Abstract
Pneumonia is a major global health problem. Prostaglandin (PG) E2 is an immunomodulatory lipid with anti-inflammatory, immunosuppressive, and pro-resolving actions. Data suggest that the E-prostanoid (EP) 2 receptor mediates immunomodulatory effects of PGE2, but the extent to which this occurs in Streptococcus pneumoniae infection is unknown. Intratracheal lung infection of C57BL/6 mice possessing (EP2+/+) or lacking (EP2−/−) the EP2 receptor was performed, as were in vitro studies of alveolar macrophage (AM) host defense functions. Bacterial clearance and survival were significantly improved in vivo in EP2−/− mice and it correlated with greater neutrophilic inflammation and higher lung IL-12 levels. Upon ex vivo challenge with pneumococcus, EP2−/−cells expressed greater amounts of TNF-α and MIP-2 than did EP2+/+ AMs, and had improved phagocytosis, intracellular killing, and reactive oxygen intermediate generation. These data suggest that PGE2-EP2 signaling may provide a novel pharmacological target for treating pneumococcal pneumonia in combination with antimicrobials.
Keywords: prostaglandins, pneumonia, bacterial infection, macrophage, innate immunity, cyclooxygenase, Streptococcus
Introduction
Pneumonia is the leading cause of infectious mortality in the United States1 and therapeutic options are limited by emerging antimicrobial resistance 2. Streptococcus pneumoniae is the most frequently isolated pathogen in community-acquired pneumonia 3 and accounts for more deaths (nearly 1.6 million per year 4) than any other bacterium 5. An improved understanding of immune defenses against S. pneumoniae is important to the discovery of improved targets for preventive and therapeutic strategies.
A critical innate immune barrier to bacterial pneumonia is the alveolar macrophage (AM) 6, which patrols gas-exchanging alveoli and removes potential pathogens through phagocytosis and intracellular killing. Furthermore, AMs alert the host to the presence of invading microbes by releasing lipid and protein mediators that activate resident cells and recruit polymorphonuclear leukocytes (neutrophils) to the focus of infection 7. This response is highly regulated, to limit self-inflicted damage to host cells and tissues 8. Emerging evidence suggests that lipid mediators are major regulators of both the amplitude and duration of infection-triggered inflammatory responses 9. For example, leukotriene (LT) B4 has potent proinflammatory effects that augment innate immune functions of the AM 10, 11 and recruit/activate neutrophils, while prostaglandins (PGs) such as PGE2 and PGI2 have opposite, anti-inflammatory actions in the context of pneumonia 12, 13. The recently-described resolvins and protectins are lipids that have been postulated to drive the resolution phase of inflammation 14.
The cyclooxygenase (COX)-derived eicosanoid PGE2 primarily down-regulates AM defense functions 12, 15, 16. PGE2 binds to four distinct G protein-coupled E-prostanoid (EP) receptors, termed EP1-4. The inhibitory effects of PGE2 primarily result from cAMP-dependent signaling processes, triggered by EP2 and/or EP4 activation 17 and likely evolved to prevent inflammatory tissue damage and promote the resolution of inflammation 18–20. Sadikot et al. demonstrated that PGE2-EP2 signaling suppressed bacterial clearance from the lungs of mice infected with Pseudomonas aeruginosa 21. In addition, Stables et al. used ex vivo human whole blood assays to demonstrate that inhibiting either PGE2 synthesis or EP2 signaling significantly improved innate immune defenses against S. pneumoniae, including antimicrobial-resistant strains 22. That important study shed new light on the potential for targeting PGE2 synthesis or signaling systems as adjunctive therapy against pneumonia. However, those studies were not designed to examine pneumococcal pneumonia in vivo 22. Thus, mechanistic details of how PGE2 regulates pulmonary host defense during pneumococcal infection remain undefined.
We hypothesized that the EP2 receptor would play a potentially maladaptive, anti-inflammatory role in severe pneumococcal pneumonia, suppressing innate host defenses to allow infection to progress. A combination of pharmacological and genetic experiments were conducted to test this hypothesis, revealing a novel and critical role for PGE2-EP2 signaling in regulating pulmonary innate immunity during pneumococcal respiratory tract infection. These data add support and understanding to the idea of targeting prostaglandin synthesis and signaling as adjunctive treatments against pneumonia.
MATERIALS AND METHODS
Animals
Mice harboring a targeted deletion of both alleles of the Ptger2 encoding the EP2 receptor were originally generated by Dr. Richard Breyer (Vanderbilt University). These six-to-eight week old female EP2-deficient (EP2−/−) mice, bred on a C57BL/6 background, and age-matched, female C57BL/6 wild type (WT) animals (EP2+/+ mice) were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred in the University of Michigan Unit for Laboratory Animal Medicine. The genotypes of mouse strains were confirmed by tail-snip DNA PCR analyses performed by Transnetyx (Cordova, TN). Animals were treated according to National Institutes of Health guidelines for the use of experimental animals with the approval of the University of Michigan Committee for the Use and Care of Animals.
Reagents
RPMI 1640 culture medium and penicillin/streptomycin/amphotericin B solution were supplied by Gibco-Invitrogen (Carlsbad, CA). Todd-Hewitt broth was purchased from Difco (Detroit, MI). Cytochalasin D, saponin, and MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) were obtained from Sigma (St. Louis, MO). 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF) was obtained from Invitrogen (Carlsbad, CA).
Pneumococcal pneumonia
S. pneumoniae serotype 3, 6303 (American Type Culture Collection, Manassas, VA) was grown to mid-log phase in Todd-Hewett broth, washed in PBS, and serially diluted in sterile saline in order to deliver 1 × 105 colony forming units (CFU) intratracheally to induce pneumococcal pneumonia as described previously 23. Bacterial growth in lung and spleen homogenates was determined 24 h after infection using serial dilutions as previously published 23.
Isolation and culture of alveolar macrophages
Mouse resident AMs were obtained via lung lavage as previously described 23, 24 and resuspended in RPMI 1640 to a final concentration of 1–4 × 106 cells/ml. Cells were adhered to tissue culture-treated plates for 1 h (37°C, 5% CO2) followed by two washes with warm medium. Cells were cultured overnight in RPMI 1640 containing 10% FBS and 1% penicillin/streptomycin/amphotericin B prior to use. The following day cells were washed 2x using warm medium to remove nonadherent cells.
In vitro macrophage assays for intracellular cAMP, nitric oxide production, and reactive oxygen intermediate generation
Macrophage nitric oxide and reactive oxygen intermediate generation were performed as recently published with modifications specified in the text 23. Cellular cAMP levels were measured from AMs ex vivo according to our previously published protocol 12.
Alveolar macrophage phagocytosis assay
The ability of mouse AMs to phagocytose S. pneumoniae was assessed using a previously published protocol for determining the internalization of fluorescent, fluorescein isothiocyanate (FITC)-labeled bacteria12, 23. Heat-killed S. pneumoniae serotype 3 were labeled with FITC as previously described (FITCS. pneumoniae) 25. 1.5 × 105 AMs were obtained from the BAL fluid of naive mice and seeded in replicates of 24 in 384-well tissue culture plates with opaque sides and optically clear bottoms (Costar, Corning Inc. Life Sciences, Lowell, MA). The next day, FITCS. pneumoniae were opsonized with 10% normal rat-derived non-immune serum. Macrophages were inoculated with FITCS. pneumoniae using a multiple of infection of 150:1 for 180 min to allow phagocytosis to occur. Trypan blue (250 μg/ml; Molecular Probes) was added for 10 min to quench the fluorescence of extracellular bacteria and fluorescence was determined using a SPECTRAMax GEMINI EM fluorometer 485ex/535em (Molecular Devices, Sunnyvale, CA). A phagocytic index was calculated as previously described in relative fluorescence units 12.
Macrophage assay of bacterial killing
The ability of bacteria to survive within AMs was determined via a tetrazolium dye reduction assay 11, 26. Bacterial growth was determined colorimetrically based on the ability of live bacteria to convert MTT to a purple formazan salt that absorbs light at 595 nm (A595). Briefly, 2 × 105/mL mouse AMs were seeded in duplicate 96-well tissue culture dishes. The next day, S. pneumoniae were opsonized with 10% normal rat-derived immune serum, as previously described 27. Macrophages were then infected with a 0.1-mL suspension of opsonized S. pneumoniae (2 × 107 CFU/mL; multiplicity of infection, 100 bacteria per AM) for 2 h to allow for phagocytosis. Bacterial killing was assessed as described 11, 26. The intensity of the A595 was directly proportional to the number of viable bacteria within macrophages 26. Results were expressed as percentage survival of ingested bacteria. The survival of ingested bacteria = 100% × A595 control (phagocytosis) plate/A595 experimental (phagocytosis + killing) plate.
Lung homogenate, bronchoalveolar lavage, and macrophage cytokine and chemokine determination
Lung homogenates, obtained from mice 24 h after pneumococcal infection, were evaluated for IL-1β, TNF-α, IL-10, IL-12 p40, IL-6, and MCP-1 by ELISA (R&D Duoset, R&D Systems). Bronchoalveolar lavage was performed to determine cytokine and chemokine levels in bronchoalveolar lavage fluid (BALF) by ELISA as reported earlier 23. Resident AMs were cultured ex vivo with heat-killed S. pneumoniae (multiplicity of infection 1000:1) for 24 h in RPMI 1640 containing 1% penicillin/streptomycin and 10% fetal calf serum (EMD Biosciences). Cell culture medium was recovered and stored at −70°C until assays were performed to determine the levels of TNF-α, IL-10, IL-12 p70, and MIP-2 by ELISA (R&D Duoset, R&D Systems). ELISAs were performed by the University of Michigan Cancer Center Cellular Immunology Core.
Histopathology
Lungs were prepared for histopathology studies 24 h following infection as published 23. Histological and immunohistochemical staining were evaluated in a blinded fashion by a board-certified veterinary pathologist (ILB) using an Olympus BX45 light microscope with a scope-mounted Olympus DP72 12.5 megapixel digital camera and Olympus DP-BSW software. Slides were evaluated separately on a numeric 0–4 scale (0: none; 0.5: minimal; 1: mild; 2: moderate; 3: severe; 4: severe and extensive) for 4 categories, specifically neutrophilic inflammation within the lung, pleural inflammation, macrophage accumulation, and presence of bacteria. Each of these categories was separately given a 1–4 score and the scores were then summed across the parameters to give a total histological score (maximal score 16). The criteria dictating the numeric scores for each parameter were as follows. For inflammatory or cell accumulation parameters: 0: none; 0.5: single or multiple small foci most evident at higher magnification; 1: single or multiple small foci evident at low magnification; 2: larger multiple to coalescing foci; 3: multiple to coalescing foci affecting 50–80% of a lobe; 4: large coalescing foci affecting >80% of a lobe. For bacteria: 0:none visible; 1: few cocci visible within 1–2 fields; 2: small numbers of cocci visible within 2 or more fields; 2: cocci immediately visible in most inflamed fields but not in high numbers; 3: cocci easily visible in high numbers or in clusters in most inflamed fields; 4: numerous easily visible clusters in all affected fields evident at low magnification).
Statistical analyses
Statistical analyses were conducted using Prism 5.0 software (GraphPad Software, La Jolla, CA). Survival was evaluated for differences using a Mantel-Cox log-rank test. Where appropriate, mean values were compared using a paired Student t-test or a one-way analysis of variance (ANOVA) followed by Bonferroni correction. Differences were considered significant if P < 0.05. Unless otherwise stated, all experiments were performed at least three separate times. Data are presented as mean values ± standard error of the mean unless noted otherwise.
RESULTS
The absence of PGE2-EP2 signaling is protective in pneumococcal pneumonia
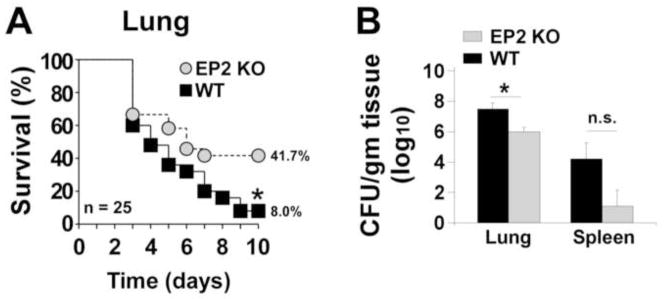
Similar to our previous study of PGE2-EP3 signaling 23, female C57BL/6 mice were noted to be susceptible to pneumococcal pneumonia, exhibiting 92% mortality after intratracheal inoculation of 1 × 105 CFU of S. pneumoniae (Fig. 1A). We have previously reported that PGE2 is an abundant mediator in the lungs of C57BL/6 mice infected with pneumococcus 28, 29. Mice unable to sense this PGE2 via the EP2 receptor (EP2−/− mice) were significantly protected from infection-related death (59.3% mortality; P < 0.05 compared with EP2+/+ mice). The increased survival of EP2−/− mice correlated with improved clearance from the lungs 24 h following infection (Fig. 1B). A non-significant trend towards reduced dissemination of bacteria from the lungs to the spleen was also observed in the EP2 null mice (Fig. 1B).
Fig. 1. Improved survival and enhanced bacterial containment after pneumococcal infection in mice lacking the EP2 receptor.
Female wild type (WT) C57BL/6 or EP2 receptor knockout (EP2 KO) mice were infected intratracheally (A) with S. pneumoniae as detailed in the Materials and Methods section. Survival was monitored for 10 days. The number of mice per group is shown. *P < 0.05 by Mantel-Cox log-rank test. (B) Bacterial loads (CFU S. pneumoniae per gram of homogenized tissue) in the lung and spleen of WT and EP2 KO mice 24 h after infection (n = 5 mice per experimental group). *P < 0.05 by Student t-test; n.s., not significant. Data from one representative experiment of two independent experiments yielding similar results are shown.
Enhanced neutrophilic inflammation in the alveoli of infected EP2−/− mice
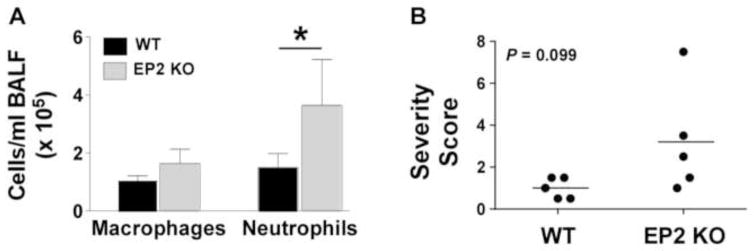
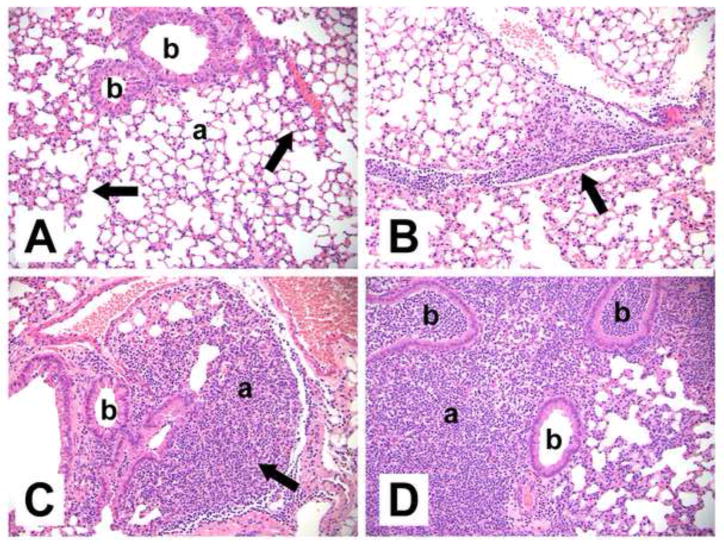
PGE2 impairs neutrophil migration to the infected lungs 30, 31 and EP2 signaling impairs cellular migration, including that of neutrophils 32, 33. Consistent with these results, the present studies demonstrated that EP2 receptor null mice had greater numbers of neutrophils in BALF 24 h following inoculation when compared to EP2+/+ animals (Fig. 2A). A cumulative severity score describing lung inflammation was obtained for infected mice (Fig. 2B). Consistent with the BALF results, there was a trend toward more severe acute inflammation in the absence of PGE2-EP2 signaling, with severity scores of 3.2 ± 1.2 in EP2−/− mice vs. 1.0 ± 0.2 in EP2+/+ mice (P = 0.099 by Student t test). This difference appeared to be due to the greater presence of neutrophils in the alveoli of EP2−/− mice (Fig. 3A–D), which was the dominant histological change. Neutrophilic inflammation predominantly involved the alveoli, with less prominent bronchiolar involvement (Fig. 3A–D). Additional changes included increased macrophages in the pulmonary interstitium and alveoli, and neutrophilic inflammation of pleural surfaces. All animals had minimally-increased macrophage cellularity, while neutrophilic inflammation was present in 5 out of 5 EP2−/− animals (minimal to moderate) but only 3 out of 5 EP2+/+ (WT) animals (minimal to mild). Pleural inflammation was present in the highest scoring EP2−/− animal.
Fig. 2. Enhanced acute inflammation in EP2 receptor deficient mice after S. pneumoniae lung infection.
(A) Bronchoalveolar lavage fluid (BALF) cell counts were determined in wild type (WT) and EP2 knockout (KO) mice as detailed in the Materials and Methods section 24 h after intratracheal inoculation with S. pneumoniae. *P < 0.05 by Student t-test (n = 5 mice per group). (B) Pulmonary inflammation was quantitated using a summary severity score calculated through blinded histopathological analysis as described in the Materials and Methods section. EP2 KO mice demonstrated a trend towards more severe acute inflammation in the lungs 24 hr after infection. *P = 0.099 by Student t-test. Data from one representative experiment of two independent experiments yielding similar results are shown
Fig. 3. Representative histopathological changes in the lungs of S. pneumoniae-infected wild type (WT) and EP2 knockout (KO) mice 24 h after inoculation.
Abbreviations: b: bronchiole, a: alveoli. (A) Infected WT mouse without significant inflammation (summary score 0.5). The majority of alveolar airspaces are clear. There are small areas of increased interstitial macrophagic cellularity (arrows). (B) Infected WT mouse with mild, focal neutrophilic inflammation (summary score 1.0), adjacent to a pulmonary blood vessel and along interlobular septae (arrow). (C) Infected EP2−/− mouse with mild-moderate inflammation (summary score 1.5) focally obscuring the alveoli (arrow). (D) Infected EP2−/− mouse with focally intense inflammation (summary score 7.5) consisting of neutrophils extensively filling alveoli and bronchioles, pleural involvement (not shown), and increased interstitial cellularity. Hematoxylin and eosin. Original magnifications x200.
Loss of PGE2-EP2 signaling results in enhanced IL-12 production in vivo during pneumonia
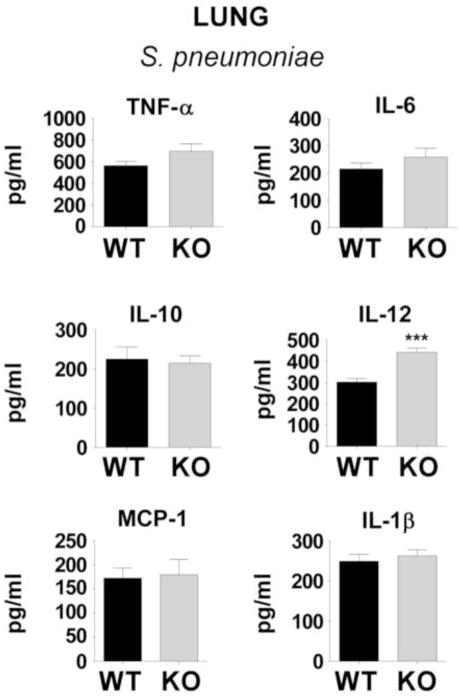
PGE2 is a potent modulator of inflammation-induced cytokine responses via the cAMP-coupled EP2 and EP4 receptors 34, 35. Studies were therefore conducted to assess the generation of inflammatory mediator generation in the lung 24 h following infections (Fig. 4). The cytokines and chemokines measured were selected based on known roles in the host response to bacterial infection and previously published evidence that their expression was regulated by PGE2. While production of TNF-α, IL-1β, IL-6, IL-10, and MCP-1 was similar in lungs of EP2−/− and EP2+/+ mice, IL-12 p40 subunit levels were significantly higher in EP2−/− lungs. Similar results were observed when these mediators were quantified in BALF, with IL-12 being significantly greater in the alveoli of EP2−/− lungs (442.6 ± 20.2 pg/ml) compared with EP2+/+ mice (302.4 ± 17.1 pg/ml; P < 0.001 by Student t test).
Fig. 4. Inflammatory mediators in whole lung homogenates of wild type (WT) C57BL/6 mice or EP2 knockout (KO) mice 24 h after inoculation.
Cytokines and chemokines were measured by ELISA as detailed in the Materials and Methods section 24 h after intratracheal inoculation with S. pneumoniae. ***P < 0.001 by Student t-test (n = 5 mice per group). IL, interleukin, MCP, monocyte chemotactic protein-1.
Alveolar macrophages from EP2−/− mice have enhanced antimicrobial activities against S. pneumoniae
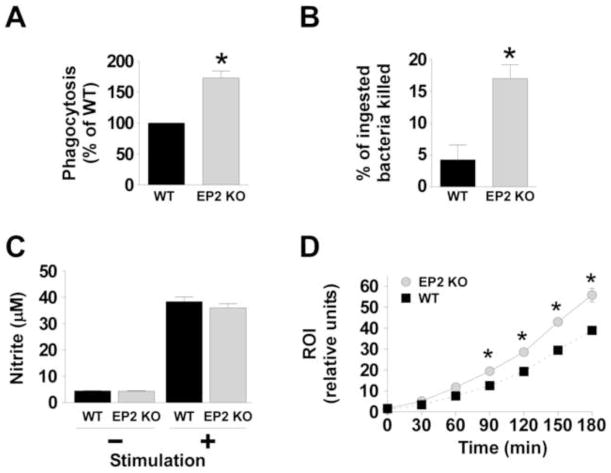
Prostaglandin E2-EP2 signaling, impairs bacterial phagocytosis by AMs 12 and inhibits the NADPH oxidase-dependent generation of reactive oxygen intermediates via cAMP-dependent signaling networks 16. We therefore tested whether similar effects were present in the context of pneumococcal infection. The intracellular cAMP concentrations in AMs obtained from uninfected EP2+/+ mice was significantly higher than that observed in EP2−/− macrophages (3.7 ± 0.31 vs. 2.5 ± 0.21 pmol/ml, P = 0.02; n = 4). As evidenced in Fig. 5A, EP2 receptor-deficient AMs phagocytosed serum-opsonized S. pneumoniae more effectively than did cells from EP2+/+ mice. The phagocytic activity of EP2−/− macrophages relative to EP2+/+ cells was 173 ± 11.2% (P = 0.02 by Student t test). In addition, bacterial killing by EP2 null macrophages was 4.25-fold more effective compared with EP2+/+ cells (P = 0.007 by Student t test; Fig. 5B).
Fig. 5. Enhanced in vitro alveolar macrophage defense functions in EP2 knockout (KO) mice compared with wild type (WT) alveolar macrophages.
Alveolar macrophages were obtained from uninfected WT and EP2 KO mice as described in the Materials and Methods section. (A) Cells were inoculated with heat-inactivated, FITC-labeled S. pneumoniae (multiple of infection 150 bacteria per macrophage) and phagocytosis was measured after 3 hrs. *P < 0.05 by Student t-test. Data represent the mean ± SEM of a minimum of 3 independent experiments performed in octuplet. (B) The survival of internalized S. pneumoniae was determined as noted in the Materials and Methods section. *P < 0.05 by Student t-test. Data represent the mean ± SEM of a minimum of 3 independent experiments performed in triplicate. (C) The capacity for alveolar macrophages to generate nitric oxide (measured as nitrite) was assessed for WT and EP2 KO cells following stimulation with or without 10 μg/ml of lipoteichoic acid and 10 ng/ml IFN-γ for 24h. (D) Alveolar macrophages from WT mice (black squares and dotted line) or EP2 KO mice (grey circles and solid line) were cultured with 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF) for 1h then stimulated with heat-killed S. pneumoniae using a multiplicity of infection of 50:1. Reactive oxygen intermediate (ROI) production was assessed fluorometrically and expressed as relative fluorescence units. The data represent the mean of 3 experiments completed in quadruplicate for each time point. *P < 0.05 by Student t-test.
It was previously shown that AMs from mice lacking the EP3 receptor also had a significantly enhanced capacity to kill intracellular pneumococci 23 and this correlated with a greater ability to generate nitric oxide during infection 23 Notably, the EP3 null mice did not have a different capacity to make reactive oxygen intermediates during infection, compared with EP3+/+ macrophages 23. In the present work we made a novel, and opposite, observation: EP2 null cells had the same ability to generate nitric oxide as EP2+/+ AMs but had a significantly greater capacity to produce reactive oxygen intermediates upon infection with S. pneumoniae (Fig.s 5C and D).
Alveolar macrophages from EP2−/− mice exhibit increased proinflammatory mediator generation in response to S. pneumoniae
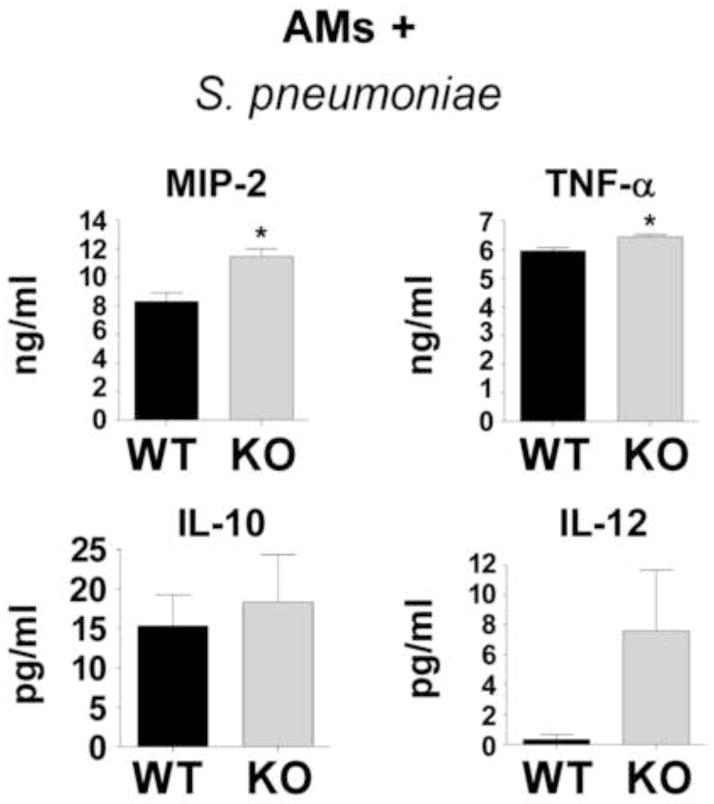
It is established that multiple AM functions are regulated by PGE2-EP2 signaling 12, 16, 29, 36, 37 but the extent to which EP2 signaling regulates the production of inflammatory mediators by AMs in response to pneumococcal infection has not been previously studied. Thus, AMs from WT and EP2−/− mice were cultured in vitro and inoculated with heat-killed pneumococci for 24 h. Inflammatory mediators were measured in the culture supernatants by ELISA (Fig. 6). Because no difference was observed in whole lung homogenates (or BALFs) in the chemokine MCP-1 (above), we assessed AM production of MIP-2, a chemoattractant for neutrophils that is involved in host defense against S. pneumoniae 38. Notably, AMs lacking EP2 showed enhanced TNF-α and MIP-2 production following stimulation with pneumococci (Fig. 6). These cells made only small amounts of IL-10 and IL-12, making comparisons difficult, but there was a trend towards greater IL-12 production by EP2−/− AMs.
Fig. 6. Alveolar macrophage cytokine and chemokine production following stimulation with S. pneumoniae.
Alveolar macrophages were obtained from uninfected WT and EP2 knockout (KO) mice and inoculated in vitro with S. pneumoniae as described in the Materials and Methods section. After 24 hrs of inoculation, bacteria- and cell-free supernatants were assayed for inflammatory mediators by ELISA. *P < 0.05 by Student t-test. Data represent the mean ± SEM of n = 3 independent experiments.
DISCUSSION
S. pneumoniae is the most common bacterial cause of community-acquired pneumonia in the world and remains a significant source of morbidity and mortality 39. This study newly defines the important contribution of PGE2-EP2 signaling to the regulation of innate pulmonary host defenses during pneumococcal pneumonia. Whereas PGE2 may serve a homeostatic function by promoting the resolution of inflammation when infection is moderate in degree 18, 40, its immunosuppressive actions might be maladaptive when an infection is overwhelming. Our data indicate that PGE2-EP2 signaling modulates the first-line innate immune processes on the respiratory surface (e.g., AM and/or epithelial cell functions) and contributes to their failure during severe infection.
Several previous studies have utilized pharmacologic inhibition or genetic deletion of COX or its isoforms and demonstrated improved outcome from infection in vivo or increased innate immune cell functions in vitro 12, 21, 22, 41–47. Although no human studies have assessed the ability of COX inhibitors to improve bacterial clearance or the outcome of localized infections of the lung or other organs, such studies in the setting of sepsis have not met with great success 48–50. Since specific prostanoids and prostanoid receptors can exert opposing biological effects, targeting the upstream enzyme COX may represent an excessively broad or nonspecific approach to immunostimulation. By contrast, greater specificity may be obtained by targeting specific downstream prostanoid receptors, such as EP2.
The anti-inflammatory (and potentially immunosuppressive) actions of PGE2 are driven by EP2- and EP4-dependent increases in cAMP generation 29, 51, 52. Recent evidence suggests that certain pathogens have evolved mechanisms to use this host response to their own advantage 21, 23, 53–56. This phenomenon appears to be important in the pathogenesis of invasive streptococcal infections 22, 29, 43, 57–61 and pharmacological targeting of PGE2 synthesis and/or signaling has gained significant interest in the treatment of severe streptococcal infections.
In 1982, Short et al. demonstrated increased survival in animals when PGE2 synthesis was inhibited during Group B Streptococcus sepsis 45. It was later reported that mice infected in the lungs with a lethal inoculum of S. pneumoniae were protected from infection and cleared the bacteria better when PGE2-EP3 signaling was genetically disrupted, possibly as a result of enhanced nitric oxide-dependent killing of the bacteria 23. In addition, Goldmann et al. noted that the inducible COX isoform COX-2 was quantifiably the most up-regulated gene in mouse macrophages infected with S. pyogenes 62, later reporting that COX-2 is also up-regulated in human and mouse tissues infected by this pathogen 43. They went on to demonstrate that PGE2 signaling via EP2 receptors suppressed host defenses against S. pyogenes 43. Abdeltawab, et al. applied an unbiased systems genetics approach that identified two PGE2 synthase enzymes as key participants mediating susceptibility to S. pyogenes infection 57.
Our studies provide new mechanistic information about PGE2 as an immunomodulator. PGE2 regulates AM functions via EP2-dependent signaling mechanisms 12, 21, but this has not been studied in the context of pneumococcal infection. We now demonstrate that genetic ablation of normal PGE2-EP2 signaling increases survival of C57BL/6 mice against a lethal model of pneumonia. This was associated with greater bacterial clearance from the lungs and exaggerated neutrophilic inflammation. It is notable that the EP2−/− mice were better able to survive infection and control bacterial growth, despite accumulating more histologically-evident inflammation. These data suggest that the greater neutrophilic immune response was beneficial, possibly because the infection was more “contained” than in the wild type mice. The trend toward reduced dissemination to the spleen in the EP2−/− mice as compared to the WT mice is also supportive of this assertion (Fig. 1B). Recent data support an important role for neutrophils in pneumococcal host defense 63. The mechanisms responsible for the increased neutrophil presence remain to be detailed. PGE2-EP2 signaling has been shown to restrict neutrophil chemoattraction 32. We also speculated that PGE2-EP2 signaling might influence chemokine production during infection. Indeed, AMs infected with pneumococcus produced large quantities of the neutrophil chemoattractant MIP-2, and this was significantly greater for EP2−/− cells (Fig. 6).
The host inflammatory response to infection is a double-edged sword that must be robust enough to clear the infection (and therefore improve outcome) but not so intense that it harms the host (and causes morbidity or mortality) 64. Our mouse model of pneumococcal pneumonia was highly lethal but the local cellular inflammatory response was patchy and relatively mild. Similar to human infection, we observed bacterial escape and dissemination from the lung during pneumonia, which possibly contributed to the mortality. Thus, enhancing innate immune responses improved local bacterial clearance to improve outcome without inducing overwhelming lung inflammation. Whether inhibiting PGE2-EP2 signaling would negatively impact host morbidity and/or mortality by promoting sepsis in a model of more severe pulmonary infection or one with a greater degree of dissemination deserves close attention in future studies.
It was also observed that AMs from EP2−/− mice expressed more TNF-α than did wild type cells upon challenge with S. pneumoniae, and there was a trend toward greater IL-12 production as well. This cytokine pattern, which suggests enhanced M1 polarization of AMs during infection, was further supported by the observation of increased IL-12 p40 levels in the lung parenchyma of infected EP2−/− mice. It is notable that the IL-12 p40 subunit has been shown to be important in host defense against S. pneumoniae 65. AMs are the principle source of this IL-12 is unclear. Our findings may also be relevant to the robust neutrophilic inflammation, since IL-12 has been shown to augment host defense against S. pneumoniae in a neutrophil-dependent manner 63. Further studies are needed to determine whether the increased neutrophils and/or IL-12 are causally responsible for the better outcome of EP2-deficient mice.
AMs from EP2−/− mice had significantly enhanced phagocytosis and intracellular killing of pneumococci and this correlated with lower levels of the second messenger molecule cAMP within the cells. Whether the difference in cAMP was causally related to the differences in host defense functions remains unknown but is consistent with what is known about PGE2-EP2 signaling and the functions of cAMP within macrophages 16, 17. Infected AMs also generated greater amounts of microbicidal reactive oxygen intermediates when PGE2-EP2 signaling was ablated. These data mechanistically differed from our work with EP3-deficient mice, where studies demonstrated a key role for the EP3 receptor in regulating AM nitric oxide generation and killing of S. pneumoniae 23. We speculate that pharmacological blockade of both EP2 and EP3 receptor subtypes might additively or synergistically improve outcome during bacterial pneumonia, and this is a subject for future research.
This study did not address cellular sources of PGE2 production during pneumococcal infection, which is a limitation. It is believed that macrophages 66 and airway epithelial cells are important sources of PGE2 during infection in vivo 59, 67. We did not measure macrophage production of PGE2 as part of our infection studies in vitro and therefore cannot rule out differences in PGE2 production between WT and EP2−/− cells.
Another limitation of these studies was the use of a single background mouse strain (C57BL/6), because genetic variation among different inbred mouse strains has been shown to affect both the susceptibility to pneumococcal infection 68 and the sensitivity to PGE2 immunoregulation 69. Future studies are needed to determine the extent to which the present findings are specific to this experimental model. Another limitation was the focus on the single eicosanoid compound PGE2, since many COX-derived lipid mediators are likely to be relevant in the setting of severe bacterial pneumonia. While the present work was specifically designed to address the role of PGE2-EP2 signaling in this setting, continued investigations are needed to identify how other prostanoids influence disease pathogenesis.
In conclusion, these studies reinforce and refine the emerging paradigm that PGE2 can impair innate immune function during severe infection and suggest that targeting specific receptors could help prevent or treat pneumococcal pneumonia.
Highlights.
EP2 receptor-deficient mice are protected from death from pneumococcal pneumonia.
EP2 null mice have enhanced inflammatory responses to S. pneumoniae in the lungs.
Alveolar macrophage phagocytosis of S. pneumoniae is suppressed by PGE2-EP2 signaling.
PGE2 restricts pneumococcal killing by alveolar macrophages via the EP2 receptor.
The EP2 receptor limits inflammatory mediator production by infected macrophages.
Acknowledgments
Funding source:
This work was supported by the National Institutes of Health [HL078727 to DMA, HL077417 to PM, HL058897 to MP-G, and T32ES007062 to EO] and the Flight Attendants Medical Research Institute [CIA-103071 to PM].
The authors wish to thank Dr. Carlos H. Serezani for his critical reading of this manuscript. We thank Joel Whitfield from the University of Michigan Cancer Center Cellular Immunology Core for technical assistance.
Footnotes
D.M. Aronoff contributed to planning experiments, analyzing data, making figures, and was the primary author of this manuscript. I.L. Bergin provided expertise in histopathology experiments, helped to analyze data, made figures, and assisted in writing the manuscript. C. Lewis, D. Goel and E. O’Brien conducted experiments, made figures, and assisted in writing the manuscript. M. Peters-Golden contributed to planning experiments, analyzing data, and editing the manuscript. P. Mancuso contributed to planning experiments, analyzing data, making figures, and writing the manuscript. All authors read an approved the final manuscript.
All the authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bernstein A, Bilheimer LT, Makuc DM. National Center for Health, S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. Hyattsville, MD: 2011. [Google Scholar]
- 2.Bosso JA, Drew RH. Application of antimicrobial stewardship to optimise management of community acquired pneumonia. Int J Clin Pract. 2011;65(7):775–83. doi: 10.1111/j.1742-1241.2011.02704.x. [DOI] [PubMed] [Google Scholar]
- 3.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 (Suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine OS, O’Brien KL, Knoll M, et al. Pneumococcal vaccination in developing countries. Lancet. 2006;367(9526):1880–2. doi: 10.1016/S0140-6736(06)68703-5. [DOI] [PubMed] [Google Scholar]
- 5.Kadioglu A, Andrew PW. The innate immune response to pneumococcal lung infection: the untold story. Trends Immunol. 2004;25(3):143–9. doi: 10.1016/j.it.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Smith AM, McCullers JA, Adler FR. Mathematical model of a three-stage innate immune response to a pneumococcal lung infection. J Theor Biol. 2011;276(1):106–16. doi: 10.1016/j.jtbi.2011.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marriott HM, Dockrell DH. The role of the macrophage in lung disease mediated by bacteria. Exp Lung Res. 2007;33(10):493–505. doi: 10.1080/01902140701756562. [DOI] [PubMed] [Google Scholar]
- 8.Pittet LA, Quinton LJ, Yamamoto K, et al. Earliest Innate Immune Responses Require Macrophage RelA during Pneumococcal Pneumonia. Am J Respir Cell Mol Biol. 2011 doi: 10.1165/rcmb.2010-0210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serhan CN, Brain SD, Buckley CD, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21(2):325–32. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mancuso P, Lewis C, Serezani CH, Goel D, Peters-Golden M. Intrapulmonary administration of leukotriene B4 enhances pulmonary host defense against pneumococcal pneumonia. Infect Immun. 2010;78(5):2264–71. doi: 10.1128/IAI.01323-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serezani CH, Aronoff DM, Jancar S, Mancuso P, Peters-Golden M. Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood. 2005;106(3):1067–75. doi: 10.1182/blood-2004-08-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol. 2004;173(1):559–65. doi: 10.4049/jimmunol.173.1.559. [DOI] [PubMed] [Google Scholar]
- 13.Aronoff DM, Peres CM, Serezani CH, et al. Synthetic prostacyclin analogs differentially regulate macrophage function via distinct analog-receptor binding specificities. J Immunol. 2007;178(3):1628–34. doi: 10.4049/jimmunol.178.3.1628. [DOI] [PubMed] [Google Scholar]
- 14.Carlo T, Levy BD. Molecular circuits of resolution in airway inflammation. ScientificWorldJournal. 2010;10:1386–99. doi: 10.1100/tsw.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strieter RM, Remick DG, Lynch JP, 3rd, et al. Differential regulation of tumor necrosis factor-alpha in human alveolar macrophages and peripheral blood monocytes: a cellular and molecular analysis. Am J Respir Cell Mol Biol. 1989;1(1):57–63. doi: 10.1165/ajrcmb/1.1.57. [DOI] [PubMed] [Google Scholar]
- 16.Serezani CH, Chung J, Ballinger MN, Moore BB, Aronoff DM, Peters-Golden M. Prostaglandin E2 suppresses bacterial killing in alveolar macrophages by inhibiting NADPH oxidase. Am J Respir Cell Mol Biol. 2007;37(5):562–70. doi: 10.1165/rcmb.2007-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. Cyclic AMP: Master Regulator of Innate Immune Cell Function. Am J Respir Cell Mol Biol. 2008;39:127–32. doi: 10.1165/rcmb.2008-0091TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez PF, Pillinger MH, Attur M, et al. Resolution of inflammation: prostaglandin E2 dissociates nuclear trafficking of individual NF-kappaB subunits (p65, p50) in stimulated rheumatoid synovial fibroblasts. J Immunol. 2005;175(10):6924–30. doi: 10.4049/jimmunol.175.10.6924. [DOI] [PubMed] [Google Scholar]
- 19.Scher JU, Pillinger MH. The Anti-Inflammatory Effects of Prostaglandins. J Investig Med. 2009 doi: 10.2310/JIM.0b013e31819aaa76. [DOI] [PubMed] [Google Scholar]
- 20.St-Onge M, Dumas A, Michaud A, Laflamme C, Dussault AA, Pouliot M. Impact of anti-inflammatory agents on the gene expression profile of stimulated human neutrophils: unraveling endogenous resolution pathways. PLoS ONE. 2009;4(3):e4902. doi: 10.1371/journal.pone.0004902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadikot RT, Zeng H, Azim AC, et al. Bacterial clearance of Pseudomonas aeruginosa is enhanced by the inhibition of COX-2. Eur J Immunol. 2007;37(4):1001–9. doi: 10.1002/eji.200636636. [DOI] [PubMed] [Google Scholar]
- 22.Stables MJ, Newson J, Ayoub SS, Brown J, Hyams CJ, Gilroy DW. Priming innate immune responses to infection by cyclooxygenase inhibition kills antibiotic susceptible and resistant bacteria. Blood. 2010 doi: 10.1182/blood-2010-05-284844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aronoff DM, Lewis C, Serezani CH, et al. E-prostanoid 3 receptor deletion improves pulmonary host defense and protects mice from death in severe Streptococcus pneumoniae infection. J Immunol. 2009;183(4):2642–9. doi: 10.4049/jimmunol.0900129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailie MB, Standiford TJ, Laichalk LL, Coffey MJ, Strieter R, Peters-Golden M. Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumoniae in association with decreased alveolar macrophage phagocytic and bactericidal activities. J Immunol. 1996;157(12):5221–4. [PubMed] [Google Scholar]
- 25.Arredouani MS, Yang Z, Imrich A, Ning Y, Qin G, Kobzik L. The macrophage scavenger receptor SR-AI/II and lung defense against pneumococci and particles. Am J Respir Cell Mol Biol. 2006;35(4):474–8. doi: 10.1165/rcmb.2006-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peck R. A one-plate assay for macrophage bactericidal activity. J Immunol Methods. 1985;82(1):131–40. doi: 10.1016/0022-1759(85)90232-7. [DOI] [PubMed] [Google Scholar]
- 27.Mancuso P, Standiford TJ, Marshall T, Peters-Golden M. 5-Lipoxygenase reaction products modulate alveolar macrophage phagocytosis of Klebsiella pneumoniae. Infect Immun. 1998;66(11):5140–6. doi: 10.1128/iai.66.11.5140-5146.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu A, Aronoff DM, Phipps J, Goel D, Mancuso P. Leptin improves pulmonary bacterial clearance and survival in ob/ob mice during pneumococcal pneumonia. Clin Exp Immunol. 2007;150(2):332–9. doi: 10.1111/j.1365-2249.2007.03491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medeiros AI, Serezani CH, Lee SP, Peters-Golden M. Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. J Exp Med. 2009;206(1):61–8. doi: 10.1084/jem.20082058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Downey GP, Gumbay RS, Doherty DE, et al. Enhancement of pulmonary inflammation by PGE2: evidence for a vasodilator effect. J Appl Physiol. 1988;64(2):728–41. doi: 10.1152/jappl.1988.64.2.728. [DOI] [PubMed] [Google Scholar]
- 31.Sordelli DO, Cerquetti MC, el-Tawil G, Ramwell PW, Hooke AM, Bellanti JA. Ibuprofen modifies the inflammatory response of the murine lung to Pseudomonas aeruginosa. Eur J Respir Dis. 1985;67(2):118–27. [PubMed] [Google Scholar]
- 32.Armstrong RA. Investigation of the inhibitory effects of PGE2 and selective EP agonists on chemotaxis of human neutrophils. Br J Pharmacol. 1995;116(7):2903–8. doi: 10.1111/j.1476-5381.1995.tb15943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagasawa SY, Takuwa N, Sugimoto N, Mabuchi H, Takuwa Y. Inhibition of Rac activation as a mechanism for negative regulation of actin cytoskeletal reorganization and cell motility by cAMP. Biochem J. 2005;385(Pt 3):737–44. doi: 10.1042/BJ20041060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinomiya S, Naraba H, Ueno A, et al. Regulation of TNFalpha and interleukin-10 production by prostaglandins I(2) and E(2): studies with prostaglandin receptor-deficient mice and prostaglandin E-receptor subtype-selective synthetic agonists. Biochemical Pharmacology. 2001;61(9):1153–60. doi: 10.1016/s0006-2952(01)00586-x. [DOI] [PubMed] [Google Scholar]
- 35.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188(1):21–8. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ratcliffe MJ, Walding A, Shelton PA, Flaherty A, Dougall IG. Activation of E-prostanoid4 and E-prostanoid2 receptors inhibits TNF-alpha release from human alveolar macrophages. Eur Respir J. 2007;29(5):986–94. doi: 10.1183/09031936.00131606. [DOI] [PubMed] [Google Scholar]
- 37.Zaslona Z, Serezani CH, Okunishi K, Aronoff DM, Peters-Golden M. Prostaglandin E2 restrains macrophage maturation via E prostanoid receptor 2/protein kinase A signaling. Blood. 2012 doi: 10.1182/blood-2011-08-374207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dallaire F, Ouellet N, Bergeron Y, et al. Microbiological and inflammatory factors associated with the development of pneumococcal pneumonia. J Infect Dis. 2001;184(3):292–300. doi: 10.1086/322021. [DOI] [PubMed] [Google Scholar]
- 39.Lynch JP, 3rd, Zhanel GG. Streptococcus pneumoniae: epidemiology and risk factors, evolution of antimicrobial resistance, and impact of vaccines. Curr Opin Pulm Med. 2010;16(3):217–25. doi: 10.1097/MCP.0b013e3283385653. [DOI] [PubMed] [Google Scholar]
- 40.Chan MM, Moore AR. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by Prostaglandin E(2)-mediated lipoxin A(4) production. J Immunol. 2010;184(11):6418–26. doi: 10.4049/jimmunol.0903816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ballinger MN, Aronoff DM, McMillan TR, et al. Critical role of prostaglandin E2 overproduction in impaired pulmonary host response following bone marrow transplantation. Journal of Immunology. 2006;177(8):5499–508. doi: 10.4049/jimmunol.177.8.5499. [DOI] [PubMed] [Google Scholar]
- 42.Butler RR, Jr, Wise WC, Halushka PV, Cook JA. Gentamicin and indomethacin in the treatment of septic shock: effects on prostacyclin and thromboxane A2 production. J Pharmacol Exp Ther. 1983;225(1):94–101. [PubMed] [Google Scholar]
- 43.Goldmann O, Hertzen E, Hecht A, et al. Inducible Cyclooxygenase Released Prostaglandin E2 Modulates the Severity of Infection Caused by Streptococcus pyogenes. J Immunol. 2010 doi: 10.4049/jimmunol.1000838. [DOI] [PubMed] [Google Scholar]
- 44.Short BL, Gardiner M, Walker RI, Jones SR, Fletcher JR. Indomethacin improves survival in gram-negative sepsis. Adv Shock Res. 1981;6:27–36. [PubMed] [Google Scholar]
- 45.Short BL, Miller MK, Fletcher JR. Improved survival in the suckling rat model of group B streptococcal sepsis after treatment with nonsteroidal anti-inflammatory drugs. Pediatrics. 1982;70(3):343–7. [PubMed] [Google Scholar]
- 46.Sordelli DO, Cerquetti MC, Fontan PA, Meiss RP. Piroxicam treatment protects mice from lethal pulmonary challenge with Pseudomonas aeruginosa. J Infect Dis. 1989;159(2):232–8. doi: 10.1093/infdis/159.2.232. [DOI] [PubMed] [Google Scholar]
- 47.Wise WC, Halushka PV, Knapp RG, Cook JA. Ibuprofen, methylprednisolone, and gentamicin as conjoint therapy in septic shock. Circ Shock. 1985;17(1):59–71. [PubMed] [Google Scholar]
- 48.Bernard GR, Wheeler AP, Russell JA, et al. The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group. New England Journal of Medicine. 1997;336(13):912–8. doi: 10.1056/NEJM199703273361303. [DOI] [PubMed] [Google Scholar]
- 49.Haupt MT, Jastremski MS, Clemmer TP, Metz CA, Goris GB. Effect of ibuprofen in patients with severe sepsis: a randomized, double-blind, multicenter study. The Ibuprofen Study Group. Crit Care Med. 1991;19(11):1339–47. doi: 10.1097/00003246-199111000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Memis D, Karamanlioglu B, Turan A, Koyuncu O, Pamukcu Z. Effects of lornoxicam on the physiology of severe sepsis. Crit Care. 2004;8(6):R474–82. doi: 10.1186/cc2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vassiliou E, Jing H, Ganea D. Prostaglandin E2 inhibits TNF production in murine bone marrow-derived dendritic cells. Cell Immunol. 2003;223(2):120–32. doi: 10.1016/s0008-8749(03)00158-8. [DOI] [PubMed] [Google Scholar]
- 52.Shi J, Johansson J, Woodling NS, Wang Q, Montine TJ, Andreasson K. The prostaglandin E2 E-prostanoid 4 receptor exerts anti-inflammatory effects in brain innate immunity. J Immunol. 2010;184(12):7207–18. doi: 10.4049/jimmunol.0903487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu W, Dubinett S, Patterson SL, Kelly KA. COX-2 inhibition affects growth rate of Chlamydia muridarum within epithelial cells. Microbes Infect. 2006;8(2):478–86. doi: 10.1016/j.micinf.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 54.Woolard MD, Hensley LL, Kawula TH, Frelinger JA. Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of gamma interferon-positive T cells. Infect Immun. 2008;76(6):2651–9. doi: 10.1128/IAI.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mittal R, Gonzalez-Gomez I, Panigrahy A, Goth K, Bonnet R, Prasadarao NV. IL-10 administration reduces PGE-2 levels and promotes CR3-mediated clearance of Escherichia coli K1 by phagocytes in meningitis. J Exp Med. 2010;207(6):1307–19. doi: 10.1084/jem.20092265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toller IM, Hitzler I, Sayi A, Mueller A. Prostaglandin E2 prevents Helicobacter- induced gastric preneoplasia and facilitates persistent infection in a mouse model. Gastroenterology. 2010;138(4):1455–67. 67 e1–4. doi: 10.1053/j.gastro.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 57.Abdeltawab NF, Aziz RK, Kansal R, et al. An unbiased systems genetics approach to mapping genetic loci modulating susceptibility to severe streptococcal sepsis. PLoS Pathog. 2008;4(4):e1000042. doi: 10.1371/journal.ppat.1000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maloney CG, Thompson SD, Hill HR, Bohnsack JF, McIntyre TM, Zimmerman GA. Induction of cyclooxygenase-2 by human monocytes exposed to group B streptococci. J Leukoc Biol. 2000;67(5):615–21. doi: 10.1002/jlb.67.5.615. [DOI] [PubMed] [Google Scholar]
- 59.N’Guessan PD, Hippenstiel S, Etouem MO, et al. Streptococcus pneumoniae-induced p38 MAPK- and NF-{kappa}B-dependent COX-2 expression in human lung epithelium. Am J Physiol Lung Cell Mol Physiol. 2006 doi: 10.1152/ajplung.00383.2005. [DOI] [PubMed] [Google Scholar]
- 60.Bennett PR, Rose MP, Myatt L, Elder MG. Preterm labor: stimulation of arachidonic acid metabolism in human amnion cells by bacterial products. Am J Obstet Gynecol. 1987;156(3):649–55. doi: 10.1016/0002-9378(87)90070-6. [DOI] [PubMed] [Google Scholar]
- 61.Rayon JI, Carver JD, Wyble LE, et al. The fatty acid composition of maternal diet affects lung prostaglandin E2 levels and survival from group B streptococcal sepsis in neonatal rat pups. J Nutr. 1997;127(10):1989–92. doi: 10.1093/jn/127.10.1889. [DOI] [PubMed] [Google Scholar]
- 62.Goldmann O, von Kockritz-Blickwede M, Holtje C, Chhatwal GS, Geffers R, Medina E. Transcriptome analysis of murine macrophages in response to infection with Streptococcus pyogenes reveals an unusual activation program. Infect Immun. 2007;75(8):4148–57. doi: 10.1128/IAI.00181-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun K, Salmon SL, Lotz SA, Metzger DW. Interleukin-12 promotes gamma interferon-dependent neutrophil recruitment in the lung and improves protection against respiratory Streptococcus pneumoniae infection. Infect Immun. 2007;75(3):1196–202. doi: 10.1128/IAI.01403-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Si-Tahar M, Touqui L, Chignard M. Innate immunity and inflammation--two facets of the same anti-infectious reaction. Clin Exp Immunol. 2009;156(2):194–8. doi: 10.1111/j.1365-2249.2009.03893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamamoto N, Kawakami K, Kinjo Y, et al. Essential role for the p40 subunit of interleukin-12 in neutrophil-mediated early host defense against pulmonary infection with Streptococcus pneumoniae: involvement of interferon-gamma. Microbes Infect. 2004;6(14):1241–9. doi: 10.1016/j.micinf.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 66.Cho WS, Chae C. Expression of cyclooxygenase-2 in swine naturally infected with Actinobacillus pleuropneumoniae. Vet Pathol. 2003;40(1):25–31. doi: 10.1354/vp.40-1-25. [DOI] [PubMed] [Google Scholar]
- 67.Park GY, Hu N, Wang X, et al. Conditional regulation of cyclooxygenase-2 in tracheobronchial epithelial cells modulates pulmonary immunity. Clin Exp Immunol. 2007;150(2):245–54. doi: 10.1111/j.1365-2249.2007.03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jeong DG, Jeong ES, Seo JH, Heo SH, Choi YK. Difference in Resistance to Streptococcus pneumoniae Infection in Mice. Lab Anim Res. 2011;27(2):91–8. doi: 10.5625/lar.2011.27.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuroda E, Yamashita U. Mechanisms of enhanced macrophage-mediated prostaglandin E2 production and its suppressive role in Th1 activation in Th2-dominant BALB/c mice. Journal of Immunology (Baltimore, Md : 1950) 2003;170(2):757–64. doi: 10.4049/jimmunol.170.2.757. [DOI] [PubMed] [Google Scholar]