Abstract
Pink salmon, Oncorhynchus gorbuscha, are the most abundant wild salmon species and are thought of as an indicator of ecosystem health. The salmon louse, Lepeophtheirus salmonis, is endemic to pink salmon habitat but these ectoparasites have been implicated in reducing local pink salmon populations in the Broughton Archipelago, British Columbia. This allegation arose largely because juvenile pink salmon migrate past commercial open net salmon farms, which are known to incubate the salmon louse. Juvenile pink salmon are thought to be especially sensitive to this ectoparasite because they enter the sea at such a small size (approx. 0.2 g). Here, we describe how ‘no effect’ thresholds for salmon louse sublethal impacts on juvenile pink salmon were determined using physiological principles. These data were accepted by environmental managers and are being used to minimize the impact of salmon aquaculture on wild pink salmon populations.
Keywords: pink salmon, salmon louse, sea lice, conservation physiology
1. Introduction
Most commercial fish stocks are currently being fished near or beyond their maximal sustainable yield and many are in decline [1]. Consequently, as human populations continue to rise, the world's fish protein requirement is increasingly being met by aquaculture. Indeed, global consumption of aquaculture-produced seafood was predicted to exceed that from wild fisheries in 2010 [2]. Net-pen salmon farming is practised around the globe, generating close to 60 per cent of the world's salmon and $1.4 billion annually [3].
Concerns regarding the impacts of net-pen aquaculture on the environment are also global, particularly the potential effects on local wild fish stocks of pathogens and parasites that have been incubated inadvertently on cultured fish [4]. For example, aquaculture has been implicated in salmon louse infections of sympatric wild salmon runs in Norway [5], Scotland [6], Ireland [7] and Canada [8]. Indeed, some but not all [9] studies have found a negative correlation between the presence of net-pen aquaculture and local wild salmon population size. Correlational studies, however, lack a direct linkage between salmon louse production and wild salmon populations, while empirical data for the large number of variables that span the time between the generation of salmon lice on farmed fish and the collapse of a wild salmon population are largely lacking for modelling purposes [10]. Thus, until empirical data are generated, uncertainty will remain regarding the direct impact of aquaculture on wild fish populations.
Conservation physiology, as defined by Seebacher & Franklin [11], can help fill this important data gap. Here, we highlight one such case study in western Canada, the Broughton Archipelago (figure 1), an area of great recent controversy regarding salmon louse impacts on pink salmon (Oncorhynchus gorbuscha) populations [14–17]. Our study quantified the direct impact of the salmon louse on relevant and sensitive life-history stages of juvenile wild pink salmon, which were then used to promote informed policy decisions for the sustainable coexistence of farmed and wild fish.
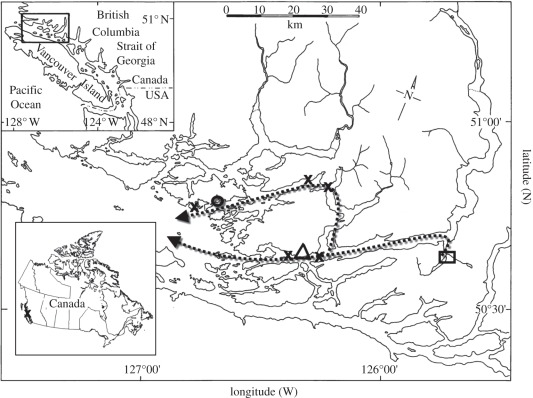
Figure 1.
The Broughton Archipelago, British Columbia, Canada, showing potential outward migration routes of juvenile pink salmon (dashed line) and the sites at which fish were collected in the studies of Sackville et al. [12] and Nendick et al. [13] which are referred to in the text. Dr. Islets field station (triangle), louse harvest sites (Wicklow Point fish farm, open circle), freshwater fish collection site (Glendale River, square) and seawater fish collection sites (crosses). (From Nendick et al. [13].)
We first provide background to the host (pink salmon), ectoparasite (salmon louse) and specific problems identified in the Broughton Archipelago. We then discuss recent findings from physiological studies that (i) provided much needed basic background information on the unique life history of juvenile pink salmon, and (ii) investigated the interaction between the salmon louse and pink salmon using a combination of laboratory and field studies. We specifically address the questions: do sea louse infections impair the fitness of juvenile pink salmon? And if so, what are the negative impacts and at what level of infection are impacts seen?
2. The host: pink salmon
The majority of Canada's wild salmon and salmon aquaculture occur in British Columbia (BC). Both wild and farmed salmon contribute substantially to the economy of Canada. The commercial wild Pacific salmon fishery is valued annually at $85–250 million (1990–1995, Anon 2002), and the recreational fishery adds another $1 billion (1995; National Survey, Anon 1995). Salmon farming contributes nearly $0.5 billion (in 2006). This juxtaposition of wild and cultured salmon presents unique, and often conflicting, challenges in striking a responsible, sustainable balance in resource management. Politically, salmon have been the focus of federal–provincial and federal–First Nations conflicts, and even international dispute (Canada versus the USA); salmon are cultural icons to the First Nations, deeply rooted in their mythology.
The abundance of wild runs of Pacific salmon, particularly in more remote regions where human impact is limited, is a testament to pristine, healthy ecosystems. As the environment is altered by human impact, habitat loss and climate change, some salmon populations will be more affected than others [18] and the status of Pacific salmon populations serve as a valuable bio-indicator of ecosystem health, resilience or collapse. Indeed, Pacific salmon are central to freshwater (FW) and marine food webs. The carcasses of returning spawners provide essential nutrients to FW and even terrestrial ecosystems [19–21]. Of the Pacific salmon, pink salmon are the most abundant and widely distributed [22], representing 66 per cent by number of Pacific salmon commercially caught by Canada, Japan, USA and Russia [22]. By virtue of their numbers, they may be the most important of the Pacific salmon as an indicator of ecosystem health. Thus, pink salmon are of utmost importance to Canada generally, and BC specifically.
Pink salmon have an unusual life history, which may make them especially sensitive to stressors early in life. Shortly after hatching, pink salmon fry emerge from their gravel nest and migrate immediately downstream to enter the ocean in February or March in the Broughton Archipelago. Thus, they are the smallest size of all salmon (0.2 g) to enter the ocean. They then spend the next three to six months foraging and migrating through the near-shore ocean environment where their small size and large numbers make them valuable prey for many marine predators. Yet, they feed gregariously and grow rapidly (3–7% body mass/day [22,23]), so that by summer's end, the juveniles enter the open ocean at sizes upwards of 3 g and begin mixing with offshore adult salmon populations. After 1 year at sea, adults return to natal rivers to spawn and die.
In contrast to pink salmon, most other salmon species spend months to years in FW and grow 10–100 times larger (2–30 g) before entering the ocean [24,25]. As a result, their body surface area to volume ratio is much lower than pink salmon. Also, most other salmon undergo a smolt stage—a physiological preparation for seawater (SW) entry while residing in FW [26]. The brief sojourn of pink salmon in FW may preclude a true smolt stage. Although smolts are pre-adapted for SW entry, the highest rate of mortality is still thought to occur within this life stage. Consequently, compared with other salmon, pink salmon may naturally enter SW with greatly reduced salinity tolerance and a reduced ability to handle the ion and water imbalance that occurs during transfer to SW. The damage to the skin caused by an ectoparasite such as the salmon louse [27] is also proportionately greater, which we reasoned would add further to the osmoregulatory challenge of SW entry. Since previous studies have suggested that salinity tolerance improves with growth to 2 g in pink salmon [28], it was imperative to better understand the baseline ionoregulatory physiology of juvenile pink salmon, while attempting to understand salmon louse impacts. Our working hypothesis was that SW entry in juvenile pink salmon is precocious relative to their hypoosmoregulatory ability and the window for salinity tolerance (and SW entry) has both genetically and physiologically timed components.
3. The parasite: salmon lice
The salmon louse, Lepeoptheirus salmonis, is an ectoparasitic copepod that is naturally associated with wild populations of adult Pacific salmon [29]. The L. salmonis life cycle consists of three planktonic stages (two uninfective nauplii and one infective copepodid) and eight parasitic stages (one copepodid, four chalimus (C1, 2, 3 and 4), two pre-adult and one adult) with no intermediate hosts [30]. The 0.7 mm copepodid stage attaches to the fish's skin and remains non-motile until it grows into a 3–4 mm motile pre-adult, which can then change host. The 1 cm adult females feed on fish mucus, epidermal tissue and blood [31] and mature females release their eggs into the water column to begin the cycle anew. The duration of this life cycle depends on environmental temperature. In BC waters, it takes three to four weeks for the egg to develop into an infective copepod, which has about a week to find a host otherwise it dies. A further four weeks of growth on a host is needed for a copepod to reach a reproductive adult [32]. The salmon louse can only complete its life history in SW with a salinity greater than 23 ppt, and the development of viable copepodids requires at least 30 ppt [32]. Therefore, juvenile pink salmon become hosts only after they enter SW.
Salmon louse infections produce epidermal lesions, ionoregulatory disruptions and secondary infections in salmon [33]. Ionoregulatory disruptions have been observed following artificial infection in larger salmonids: brown trout smolts (Salmo trutta, 40 g [34], 90 g [35]), Atlantic salmon post-smolts (Salmo salar, 40–60 g [36–38]) and Atlantic salmon adults (S. salar, 600 g [39]). Thus, the working hypothesis for pink salmon that were 100- to 1000-times smaller was that the physiological mechanism(s) through which juvenile pink salmon are affected by the salmon louse has its origin as an ionoregulatory disturbance. Furthermore, the extent of this ionoregulatory disturbance would be in proportion to the parasite load on the fish, i.e. the greater the number of parasites, the greater the damage to the epidermal barrier and the greater the ionoregulatory disturbance.
4. The problem in the Broughton Archipelago: a case study
The Canadian aquaculture industry, which is dominated by the production of salmon and trout, currently accounts for about 7 per cent of the global salmonid production [40]. Over half of Canada's farmed salmon production occurs in BC, and much of this is located in the approximately 20 active net-pen farms in the Broughton Archipelago. Salmon aquaculture in this region grew steadily over a 15-year period, reaching its current annual tonnage in 2000. In 1995, the provincial government placed a moratorium on new tenures for salmon aquaculture. From the perspective of many non-governmental organizations and the general public, the largest issue threatening the Broughton Archipelago ecosystem health is the impact of aquaculture on survival of wild pink salmon populations. At the centre of this debate is whether salmon lice, for which fish farms are potentially a point source [9], are killing wild juvenile pink salmon in sufficient numbers to threaten pink salmon populations. This concern is largely fuelled by the observation that following a record return of adult pink salmon to the Broughton Archipelago in 2000 and 2001, adult returns were reduced by 97 and 88 per cent in 2002 and 2003, respectively [9]. Moreover, more than 90 per cent of the out-migrating juveniles in 2001 (which correspond to the 2002 adult return) were infected with one or more salmon lice [41].
Despite the lack of information on the direct effect of salmon lice on juvenile pink salmon, a model was developed that predicted imminent collapse of wild pink populations in the region owing to louse-induced mortality [16]. This model and output were challenged [14,17] and the challenge rebutted [15], but the populations did not collapse suggesting something had changed and/or certain assumptions were invalid. More recently, Marty et al. [9] analysed 10–20 years of fish farm data and 60 years of pink salmon data and concluded that wild salmon productivity is not associated with fish farm production or farm louse numbers. Further analysis of the same dataset but using a different model yielded the opposite conclusion [42]. Clearly, implying cause-and-effect from indirect associations is always problematic especially when inherently limited by a vast number of assumptions required to model such a complex system. While salmon louse infection remains a possibility for low pink salmon returns, it is only one factor that may be affecting pink salmon stocks. Reducing the number of assumptions is critical to moving forward in understanding this complex system.
Our understanding of the biology of juvenile pink salmon and how they interact with the salmon louse is incomplete. The direct physiological effect of salmon louse infection and the specific mechanism(s) of impact on juvenile pink salmon were previously unknown. Among the critical questions that lacked clear answers were: Do sea louse infections impair the fitness of juvenile pink salmon? And if so, what are the negative impacts and at what level of infection are impacts seen? These questions can only be properly addressed with carefully controlled infection experiments in which impacts can be unequivocally linked to the infection and then on to fitness. This requirement creates several challenges. While laboratory infection experiments afford the necessary control to study cause–effect relationships, the laboratory environment could easily modify louse–salmon interactions. On the other hand, while field experiments create natural exposure conditions, they relax the influence of extrinsic variables, making cause–effect relationships more difficult to establish. An additional problem is linking sublethal effects in juveniles with fitness per se. This problem dictates careful selection of variables that assess louse impacts and still relate to fitness. Compounding matters further is the absence of baseline information on juvenile pink salmon from which to make assessments of resilience to the salmon louse. Extrapolation from other salmonid species is particularly unwise given the relatively small size of juvenile pink salmon at time of SW entry. The following are some of the physiological experiments that we have recently conducted to address these issues, and that have resulted in recommendations to management.
5. The baseline physiology of pink salmon: is bigger better for seawater entry?
Surprisingly little is known about the basic physiology of the early life stages of juvenile pink salmon [43], information which is critical in interpreting the impact of salmon louse infection. Laboratory studies have shown that juvenile pink salmon are less affected by the salmon louse once they reach 0.7 g and have developed scales [44]. This introduces the possibility that delayed SW entry may confer reduced sensitivity to louse parasitism if fish are larger when they first encounter salmon lice. However, bigger is not necessarily better for SW entry in other salmonids, because the window of salinity tolerance is very much dictated by the process of smoltification. Sullivan et al. [43] observed increased plasma thyroxine and gill Na+,K+-ATPase (NKA) activity (the driving force for ion excretion in SW fish) when pink salmon emerged from gravel, and hypothesized that this indicated a form of smoltification. Thus, if pink salmon go through smoltification, delayed SW entry to increase body size would not be a useful management strategy to mitigate salmon louse effects.
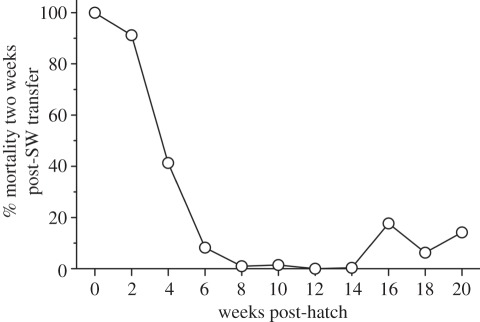
To investigate this, we assessed salinity tolerance of pink salmon throughout development from immediately post-hatch and well beyond the time that they would normally enter SW. In juvenile pink salmon transferred directly from FW to SW at the time of their out-migration, a 200–300% increase in whole body ion levels was observed that gradually returned to pre-transfer values within 8–10 weeks [23]. During this period, the development of hypoosmoregulatory ability continued for nearly eight weeks, as indicated by an increase in gill NKA activity [23]. This was the first direct evidence that pink salmon normally enter SW in a precocious ionoregulatory state. Furthermore, large whole body ion disturbance associated with SW entry indicates that juvenile pink salmon may be especially sensitive to additional ionoregulatory stressors, especially those that may be induced by the salmon louse. To better clarify salinity tolerance in relation to development, pink salmon were transferred from FW directly to SW every two weeks while measuring subsequent fish survival and gill NKA activity and mRNA isoform expression. Several salmonid species upregulate the α1b gill NKA isoform (referred to as a SW isoform) and reduce the α1a gill NKA isoform (referred to as a FW isoform [45]) as well as increase overall gill NKA activity when they are transferred from FW to SW, and when smoltification occurs in FW prior to SW entry [46–49]. As indicated in figure 2, pink salmon appear to have a window of salinity tolerance where mortality drops to near zero following SW transfer [50]. This window correlates with complete yolk absorption, which occurs at the normal time of emergence and out-migration of this population of pink salmon. Delayed SW transfer was associated with an increase in mortality defining an end to the window of salinity tolerance [50]. Furthermore, when fish were held in FW, this window corresponded to an increase in the ratio of the SW to the FW isoforms of gill NKA, which is indicative of an innate smoltification process. The concept of precocious SW entry was reinforced, however, by the observation that SW transfer always increased the ratio of the SW to the FW isoforms of gill NKA beyond that for fish of the same stage held in FW. Taken together, these data indicate that pink salmon do go through a process of limited smoltification, one that differs from smolts of other salmonids [50]. Pink salmon enter SW precociously in that a large increase in whole body ions is observed following SW entry, which is gradually corrected probably as a result of increased gill NKA expression which increasingly favours the SW isoform over the FW isoform. The management implication of these results is fairly obvious. Deliberately delaying SW entry of pink salmon to potentially increase body size and minimize salmon louse impacts is unlikely to be useful as a management tool because mortality would increase independent of salmon louse infection once juvenile pink salmon pass their smoltification window.
Figure 2.
Percent mortality in juvenile pink salmon within two weeks following transfer to SW from FW. Groups of developing pink salmon were transferred to SW from FW every two weeks starting at hatch (0 weeks). Fish were held at 5°C throughout and completed yolk absorption at 12 weeks post-hatch. Pink salmon of the same cohort naturally emerged from gravel to enter SW at a developmental state similar to 12 weeks post-hatch. (From Gallagher [50].)
6. The impact of salmon lice on ionoregulation: how many is too many?
Ionoregulation is a vital homeostatic process of all teleost fishes. Failure to maintain hydromineral balance has devastating effects at the cellular, tissue and organismal levels. For example, ionic disruptions sustained through premature (pre-smoltification) SW entry can cause death within days [51], as well as impair growth [52] and swimming ability [53] in survivors. We hypothesized that salmon louse infection impaired ionic homeostasis of juvenile pink salmon. This hypothesis was founded on the known physical disruption to fish skin of attached lice and previous reports of increased plasma osmolarity in other but larger louse-infected salmonids (see above). Furthermore, we reasoned that the precocious SW entry and small body size of out-migrating pink fry would make them particularly sensitive to the ionic challenge posed by louse infection.
To test these ideas, ionoregulatory status was measured in juvenile pink salmon that had been infected artificially under controlled, laboratory conditions, and those that had been captured with natural infections in the wild [12]. For the laboratory study, the Glendale River was selected as a fish source because it is highly representative of the Broughton pink salmon population, accounting for more than 35 and 85 per cent of total regional pink salmon in even and odd years, respectively [14]. The use of out-migrating, river-caught fish also ensured that fish had no prior exposure to salmon lice, had naturally entered their smolt window, and represented the earliest and presumably most-sensitive life stage that could become infected in the wild. Glendale River fish were captured during their natural out-migration and transferred to a field site in the Broughton Archipelago (Dr Islets field station; figure 1) where they were held in SW prior to controlled copepodid infections [12]. These fish were reared for up to five weeks, during which both the salmon and salmon lice developed together, the latter through to pre-adult stage. Fish infected naturally in the wild were larger and were collected at various points along their SW migration route within the Broughton Archipelago. Naturally infected, wild fish were used to validate the laboratory findings. Uninfected wild fish were collected simultaneously to serve as controls.
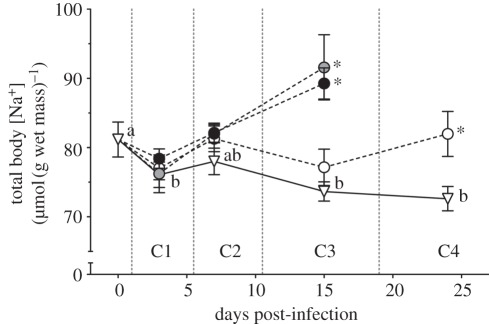
For the controlled infection study with L. salmonis copepodids, ionoregulatory status of approximately 0.2 g fish was monitored for 24 days as the lice grew and developed through four chalimus stages [12]. Ecologically relevant infection loads of one to three lice/fish were compared. Mortality of fish initially infected with one to three lice was only 2.4 per cent during the nearly one-month infection/observation period, during which fish mass doubled to 0.4 g. A significant ionic burden was triggered by a single salmon louse at day 24 (chalimus 4; a 12% increase in total body Na+; figure 3) and by two to three lice at day 15 (chalimus 3; a 23% increase in total body Na+; figure 3). Therefore, as predicted, a salmon louse created an ionic burden for tiny pink salmon once the louse approached the pre-adult stage. Furthermore, this burden increased in intensity and occurred earlier with additional lice. Following this, ionic impact through to the adult stage of the louse was precluded by the ability of pink salmon to shed salmon lice (discussed further below).
Figure 3.
Total body [Na+] relative to fish wet weight of Glendale River-caught fish artificially infected with L. salmonis copepodids (triangles, control; white circles, one louse; grey circles, two lice; black circles, three lice). Fish were infected one week following transfer to seawater, and sampled over 24 days as both fish and lice developed together. Fish were sampled at times corresponding to each of the four chalimus developmental stages of lice (C1–C4). Louse moulting events are marked by broken vertical lines. Data points represent mean ± s.e.m., n = 10. Asterisks indicate statistically significant differences from time-matched controls; different letters indicate statistically significant differences within the control group (p ≤ 0.05). (From Sackville et al. [12].)
Naturally infected fish were collected from SW over a larger and overlapping size range (approx. 0.3–2.0 g) but never in sufficient numbers with more than one salmon louse or beyond chalimus life stages (1–4). Total body Na+ for fish infected with one louse did not differ from uninfected controls. Therefore, it was then possible to combine the controlled and natural infection datasets, while recognizing the dependence of total body Na+ on body mass for uninfected fish. This analysis revealed a threshold fish mass of 0.5 g beyond which one chalimus 4 louse no longer significantly elevated total body Na+. This 0.5 g size threshold for maintaining ionic homeostasis while carrying a load of one salmon louse corresponds with the body size when pink salmon increase their hypo-osmoregulatory capacity [23], and closely approximates the 0.7 g size threshold when louse resistance increases owing to epidermal and immune system development [44]. These findings show for the first time that louse infection does impair ionic homeostasis in small juvenile pink salmon, and suggest that fish are particularly sensitive to louse infection as homeostatic systems (ionic, physical barriers and immune defence) develop from precocial states at SW entry. However, the degree to which the observed sublethal ionic disturbance affects fish fitness is unclear.
7. An impact of salmon lice on fitness: can pink salmon swim normally?
Exercise is vital for fish survival, whether to escape predators, capture prey, compete for resources or migrate. Maximum exercise forces the respiratory, cardiovascular and metabolic systems to be efficiently integrated such that physiological disturbances owing to environmental change, disease and toxicants are revealed in premature fatigue and ionic disturbances [54]. Conversely, healthy salmonids can maintain homeostasis when swum to a maximum aerobic swimming velocity and can even repeat the same level of performance with only a short (less than an hour) recovery period [55–58]. In contrast, unhealthy fish and poorly prepared smolts have ionoregulatory disturbances at fatigue and repeat swimming performance is reduced by up to 33 per cent [55,56]. Therefore, exercise capacity is used as a tool to assess physical condition that is thought to have ecological relevance [59] and therefore may be a useful indicator of ecological fitness [13].
It is not surprising that at some point salmon louse will impair swimming performance; however, whether this impairment is seen at ecologically relevant levels of infection is of primary interest. Again, the life histories of both the salmon louse and pink salmon are complex and thus the possibility for types of infection vary widely, from juveniles being infected with adult salmon lice resulting in immediate effects, to those where pinks and salmon lice interact early in development and then grow together over a long duration.
Wild juvenile pink salmon (possibly about 2 g based on a reported fork length of 5–6 cm and the mass:length relationship reported in Grant et al. [23]) that were captured with salmon louse infections with a mean of 1.3 motile male lice per fish had a similar swimming endurance relative to control uninfected fish [60]. As discussed above, a single louse in naturally infected juvenile pink fry did not exhibit ionoregulatory disturbances [12] and therefore might not be expected to reduce swimming performance. However, when similar-sized fish were artificially infected with up to four mature female salmon lice, swimming endurance was significantly reduced 36–48 h following infection [60], but not with a simple dependence on louse load; four lice per fish produce a large decrease in endurance, but one to three lice per fish differed very little if at all in their effect on endurance.
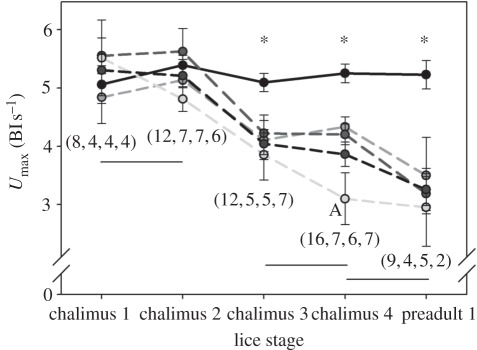
To examine a broader range of fish sizes, including the size threshold at which ionic disturbances are observed in resting fish (i.e. less than 0.5 g [12]), and a range of earlier salmon louse developmental stages, swimming performance was measured in the companion fish of Sackville et al. [12] (see above) that had been artificially infected under a controlled, laboratory setting and those that had been captured with natural infections in the wild. A repeat-maximal swimming performance (Umax) protocol that had been specifically developed for juvenile pink salmon [57] was used to assess the effect of different salmon louse loads and developmental stages on swimming performance. Umax was significantly reduced by a single sea louse once it reached chalimus 3 stage (where both louse and salmon developed together) provided the pink salmon was less than 0.34 g. Impaired Umax in infected fish was associated with elevated whole body ion levels, indicating an ionoregulatory disturbance [13]. However, the impairment of Umax was not related to louse load as predicted, because there was no statistically significant difference in Umax among salmon louse loads of 1, 2, 3 or more lice/fish. Even so, the magnitude of the reduction in Umax increased from 20 per cent for chalimus 3 to almost 40 per cent for pre-adults (figure 4), suggesting that physiological rather than physical (drag) effects predominated in the impairment of swimming speed. The clear sublethal effect on swimming performance up to a body mass of 0.34 g correlates well with the mass threshold for physiological disturbances noted above for resting fish [46]. Furthermore, the mortality of infected fish during this swimming study was again low (approx. 1% [54]). Given that 10 per cent of wild juvenile pink salmon were infected with salmon lice at an intensity of about one for the past several years [61], the management implication of this work is that up to 10 per cent of the juvenile pink salmon in the Broughton Archipelago may exhibit sublethal effects of salmon louse exposure. However, what this ultimately means in terms of fish survival remains unknown [13].
Figure 4.
Maximum swimming performance (Umax) of Glendale River-caught fish artificially infected with L. salmonis copepodids. Control fish (black solid line) and fish with salmon louse infection intensity of one (mid-grey, dashed), two (dark grey), three (light grey) and all infection intensities pooled (black dashed line). An asterisk (*) denotes significant differences between control and infected (pooled or individual) values, while discontinuous horizontal lines denote a difference among the louse development stages (independent of louse intensity) and an uppercase letter indicates the single difference among louse intensity for a given louse development stage. Numbers within parentheses are the number of fish used (reading left to right) for control, 1 louse, 2 and 3 lice per fish, respectively. Values are mean ± standard error of the mean (s.e.m.). (From Nendick et al. [13]).
8. A complicating factor: shedding of salmon lice
Shedding of salmon lice by juvenile salmon is well documented [44,62–66] and is influenced by both biotic and abiotic factors, including parasite mortality, immune response by the host, water temperature and water salinity [32,44,63]. Thus, all controlled infection studies of Pacific and Atlantic salmon performed to date and designed to investigate the effect of fixed levels of infection have been confounded by the intensity of salmon louse infection decreasing with time. In our studies, which were conducted on the same population of fish [12,13], we can calculate shedding rates. At 14 days post-infection (DPI), the abundance of salmon lice (no. of lice/no. of fish) decreased to 0.19 (n = 90), 0.46 (n = 72), 0.67 (n = 49) and 1.0 (n = 170) for fish that originally all had 1, 2 and 3 and 4+ lice/fish, respectively. Therefore, a large group of artificially infected pink salmon were able to shed 75–80% of the initial number of attached lice after 14 days, while some individuals shed four or more salmon lice. Thus, reporting the physiological effects of salmon louse infection relative to the infection intensity at the time of sampling, which we do [12,13], is conservative in that many of those fish may have been infected with a higher intensity at some point.
The level of shedding we observed is not unusual compared with previous studies where pink salmon were artificially infected (abundance was reduced within 5 to 12 DPI; [44]) or naturally infected pink salmon were held [66,67]. A high rate of louse shedding is also of importance in mathematical models, where the incorporation of realistic shedding rates [67] reduces the predicted mortality of pink salmon owing to salmon louse infection by 95% relative to an earlier model where shedding was not considered [10]. Collectively, these findings of high rates of shedding of attached lice suggest that the majority of the salmon lice that successfully infect a fish will be shed before they reach motile and reproductive stages. Clearly, the interactions between salmon lice, juvenile pink salmon and their environment are extremely complex and change temporally as both salmon louse and fish develop and the implications of this need to be considered in relation to conservation efforts.
9. Recommendations: a conservative no-effect body mass threshold
Salmon lice are endemic to the Broughton Archipelago, and are brought back to the system by returning adult salmon. Whether out-migrating juvenile pink salmon have been historically exposed to the salmon louse at such a high level of prevalence and intensity as was observed in 2001 is unclear. The necessary detailed records simply do not exist, but given the decline to the current levels of salmon louse infection (10% of juvenile pink salmon are infected with one louse [61]), we are left with three possibilities: an unusual epizooitic, a high infection driven by incubation on salmon in net-pens, or a combination.
Although it is possible that the salmon louse may be a food source for this stage of pink salmon [9,68], negative effects on performance and ultimately fitness will certainly occur at some level of infection intensity and pink salmon size. The physiological studies conducted to date on artificially and naturally infected juvenile pink salmon indicate that a single chalimus results in an ionoregulatory disturbance and impairment in exercise capacity (and its implied fitness consequence) well before any large incidence of mortality is observed. When pink salmon are less than 0.4 g, a significant ionoregulatory disturbance developed when a single chalimus reached stage 4, about 24 days after a copepodid attached to the skin. When two to three copepodids attach, a significant effect occurs after 15 days when the chalimus 3 stage is reached [12]. For the same cohort of pink salmon, infection with a single copepodid reduced Umax after 15 days when the chalimus 3 stage is reached [13]. However, none of these sublethal effects is evident once pink salmon reach about 0.5 g, which would typically take about one to two months of normal growth. The ‘no effect’ threshold for sublethal disturbance defined by Sackville et al. [12] of 0.5 g with one chalimus 4 is consistent with the developmental stage at which pink salmon develop scales and exhibit a heightened immunocompetence [44].
These results have been rapidly adopted by managers, perhaps because they have a mechanistic basis and the results for the different physiological endpoints show some consistency. A co-ordinated area management plan (CAMP) has evolved out of the interaction between government (Provincial and Federal) and academia, spearheaded through the BC Pacific Salmon Forum [69]. The CAMP has recommended that no more than 3 per cent of out-migrating wild juvenile pink salmon less than 0.5 g can have one or more pre-adult lice between March 1 and May 31, which corresponds with the timing of their natural out-migration through the Broughton Archipelago. Industry has voluntarily complied with this recommendation and fallowed farms accordingly. In a recent study to investigate the benefit of fallowing fish farms in the Broughton Archipelago, it was determined that fallowing reduces salmon louse levels around the farm and reduces juvenile pink infection levels to background levels [70], indicating that this recommendation and voluntary compliance may mitigate salmon louse effects on the most sensitive stages of pink salmon.
Perhaps the only study that has truly considered the impact of salmon louse infection on juvenile salmon is the 10-year study of the return of Atlantic salmon smolts that had been treated with SLICE (emamectin benzoate) to protect them from salmon louse infection for the first 90 days of their outward migration, a period which easily extended beyond the contact with net-pen aquaculture and associated salmon lice. Remarkably, a comparison with non-treated fish revealed that protection of juveniles from salmon louse infection represented a minor component to overall marine survival. Indeed, during the 10-year study, adult Atlantic salmon returns fell a similar 10-fold in both treated and non-treated fish [71]. A similar study on juvenile pink salmon treated with SLICE in the Broughton Archipelago could be very revealing in assessing the true impact of sea lice on pink salmon fitness.
Acknowledgements
We thank two anonymous reviewers for their comments which improved this manuscript. This work was supported by a Natural Sciences and Engineering Research Council (NSERC) Strategic Grant to C.J.B. and A.P.F. M.S. was supported by an NSERC CGSD and L.N. by an NSERC PGSM.
References
- 1.Pauly D., Watson R., Alder J. 2005. Global trends in world fisheries: impacts on marine ecosystems and food security. Phil. Trans. R. Soc. B 360, 5–12 10.1098/rstb.2004.1574 (doi:10.1098/rstb.2004.1574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naylor R. L., et al. 2009. Feeding aquaculture in an era of finite resources. Proc. Natl Acad. Sci. USA 106, 15 103–15 110 10.1073/pnas.0905235106 (doi:10.1073/pnas.0905235106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FAO Fisheries and Aquaculture Information and Statistics Service 2011. FAO yearbook. Fishery and Aquaculture Statistics 2009. Rome, Italy: Food and Agriculture Organization
- 4.Costello M. J. 2009. How sea lice from salmon farms may cause wild salmonid declines in Europe and North America and be a threat to fishes elsewhere. Proc. R. Soc. B 276, 3385–3394 10.1098/rspb.2009.0771 (doi:10.1098/rspb.2009.0771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjorn P. A., Finstad B. 2002. Salmon lice, Lepeophtheirus salmonis (Krøyer), infestation in sympatric populations of Arctic char, Salvelinus alpinus (L.), and sea trout, Salmo trutta (L.), in areas near and distant from salmon farms. ICES J. Mar. Sci. 59, 131–139 10.1006/jmsc.2001.1143 (doi:10.1006/jmsc.2001.1143) [DOI] [Google Scholar]
- 6.MacKenzie K., Longshaw M., Begg G. S., McVicar A. H. 1998. Sea lice (Copepoda: Caligidae) on wild sea trout (Salmo trutta L.) in Scotland. ICES J. Mar. Sci. 55, 151–162 10.1006/jmsc.1997.0362 (doi:10.1006/jmsc.1997.0362) [DOI] [Google Scholar]
- 7.Tully O., Gargan P., Poole W. R., Whelan K. F. 1999. Spatial and temporal variation in the infestation of sea trout (Salmo trutta L.) by the caligid copepod Lepeophtheirus salmonis (Krøyer) in relation to sources of infection in Ireland. Parasitology 119, 41–51 10.1017/S003118209900445X (doi:10.1017/S003118209900445X) [DOI] [PubMed] [Google Scholar]
- 8.Morton A., Routledge R., Peet C., Ladwig A. 2004. Sea lice (Lepeophtheirus salmonis) infection rates on juvenile pink (Oncorhynchus gorbuscha) and chum (Oncorhynchus keta) salmon in the nearshore marine environment of British Columbia, Canada. Can. J. Fish. Aqua. Sci. 61, 147–157 10.1139/f04-016 (doi:10.1139/f04-016) [DOI] [Google Scholar]
- 9.Marty G. D., Saksida S. M., Quinn T. J. 2010. Relationship of farm salmon, sea lice, and wild salmon populations. Proc. Natl Acad. Sci. USA 107, 22 599–22 604 10.1073/pnas.1009573108 (doi:10.1073/pnas.1009573108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krkosek M., Lewis M. A., Morton A., Frazer N. L., Volpe J. P. 2006. Epizootics of wild fish induced by farm fish. Proc. Natl Acad. Sci. USA 103, 15 506–15 510 10.1073/pnas.0603525103 (doi:10.1073/pnas.0603525103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seebacher F., Franklin C. E. 2012. Determining environmental causes of biological effects: the need for a mechanistic physiological dimension in conservation biology. Phil. Trans. R. Soc. B 367, 1607–1614 10.1098/rstb.2011.0036 (doi:10.1098/rstb.2011.0036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sackville M., Tang S., Nendick L., Farrell A. P., Brauner C. J. 2011. Pink salmon (Oncorhynchus gorbuscha) osmoregulatory development plays a key role in sea louse (Lepeophtheirus salmonis) tolerance. Can. J. Fish. Aqua. Sci. 68, 1087–1096 10.1139/f2011-037 (doi:10.1139/f2011-037) [DOI] [Google Scholar]
- 13.Nendick L., Sackville M., Tang S., Brauner C. J., Farrell A. P. 2011. Sea lice infection of juvenile pink salmon (Oncorhynchus gorbuscha): effects on swimming performance and postexercise ion balance. Can. J. Fish. Aqua. Sci. 68, 241–249 10.1139/F10-150 (doi:10.1139/F10-150) [DOI] [Google Scholar]
- 14.Brooks K. M., Jones S. R. M. 2008. Perspectives on pink salmon and sea lice: scientific evidence fails to support the extinction hypothesis. Rev. Fish. Sci. 16, 403–412 10.1080/10641260801937131 (doi:10.1080/10641260801937131) [DOI] [Google Scholar]
- 15.Krkosek M., Ford J. S., Morton A., Lele S., Lewis M. A. 2008. Response to comment on ‘declining wild salmon populations in relation to parasites from farm salmon’. Science 322, 1790. 10.1126/science.1156578 (doi:10.1126/science.1156578)19095926 [DOI] [Google Scholar]
- 16.Krkosek M., Ford J. S., Morton A., Lele S., Myers R. A., Lewis M. A. 2007. Declining wild salmon populations in relation to parasites from farm salmon. Science 318, 1772–1775 10.1126/science.1148744 (doi:10.1126/science.1148744) [DOI] [PubMed] [Google Scholar]
- 17.Riddell B. E., Beamish R. J., Richards L. J., Candy J. R. 2008. Comment on ‘Declining wild salmon populations in relation to parasites from farm salmon’. Science 322, 1790. 10.1126/science.1156341 (doi:10.1126/science.1156341) [DOI] [PubMed] [Google Scholar]
- 18.Cooke S. J., et al. 2012. Conservation physiology in practice: how physiological knowledge has improved our ability to sustainably manage Pacific salmon during up-river migration. Phil. Trans. R. Soc. B 367, 1757–1769 10.1098/rstb.2012.0022 (doi:10.1098/rstb.2012.0022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Field R. D., Reynolds J. D. 2011. Sea to sky: impacts of residual salmon-derived nutrients on estuarine breeding bird communities. Proc. R. Soc. B 278, 3081–3088 10.1098/rspb.2010.2731 (doi:10.1098/rspb.2010.2731) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helfield J. M., Naiman R. J. 2001. Effects of salmonid-derived nitrogen on riparian forest growth and implications for stream productivity. Ecology 82, 2403. 10.1890/0012-9658(2001)082[2403:EOSDNO]2.0.CO;2 (doi:10.1890/0012-9658(2001)082[2403:EOSDNO]2.0.CO;2) [DOI] [Google Scholar]
- 21.Gende S. M., Quinn T. P., Wilson M. F., Heintz R., Scott T. M. 2004. Magnitude and fate of salmon-derived nutrients and energy in a coastal stream ecosystem. J. Freshw. Ecol. 19, 149–160 10.1080/02705060.2004.9664522 (doi:10.1080/02705060.2004.9664522) [DOI] [Google Scholar]
- 22.Heard W. R. 1991. Life history of pink salmon (Oncorhynchus gorbuscha). In Pacific Salmon life histories (eds Groot C., Margolis L.), pp. 119–230 Vancouver, Canada: University of British Columbia Press [Google Scholar]
- 23.Grant A., Gardner M., Nendick L., Sackville M., Farrell A. P., Brauner C. J. 2009. Growth and ionoregulatory ontogeny of wild and hatchery-raised juvenile pink salmon (Oncorhynchus gorbuscha). Can. J. Zool. 87, 221–228 10.1139/Z08-149 (doi:10.1139/Z08-149) [DOI] [Google Scholar]
- 24.Hoar W. S. 1976. Smolt transformation: evolution, behavior, and physiology. J. Fish. Res. Board Can. 33, 1234–1252 [Google Scholar]
- 25.Rounsefell G. A. 1958. Anadromy in North American salmonidae. U.S. Fish Wildl. Fish. Bull. 58, 171–185 [Google Scholar]
- 26.McCormick S. D., Saunders R. L. 1987. Preparatory physiological adaptations for marine life of salmonids: osmoregulation, growth and metabolism. T. Am. Fish. Soc. 1, 211–229 [Google Scholar]
- 27.Brandal P. O., Egidius E., Romslo I. 1976. Host blood: a major food component for the parasitic copepod Lepeophtheirus salmonis (Kroyer, 1837) (Crustacea: Caligidae). Norw. J. Zool. 24, 341–343 [Google Scholar]
- 28.Clarke W. C., Hirano T. 1995. Osmoregulation. In Physiological ecology of Pacific salmon (eds Groot C., Margolis L., Clarke W. C.), pp. 317–378 Vancouver, Canada: University of British Columbia Press [Google Scholar]
- 29.Beamish R. J., Neville C. M., Sweeting R. M., Ambers N. 2005. Sea lice on adult Pacific salmon in coastal waters of Central British Columbia, Canada. Fish. Res. 76, 198–208 10.1016/j.fishres.2005.06.007 (doi:10.1016/j.fishres.2005.06.007) [DOI] [Google Scholar]
- 30.Johnson S. C., Albright L. J. 1991. The developmental stages of Lepeophtheirus salmonis (Kroyer, 1837) (Copepoda: Caligidae). Can. J. Zool. 69, 929–950 10.1139/z91-138 (doi:10.1139/z91-138) [DOI] [Google Scholar]
- 31.Johnson S. C., Blaylock R. B., Elphick J., Hyatt K. D. 1996. Disease induced by the sea louse (Lepeophtheirus salmonis) (Copepoda: Caligidae) in wild sockeye salmon (Oncorhynchus nerka) stocks of Alberni Inlet, British Columbia. Can. J. Fish. Aqua. Sci. 53, 2888–2897 10.1139/f96-226 (doi:10.1139/f96-226) [DOI] [Google Scholar]
- 32.Johnson S. C., Albright L. J. 1991. Development, growth, and survival of Lepeophtheirus salmonis (Copepoda: Caligidae) under laboratory conditions. J. Mar. Biol. Assoc. UK 71, 425–436 10.1017/S0025315400051687 (doi:10.1017/S0025315400051687) [DOI] [Google Scholar]
- 33.Wagner G. N., Fast M. D., Johnson S. C. 2008. Physiology and immunology of Lepeophtheirus salmonis infections of salmonids. Trends Parasitol. 24, 176–183 10.1016/j.pt.2007.12.010 (doi:10.1016/j.pt.2007.12.010) [DOI] [PubMed] [Google Scholar]
- 34.Wells A., et al. 2006. Physiological effects of simultaneous, abrupt seawater entry and sea lice (Lepeophtheirus salmonis) infestation of wild, sea-run brown trout (Salmo trutta) smolts. Can. J. Fish. Aqua. Sci. 63, 2809–2821 10.1139/f06-160 (doi:10.1139/f06-160) [DOI] [Google Scholar]
- 35.Bjørn P. A., Finstad B. 1997. The physiological effects of salmon lice infection on sea trout post smolts. Nord. J. Freshw. Res. 73, 60–72 [Google Scholar]
- 36.Finstad B., Bjørn P. A., Grimnes A., Hvidsten N. A. 2000. Laboratory and field investigations of salmon lice [Lepeophtheirus salmonis (Krøyer)] infestation on Atlantic salmon (Salmo salar L.) post-smolts. Aquac. Res. 31, 795–803 10.1046/j.1365-2109.2000.00511.x (doi:10.1046/j.1365-2109.2000.00511.x) [DOI] [Google Scholar]
- 37.Grimnes A., Jakobsen P. J. 1996. The physiological effects of salmon lice on post-smolt Atlantic salmon. J. Fish Biol. 48, 1179–1194 10.1111/j.1095-8649.1996.tb01813.x (doi:10.1111/j.1095-8649.1996.tb01813.x) [DOI] [Google Scholar]
- 38.Nolan D. T., Reilly P., Wendelaar Bonga S. E. 1999. Infection with low numbers of the sea louse Lepeophtheirus salmonis induces stress-related effects in post-smolt Atlantic salmon (Salmo salar). Can. J. Fish. Aqua. Sci. 56, 947–959 [Google Scholar]
- 39.Wagner G. N., McKinley R. S., Bjørn P. A., Finstad B. 2003. Physiological impact of sea lice on swimming performance of Atlantic salmon. J. Fish Biol. 62, 1000–1009 10.1046/j.1095-8649.2003.00091.x (doi:10.1046/j.1095-8649.2003.00091.x) [DOI] [Google Scholar]
- 40.Tacon A. G. J. 2005. Salmon aquaculture dialogue: status of information on salmon aquaculture feed and the environment. Int. Aquafeed. 8, 22–37 [Google Scholar]
- 41.Morton A. B., Williams R. 2003. Infestation of the sea louse Lepeophtheirus salmonis (Krøyer) on juvenile pink salmon Oncorhynchus gorbuscha (Walbaum) in British Columbia. Can. Field Nat. 117, 634–641 [Google Scholar]
- 42.Krkosek M., Connors B. M., Morton A., Lewis M. A., Dill L. M., Hilborn R. 2011. Effects of parasites from salmon farms on productivity of wild salmon. Proc. Natl Acad. Sci. USA 108, 14 700–14 704 10.1073/pnas.1101845108 (doi:10.1073/pnas.1101845108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sullivan C. V., Brewer S. D., Johnston G. P., Dickhoff W. W. 1983. Plasma thyroid hormone concentrations and gill Na+,K+-ATPase activities in post-emergent pink salmon. T. Am. Fish. Soc. 112, 825–829 10.1577/1548-8659(1983)112%3C825:PTCAGN%3E2.0.CO;2 (doi:10.1577/1548-8659(1983)112<825:PTCAGN>2.0.CO;2) [DOI] [Google Scholar]
- 44.Jones S. R. M., Kim E., Bennett W. 2008. Early development of resistance to the salmon louse, Lepeophtheirus salmonis (Kroyer), in juvenile pink salmon, Oncorhynchus gorbuscha (Walbaum). J. Fish Dis. 31, 591–600 10.1111/j.1365-2761.2008.00933.x (doi:10.1111/j.1365-2761.2008.00933.x) [DOI] [PubMed] [Google Scholar]
- 45.Richards J. G., Semple J. W., Bystriansky J. S., Schulte P. M. 2003. Na+/K+-ATPase α-isoform switching in gills of rainbow trout (Oncorhynchus mykiss) during salinity transfer. J. Exp. Biol. 206, 4475–4486 10.1242/jeb.00701 (doi:10.1242/jeb.00701) [DOI] [PubMed] [Google Scholar]
- 46.Stefansson S. O., Nilsen T. O., Ebbesson L. O. E., Wargelius A., Madsen S. S., Björnsson B. T., McCormick S. D. 2007. Molecular mechanisms of continuous light inhibition of Atlantic salmon parr-smolt transformation. Aquaculture 273, 235–245 10.1016/j.aquaculture.2007.10.005 (doi:10.1016/j.aquaculture.2007.10.005) [DOI] [Google Scholar]
- 47.Bystriansky J. S., Richards J. G., Schulte P. M., Ballantyne J. S. 2006. Reciprocal expression of gill Na+/K+-ATPase α-subunit isoforms α1a and α1b during seawater acclimation of three salmonid fishes that vary in their salinity tolerance. J. Exp. Biol. 209, 1848–1858 10.1242/jeb.02188 (doi:10.1242/jeb.02188) [DOI] [PubMed] [Google Scholar]
- 48.Nilsen T. O., Ebbesson L. O. E., Madsen S. S., McCormick S. D., Andersson E., Bjornsson B. T., Prunet P., Stefansson S. O. 2007. Differential expression of gill Na+,K+-ATPase α- and β-subunits, Na+,K+,2Cl- cotransporter and CFTR anion channel in juvenile anadromous and landlocked Atlantic salmon Salmo salar. J. Exp. Biol. 210, 2885–2896 10.1242/jeb.002873 (doi:10.1242/jeb.002873) [DOI] [PubMed] [Google Scholar]
- 49.McCormick S. D. 2009. Evolution of the hormonal control of animal performance: insights from the seaward migration of salmon. Integr. Comp. Biol. 49, 408–422 10.1093/icb/icp044 (doi:10.1093/icb/icp044) [DOI] [PubMed] [Google Scholar]
- 50.Gallagher Z. S. 2011. The development of salinity tolerance in juvenile pink salmon (Oncorhynchus gorbuscha). MSc Thesis, University of British Columbia, Vancouver, Canada [Google Scholar]
- 51.Boeuf G., Harache Y. 1982. Criteria for adaptation of salmonids to high salinity seawater in France. Aquaculture 28, 163–176 10.1016/0044-8486(82)90019-9 (doi:10.1016/0044-8486(82)90019-9) [DOI] [Google Scholar]
- 52.Folmar L. C., Dickhoff W. W., Mahnken C. V. W., Waknitz F. W. 1982. Stunting and parr-reversion during smoltification of coho salmon (Oncorhynchus kisutch). Aquaculture 28, 91–104 10.1016/0044-8486(82)90012-6 (doi:10.1016/0044-8486(82)90012-6) [DOI] [Google Scholar]
- 53.Brauner C. J., Shrimpton J. M., Randall D. J. 1992. Effect of short-duration seawater exposure on plasma ion concentrations and swimming performance in coho salmon (Oncorhynchus kisutch) parr. Can. J. Fish. Aqua. Sci. 49, 2399–2405 10.1139/f92-265 (doi:10.1139/f92-265) [DOI] [Google Scholar]
- 54.Randall D. J., Brauner C. J. 1991. Effects of environmental factors on exercise in fish. J. Exp. Biol. 160, 113–126 [Google Scholar]
- 55.Brauner C. J., Iwama G. K., Randall D. J. 1994. The effect of short-duration seawater exposure on the swimming performance of wild and hatchery-reared juvenile coho salmon (Oncorhynchus kisutch) during smoltification. Can. J. Fish. Aqua. Sci. 51, 2188–2194 10.1139/f94-220 (doi:10.1139/f94-220) [DOI] [Google Scholar]
- 56.Jain K. E., Birtwell I. K., Farrell A. P. 1998. Repeat swimming performance of mature sockeye salmon following a brief recovery period: a proposed measure of fish health and water quality. Can. J. Zool. 76, 1488–1496 10.1139/z98-079 (doi:10.1139/z98-079) [DOI] [Google Scholar]
- 57.Nendick L., Grant A., Gardner M., Sackville M., Brauner C. J., Farrell A. P. 2009. Swimming performance and associated ionic disturbance of juvenile pink salmon Oncorhynchus gorbuscha determined using different acceleration profiles. J. Fish Biol. 75, 1626–1638 10.1111/j.1095-8649.2009.02388.x (doi:10.1111/j.1095-8649.2009.02388.x) [DOI] [PubMed] [Google Scholar]
- 58.Randall D. J., Mense D., Boutilier R. G. 1987. The effects of burst swimming on aerobic swimming in chinook salmon (Oncorhynchus tshawytscha). Mar. Behav. Physiol. 13, 77–88 10.1080/10236248709378664 (doi:10.1080/10236248709378664) [DOI] [Google Scholar]
- 59.Plaut I. 2001. Critical swimming speed: its ecological relevance. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 131, 41–50 10.1016/S1095-6433(01)00462-7 (doi:10.1016/S1095-6433(01)00462-7) [DOI] [PubMed] [Google Scholar]
- 60.Mages P. A., Dill L. M. 2010. The effect of sea lice (Lepeophtheirus salmonis) on juvenile pink salmon (Oncorhynchus gorbuscha) swimming endurance. Can. J. Fish. Aqua. Sci. 67, 2045–2051 10.1139/F10-121 (doi:10.1139/F10-121) [DOI] [Google Scholar]
- 61.Jones S. R. M., Hargreaves N. B. 2009. Infection threshold to estimate Lepeophtheirus salmonis-associated mortality among juvenile pink salmon. Dis. Aquat. Org. 84, 131–137 10.3354/dao02043 (doi:10.3354/dao02043) [DOI] [PubMed] [Google Scholar]
- 62.Fast M. D., Ross N. W., Mustafa A., Sims D. E., Johnson S. C., Conboy G. A., Speare D. J., Johnson G., Burka J. F. 2002. Susceptibility of rainbow trout (Oncorhynchus mykiss), Atlantic salmon (Salmo salar) and coho salmon (Oncorhynchus kisutch) to experimental infection with sea lice (Lepeophtheirus salmonis). Dis. Aquat. Org. 52, 57–68 10.3354/dao052057 (doi:10.3354/dao052057) [DOI] [PubMed] [Google Scholar]
- 63.Johnson S. C., Albright L. J. 1992. Effects of cortisol implants on the susceptibility and the histopathology of the responses of naive coho salmon (Oncorhynchus kisutch) to experimental infection with Lepeophtheirus salmonis (Copepoda: Caligidae). Dis. Aquat. Org. 14, 195–205 10.3354/dao014195 (doi:10.3354/dao014195) [DOI] [Google Scholar]
- 64.Jones S. R. M., Fast M. D., Johnson S. C. 2008. Influence of reduced feed ration on Lepeophtheirus salmonis infestation and inflammatory gene expression in juvenile pink salmon (Oncorhynchus gorbuscha). J. Aquat. Anim. Health. 20, 103–109 10.1577/H07-014.1 (doi:10.1577/H07-014.1) [DOI] [PubMed] [Google Scholar]
- 65.Jones S. R. M., Hargreaves N. B. 2007. The abundance and distribution of Lepeoptheirus salmonis (Copepoda: Caligidae) on pink (Oncorhynchus gorbuscha) and chum (O. keta) salmon in coastal British Columbia. J. Parasitol. 93, 1324–1331 10.1645/GE-1252.1 (doi:10.1645/GE-1252.1) [DOI] [PubMed] [Google Scholar]
- 66.Morton A., Routledge R. 2005. Mortality rates for juvenile pink (Oncorhynchus gorbuscha) and chum (O. keta) salmon infested with sea lice (Lepeophtheirus salmonis) in the Broughton Archipelago. Alaska Fish. Res. Bull. 11, 146–152 [Google Scholar]
- 67.Krkosek M., Morton A., Volpe J. P., Lewis M. A. 2009. Sea lice and salmon population dynamics: effects of exposure time for migratory fish. Proc. R. Soc. B 276, 2819–2828 10.1098/rspb.2009.0317 (doi:10.1098/rspb.2009.0317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang S., Lewis A. G., Sackville M., Nendick L., DiBacco C., Brauner C. J., Farrell A. P. 2011. Diel vertical distribution of early marine phase juvenile pink salmon (Oncorhynchus gorbuscha) and behaviour when exposed to salmon louse (Lepeophtheirus salmonis). Can. J. Zool. 89, 796–807 10.1139/z11-049 (doi:10.1139/z11-049) [DOI] [Google Scholar]
- 69.Pacific Salmon Form 2009. BC Pacific Salmon Forum Final Report & Recommendations to the Government of British Columbia. British Columbia Provincial Government Technical Report. British Columbia Pacific Salmon Forum
- 70.Morton A., Routledge R., McConnell A., Krkosek M. 2011. Sea lice dispersion and salmon survival in relation to salmon farm activity in the Broughton Archipelago. ICES J. Mar. Sci. 68, 144–156 10.1093/icesjms/fsq146 (doi:10.1093/icesjms/fsq146) [DOI] [Google Scholar]
- 71.Jackson D., et al. 2011. An evaluation of the impact of early infestation with the salmon louse Lepeophtheirus salmonis on the subsequent survival of outwardly migrating Atlantic salmon, Salmo salar L., smolts. Aquaculture 320, 159–163 10.1016/j.aquaculture.2011.03.029 (doi:10.1016/j.aquaculture.2011.03.029) [DOI] [Google Scholar]