angiotensin-converting enzyme (ACE) 2, the main Ang-(1-7)-forming enzyme, also improves the baroreflex responses (Diz et al., 2008b; Yamazato et al., 2011). Regarding the chemoreflex, it has been demonstrated that Ang II has an important role in the carotid body function by increasing the sensibility of the chemoreceptor cells and the sympathetic activity (Allen, 1998; Li et al., 2006; Ding et al., 2011).
Autonomic stimulation alters the myocardial mechanical performance and it has been suggested that CAN may be responsible for the cardiac dysfunction in diabetes (Scognamiglio et al., 1998; Poirier et al., 2003; Sacre et al., 2010). Furthermore, imbalance of the autonomic system along with the impairment of the neural control of the cardiovascular system is related to poor prognosis and mortality in diabetes (Maser et al., 2003; Freeman, 2005). Thus, considering the beneficial effects induced by the activation of the ACE2/Ang-(1-7)/Mas axis on the cardiovascular function and the lack in the literature of studies evaluating the role of ACE2 in CAN caused by diabetes, in the present study we hypothesized that the chronic activation of ACE2 would improve the cardiac and cardiovascular autonomic dysfunction observed in diabetic rats. To test this hypothesis, we conducted, for the first time, an oral treatment with the ACE2 activator 1-[(2-dimethylamino)ethylamino]-4-(hydroxymethyl)-7-[(4-methylphenyl) sulfonyl oxy]-9H-xanthene-9-one (XNT) in diabetic rats. XNT is a newly discovered ACE2 activator (Hernandez Prada et al., 2008) and previous studies have demonstrated that activation of this enzyme is a promising strategy to treat cardiovascular and related diseases (Hernandez Prada et al., 2008; Ferreira et al., 2009; Fraga-Silva et al., 2010; Ferreira et al., 2011b).
Methods
Ethical approval
This study was conducted in 39 male Wistar rats (180–200 g) from CEBIO-Federal University of Minas Gerais (Belo Horizonte, MG, Brazil). Whenever possible the same animal was used in all protocols. The animals were housed in a temperature-controlled room (22–23°C) with a 12–12h light-dark cycle. Water and food were available ad libitum. All experimental protocols were performed in accordance with the Federal University of Minas Gerais (Brazil) Institutional Animal Care and Use Committee, which is in compliance with the NIH guidelines.
Diabetes induction and XNT treatment
Briefly, the animals were fasted for approximately 16h. After anesthesia with a ketamine/xylazine mixture (60:6mg/kg, i.p.), the animals were injected with streptozotocin (STZ; 50mg/kg i.v., Sigma, MO, USA) in sodium citrate buffer (10mmol/L, pH 4.5). Control non-diabetic (CTL) rats were injected with ~0.2mL of sodium citrate buffer (10mmol/L, pH 4.5, i.v.). Ten days after diabetes induction, the rats were assessed for blood glucose levels (CTL: n=10; XNT: n=9; STZ: n=10; STZ+XNT: n=10) using a glucometer (Accu-Chek® Compact Plus; Roche, IN, USA). The animals with fasting blood glucose concentration over 126mg/dL were considered diabetic. The treatment with XNT (1mg/kg/day, 0.1mL/100g of rat, gavage) or vehicle (saline pH 2–2.5; equivalent volume, gavage) was initiated ten days after diabetes induction and conducted for 30 days. Pilot experiments were performed in order to determine the lowest dose of XNT able to improve the baroreflex and chemoreflex activities in diabetic animals. Thus, after testing the doses of 0.6mg/kg/day and 1mg/kg/day, we chose the dose of 1mg/kg/day based on the effects observed in the baroreflex and chemoreflex activities. We have demonstrated in previous studies that XNT is able to activate ACE2 both in vitro (Hernandez Prada et al., 2008) and in vivo (Ferreira et al., 2009; Fraga-Silva et al., 2010; Ferreira et al., 2011b).
Cardiovascular parameters analysis
Under anesthesia with a ketamine/xylazine mixture (60:6 mg/kg, i.p.) a catheter (PE-10 connected to a PE-50) was inserted into the femoral artery (until abdominal aorta) and vein for blood pressure measurement and drug injections, respectively. The catheters were tunneled subcutaneously into the back of the neck to allow access when the animal was awake. After 24h of recovery, basal (CTL: n=10; XNT: n=9; STZ: n=10; STZ+XNT: n=10) and autonomic cardiovascular parameters were evaluated in unanesthetized animals. The arterial catheter was connected to a strain-gauge transducer coupled to a computer-based data acquisition system (MP100A, Biopac Systems Inc., CA, USA) in order to record pulsate arterial pressure (PAP). Mean arterial pressure (MAP) and HR were simultaneously calculated by the software Acqknowledge (Biopac Systems Inc., CA, USA) and continuously displayed.
Baroreflex control of the HR was determined by recording the reflex changes in the HR in response to transient increases in MAP produced by bolus injections of phenylephrine (1.0 μg/0.1mL/rat, i.v.) (CTL: n=7; XNT: n=5; STZ: n=7; STZ+XNT: n=7) or decreases in MAP induced by sodium nitroprusside (1.0 μg/0.1mL/rat, i.v.) (CTL: n=10; XNT: n=7; STZ: n=6; STZ+XNT: n=6), as previously described (Campagnole-Santos et al., 1988). Control injections were performed with 0.1mL of isotonic sodium chloride (saline). The peak reflex changes of the HR corresponding to the maximum change in the MAP, which occurred immediately after the injections, were converted to changes in pulse interval (ΔPI=60.000/HR). For each animal, the ratio between changes in HR (as pulse interval, ΔPI, ms) and changes in MAP (ΔMAP, mmHg) was calculated (baroreceptor sensitivity index - BSI).
The chemoreflex was activated by intravenous injection of potassium cyanide (KCN: 40 μg/0.1mL) (CTL: n=7; XNT: n=7; STZ: n=7; STZ+XNT: n=8), in accordance with previous studies (Franchini & Krieger, 1993). The changes in MAP and HR (ΔMAP, mmHg and ΔHR, bpm) were analyzed at the peak of the responses.
The autonomic function was evaluated using sequential and alternated pharmacological blockage of muscarinic and β-adrenergic receptors with methyl-atropine (3mg/kg/0.2mL) and atenolol (8mg/kg/0.2mL), respectively, with an interval of 15 minutes between the injections. The intrinsic heart rate (IHR) was evaluated after 15 minutes of the double blockage with methyl-atropine and atenolol and it was used for calculation of the parasympathetic (IHR minus minimum HR) (CTL: n=8; XNT: n=7; STZ: n=6; STZ+XNT: n=7) and sympathetic (maximum HR minus IHR) (CTL: n=8; XNT: n=7; STZ: n=6; STZ+XNT: n=7) tones (Negrao et al., 1992).
Isolated heart preparation
At the end of the invasive cardiovascular analysis, the rats (CTL: n=8; XNT: n=8; STZ: n=7; STZ+XNT: n=6) were heparinized (400IU, i.p.) and decapitated. The thorax was opened and the heart was carefully dissected, removed and placed in a cold Krebs-Ringer Solution (KRS: 118.4mM NaCl, 4.7mM KCl, 1.2mM KH2PO4, 1.2mM, MgSO4.7H2O, 2.5mM CaCl2.2H2O, 11.7mM glucose and 26.5mM NaHCO3) to preserve the heart before the perfusion. As described previously (Goes et al., 1993), the hearts were perfused through an aortic stump with KRS at 37±1°C in a Langendorff system with constant pressure (65 mmHg) and oxygenation (5% CO2 and 95%O2). A force transducer (TSD 104 A, Biopac Systems Inc., CA, USA) was attached through a heart clip to the apex of the ventricles to record the contractile force using a data-acquisition system (Acqknowledge, Biopac Systems Inc., CA, USA). A diastolic tension of 1.0±0.2g was applied to the hearts. Coronary flow was measured by collecting the perfusate over a period of 1 minute at regular intervals. HR and ±dT/dt were calculated from the contractile tension recordings. After 30 minutes of stabilization, the functional parameters were recorded for an additional period of 30 minutes.
Statistical analysis
Data are expressed as mean ± SEM. Statistical analysis was performed using Student’s unpaired t Test (glycemia) or one-way ANOVA followed by the Newman-Keuls post-test. P<0.05 was considered statistically significant.
Results
Effects of XNT on hyperglycemia
As expected, ten days after induction of diabetes with streptozotocin (day 10 - D10), the animals presented hyperglycemia [80±3 mg/dL in control rats (CTL and XNT groups), n=19 vs. 289±23 mg/dL in diabetic rats (STZ and STZ+XNT groups), n=20, P<0.05]. At the end of the treatment (day 40 - D40), no further increase in glycemia was observed in diabetic animals treated with XNT when compared with diabetic rats treated with saline (STZ: 434±51 mg/dL, n=10 vs. STZ+XNT: 274±42 mg/dL, n=10, P<0.05).
Effects of diabetes and XNT on baseline and autonomic cardiovascular function
The baseline MAP and HR and autonomic cardiovascular parameters were assessed 24h after vascular catheterization in unanesthetized and freely moving animals. At baseline conditions, i.e. after adaptation of the animals to the experimental condition and before the evaluation of the reflexes and autonomic function, no significant effects induced by diabetes or by the XNT treatment were observed in MAP. However, diabetes caused a reduction in HR and the treatment of diabetic animals with XNT did not alter this parameter (Table 1).
Table 1.
Effects of diabetes and XNT on mean arterial pressure and heart rate of rats.
| CTL | XNT | STZ | STZ+XNT | |
|---|---|---|---|---|
| MAP (mmHg) | 112 ± 2 | 111 ± 1 | 109 ± 3 | 105 ± 2 |
| HR (bpm) | 330 ± 5 | 329 ± 7 | 299 ± 9* | 298 ± 9* |
P<0.05 compared to control group (CTL). One-way ANOVA followed by the Newman-Keuls post-test.
MAP: mean arterial pressure and HR: heart rate.
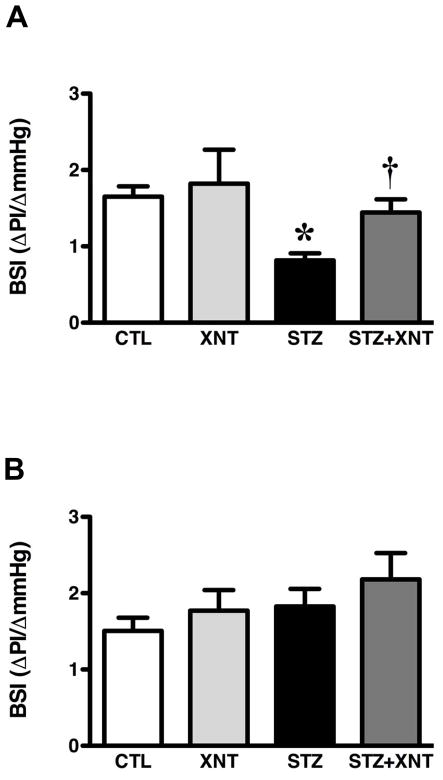
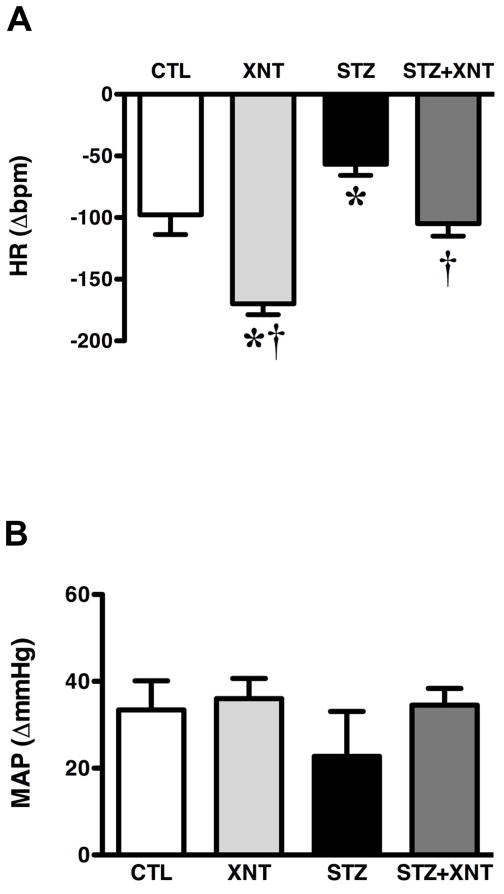
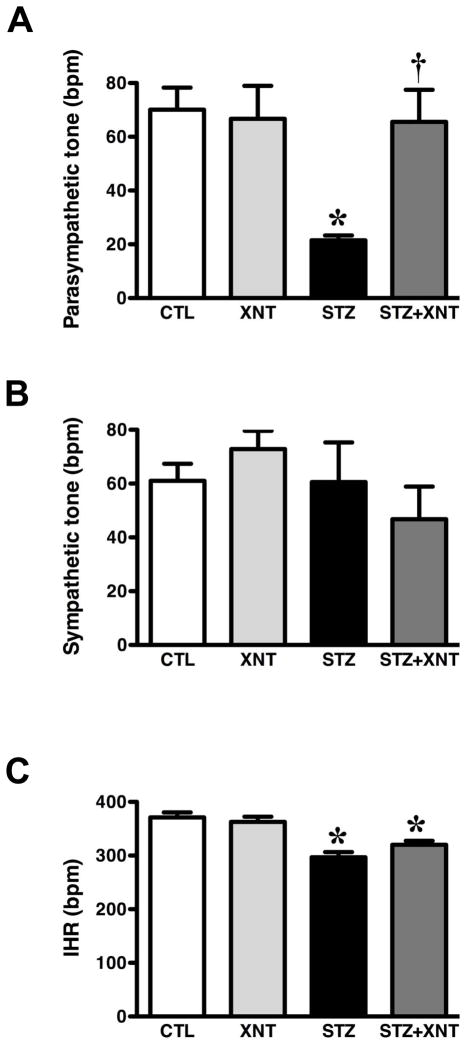
Next, we evaluated the effects of chronic ACE2 activation on the impairment of the baroreflex, chemoreflex and autonomic tone induced by diabetes. Diabetic rats presented a reduced baroreflex bradycardia sensibility and the treatment of these animals with XNT improved this effect (STZ: 0.82±0.09 vs. STZ+XNT: 1.44±0.17 ΔPI/ΔmmHg, Fig. 1A). In control animals, XNT administration had no effect on the baroreflex bradycardia sensibility. Furthermore, no significant changes were observed in the baroreflex tachycardia sensibility among any of the groups (Fig. 1B). Regarding the chemoreflex, diabetic animals showed a decreased chemoreflex chronotropic response, which was completely reversed by the XNT treatment (STZ: −57±9 vs. STZ+XNT: −105±10 Δbpm, Fig. 2A). Interestingly, the treatment of control rats with XNT significantly enhanced the chemoreflex bradycardia (Fig. 2A). No significant changes were observed in the chemoreflex pressor response among any of the groups (Fig. 2B). In agreement with the impairment of the cardiovascular reflexes, diabetes induced an expressive attenuation of the parasympathetic tone and the chronic ACE2 activation efficiently normalized this effect (STZ: 22±2 vs. STZ+XNT: 66±12 bpm, Fig. 3A). Also, no significant effects were seen in the sympathetic tone among any of the groups (Fig. 3B). The IHR was reduced in diabetic rats and ACE2 activation did not alter this effect (Fig. 3C).
FIGURE 1.
Effects of diabetes and XNT on the baroreflex sensitivity. Sensibility index (ΔPI/ΔmmHg) of (A) baroreflex bradycardia and (B) baroreflex tachycardia of control and diabetic animals treated with saline (A - CTL: n=7 and STZ: n=7; B - CTL: n=10 and STZ: n=6) or with XNT (A - XNT: n=5 and STZ+XNT: n=7; B - XNT: n=7 and STZ+XNT: n=6). (*)P<0.05 compared to control group treated with saline (CTL) and (†)P<0.05 compared to diabetes treated with saline (STZ). (One-way ANOVA followed by the Newman-Keuls post-test). BSI: baroreceptor sensibility index.
FIGURE 2.
Effects of diabetes and XNT on the chemoreflex activity. (A) Chronotropic (ΔHR) and (B) pressor responses (ΔmmHg) of the chemoreflex of control and diabetic animals treated with saline (CTL: n=7 and STZ: n=7) or with XNT (XNT: n=7 and STZ+XNT: n=8). (*)P<0.05 compared to control group treated with saline (CTL) and (†)P<0.05 compared to diabetes treated with saline (STZ). (One-way ANOVA followed by the Newman-Keuls post-test).
FIGURE 3.
Effects of diabetes and XNT on autonomic tone and intrinsic heart rate (IHR). (A) Parasympathetic tone (bpm); (B) sympathetic tone (bpm); and (C) IHR (bpm) of control and diabetic animals treated with saline (A - CTL: n=8 and STZ: n=6; B - CTL: n=8 and STZ: n=7; C - CTL: n=8 and STZ: n=8) or with XNT (A - XNT: n=7 and STZ+XNT: n=7; B - XNT: n=7 and XNT+STZ: n=5; C - XNT: n=8 and STZ+XNT: n=7). (*)P<0.05 compared to control group treated with saline (CTL) and (†)P<0.05 compared to diabetes treated with saline (STZ). (One-way ANOVA followed by the Newman-Keuls post-test).
Effects of diabetes and XNT on cardiac function
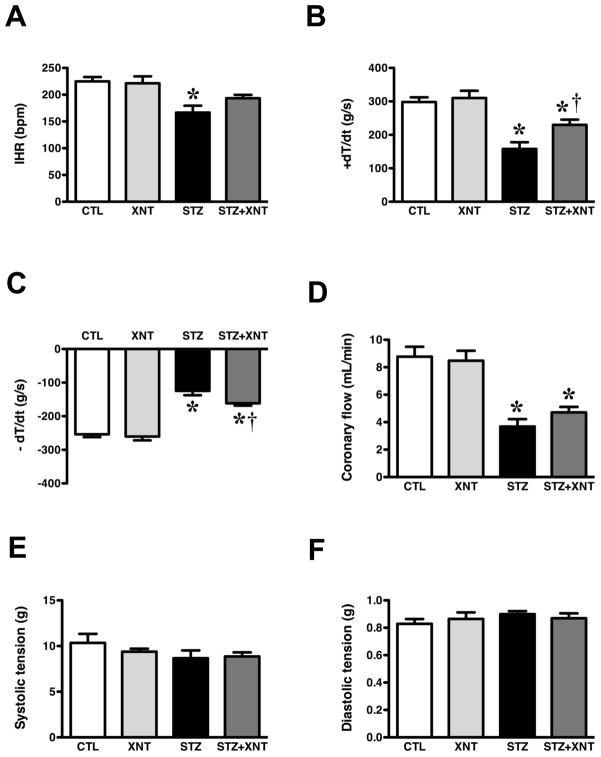
After the in vivo studies, the animals were sacrificed and the cardiac function was evaluated using an isolated heart preparation. Chronic activation of ACE2 improved the reduction in the IHR induced by diabetes (Fig. 4A). Furthermore, the decrease in the ±dT/dt observed in hearts from diabetic animals was significantly attenuated by the XNT treatment (+dT/dt, STZ: 158±20 vs. STZ+XNT: 230±16 g/s, Fig. 4B and −dT/dt, STZ: 125±13 vs. STZ+XNT: 161±7 g/s, Fig. 4C). Concerning the coronary flow, we observed a decrease in diabetic animals treated or not with XNT, indicating that XNT did not affect the coronary vasoconstriction induced by diabetes (Fig. 4D). No significant changes were observed in systolic (Fig. 4E) and diastolic (Fig. 4F) tension among any of the groups.
FIGURE 4.
Effects of diabetes and XNT on the cardiac function of isolated hearts. (A) Intrinsic heart rate (IHR, bpm); (B) +dT/dt (g/s); (C) −dT/dt (g/s); (D) coronary flow (mL/min); (E) systolic pressure (g); and (F) diastolic pressure (g) of control and diabetic animals treated with saline (CTL: n=7 and STZ: n=7) or with XNT (XNT: n=8 and STZ+XNT: n=6). (*)P<0.05 compared to control group treated with saline (CTL) and (†)P<0.05 compared to diabetes treated with saline (STZ). (One-way ANOVA followed by the Newman-Keuls post-test).
Discussion
The present study was designed to evaluate the effects of chronic activation of intrinsic ACE2 on autonomic cardiovascular function of unanesthetized diabetic animals, as well as on cardiac function. The main findings of this study were: i) XNT treatment improved the baroreflex bradycardia sensibility, the chemoreflex chronotropic response and the parasympathetic tone of diabetic animals; ii) chronic ACE2 activation increased the bradycardia induced by the chemoreflex activation in non-diabetic animals; and iii) the cardiac function of diabetic rats was ameliorated by XNT treatment.
The CAN diagnosis in humans has been essentially functional and studies have been designed to understand its neural mechanisms. In this study, the XNT treatment normalized the parasympathetic and cardiovascular reflex dysfunction triggered by diabetes, suggesting a protective role of ACE2 in the parasympathetic neurotransmission in the heart and in the baroreflex and chemoreflex pathways. Several studies have shown that the neuronal alterations caused by diabetes in the autonomic system comprise both structural and functional changes. Li and co-workers (2010) have reported structural atrophy of vagal aortic afferent and cardiac efferent axons and terminals in chronic diabetes. Furthermore, it has been suggested that diabetes induces depression of the baroreceptor sensorial input, impairment of the ganglionic transmission and loss of preganglionic vagal efferent nerve fibers of the heart (Dall’Ago et al., 1997; Mabe & Hoover, 2011). Regarding the central control of the autonomic nervous system, it seems that diabetic animals may have dysfunctional neurons in the nucleus ambiguous and central deficit in the baroreflex arc (Gu et al., 2008; Yan et al., 2009; Ai et al., 2010). In addition, activation of Ang II/AT1 inhibits the parasympathetic neurotransmission in the heart (Potter, 1982; Du et al., 1998; Kawada et al., 2007). Indeed, the brain RAS and the local balance between the ACE/Ang II/AT1 and ACE2/Ang-(1-7)/Mas axes have been established as an important regulator of the cardiovascular function (Crackower et al., 2002; Xu et al., 2011). Some studies targeting both axes demonstrated beneficial effects on the baroreflex activity and neurogenic hypertension by shifting the balance between the central ACE/Ang II/AT1 and ACE2/Ang-(1-7)/Mas axes toward this latter branch (Yamazato et al., 2007; Diz et al., 2008a; Diz et al., 2008b; Xia et al., 2009; Feng et al., 2010; Yamazato et al., 2011). Thus, based on these evidences and that XNT is an ACE2 activator, this compound may decrease the Ang II levels and/or increase the Ang-(1-7) concentration, leading to modulation of peripheral pathways and of the central integration of the cardiovascular reflexes and autonomic function.
In addition to the effects of the ACE2 activation in diabetic animals, the chemoreflex bradycardia was exacerbated in non-diabetic rats treated with XNT. Considering that the effects of XNT in diabetic animals were observed in both baroreflex and chemoreflex cardiovagal component and that the baroreflex bradycardia was unchanged in non-diabetic animals, these data suggest that this compound increases the hypoxic sensibility of the chemoreceptors and/or enhances the central regulation of the chemoreflex independent of the presence of diabetes. The carotid body is the main oxygen peripheral arterial chemoreceptor in mammals. It has been described a local RAS in the carotid body (Lam & Leung, 2002). In pathological conditions associated to hypoxia, such as heart failure, it has been evidenced that the Ang II/AT1 axis is hyperactive in the carotid body, thereby suggesting an enhancement of the chemoreceptor cells sensibility with consequent increase of the sympathetic activity (Allen, 1998; Li et al., 2006; Ding et al., 2011). Altogether, it is plausible to speculate that ACE2 might play a role in the central regulation of the baseline chemoreflex, preventing the impairment of this reflex in diabetic animals.
Looking at the results of the isolated heart preparation, XNT partially improved the cardiac dysfunction induced by diabetes. It has been reported that CAN may trigger cardiac dysfunction in diabetes (Scognamiglio et al., 1998; Poirier et al., 2003; Sacre et al., 2010). Our findings indicated that the main target of XNT was the autonomic system and not the heart. This is supported by the observation that diabetic animals treated with XNT presented autonomic function comparable to control animals while the cardiac function was only partially improved. However, it is important to point out that the cardiac beneficial effects of XNT have been previously demonstrated. We have found that ACE2 activation induced by XNT improves the cardiac function and reduces the myocardial fibrosis in spontaneously hypertensive rats (SHR) by a mechanism involving ERK1/2 phosphorylation (Hernandez Prada et al., 2008; Ferreira et al., 2011b). In this way, further investigations using different approaches are needed to better understand the effects of ACE2 activation on diabetes-induced cardiac dysfunction. Also, at the present time, we can not exclude the possibility that the actions of XNT in the autonomic function were, at least in part, due to its possible beneficial effects on the cardiac structure.
Although the treatment with XNT improved the cardiac dysfunction in diabetic rats, no significant effects were observed in the reduction of the coronary flow observed in these animals. Classically, the effect of Ang II in vascular beds is vasoconstriction while the heptapeptide Ang-(1-7) causes vasodilation. However, it has been demonstrated that these effects depend on the dose and the vascular bed evaluated. For instance, Ang II at concentrations of 1–30 μM induces vasodilation (Toda & Miyazaki, 1981; Fukada et al., 2005) whereas Ang-(1-7) at high concentrations (27–210nM) reduces the coronary flow in isolated rat hearts (Neves et al., 1997). Thus, considering these evidences, it is pertinent to suppose that the lack of effects in the coronary bed of XNT-treated animals may be related to the available amount of Ang II and/or Ang-(1-7) after ACE2 activation.
Resting bradycardia is an usual finding in streptozotocin-induced diabetic rats (Howarth et al., 2007). In the present study, we observed resting bradycardia along with reduced IHR in unanesthetized diabetic rats, as well as in isolated diabetic rat hearts. Altogether, these data suggest that the bradycardia observed in diabetic hearts was mediated by alterations in the sinoatrial node (SAN) function. Importantly, isolated hearts from diabetic animals treated with XNT presented a slight improvement in the HR, suggesting that ACE2 has a role in the SAN. In fact, all components of the ACE2/Ang-(1-7)/Mas branch are expressed in the SAN of rats (Ferreira et al., 2011a) and Ang II may induce apoptosis in cells of the SAN (Vongvatcharanon et al., 2004).
Of note, chronic ACE2 activation prevented further increase in glycemia in diabetic rats, indicating a possible effect of XNT on pancreatic function. The role of ACE2 in the glucose homeostasis was recently reported by Bindom and co-workers (2010). ACE2 overexpression in the pancreas elicited an improvement in the fasting glycemia, glucose tolerance and islet insulin content along with an enhancement of the beta cells viability in type 2 diabetic mice (Bindom et al., 2010). In addition, several studies have demonstrated that the major substrate for ACE2, Ang II, causes oxidative stress, inflammation and apoptosis in pancreatic beta cells (Tsang et al., 2004; Lupi et al., 2006; Kamper et al., 2010; Saitoh et al., 2010; Yuan et al., 2010).
Along with the potential effects of XNT on the peripheral pathways and on the central integration of the cardiovascular reflexes and autonomic function, a further suitable mechanism of XNT action in diabetic animals could be the modulation of the inflammatory process elicited by the ACE/Ang II/AT1 axis. Many studies have shown that the autonomic nervous system is able to modulate the immune response and the progression of inflammatory diseases. Stimulation of the vagus nerve may suppress innate immune responses and downregulate the expression of pro-inflammatory cytokines (Borovikova et al., 2000; Tracey, 2002, 2007). On the other hand, it has been established that the ACE2/Ang-(1-7)/Mas axis possesses a anti-inflammatory role (Thomas et al., 2010; Thatcher et al., 2011; Zhong et al., 2011) and that the chronic treatment with XNT can modulate the ACE activity and inhibit the expression of inflammatory cytokines (Ferreira et al., 2009). Therefore, it is tempting to speculate that the prevention of further increase in glycemia along with the improvement in the parasympathetic tone, leading to a potential reduction in the inflammatory process, may represent a cardiovascular protective mechanism in diabetic animals treated with XNT.
It should be noted that XNT is a non-FDA-approved drug. This compound was initially described as an ACE2 activator in 2008 based on a virtual screening of its crystal structure (Hernandez Prada et al., 2008). Since then it has been used to prove the concept that activation of endogenous ACE2 is a feasible strategy to treat cardiovascular and related diseases. Thus, XNT is a lead compound which the main objective is to serve as a “proof-of-the-concept”. Certainly, other compounds can be synthesized based on the XNT’s structure or may be discovered as an alternative to XNT for human testing. In fact, other ACE2 activators have been described (Kulemina & Ostrov, 2011).
In summary, in this study we demonstrated that chronic activation of endogenous ACE2 through oral administration of XNT protects against diabetes-induced cardiovascular autonomic and cardiac dysfunction. Therefore, ACE2 activation might be a feasible therapeutic strategy to prevent CAN and an adjuvant player in the treatment of the diabetic cardiomyopathy.
Acknowledgments
This work was supported in part by FAPEMIG-Brazil (Fundação de Amparo à Pesquisa do Estado de Minas Gerais), CNPq-Brazil (Conselho Nacional de Desenvolvimento Cientifíco e Tecnólogico), CAPES-Brazil (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and NIH grants HL56921 and HL33610.
Footnotes
No potential conflicts of interest were reported.
References
- Ai J, Wang LH, Zhang R, Qiao GF, Wang N, Sun LH, Lu GY, Sun C, Yang BF. Protective effect of the daming capsule on impaired baroreflexes in STZ-induced diabetic rats with hyperlipoidemia. BMC Complement Altern Med. 2010;10:80. doi: 10.1186/1472-6882-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AM. Angiotensin AT1 receptor-mediated excitation of rat carotid body chemoreceptor afferent activity. J Physiol. 1998;510 (Pt 3):773–781. doi: 10.1111/j.1469-7793.1998.773bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AM, O’Callaghan EL, Chen D, Bassi JK. Central neural regulation of cardiovascular function by angiotensin: a focus on the rostral ventrolateral medulla. Neuroendocrinology. 2009;89:361–369. doi: 10.1159/000197863. [DOI] [PubMed] [Google Scholar]
- Averill DB, Diz DI. Angiotensin peptides and baroreflex control of sympathetic outflow: pathways and mechanisms of the medulla oblongata. Brain Res Bull. 2000;51:119–128. doi: 10.1016/s0361-9230(99)00237-3. [DOI] [PubMed] [Google Scholar]
- Bennett T, Hosking DJ, Hampton JR. Cardiovascular control in diabetes mellitus. Br Med J. 1975;2:585–587. doi: 10.1136/bmj.2.5971.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi L, Rossi M, Leuzzi S, Mevio E, Fornasari G, Calciati A, Orlandi C, Fratino P. Reduction of 0.1 Hz microcirculatory fluctuations as evidence of sympathetic dysfunction in insulin-dependent diabetes. Cardiovasc Res. 1997;34:185–191. doi: 10.1016/s0008-6363(97)00017-5. [DOI] [PubMed] [Google Scholar]
- Bindom SM, Hans CP, Xia H, Boulares AH, Lazartigues E. Angiotensin I-converting enzyme type 2 (ACE2) gene therapy improves glycemic control in diabetic mice. Diabetes. 2010;59:2540–2548. doi: 10.2337/db09-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Britto RR, Santos RA, Fagundes-Moura CR, Khosla MC, Campagnole-Santos MJ. Role of angiotensin-(1–7) in the modulation of the baroreflex in renovascular hypertensive rats. Hypertension. 1997;30:549–556. doi: 10.1161/01.hyp.30.3.549. [DOI] [PubMed] [Google Scholar]
- Campagnole-Santos MJ, Diz DI, Ferrario CM. Baroreceptor reflex modulation by angiotensin II at the nucleus tractus solitarii. Hypertension. 1988;11:I167–171. doi: 10.1161/01.hyp.11.2_pt_2.i167. [DOI] [PubMed] [Google Scholar]
- Campagnole-Santos MJ, Heringer SB, Batista EN, Khosla MC, Santos RA. Differential baroreceptor reflex modulation by centrally infused angiotensin peptides. Am J Physiol. 1992;263:R89–94. doi: 10.1152/ajpregu.1992.263.1.R89. [DOI] [PubMed] [Google Scholar]
- Chaves GZ, Caligiorne SM, Santos RA, Khosla MC, Campagnole-Santos MJ. Modulation of the baroreflex control of heart rate by angiotensin-(1–7) at the nucleus tractus solitarii of normotensive and spontaneously hypertensive rats. J Hypertens. 2000;18:1841–1848. doi: 10.1097/00004872-200018120-00019. [DOI] [PubMed] [Google Scholar]
- Chessa M, Butera G, Lanza GA, Bossone E, Delogu A, De Rosa G, Marietti G, Rosti L, Carminati M. Role of heart rate variability in the early diagnosis of diabetic autonomic neuropathy in children. Herz. 2002;27:785–790. doi: 10.1007/s00059-002-2340-4. [DOI] [PubMed] [Google Scholar]
- Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- Dall’Ago P, Fernandes TG, Machado UF, Bello AA, Irigoyen MC. Baroreflex and chemoreflex dysfunction in streptozotocin-diabetic rats. Braz J Med Biol Res. 1997;30:119–124. doi: 10.1590/s0100-879x1997000100018. [DOI] [PubMed] [Google Scholar]
- de Moura MM, dos Santos RA, Campagnole-Santos MJ, Todiras M, Bader M, Alenina N, Haibara AS. Altered cardiovascular reflexes responses in conscious Angiotensin-(1–7) receptor Mas-knockout mice. Peptides. 2010;31:1934–1939. doi: 10.1016/j.peptides.2010.06.030. [DOI] [PubMed] [Google Scholar]
- Ding Y, Li YL, Schultz HD. Role of blood flow in carotid body chemoreflex function in heart failure. J Physiol. 2011;589:245–258. doi: 10.1113/jphysiol.2010.200584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz DI, Garcia-Espinosa MA, Gallagher PE, Ganten D, Ferrario CM, Averill DB. Angiotensin-(1–7) and baroreflex function in nucleus tractus solitarii of (mRen2)27 transgenic rats. J Cardiovasc Pharmacol. 2008a;51:542–548. doi: 10.1097/FJC.0b013e3181734a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz DI, Garcia-Espinosa MA, Gegick S, Tommasi EN, Ferrario CM, Ann Tallant E, Chappell MC, Gallagher PE. Injections of angiotensin-converting enzyme 2 inhibitor MLN4760 into nucleus tractus solitarii reduce baroreceptor reflex sensitivity for heart rate control in rats. Exp Physiol. 2008b;93:694–700. doi: 10.1113/expphysiol.2007.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du XJ, Cox HS, Dart AM, Esler MD. Depression of efferent parasympathetic control of heart rate in rats with myocardial infarction: effect of losartan. J Cardiovasc Pharmacol. 1998;31:937–944. doi: 10.1097/00005344-199806000-00019. [DOI] [PubMed] [Google Scholar]
- Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: mechanisms to management. Pharmacol Ther. 2008;120:1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RA, Speth RC, Sigmund CD, Lazartigues E. Brain-selective overexpression of human Angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ Res. 2010;106:373–382. doi: 10.1161/CIRCRESAHA.109.208645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira AJ, Moraes PL, Foureaux G, Andrade AB, Santos RA, Almeida AP. The angiotensin-(1–7)/Mas receptor axis is expressed in sinoatrial node cells of rats. J Histochem Cytochem. 2011a;59:761–768. doi: 10.1369/0022155411411712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira AJ, Shenoy V, Qi Y, Fraga-Silva RA, Santos RA, Katovich MJ, Raizada MK. Angiotensin-converting enzyme 2 activation protects against hypertension-induced cardiac fibrosis involving extracellular signal-regulated kinases. Exp Physiol. 2011b;96:287–294. doi: 10.1113/expphysiol.2010.055277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira AJ, Shenoy V, Yamazato Y, Sriramula S, Francis J, Yuan L, Castellano RK, Ostrov DA, Oh SP, Katovich MJ, Raizada MK. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:1048–1054. doi: 10.1164/rccm.200811-1678OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga-Silva RA, Sorg BS, Wankhede M, Dedeugd C, Jun JY, Baker MB, Li Y, Castellano RK, Katovich MJ, Raizada MK, Ferreira AJ. ACE2 activation promotes antithrombotic activity. Mol Med. 2010;16:210–215. doi: 10.2119/molmed.2009.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini KG, Krieger EM. Cardiovascular responses of conscious rats to carotid body chemoreceptor stimulation by intravenous KCN. J Auton Nerv Syst. 1993;42:63–69. doi: 10.1016/0165-1838(93)90342-r. [DOI] [PubMed] [Google Scholar]
- Frattola A, Parati G, Gamba P, Paleari F, Mauri G, Di Rienzo M, Castiglioni P, Mancia G. Time and frequency domain estimates of spontaneous baroreflex sensitivity provide early detection of autonomic dysfunction in diabetes mellitus. Diabetologia. 1997;40:1470–1475. doi: 10.1007/s001250050851. [DOI] [PubMed] [Google Scholar]
- Freeman R. Autonomic peripheral neuropathy. Lancet. 2005;365:1259–1270. doi: 10.1016/S0140-6736(05)74815-7. [DOI] [PubMed] [Google Scholar]
- Fukada SY, Tirapelli CR, de Godoy MA, de Oliveira AM. Mechanisms underlying the endothelium-independent relaxation induced by angiotensin II in rat aorta. J Cardiovasc Pharmacol. 2005;45:136–143. doi: 10.1097/01.fjc.0000151929.34896.c3. [DOI] [PubMed] [Google Scholar]
- Giunti S, Bruno G, Lillaz E, Gruden G, Lolli V, Chaturvedi N, Fuller JH, Veglio M, Cavallo-Perin P, Group EICS. Incidence and risk factors of prolonged QTc interval in type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetes Care. 2007;30:2057–2063. doi: 10.2337/dc07-0063. [DOI] [PubMed] [Google Scholar]
- Goes S, Freire-Maia L, Almeida AP. Effects of anesthetics on the incidence and duration of reperfusion arrhythmias in isolated rat heart. Braz J Med Biol Res. 1993;26:1091–1095. [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiological Reviews. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Gu H, Epstein PN, Li L, Wurster RD, Cheng ZJ. Functional changes in baroreceptor afferent, central and efferent components of the baroreflex circuitry in type 1 diabetic mice (OVE26) Neuroscience. 2008;152:741–752. doi: 10.1016/j.neuroscience.2007.11.030. [DOI] [PubMed] [Google Scholar]
- Haibara AS, Colombari E, Chianca DA, Jr, Bonagamba LG, Machado BH. NMDA receptors in NTS are involved in bradycardic but not in pressor response of chemoreflex. Am J Physiol. 1995;269:H1421–1427. doi: 10.1152/ajpheart.1995.269.4.H1421. [DOI] [PubMed] [Google Scholar]
- Hernandez Prada JA, Ferreira AJ, Katovich MJ, Shenoy V, Qi Y, Santos RA, Castellano RK, Lampkins AJ, Gubala V, Ostrov DA, Raizada MK. Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension. 2008;51:1312–1317. doi: 10.1161/HYPERTENSIONAHA.107.108944. [DOI] [PubMed] [Google Scholar]
- Howarth FC, Al-Sharhan R, Al-Hammadi A, Qureshi MA. Effects of streptozotocin-induced diabetes on action potentials in the sinoatrial node compared with other regions of the rat heart. Mol Cell Biochem. 2007;300:39–46. doi: 10.1007/s11010-006-9366-5. [DOI] [PubMed] [Google Scholar]
- Hume L, Oakley GD, Boulton AJ, Hardisty C, Ward JD. Asymptomatic myocardial ischemia in diabetes and its relationship to diabetic neuropathy: an exercise electrocardiography study in middle-aged diabetic men. Diabetes Care. 1986;9:384–388. doi: 10.2337/diacare.9.4.384. [DOI] [PubMed] [Google Scholar]
- IDF. IDF Diabetes Atlas. International Diabetes Federation; Brussels: 2011. p. 2011. [Google Scholar]
- Ieda M, Kimura K, Kanazawa H, Fukuda K. Regulation of cardiac nerves: a new paradigm in the management of sudden cardiac death? Curr Med Chem. 2008;15:1731–1736. doi: 10.2174/092986708784872339. [DOI] [PubMed] [Google Scholar]
- Kamper M, Tsimpoukidi O, Chatzigeorgiou A, Lymberi M, Kamper EF. The antioxidant effect of angiotensin II receptor blocker, losartan, in streptozotocin-induced diabetic rats. Transl Res. 2010;156:26–36. doi: 10.1016/j.trsl.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Kawada T, Yamazaki T, Akiyama T, Li M, Zheng C, Shishido T, Mori H, Sugimachi M. Angiotensin II attenuates myocardial interstitial acetylcholine release in response to vagal stimulation. Am J Physiol Heart Circ Physiol. 2007;293:H2516–2522. doi: 10.1152/ajpheart.00424.2007. [DOI] [PubMed] [Google Scholar]
- Kennedy WR, Navarro X, Sutherland DE. Neuropathy profile of diabetic patients in a pancreas transplantation program. Neurology. 1995;45:773–780. doi: 10.1212/wnl.45.4.773. [DOI] [PubMed] [Google Scholar]
- Kirchheim HR. Systemic arterial baroreceptor reflexes. Physiol Rev. 1976;56:100–177. doi: 10.1152/physrev.1976.56.1.100. [DOI] [PubMed] [Google Scholar]
- Kulemina LV, Ostrov DA. Prediction of off-target efefects on angiotensin-converting enzyme 2. J Biomol Screen. 2011;16:878–885. doi: 10.1177/1087057111413919. [DOI] [PubMed] [Google Scholar]
- Lam SY, Leung PS. A locally generated angiotensin system in rat carotid body. Regul Pept. 2002;107:97–103. doi: 10.1016/s0167-0115(02)00068-x. [DOI] [PubMed] [Google Scholar]
- Lefrandt JD, Hoogenberg K, van Roon AM, Dullaart RP, Gans RO, Smit AJ. Baroreflex sensitivity is depressed in microalbuminuric Type I diabetic patients at rest and during sympathetic manoeuvres. Diabetologia. 1999;42:1345–1349. doi: 10.1007/s001250051448. [DOI] [PubMed] [Google Scholar]
- Li L, Huang C, Ai J, Yan B, Gu H, Ma Z, Li AY, Xinyan S, Harden SW, Hatcher JT, Wurster RD, Cheng ZJ. Structural remodeling of vagal afferent innervation of aortic arch and nucleus ambiguus (NA) projections to cardiac ganglia in a transgenic mouse model of type 1 diabetes (OVE26) J Comp Neurol. 2010;518:2771–2793. doi: 10.1002/cne.22363. [DOI] [PubMed] [Google Scholar]
- Li YL, Xia XH, Zheng H, Gao L, Li YF, Liu D, Patel KP, Wang W, Schultz HD. Angiotensin II enhances carotid body chemoreflex control of sympathetic outflow in chronic heart failure rabbits. Cardiovasc Res. 2006;71:129–138. doi: 10.1016/j.cardiores.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Lupi R, Del Guerra S, Bugliani M, Boggi U, Mosca F, Torri S, Del Prato S, Marchetti P. The direct effects of the angiotensin-converting enzyme inhibitors, zofenoprilat and enalaprilat, on isolated human pancreatic islets. Eur J Endocrinol. 2006;154:355–361. doi: 10.1530/eje.1.02086. [DOI] [PubMed] [Google Scholar]
- Mabe AM, Hoover DB. Remodeling of cardiac cholinergic innervation and control of heart rate in mice with streptozotocin-induced diabetes. Auton Neurosci. 2011;162:24–31. doi: 10.1016/j.autneu.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Mancini M, Filippelli M, Seghieri G, Iandelli I, Innocenti F, Duranti R, Scano G. Respiratory muscle function and hypoxic ventilatory control in patients with type I diabetes. Chest. 1999;115:1553–1562. doi: 10.1378/chest.115.6.1553. [DOI] [PubMed] [Google Scholar]
- Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 2003;26:1895–1901. doi: 10.2337/diacare.26.6.1895. [DOI] [PubMed] [Google Scholar]
- Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328:1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- Negrao CE, Moreira ED, Brum PC, Denadai ML, Krieger EM. Vagal and sympathetic control of heart rate during exercise by sedentary and exercise-trained rats. Braz J Med Biol Res. 1992;25:1045–1052. [PubMed] [Google Scholar]
- Neves LA, Almeida AP, Khosla MC, Santos RAS. Metabolism of angiotensin I in isolated rat hearts. Effect of angiotensin converting enzyme inhibitors. Biochem Pharmacol. 1995;50:1451–1459. doi: 10.1016/0006-2952(95)02049-7. [DOI] [PubMed] [Google Scholar]
- Oliveira DR, Santos RA, Santos GF, Khosla M, Campagnole-Santos MJ. Changes in the baroreflex control of heart rate produced by central infusion of selective angiotensin antagonists in hypertensive rats. Hypertension. 1996;27:1284–1290. doi: 10.1161/01.hyp.27.6.1284. [DOI] [PubMed] [Google Scholar]
- Paton JF, Kasparov S. Differential effects of angiotensin II on cardiorespiratory reflexes mediated by nucleus tractus solitarii - a microinjection study in the rat. J Physiol. 1999;521(Pt 1):213–225. doi: 10.1111/j.1469-7793.1999.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MI, de Oliveira EM. Brain renin angiotensin in disease. J Mol Med (Berl) 2008;86:715–722. doi: 10.1007/s00109-008-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MI, Sumners C. Angiotensin II in central nervous system physiology. Regul Pept. 1998;78:1–11. doi: 10.1016/s0167-0115(98)00122-0. [DOI] [PubMed] [Google Scholar]
- Poirier P, Bogaty P, Philippon F, Garneau C, Fortin C, Dumesnil JG. Preclinical diabetic cardiomyopathy: relation of left ventricular diastolic dysfunction to cardiac autonomic neuropathy in men with uncomplicated well-controlled type 2 diabetes. Metabolism. 2003;52:1056–1061. doi: 10.1016/s0026-0495(03)00091-x. [DOI] [PubMed] [Google Scholar]
- Pop-Busui R. Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care. 2010;33:434–441. doi: 10.2337/dc09-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter EK. Angiotensin inhibits action of vagus nerve at the heart. Br J Pharmacol. 1982;75:9–11. doi: 10.1111/j.1476-5381.1982.tb08752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacre JW, Franjic B, Jellis CL, Jenkins C, Coombes JS, Marwick TH. Association of cardiac autonomic neuropathy with subclinical myocardial dysfunction in type 2 diabetes. JACC Cardiovasc Imaging. 2010;3:1207–1215. doi: 10.1016/j.jcmg.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Saitoh Y, Hongwei W, Ueno H, Mizuta M, Nakazato M. Candesartan attenuates fatty acid-induced oxidative stress and NAD(P)H oxidase activity in pancreatic beta-cells. Diabetes Res Clin Pract. 2010;90:54–59. doi: 10.1016/j.diabres.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Schonauer M, Thomas A, Morbach S, Niebauer J, Schonauer U, Thiele H. Cardiac autonomic diabetic neuropathy. Diab Vasc Dis Res. 2008;5:336–344. doi: 10.3132/dvdr.2008.047. [DOI] [PubMed] [Google Scholar]
- Scognamiglio R, Avogaro A, Casara D, Crepaldi C, Marin M, Palisi M, Mingardi R, Erle G, Fasoli G, Dalla Volta S. Myocardial dysfunction and adrenergic cardiac innervation in patients with insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1998;31:404–412. doi: 10.1016/s0735-1097(97)00516-0. [DOI] [PubMed] [Google Scholar]
- Tantucci C, Scionti L, Bottini P, Dottorini ML, Puxeddu E, Casucci G, Sorbini CA. Influence of autonomic neuropathy of different severities on the hypercapnic drive to breathing in diabetic patients. Chest. 1997;112:145–153. doi: 10.1378/chest.112.1.145. [DOI] [PubMed] [Google Scholar]
- Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P Toronto Diabetic Neuropathy Expert G. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher SE, Zhang X, Howatt DA, Lu H, Gurley SB, Daugherty A, Cassis LA. Angiotensin-converting enzyme 2 deficiency in whole body or bone marrow-derived cells increases atherosclerosis in low-density lipoprotein receptor−/− mice. Arterioscler Thromb Vasc Biol. 2011;31:758–765. doi: 10.1161/ATVBAHA.110.221614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MC, Pickering RJ, Tsorotes D, Koitka A, Sheehy K, Bernardi S, Toffoli B, Nguyen-Huu TP, Head GA, Fu Y, Chin-Dusting J, Cooper ME, Tikellis C. Genetic Ace2 deficiency accentuates vascular inflammation and atherosclerosis in the ApoE knockout mouse. Circ Res. 2010;107:888–897. doi: 10.1161/CIRCRESAHA.110.219279. [DOI] [PubMed] [Google Scholar]
- Toda N, Miyazaki M. Angiotensin-induced relaxation in isolated dog renal and cerebral arteries. Am J Physiol. 1981;240:H247–254. doi: 10.1152/ajpheart.1981.240.2.H247. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang SW, Cheng CH, Leung PS. The role of the pancreatic renin-angiotensin system in acinar digestive enzyme secretion and in acute pancreatitis. Regul Pept. 2004;119:213–219. doi: 10.1016/j.regpep.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Vongvatcharanon U, Vongvatcharanon S, Radenahmad N, Kirirat P, Intasaro P, Sobhon P, Parker T. Angiotensin II may mediate apoptosis via AT1-receptors in the rat cardiac conduction system. J Renin Angiotensin Aldosterone Syst. 2004;5:135–140. doi: 10.3317/jraas.2004.030. [DOI] [PubMed] [Google Scholar]
- Wright S. Further observations on the mode of oxygen lack on respiration. Experimental Physiology. 1936;26:63–77. [Google Scholar]
- Xia H, Feng Y, Obr TD, Hickman PJ, Lazartigues E. Angiotensin II type 1 receptor-mediated reduction of angiotensin-converting enzyme 2 activity in the brain impairs baroreflex function in hypertensive mice. Hypertension. 2009;53:210–216. doi: 10.1161/HYPERTENSIONAHA.108.123844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Sriramula S, Lazartigues E. ACE2/ANG-(1–7)/Mas pathway in the brain: the axis of good. Am J Physiol Regul Integr Comp Physiol. 2011;300:R804–817. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazato M, Ferreira AJ, Yamazato Y, Diez-Freire C, Yuan L, Gillies R, Raizada MK. Gene transfer of angiotensin-converting enzyme 2 in the nucleus tractus solitarius improves baroreceptor heart rate reflex in spontaneously hypertensive rats. J Renin Angiotensin Aldosterone Syst. 2011 doi: 10.1177/1470320311412809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension. 2007;49:926–931. doi: 10.1161/01.HYP.0000259942.38108.20. [DOI] [PubMed] [Google Scholar]
- Yan B, Li L, Harden SW, Epstein PN, Wurster RD, Cheng ZJ. Diabetes induces neural degeneration in nucleus ambiguus (NA) and attenuates heart rate control in OVE26 mice. Exp Neurol. 2009;220:34–43. doi: 10.1016/j.expneurol.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Yuan L, Li X, Xu GL, Qi CJ. Effects of renin-angiotensin system blockade on islet function in diabetic rats. J Endocrinol Invest. 2010;33:13–19. doi: 10.1007/BF03346544. [DOI] [PubMed] [Google Scholar]
- Zhong J, Guo D, Chen CB, Wang W, Schuster M, Loibner H, Penninger JM, Scholey JW, Kassiri Z, Oudit GY. Prevention of angiotensin II-mediated renal oxidative stress, inflammation, and fibrosis by angiotensin-converting enzyme 2. Hypertension. 2011;57:314–322. doi: 10.1161/HYPERTENSIONAHA.110.164244. [DOI] [PubMed] [Google Scholar]