Abstract
A recently developed integrative framework proposes that the vulnerability of a species to environmental change depends on the species' exposure and sensitivity to environmental change, its resilience to perturbations and its potential to adapt to change. These vulnerability criteria require behavioural, physiological and genetic data. With this information in hand, biologists can predict organisms most at risk from environmental change. Biologists and managers can then target organisms and habitats most at risk. Unfortunately, the required data (e.g. optimal physiological temperatures) are rarely available. Here, we evaluate the reliability of potential proxies (e.g. critical temperatures) that are often available for some groups. Several proxies for ectotherms are promising, but analogous ones for endotherms are lacking. We also develop a simple graphical model of how behavioural thermoregulation, acclimation and adaptation may interact to influence vulnerability over time. After considering this model together with the proxies available for physiological sensitivity to climate change, we conclude that ectotherms sharing vulnerability traits seem concentrated in lowland tropical forests. Their vulnerability may be exacerbated by negative biotic interactions. Whether tropical forest (or other) species can adapt to warming environments is unclear, as genetic and selective data are scant. Nevertheless, the prospects for tropical forest ectotherms appear grim.
Keywords: climate change, ectotherms, endotherms, temperature, thermoregulation, tropics
1. Introduction
Climate warming presents major challenges to organisms [1,2]. Consequently, biologists are endeavouring to develop robust ways to evaluate the differential vulnerability of organisms to climate change [3–10] and then to evaluate and implement management strategies tailored for species judged most at risk [11].
Attempts to evaluate vulnerability and to develop management strategies should be based on relevant biological foundations. Williams et al. [5] developed an integrative framework for assessing traits that promote vulnerability. They proposed that the vulnerability of a species depends on its sensitivity to environmental change, its exposure to that change, its resilience or ability to recover from perturbations and its potential to adapt to change. That framework is appealing, but implementing it will be challenging because each of these vulnerability traits requires extensive biological information.
Here, we address implementation of that model. We begin by reviewing how and why the above factors influence organismal vulnerability [5]. Then we outline the biological data necessary to evaluate each factor. However, because such data are rarely available, we suggest and evaluate ‘quick-and-dirty’ proxies. We focus on terrestrial organisms, especially ‘dry-skinned’ ectotherms such as lizards or insects, for which the critical data (natural history, physiology, behaviour) are relatively rich. We do not discuss aquatic organisms, for which temperature impacts are complicated by interactions with O2, CO2 and salinity [12–14], intertidal organisms, which live in rapidly fluctuating environments and sometimes have few options for behavioural thermoregulation [14–17], and ‘wet-skinned’ organisms such as amphibians, where temperature impacts are complicated by sensitivity to moisture [18] and to fungal infections [19].
Many aspects of climate are changing in diverse environments, but we focus on the direct effects of increased air temperature (Ta) on the thermal biology of terrestrial animals, especially ectotherms. Changes in environmental temperature are the most direct and predictable outcome of increased greenhouse gas emissions [2]. Such changes can alter body temperatures (Tb) of ectotherms, and thus their physiological performance and vulnerability [8,10,20–24], or heat loads on endotherms [25–28], and thus their energy and water balances.
An organism's vulnerability also depends on factors other than temperature (e.g. disease, food, rainfall, cloud cover, CO2 and O2) as well as on additional stressors (e.g. habitat destruction and fragmentation, fire, pollutants, invasive species) that can interact with climate warming [7,29–39]. Furthermore, no organism is an ecological island; and so its vulnerability will also depend on how climate change alters its interactions with competitors, predators, parasites, diseases and mutualists [9,17,40–45]. Despite these complexities, attempts to understand an organism's vulnerability to climate warming must build from a robust understanding of its sensitivity and response to temperature.
2. Physiological sensitivity
(a). Physiological traits dictating sensitivity
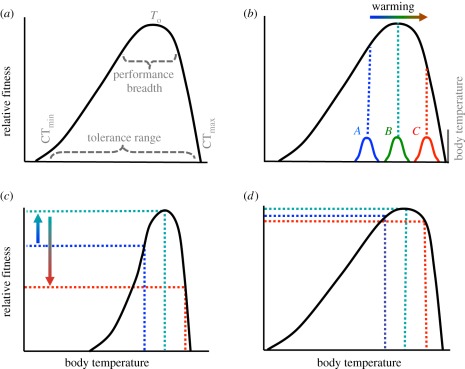
A thermal ‘performance’ or ‘fitness’ curve serves as a convenient descriptor of how a change in body temperature (Tb) influences physiological sensitivity and fitness of ectotherms [22,46,47]. Very low and high Tb reduce an ectotherm's performance and can be lethal in the extreme: these endpoint Tb are called the ‘critical temperatures’ (figure 1a; CTmax, CTmin). Within those critical limits, performance reaches a maximum at an optimal temperature region (To), and then typically plummets at higher Tb [46,49,50]. Thermal performance curves can, however, shift somewhat depending on the trait, acclimation and time of temperature exposure [48,51–53].
Figure 1.
(a) Thermal fitness (performance) curve for a hypothetical ectotherm, with key descriptive parameters CTmin, CTmax, tolerance range, performance breadth and optimal temperature (To) identified (adapted from Huey [48]). (b) With climate warming, realized Tb distributions can shift higher. If warming results in Tb that are closer to To of a species (for example Tb shift from A to B), then warming should enhance fitness; but if warming raises Tb above To (e.g. if Tb shifts from B to C), fitness will be reduced (see text). (c,d) Step increases in Tb distributions from warming can have much bigger effects on (c) thermal specialists than on (d) thermal generalists.
An organism's integrated performance or fitness over some time interval depends on its performance curve, weighted by the Tb it experiences during that interval [22,50,54–56]. Thus, the physiological impact of warming depends primarily on an organism's field Tb (at the commencement of warming) relative to its To. If pre-warming Tb is less than To (‘A’ in figure 1b), then warming-induced increases in Tb will enhance fitness. If pre-warming Tb are similar to To (‘B’ in figure 1b), then modest warming will have little impact (at least initially); but if Tb rises significantly above To (‘C’ in figure 1b), then warming should cause catastrophic effects, because fitness drops rapidly at Tb greater than To.
Sensitivity to climate warming also depends on whether a species is a thermal generalist or specialist [6,47,49,54,57,58]. A given increase in Tb from warming will usually have a larger impact on a thermal specialist (figure 1c) than on a thermal generalist (figure 1d).
In contrast to ectotherms, most endotherms (birds and mammals) are homeothermic and use behavioural, morphological and, especially, physiological adjustments to maintain a high and relatively constant body temperature (approx. 35–40°C being the ‘set-point’) under diverse environmental conditions [59,60]. Endotherms with body temperatures only a few degrees below or above the set-point temperature range can be physiologically stressed [28]. Most endotherms can be thus considered extreme thermal specialists (with respect to Tb).
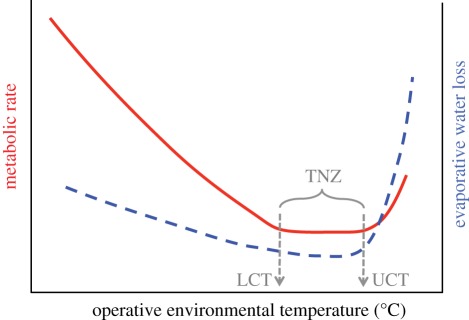
When exposed to changing environmental temperatures, endotherms typically defend body temperature (figure 2). For example, if environmental temperature falls below an endotherm's ‘thermoneutral zone’ (TNZ), a ‘comfort zone’ within which endotherms can maintain a low metabolic rate, endotherms increase metabolic heat to offset increased heat loss. But if environmental temperatures rise above the TNZ, endotherms usually defend their set-point temperature primarily by increasing rates of evaporative cooling (panting, sweating). Temperatures bounding the TNZ are called the ‘lower critical temperature’ (LCT) and ‘upper critical temperature’ (UCT). The width and position of the TNZ partly reflect measurement conditions, but mainly reflect the size, shape and pelage of the endotherm, and its ability to control heat loss through postural adjustments, fur or feather erection and altered blood flow [26,61].
Figure 2.
Effects of environmental temperature on rates of metabolic heat production (red) and of evaporative water loss (blue) of endotherms. At low environmental temperatures, energy expenditures and thus heat production are elevated to balance heat loss. At high ambient temperatures, rates of evaporative water loss are elevated to dump excess heat. Indicated are the thermal neutral zone (TNZ), and the lower (LCT) and upper (UCT) critical temperatures, beyond which metabolic rates increase.
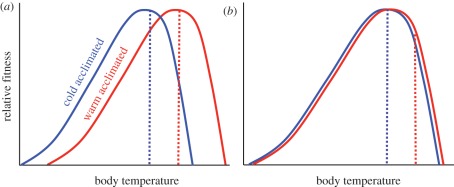
Finally, thermal sensitivities of both ectotherms and endotherms can shift somewhat depending on its recent or anticipated environmental experience [62]. (Such phenotypic plasticity of physiology is often called ‘acclimatization’ (for natural shifts) or ‘acclimation’ (for shifts in laboratory studies).) In ectotherms, for example, recent exposure to elevated temperatures sometimes enhances thermal performance at higher temperatures ([63], but see [64,65]). Such ‘beneficial’ acclimation [64] can help buffer physiological impacts of climate change (figure 3a) [22,57,62,66,67] relative to the case of limited acclimation (figure 3b). Endotherms can also acclimatize, sometimes by changing insulation or posture [26]. They can also shift their ‘set-point’ body temperatures: torpor and hibernation are extreme cases.
Figure 3.
Acclimatization to low (blue dotted line) versus high (red dotted line) Tb (simulating an acute climate shift) sometimes induces a phenotypic shift in an ectotherm's thermal fitness curve. (a) Depicts an ectotherm with marked acclimatization capacities. Its elevated To (red) provides some physiological buffering against climate warming (‘Beneficial Acclimation’). (b) Shows an ectotherm with a relatively limited response. If its Tb is elevated by climate warming (red dotted line), its performance will decline.
(b). Which organisms are physiologically sensitive to climate warming?
The above considerations suggest indicator traits that may predict species most vulnerable to climate warming: specifically, vulnerable species are likely to be thermal specialists, to be active at Tb that are near (or even above) To [6,9,68,69] and to have limited capacity to acclimatize to changing Tb. Where geographically do such organisms typically occur? In a seminal paper, Janzen [57] predicted that thermal specialists and species with limited acclimation capacity should occur in the lowland tropics because temperature variation (daily or seasonal) is relatively limited there.
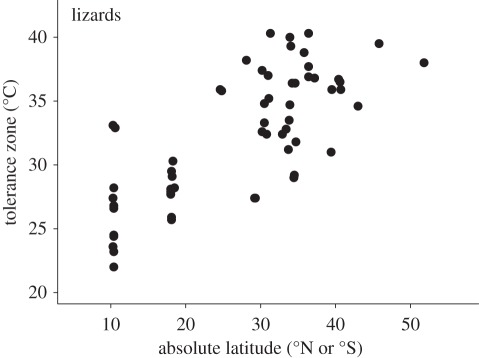
Janzen's prediction was largely based on intuition, but has been supported subsequently by empirical studies on diverse ectotherms [6,37,70–75]. Indeed, tolerance ranges (figure 1a) increase with latitude in many taxa including frogs [76], insects [77,78] and lizards (figure 4) [37,68,79]. Interestingly, the increased tolerance range at high latitude consistently results from a much greater shift in CTmin than in CTmax [37,68,74,76,77,79], probably because minimum yearly (ambient) temperatures drop rapidly with latitude, whereas maximum yearly temperatures are relatively independent of latitude (except at extreme latitudes [37,72,77,80]).
Figure 4.
Tolerance zones (CTmax–CTmin; see figure 1a) of lizards increase with absolute latitude. The increase is due primarily to a shift in CTmin (rather than CTmax) with latitude (see text). Note: see Huey et al. [68] for a phylogenetic analyses.
Although the tolerance range (CTmax–CTmin) is relatively narrow for most tropical ectotherms (figure 4), thermal performance breadth (figure 1a) for sprinting is surprisingly independent of latitude in lizards [79], at least at high performance levels (e.g. 85% of maximum). Whether this holds for other ectotherm performance traits needs to be determined.
Comparative levels of thermal specialization in endotherms are not as well described as those of ectotherms. The width of the TNZ (figure 2) is a potential index of endotherm thermal specialization; but we see two concerns here. First, laboratory measurements of TNZ are sensitive to the specific experimental conditions, which will differ from the field [26]. Second, TNZ itself is rarely measured. Even so, the width of the TNZ should be (roughly) inversely proportional to the LCT (figure 2) simply because the UCT (figure 2) appears much less variable than the LCT [81]. Hence, we focus our discussion on LCT.
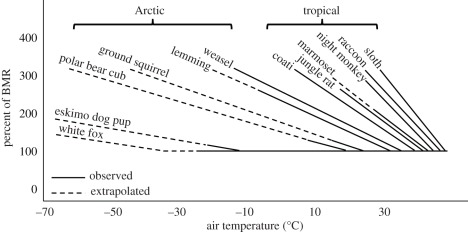
In a pioneering paper, Scholander et al. [61] showed that the LCT of tropical mammals was much higher than that of non-tropical species (figure 5), primarily because they had less dense and less deep pelage. Thus TNZ is directly related to latitude, such that tropical species have a relatively narrow TNZ. Nevertheless, the impact of climate warming on endotherms in warm environments should be more sensitive to the UCT than to the LCT. However, because interspecific (or phenotypic) variation in UCT appears minor [81], endotherms may have limited capacity to shift UCT by physiological or morphological adjustments. Torpor may be an endotherm's primary way of dealing with stressfully warm environments [83], even in the tropics [84].
Figure 5.
Metabolic rate as a percentage of basal metabolic rate (BMR) versus air temperature for Arctic and tropical mammals. Arctic mammals have relatively low LCTs, and thus relatively broad TNZs, assuming that UCTs are independent of latitude. Adapted from Porter & Kearney [26], which was based on the study of Scholander et al. [61,82].
Janzen [57] also predicted that tropical organisms would have limited acclimatization capacities (figure 3b). Evidence for ectotherms is limited but is generally consistent with this expectation (reviewed in [72,75], but see [85]). One counterexample involves a tropical crocodile, in which sustained swimming speed showed perfect compensation for acclimation temperature [63]. In contrast, diving metabolic rate did not compensate adequately in another tropical crocodile [86]. Also, a recent study shows rapid cross-generation acclimation of aerobic scope to temperature by a tropical reef fish [87].
Comparative (latitudinal) evidence for endotherm acclimation capacities is even more scanty. One relevant index could be the magnitude of seasonal or environmental shifts in the position or width of TNZ [88,89], especially shifts of UCT. However, we know of no comprehensive review on this topic. Clearly, a comprehensive analysis and review of acclimatization responses of tropical versus temperate zone species (ectotherms and endotherms) to warming temperatures is needed.
(c). Quantifying physiological traits and proxies
Ideally, one should measure the full thermal sensitivity (figure 1a) of fitness or of key functional traits (see fig. 6 in [47]) [48,90,91] of the ectotherm of interest. Moreover, the particular performance traits selected should be tailored to the ecology of the species under study [46] but generally might include sprint speed, prey capture ability, development time, reproductive rate, growth rate or net energy gain [46,56,91,92]. Performance of such traits can be linked to survival and fitness [93–96], but establishing links among thermal performance curves, environmental variation and fitness is challenging [97]. For some species with short generation times, one can measure the thermal sensitivity of Darwinian fitness (e.g. intrinsic rate of population growth or net reproductive rate [51,98–102]), but the relevance of different fitness measures is demography-dependent [51].
Importantly, performance curves depend to some extent on experimental methodology [53]. This is apparent in estimates of CTmax, which can vary with heating rate [103–105] or with the specific index of CTmax [106,107]. Thus, caution is appropriate when compiling data from independent studies [37,68,108,109], though the biological signal is often large enough to swamp these issues, at least in some taxa [68,110].
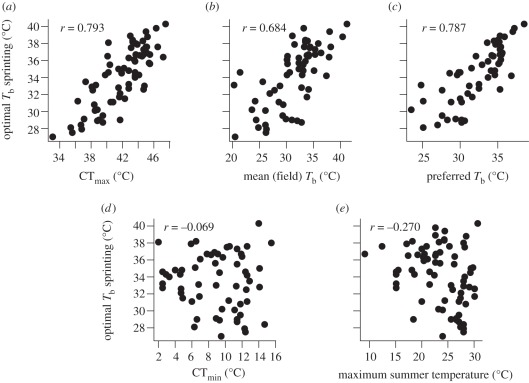
Because measuring a full thermal performance curve of an ectotherm is often impractical, a search among available proxies (e.g. critical temperatures, figure 1a) might provide climate workers with robust clues as to the position and shape of a species' performance curve. The thermal dependence of sprint speed has been quantified for many lizards and thus offers an opportunity to evaluate the ability of several potential proxies to predict To for sprint speed [68]. We find that CTmax, mean Tb of lizards active in the field, and preferred body temperature in laboratory thermal gradients all predict To, at least roughly (figure 6a–c). However, CTmin and mean maximum daily temperature in summer do not (figure 6d,e). Nevertheless, considerable scatter is evident, even for the significant proxies (figure 6a–c); and this scatter may reflect phylogenetic influences [111], methodological differences [103] or experimental error. Therefore, these indices should be used with caution [112]. Furthermore, even significant relationships are not scaled 1 : 1 (figure 6a–c), and so regression approaches will be required to predict To from proxies.
Figure 6.
Potential proxies for optimal temperatures (To) with lizard data as exemplars: (a) CTmax, (b) mean Tb (field), (c) preferred Tb in laboratory thermal gradients, (d) CTmin and (e) mean maximum daily temperature for the three warmest months. CTmax, Tb and Tp predict To (a–c), but CTmin and maximum summer temperatures do not (d,e). Data source: Huey et al. [68].
The thermal dependence of metabolic rate also provides opportunities to quantify thermal endpoints. Metabolic rate increases exponentially with temperature, but eventually plateaus and then drops as an animal approaches a critical or mortal temperature [113]. Lighton & Turner [114] showed with ants that the temperature at which metabolic rate starts to drop correlates closely with the temperature at which locomotion ceased. Similarly, the upper temperature at which metabolic rate starts to drop has been used to index the onset of temperature stress in salamanders, and Bernardo & Spotila [115] argue that the magnitude of that drop is also a physiologically meaningful index of vulnerability to warming.
For endotherms, potential proxies are currently less clear. Endotherms have high physiological capacities to buffer environmental variations [60], but are not immune to extreme heat waves [38,116,117], which may increase in frequency, intensity and duration as climate warms [118]. For endotherms facing such conditions, perhaps the UCT (figure 2)—or perhaps ambient temperature at the onset of panting—might be useful proxies of risk. However, we know of no recent comparative study that quantifies these temperatures as a function of latitude or climate.
Body size will undoubtedly affect endotherm vulnerability, but its roles are complex [26]. For example, large endotherms will have thermal inertia and greater reserves (e.g. fuel and water), and thus should have longer survival times during extreme heat waves [117]; but small endotherms may have more thermoregulatory opportunities (e.g. access to cool burrows or small patches of shade).
Diurnality is an obvious risk factor for endotherms, as day-active species may be potentially exposed to high heat loads as well as to water stress [117]. Because these stresses may force them to restrict activity times [119,120], their energy budgets may be constrained. As noted above, some endotherms can escape the heat by becoming torpid during warm spells [83,84], but only if they have access to cool retreats (e.g. deep burrows).
Overall, an evaluation of vulnerability proxies for endotherms requires further investigation. Perhaps the first step is developing a full mechanistic understanding of factors that increase endotherm vulnerability to warming [26,27].
(d). Estimating relevant environmental temperatures
Predictions of the physiological impacts of climate warming on physiology require reliable estimates of how the distribution of environmental temperatures will shift with warming. However, climate models [2] typically predict only mean annual air temperatures. Unfortunately, mean annual temperatures have limited physiological relevance because they eliminate within-site temperature variation (large in temperate zones) that drives physiological activity and performance [37,121–123]. Such metrics also completely obscure chronic or acute thermal events, which sometimes dominate selection [9,37,52,124,125].
Air temperatures have limited physiological relevance. ‘Operative body temperature’ (Te) is a more relevant index because Te approximates the equilibrium Tb of an ectotherm at a given time and place. Te can differ substantially from air temperature (especially for large organisms) because Te is determined not only by air temperatures (convection) but also by radiation, conduction, evaporation and metabolism [121,126]. Fortunately, biophysical and climate models can be combined to predict Te in the future [4,8,127].
Te of ectotherms can be estimated in two ways. First, one can construct physical models that approximate size, shape and colour of a given animal, implant a thermocouple and then measure Te [121,126]. These models are typically hollow and equilibrate quickly, and the resulting Te can be within 1°C of actual Tb at the same site [128,129]. (Note: adjustments need to be made for large ectotherms, which have substantial thermal inertia [130–132], and amphibians, which can have high rates of evaporative water loss [18,133]). Second, one can measure the key environmental variables (e.g. air temperature, wind speed, radiation) in a particular micro-environment, as well as animal properties (size, shape, reflectivity and behaviour), and then use a mathematical model to calculate Te [26,121,134]. Given spatially explicit data on climate and terrain, one can use these latter models to estimate Te at any spot or time [8,127] or the distribution of Te at a site [128]. These approaches are complementary: physical models are convenient for mapping microclimate variation on a fine scale [128,135], whereas mathematical models enable ‘what if’ simulations as well as an understanding of the physical basis of Te [8,26,127].
Estimating environmental heat loads on endotherms is complicated because endotherms metabolically generate high internal-heat loads, have insulation and can achieve high rates of evaporative heat loss. To index thermal stresses on endotherms, biophysical ecologists [136] estimate ‘standard operative temperature’ (Tes). As with Te, Tes can be calculated or approximated using physical models. Heated models are preferred [136,137], but even unheated ones can provide useful predictions of activity and behaviour [138]. Standardized heat-generating objects can be placed in different retreat-sites, such as tree hollows, to assess the interaction between insulation of the retreat-site and heat production by the organism [139]. Indeed, metabolic heat production can be sufficient to cause significant heat stress to endotherms inside well-insulated retreats [139,140].
(e). Behavioural temperature regulation as a buffer
In most terrestrial habitats, a range of potential Te (or Tes) exists at any time of day, and that range will shift over the day and seasons [121]. As has been known for decades [141,142], mobile animals can behaviourally exploit that thermal heterogeneity and thus control Tb within relatively narrow ranges. For example, they might move to a sunny spot (relatively high Te) early in the morning or late in the afternoon, bask and thus use solar radiation to drive Tb higher. Then they might move to shade at midday, avoiding solar radiation and taking advantage of convective cooling to keep their Tb from rising excessively. Regulation of time of activity, posture and retreat-site selection are classic methods of behavioural thermoregulation [142–145].
Behavioural thermoregulation can thus buffer the impact of climate warming [8,146,147]. However, behavioural thermoregulation is feasible only if the thermal environment is heterogeneous [8], as evaluated by the distribution of Te [135,148,149]. If all accessible microhabitats have similar Te, then microhabitat selection can have little or no impact on Te and thus Tb. Thermal heterogeneity is limited at night and at all times beneath the canopy in heavily forested areas [128,150], except in clearings and in sun flecks. Not surprisingly, nocturnal and forest ectotherms must behaviourally control Tb primarily by regulating the time of activity (i.e. becoming active only when Te are suitable). Thermal heterogeneity can also be limited even in very open habitats at midday, when most of the ground surface is fully exposed to solar radiation [8,135,144].
(f). Complications from diverse life-cycle stages
Terrestrial ectotherms typically have a sessile egg stage and mobile juvenile and adult stages. Many insects have more complex life cycles: their stages (eggs, larvae, pupae, adults) can live in dramatically different habitats and can differ in vagility [125,151]. For instance, a butterfly may have a sessile egg phase on one host plant, a motile larval phase on the same plant, a sessile pupal phase in the soil and a flying adult phase. Each life-cycle stage may thus experience different thermal environments (especially if stages are non-overlapping in time [152]) and may even have quite different thermal sensitivities and behavioural options [125,151,153–157]. For such species, predictions of the impact of climate warming thus require consideration of the vulnerability of each life-cycle stage [52,125,158], especially on development [47].
Sessile stages (especially eggs) are incapable of behavioural buffering (but see [159]). Yet the thermal environment of embryos (e.g. eggs) can have a major effect on subsequent survival, development rates, adult size and morphology, physiological capacities, and even gender [22,127,160–163]. Although eggs cannot move, the mother's choice of the oviposition site may provide some buffering ([127,161,164–166], but see [167]). For example, a female can control the thermal conditions her eggs will experience simply by altering the depth of her nest or its shading [127,161,168,169], by changing season of laying [170,171] or by using buffered microhabitats such as termite mounds [172] or transpiring leaves [166]. Even so, females can only potentially choose from among the available temperatures, which may not be suitable [127,161]. Egg retention and viviparity can also enable a female to control the thermal environment of her embryos [173].
(g). Genetic responses to climate warming
If climate warming increases Tb, such that performance and fitness is reduced (b to c in figure 1b), selection will favour genotypes that perform relatively well at higher temperatures [9,52,56,174–176]. If the selective response can keep pace with the change in Tb, overall fitness will not be affected dramatically by climate change [52,58,124,176–179]. Furthermore, gene exchange between genetically differentiated populations may enable persistence [10].
Modelling selection and the response to selection on thermal sensitivity is complex [9,52,56,58,67,177,179–181], but we can summarize factors that will be influential. The simple response to selection is characterized in the familiar ‘breeder's equation’ response = (selection differential) × (heritability). Response will be relatively fast in species that have short generation times, pronounced heritable variation in thermal sensitivity, large population size, limited inbreeding and thermally specialized physiologies [58,67,180,181]. Unfortunately, the genetic architecture of ectotherm thermal sensitivity is largely unknown, but recent studies suggest that some lizards [9] and even some Drosophila [52] have limited capacity to keep pace with warming. We return to genetic issues below.
3. Temporal responses to climate warming
We are now in a position to evaluate the temporal pattern of the impact of global warming on an organism. We focus on a case in which an ectotherm initially lives in an environment where most Te are initially at or near To (‘B’ in figure 1b). Thus, any warming can potentially reduce performance and fitness.
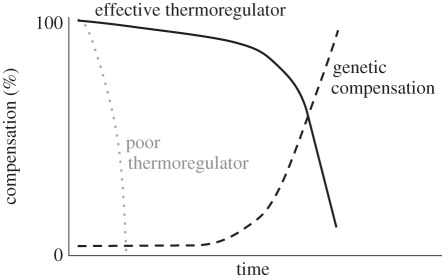
Whether warming does reduce fitness depends in part on the organism's capacity for behavioural buffering and acclimatization, as noted above. An appreciation that behaviour can buffer environmental change traces to Charles Bogert ([182], see also [183]), and is called the ‘Bogert Effect’ [146]. Consider two extremes. If a species has well-developed capacity for behavioural thermoregulation (or for acclimatization) and if it lives in a thermally heterogeneous environment, then it can achieve Tb near To for an extended period of warming before reaching the limits of behavioural buffering (figure 7, black line). In other words, it evades the force of selection for some time. However, if warming continues, it will eventually reach the limits of behavioural buffering, and its Tb will rise significantly above To. Its survival will then depend on its capacity to respond to selection (black dashed line). Alternatively, if a species lives in a thermally homogeneous environment and thus has limited opportunities for behavioural compensation (figure 7, grey dotted line), its Tb will soon be forced above To. Consequently, it will rapidly experience selection for increased heat tolerance [151]. (Note: the impact of Tb shifts will also depend on an organism's acclimatization capacity [67] and on the availability of water, which provides a physiological buffer via evaporative cooling.)
Figure 7.
Consequences of differences in ability to compensate behaviourally (or via acclimatization) for climate warming. At the start, assume that mean Te equals mean To, so that the thermal environment is ideal. If Te increases from warming, a species with strong behavioural capacity to thermoregulate (black line) will not experience a marked shift in Tb (and thus no associated selection on thermal sensitivity) for some time. But if warming continues, the species will eventually exceed the limit of behavioural (or physiological) compensation and will then begin to rely on genetic compensatory changes (dashed line), or to go extinct. However, if a species has limited thermoregulatory capacity (dotted grey line), it will soon need to rely on genetic compensation to survive (note: the earlier onset of selection for genetic compensation is not depicted).
We can also consider the temporal pattern of warming-induced changes in Tb for a species that is initially living in an environment in which Te are generally less than To (‘A’ in figure 1b), as may apply to many high-latitude ectotherms [6,8,180]. As warming proceeds, a thermoregulating animal will generally select the warmest available microenvironments, and consequently its Tb and thus its fitness may increase as Te increase [6,8]. But once Te begin to exceed To, the animal will begin to pick the coolest microenvironments; thus behavioural buffering will reduce the rate of fitness decline relative to the rate of change in the thermal environment (figure 6) by the Bogert Effect. In other words, behaviour allows organisms living in cool environments not only to take advantage of the initial stages of warming, but then potentially to use behaviour to evade further warming.
What kinds of terrestrial animals have limited capacities for behavioural buffering? As noted above, these will be species living in thermally homogeneous environments. They are typically (below-canopy) forest species living in the lowland tropics. These ectotherms may be additionally vulnerable because they have limited acclimatization capacities [72], low heat tolerance [68,78,184] and already live in environments that are warm relative to To [6,47,68,123,185,186]. Their vulnerability will only be exacerbated if climate change induces a dieback of tropical forests [187].
The proposal that many tropical ectotherms are vulnerable to warming [6,8,9,47,68,123,186,188] has been challenged recently. For example, Hoffmann [52] and Clusella-Trullas et al. [37] have suggested that selection on heat tolerance is dominated by extreme events, which were supposedly ignored in prior studies [6,68] and which can sometimes be accentuated in mid-latitude areas [15,52]. We agree that extreme temperatures will sometimes be important, but demographic persistence may often be governed by warming-induced energetic limitations. Sinervo et al. [9] discovered that extinctions of lizard populations were correlated with spring warming, not summer warming. They proposed that spring warming led to a demographic collapse by reducing foraging time and energy gain of females during the critical breeding season, even though spring temperatures are far from extreme. Undoubtedly, both extreme events and demographic collapse are important; and further work should be focused on establishing their relative importance.
In a large comparative analysis, Clusella Trullas et al. [37] found that preferred body temperatures of reptiles have stronger correlations with rainfall than with air temperature variables. They suggested that increased tropical rainfall or cloud cover will mitigate the impact of warming on tropical ectotherms [37,39]. We agree but note that current predictions about rainfall have high uncertainty [187].
Third, all authors agree that ectotherms from mid-latitudes—not just ones from the tropics—are also vulnerable. In particular, mid-latitude desert species live in extreme environments [8,135], can have negative thermal safety margins (fig. 2 in [68]), be in negative energy balance [9] and are thus at risk of stress and extinction [8,21]. Indeed, lizard extinctions have already been observed at mid-latitude sites [9].
Predicting geographical patterns of ectotherm responses to climate change is difficult, as the number of involved biological factors—and the environmental uncertainties—is large. Extinctions will occur widely [9]. Even so, the total biotic impact of climate warming may probably be largest in the tropics, because that is where most ectotherms live [6,185].
(a). Asymmetry of biotic interactions
Our paper has thus far focused on impacts of warming on single species, but warming will also affect biotic interactions among species, greatly complicating predictions of warming impacts [17,44,45,124,189]. Importantly, the impact of climate warming may be decidedly asymmetric on warm- versus cold-adapted species. Consider a tropical locality with both forest-habitat and open-habitat lizards. Forest lizards typically encounter warm (but not hot) Te, are usually thermoconformers (except species that use sun flecks) and typically have low To and CTmax relative to open-habitat species [68,184]. Prior to climate warming, Te inside lowland forests can be ideal (i.e. near To) for lowland forest species [48,148], but may be too cool for the open-habitat species, which have higher To [190]. Because thermoregulatory options are limited for forest species (above), climate warming will necessarily increase Te and thus Tb: if so, heat stress is inevitable. However, that same rise in Te will make the forest increasingly suitable for the open-habitat species. If warming continues, the open-habitat species will be able to invade the forest, at least at warm times of the year [68]. They may even begin to use the forest as a thermal refuge.
The forest species is thus likely to be in ‘double trouble’: warming will not only induce heat stress, but also induce increased biotic stress [68]. This combination could induce strong selection on thermal sensitivity of the forest species, but might nonetheless overwhelm their adaptive capacities. If so, tropical forest species are at amplified risk from warming.
These suggestions assume that forest structure and other environmental variables will be unaffected by warming, such that the rise in Te beneath the canopy is driven only by Ta. However, climate warming may reduce plant productivity and growth by increasing respiratory costs relative to photosynthetic gains (but see [33,187,191,192]). If this causes canopies to open, solar radiation will penetrate and raise Te even further [193]. Moreover, humidity will drop, adding novel water stress to the woes of forest animals, especially those that are moisture-sensitive [179,194]. The increasing threat of forest disturbance or clearing [34] and declines in rainfall and cloud cover [124,195] potentially add another synergistic threat to closed forest species by opening up the canopy, adding the cascading threat of drying and fire [29], reducing thermal buffering and refugia, and facilitating invasion by the open-habitat species.
A similar effect may occur for species arrayed along an altitudinal gradient. Warming may force some species to retreat uphill [115,196–200], which can lead to community disassembly, especially in the tropics [201]. However, shifts may depend on precipitation regimes as well [202]. As warm-adapted species move uphill, cold-adapted (thus high-altitude) species may be exposed to novel competitors as well as reduced range size, potentially increasing their extinction risk [9].
4. Concluding remarks
We have outlined some of the issues that are relevant to predictions as to whether climate warming will harm or benefit organisms. Physiological information is fundamental here [4–6,24,39,124,203,204]: one needs to know whether a species is a thermal specialist or a thermal generalist, is warm versus cold-adapted, has marked acclimation capacities [67] or is sensitive to other physiological variables [37,52]. One also needs to know which stage of the life cycle (e.g. eggs versus adults) is most vulnerable [52,125,202]. Laboratory estimates of an organism's thermal sensitivity are useful in predicting phenotypic effects of warming (figure 1a), but some proxies for thermal sensitivity (e.g. CTmax, field Tb) may sometimes be adequate (figure 6b). Information on operative temperatures (level and heterogeneity) is also needed to evaluate whether operative temperatures (at the initiation of warming) are at or below a species' thermal optimum [6] and whether spatial and temporal heterogeneity in operative temperatures will facilitate behavioural buffering [8]. We need more insight into whether selection is driven primarily by extreme events [37,52] or by chronic pressures [9]. Genetic information [67,124,179] will be necessary to anticipate whether species are genetically capable of keeping pace with shifting climates (a ‘Red Queen’ scenario), or whether they will increasingly lag behind (a ‘moon-walk’ scenario) and ultimately go extinct. Genetic approaches require estimates of selection differentials in nature and of heritabilities for fitness traits [22,58,67,177,178,181,205]. Unfortunately, those are rarely available [9,51,56,179,181], making current predictive attempts unreliable. We have much to learn and little time to work.
Our review reinforces the recent view [5,6,8,68,185,186,206] that tropical forest ectotherms are at risk from warming and that they may have limited genetic variation and thus not be able to adapt rapidly [52,207,208]. If this expectation is correct, then the ecological impact of climate warming will be devastating, because tropical forests are the centre of diversity of most terrestrial ectotherm taxa.
We do not deny that many organisms elsewhere also appear in trouble from warming [8,9,21,37,52,68,124]. In fact, many lizard populations outside the tropics may have already gone locally extinct from climate warming; and many more are projected to go extinct [9]. Behavioural buffering can help [8,146], but may only slow or delay the march towards extinction.
Acknowledgements
This paper emerged from a workshop (‘Predicting climate change impacts on biodiversity: the way forward’) held in Daintree, Queensland, Australia (17–21 November 2008). This workshop was organized by the Centre for Tropical Biodiversity and Climate Change at James Cook University and funded by the MTSRF. We thank other participants for ideas that emerged during discussions, B. Sinervo and S. Clusella-Trullas for comments, and C. Franklin and F. Seebacher for the opportunity to participate in this volume. R.B.H. was supported by NSF grant IBN-0416843. M.R.K. was supported by an ARC Australian Research Fellowship DP110101776.
References
- 1.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Syst. 37, 637–669 10.1146/annurev.ecolsys.37.091305.110100 (doi:10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 2.IPCC 2007. Climate change 2007: the physical science basis. Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Bernardo J., Ossola R. J., Spotila J., Crandall K. A. 2007. Interspecies physiological variation as a tool for cross-species assessments of global warming-induced endangerment: validation of an intrinsic determinant of macroecological and phylogeographic structure. Biol. Lett. 3, 695–699 10.1098/rsbl.2007.0259 (doi:10.1098/rsbl.2007.0259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckley L. B. 2008. Linking traits to energetics and population dynamics to predict lizard ranges in changing environments. Am. Nat. 171, E1–E19 10.1086/523949 (doi:10.1086/523949) [DOI] [PubMed] [Google Scholar]
- 5.Williams S. E., Shoo L. P., Isaac J. L., Hoffmann A. A., Langham G. 2008. Towards an integrated framework for assessing the vulnerability of species to climate change. PLoS Biol. 6, 2621–2626 10.1371/journal.pbio.0060325 (doi:10.1371/journal.pbio.0060325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deutsch C. A., Tewksbury J. J., Huey R. B., Sheldon K. S., Ghalambor C. K., Haak D. C., Martin P. R. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672 10.1073/pnas.0709472105 (doi:10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wake D. B., Vredenburg V. T. 2008. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl Acad. Sci. USA 105, 11 466–11 473 10.1073/pnas.0801921105 (doi:10.1073/pnas.0801921105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kearney M., Shine R., Porter W. P. 2009. The potential for behavioral thermoregulation to buffer ‘cold-blooded’ animals against climate warming. Proc. Natl Acad. Sci. USA 106, 3835–3840 (doi:10.1073/pnas.0808913106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinervo B., et al. 2010. Erosion of lizard diversity by climate change and altered thermal niches. Science 328, 894–899 10.1126/science.1184695 (doi:10.1126/science.1184695) [DOI] [PubMed] [Google Scholar]
- 10.Moritz C., Langham G., Kearney M., Krockenberger A., VanDerWal J., Williams S. 2012. Integrating phylogeography and physiology reveals divergence of thermal traits between central and peripheral lineages of tropical rainforest lizards. Phil. Trans. R. Soc. B 367, 1680–1687 10.1098/rstb.2012.0018 (doi:10.1098/rstb.2012.0018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee T. M., Jetz W. 2008. Future battlegrounds for conservation under global change. Proc. R. Soc. B 275, 1261–1270 10.1098/rspb.2007.1732 (doi:10.1098/rspb.2007.1732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pörtner H. O., Kunst R. 2007. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315, 95–97 10.1126/science.1135471 (doi:10.1126/science.1135471) [DOI] [PubMed] [Google Scholar]
- 13.Pörtner H. O., Farrell A. P. 2008. Physiology and climate change. Science 322, 690–692 10.1126/science.1163156 (doi:10.1126/science.1163156) [DOI] [PubMed] [Google Scholar]
- 14.Evans T. G., Hofmann G. E. 2012. Defining the limits of physiological plasticity: how gene expression can assess and predict the consequences of ocean change. Phil. Trans. R. Soc. B 367, 1733–1745 10.1098/rstb.2012.0019 (doi:10.1098/rstb.2012.0019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helmuth B., Harley C. D. G., Halpin P. M., O'Donnell M., Hofmann G. E., Blanchete C. A. 2002. Climate change and latitudinal patterns of intertidal thermal stress. Science 298, 1015–1017 10.1126/science.1076814 (doi:10.1126/science.1076814) [DOI] [PubMed] [Google Scholar]
- 16.Huey R. B., Carlson M., Crozier L., Frazier M., Hamilton H., Harley H., Honag A., Kingsolver J. G. 2002. Plants versus animals: do they deal with stress in different ways? Integr. Comp. Biol. 42, 415–423 10.1093/icb/42.3.415 (doi:10.1093/icb/42.3.415) [DOI] [PubMed] [Google Scholar]
- 17.Harley C. D. G. 2011. Climate change, keystone predation, and biodiversity loss. Science (Wash.) 334, 1124–1127 10.1126/science.1210199 (doi:10.1126/science.1210199) [DOI] [PubMed] [Google Scholar]
- 18.Tracy C. R., Christian K. A., Tracy C. R. 2010. Not just small, wet, and cold: interacting effects of body size, skin resistance, and microclimate on thermoregulation and arboreality in frogs. Ecology 91, 1477–1484 10.1890/09-0839.1 (doi:10.1890/09-0839.1) [DOI] [PubMed] [Google Scholar]
- 19.Blaustein A. R., Gervasi S. S., Johnson P. T. J., Hoverman J. T., Belden L. K., Bradley P. W., Xie G. Y. 2012. Ecophysiology meets conservation: understanding the role of disease in amphibian population declines. Phil. Trans. R. Soc. B 367, 1688–1707 10.1098/rstb.2012.0011 (doi:10.1098/rstb.2012.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huey R. B. 1991. Physiological consequences of habitat selection. Am. Nat. 137, S91–S115 10.1086/285141 (doi:10.1086/285141) [DOI] [Google Scholar]
- 21.Dunham A. E. 1993. Population responses to environmental change: physiologically structured models, operative environments, and population dynamics. In Biotic interactions and global change (eds Kareiva P. M., Kingsolver J. G., Huey R. B.), pp. 95–119 Sunderland, MA: Sinauer Associates [Google Scholar]
- 22.Angilletta M. J., Jr 2009. Thermal adaptation: a theoretical and empirical synthesis. Oxford, UK: Oxford University Press [Google Scholar]
- 23.Sinervo B., Huey R. B. 1990. Allometric engineering: an experimental test of the causes of interpopulation differences in performance. Science 248, 1106–1109 10.1126/science.248.4959.1106 (doi:10.1126/science.248.4959.1106) [DOI] [PubMed] [Google Scholar]
- 24.Seebacher F., Franklin C. E. 2012. Determining environmental causes of biological effects: the need for a mechanistic physiological dimension in conservation biology. Phil. Trans. R. Soc. B 367, 1607–1614 10.1098/rstb.2012.0036 (doi:10.1098/rstb.2012.0036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter W. P., Budaraju S., Stewart W. E., Ramankutty N. 2000. Calculating climate effects on birds and mammals: impacts on biodiversity, conservation, population parameters, and global community structure. Am. Zool. 40, 1175–1176 10.1093/icb/40.4.597 (doi:10.1093/icb/40.4.597) [DOI] [Google Scholar]
- 26.Porter W. P., Kearney M. 2009. Size, shape and the thermal niche of endotherms. Proc. Natl Acad. Sci. USA 106, 19 666–19 672 10.1073/pnas.0907321106 (doi:10.1073/pnas.0907321106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kearney M. R., Wintle B. A., Porter W. P. 2010. Correlative and mechanistic models of species distribution provide congruent forecasts under climate change. Conserv. Lett. 3, 203–213 10.1111/j.1755-263X.2010.00097.x (doi:10.1111/j.1755-263X.2010.00097.x) [DOI] [Google Scholar]
- 28.Angilletta M. J., Cooper B. S., Schuler M. S., Boyles J. G. 2010. The evolution of thermal physiology in endotherms. Front Biosci. E2, 861–881 10.2741/E148 (doi:10.2741/E148) [DOI] [PubMed] [Google Scholar]
- 29.Laurance W. F., Williamson G. B. 2001. Positive feedbacks among forest fragmentation, drought, and climate change in the Amazon. Conserv Biol. 15, 1529–1535 10.1046/j.1523-1739.2001.01093.x (doi:10.1046/j.1523-1739.2001.01093.x) [DOI] [Google Scholar]
- 30.Travis J. M. J. 2003. Climate change and habitat destruction: a deadly anthropogenic cocktail. Proc. R. Soc. Lond. B 270, 467–473 10.1098/rspb.2002.2246 (doi:10.1098/rspb.2002.2246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright S. J. 2005. Tropical forests in a changing environment. Trends Ecol. Evol. 20, 553–560 10.1016/j.tree.2005.07.009 (doi:10.1016/j.tree.2005.07.009) [DOI] [PubMed] [Google Scholar]
- 32.Pounds J. A., et al. 2006. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439, 161–167 10.1038/nature04246 (doi:10.1038/nature04246) [DOI] [PubMed] [Google Scholar]
- 33.Lewis S. L. 2006. Tropical forests and the changing earth system. Phil. Trans. R. Soc. B 361, 195–210 10.1098/rstb.2005.1711 (doi:10.1098/rstb.2005.1711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malhi Y., Roberts J. T., Betts R. A., Killeen T. J., Nobre C. A. 2007. Climate change, deforestation, and the fate of the Amazon. Science 319, 169–172 10.1126/science.1146961 (doi:10.1126/science.1146961) [DOI] [PubMed] [Google Scholar]
- 35.Brook B. W., Sodhi N. S., Bradshaw C. J. A. 2008. Synergies among extinction drivers under global change. Trends Ecol. Evol. 23, 453–460 10.1016/j.tree.2008.03.011 (doi:10.1016/j.tree.2008.03.011) [DOI] [PubMed] [Google Scholar]
- 36.Bonebrake T. C., Mastrandrea M. D. 2010. Tolerance adaptation and precipitation changes complicate latitudinal patterns of climate change impacts. Proc. Natl Acad. Sci. USA 107, 12 581–12 886 10.1073/pnas.0911841107 (doi:10.1073/pnas.0911841107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clusella-Trullas S., Blackburn T. M., Chown S. L. 2011. Climatic predictors of temperature performance curve parameters in ectotherms imply complex responses to climate change. Am. Nat. 177, 738–751 10.1086/660021 (doi:10.1086/660021) [DOI] [PubMed] [Google Scholar]
- 38.Krockenberger A. K., Edwards W., Kanowski J. 2012. The limit to distribution of a rainforest marsupial folivore is consistent with thermal intolerance hypothesis. Oecologia. 168, 889–899 [DOI] [PubMed] [Google Scholar]
- 39.Chown S. L. 2012. Trait-based approaches to conservation physiology: forecasting environmental change risks from the bottom up. Phil. Trans. R. Soc. B 367, 1615–1627 10.1098/rstb.2011.0422 (doi:10.1098/rstb.2011.0422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ives A. R., Gilchrist G. W. 1993. Climate change and ecological interactions. In Biotic interactions and global change (eds Kareiva P., Kingsolver J. G., Huey R. B.). Sunderland, MA: Sinauer Associates [Google Scholar]
- 41.Buckley L. B., Roughgarden J. 2006. Climate, competition, and the coexistence of island lizards. Funct. Ecol. 20, 315–322 10.1111/j.1365-2435.2006.01095.x (doi:10.1111/j.1365-2435.2006.01095.x) [DOI] [Google Scholar]
- 42.Lister B. C., Garcia A. 1992. Seasonality, predation, and the behaviour of a tropical mainland anole. J. Anim. Ecol. 61, 717–733 10.2307/5626 (doi:10.2307/5626) [DOI] [Google Scholar]
- 43.Pincebourde S., Casas J. 2006. Multitrophic biophysical budgets: thermal ecology of an intimate herbivore insect–plant interaction. Ecol. Monogr. 76, 175–194 10.1890/0012-9615(2006)076[0175:MBBTEO]2.0.CO;2 (doi:10.1890/0012-9615(2006)076[0175:MBBTEO]2.0.CO;2) [DOI] [Google Scholar]
- 44.Gilman S. E., Urban M. C., Tewksbury J., Gilchrist G. W., Holt R. D. 2010. A framework for community interactions under climate change. Trends Ecol. Evol. 25, 325–331 10.1016/j.tree.2010.03.002 (doi:10.1016/j.tree.2010.03.002) [DOI] [PubMed] [Google Scholar]
- 45.Adamo S. A., Lovett M. M. E. 2011. Some like it hot: the effects of climate change on reproduction, immune function and disease resistance in the cricket Gryllus texensis. J. Exp. Biol. 214, 1997–2004 10.1242/jeb.056531 (doi:10.1242/jeb.056531) [DOI] [PubMed] [Google Scholar]
- 46.Huey R. B., Stevenson R. D. 1979. Integrating thermal physiology and ecology of ectotherms: a discussion of approaches. Am. Zool. 19, 357–366 10.1093/icb/19.1.357 (doi:10.1093/icb/19.1.357) [DOI] [Google Scholar]
- 47.Amarasekare P., Savage V. 2012. A framework for elucidating the temperature dependence of fitness. Am. Nat. 179, 179–191 10.1086/663677 (doi:10.1086/663677) [DOI] [PubMed] [Google Scholar]
- 48.Huey R. B. 1982. Temperature, physiology, and the ecology of reptiles. In Biology of the Reptilia, physiology (C), vol. 12 (eds Gans C., Pough F. H.), pp. 25–91 London, UK: Academic Press [Google Scholar]
- 49.Gilchrist G. W. 1995. Specialists and generalists in changing environments. I. Fitness landscapes of thermal sensitivity. Am. Nat. 146, 252–270 10.1086/285797 (doi:10.1086/285797) [DOI] [Google Scholar]
- 50.Martin T. L., Huey R. B. 2008. Why suboptimal is optimal: Jensen's inequality and ectotherm thermal preferences. Am. Nat. 171, E102–E118 10.1086/527502 (doi:10.1086/527502) [DOI] [PubMed] [Google Scholar]
- 51.Huey R. B., Berrigan D. 2001. Temperature, demography, and ectotherm fitness. Am. Nat. 158, 204–210 10.1086/321314 (doi:10.1086/321314) [DOI] [PubMed] [Google Scholar]
- 52.Hoffmann A. A. 2010. Physiological climatic limits in Drosophila: patterns and implications. J. Exp. Biol. 213, 870–880 10.1242/jeb.037630 (doi:10.1242/jeb.037630) [DOI] [PubMed] [Google Scholar]
- 53.Schulte P. M., Healy T. M., Fangue N. A. 2011. Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr. Comp. Biol. 51, 691–702 10.1093/icb/icr097 (doi:10.1093/icb/icr097) [DOI] [PubMed] [Google Scholar]
- 54.Huey R. B., Slatkin M. 1976. Costs and benefits of lizard thermoregulation. Q. Rev. Biol. 51, 363–384 10.1086/409470 (doi:10.1086/409470) [DOI] [PubMed] [Google Scholar]
- 55.Tracy C. R., Christian K. A. 1983. Ecological relations among space, time and thermal niche axes. Ecology 67, 609–615 10.2307/1937684 (doi:10.2307/1937684) [DOI] [Google Scholar]
- 56.Kingsolver J. G., Ragland G. J., Shlichta J. G. 2004. Quantitative genetics of continuous reaction norms: thermal sensitivity of caterpillar growth rates. Evolution 58, 1521–1529 [DOI] [PubMed] [Google Scholar]
- 57.Janzen D. H. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 233–249 10.1086/282487 (doi:10.1086/282487) [DOI] [Google Scholar]
- 58.Huey R. B., Kingsolver J. G. 1993. Evolution of resistance to high temperature in ectotherms. Am. Nat. 142, S21–S46 10.1086/285521 (doi:10.1086/285521) [DOI] [Google Scholar]
- 59.Scholander P. F. 1955. Evolution of climatic adaptation in homeotherms. Evolution 9, 15–26 10.2307/2405354 (doi:10.2307/2405354) [DOI] [Google Scholar]
- 60.Porter W. P., Gates D. M. 1969. Thermodynamic equilibria of animals with environment. Ecol. Monogr. 39, 227–244 10.2307/1948545 (doi:10.2307/1948545) [DOI] [Google Scholar]
- 61.Scholander P. F., Hock R., Walters V., Johnson F., Irving L. 1950. Heat regulation in some arctic and tropical mammals and birds. Biol. Bull. 99, 237–258 10.2307/1538741 (doi:10.2307/1538741) [DOI] [PubMed] [Google Scholar]
- 62.Somero G. N. 2010. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 213, 912–920 10.1242/jeb.037473 (doi:10.1242/jeb.037473) [DOI] [PubMed] [Google Scholar]
- 63.Glanville E. J., Seebacher F. 2006. Compensation for environmental change by complementary shifts of thermal sensitivity and thermoregulatory behaviour in an ectotherm. J. Exp. Biol. 209, 4869–4877 10.1242/jeb.02585 (doi:10.1242/jeb.02585) [DOI] [PubMed] [Google Scholar]
- 64.Leroi A. M., Bennett A. F., Lenski R. E. 1994. Temperature acclimation and competitive fitness: an experimental test of the beneficial acclimation assumption. Proc. Natl Acad. Sci. USA 91, 1917–1921 10.1073/pnas.91.5.1917 (doi:10.1073/pnas.91.5.1917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huey R. B., Berrigan D., Gilchrist G. W., Herron J. C. 1999. Testing the adaptive significance of acclimation: a strong inference approach. Am. Zool. 39, 135–148 10.1093/icb/39.2.323 (doi:10.1093/icb/39.2.323) [DOI] [Google Scholar]
- 66.Stillman J. H. 2003. Acclimation capacity underlies susceptibility to climate change. Science 301, 65. 10.1126/science.1083073 (doi:10.1126/science.1083073) [DOI] [PubMed] [Google Scholar]
- 67.Chevin L.-M., Lande R., Mace G. M. 2010. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357. 10.1371/journal.pbio.1000357 (doi:10.1371/journal.pbio.1000357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huey R. B., Deutsch C. A., Tewksbury J. J., Vitt L. J., Hertz P. E., Álvarez H. J. 2009. Why tropical forest lizards are vulnerable to climate warming. Proc. R. Soc. B 276, 1939–1948 10.1098/rspb.2008.1957 (doi:10.1098/rspb.2008.1957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beaumont L. J., Pitman A., Perkins S., Zimmermann N. E., Yoccoz N. G., Thuiller W. 2011. Impacts of climate change on the world's most exceptional ecoregions. Proc. Natl Acad. Sci. USA 108, 2306–2311 10.1073/pnas.1007217108 (doi:10.1073/pnas.1007217108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wake D. B., Lynch J. F. 1976. The distribution, ecology, and evolutionary history of plethodontid salamanders in tropical America. Natur. Hist. Mus. Los Angeles Co. Sci. Bull. 25, 1–65 [Google Scholar]
- 71.Huey R. B. 1978. Latitudinal pattern of between-altitude faunal similarity: mountains might be ‘higher’ in the tropics. Am. Nat. 112, 225–229 10.1086/283262 (doi:10.1086/283262) [DOI] [Google Scholar]
- 72.Ghalambor C. K., Huey R. B., Martin P. R., Tewksbury J. J., Wang G. 2006. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integr. Comp. Biol. 46, 5–17 10.1093/icb/icj003 (doi:10.1093/icb/icj003) [DOI] [PubMed] [Google Scholar]
- 73.McCain C. M. 2009. Vertebrate range sizes indicate that mountains may be ‘higher’ in the tropics. Ecol. Lett. 12, 550–560 10.1111/j.1461-0248.2009.01308.x (doi:10.1111/j.1461-0248.2009.01308.x) [DOI] [PubMed] [Google Scholar]
- 74.Sunday J. M., Bates A. E., Dulvy N. K. 2010. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823–1830 10.1098/rspb.2010.1295 (doi:10.1098/rspb.2010.1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitchell K. A., Sgrò C. M., Hoffmann A. A. 2011. Phenotypic plasticity in upper thermal limits is weakly related to Drosophila species distributions. Funct. Ecol. 25, 661–670 10.1111/j.1365-2435.2010.01821.x (doi:10.1111/j.1365-2435.2010.01821.x) [DOI] [Google Scholar]
- 76.Snyder G. K., Weathers W. W. 1975. Temperature adaptations in amphibians. Am. Nat. 109, 93–101 10.1086/282976 (doi:10.1086/282976) [DOI] [Google Scholar]
- 77.Addo-Bediako A., Chown S. L., Gaston K. J. 2000. Thermal tolerance, climatic variability and latitude. Proc. R. Soc. Lond. B 267, 739–745 10.1098/rspb.2000.1065 (doi:10.1098/rspb.2000.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kimura M. T. 2004. Cold and heat tolerance of Drosophilid flies with reference to their latitudinal distributions. Oecologia 140, 442–449 10.1007/s00442-004-1605-4 (doi:10.1007/s00442-004-1605-4) [DOI] [PubMed] [Google Scholar]
- 79.van Berkum F. H. 1988. Latitudinal patterns of the thermal sensitivity of sprint speed in lizards. Am. Nat. 132, 327–343 10.1086/284856 (doi:10.1086/284856) [DOI] [Google Scholar]
- 80.Bradshaw W. E., Holzapfel C. M. 2006. Evolutionary response to rapid climate change. Science 312, 1477–1478 10.1126/science.1127000 (doi:10.1126/science.1127000) [DOI] [PubMed] [Google Scholar]
- 81.Vaughan T. A., Ryan J. M., Czaplewski N. J. 2011. Mammology. Sudbury, MA: Jones and Bartlett Publishers [Google Scholar]
- 82.Scholander P. F., Walters V., Hock R., Irving L. 1950. Body insulation of some arctic and tropical mammals and birds. Biol. Bull. 99, 225–236 10.2307/1538740 (doi:10.2307/1538740) [DOI] [PubMed] [Google Scholar]
- 83.Geiser F., Turbill C. 2009. Hibernation and daily torpor minimize mammalian extinctions. Naturwissenschaften 96, 1235–1240 10.1007/s00114-009-0583-0 (doi:10.1007/s00114-009-0583-0) [DOI] [PubMed] [Google Scholar]
- 84.Geiser F., Stawski C. 2011. Hibernation and torpor in tropical and subtropical bats in relation to energetics, extinctions, and the evolution of endothermy. Integr. Comp. Biol. 51, 337–348 10.1093/icb/icr042 (doi:10.1093/icb/icr042) [DOI] [PubMed] [Google Scholar]
- 85.Overgaard J., Kristensen T. N., Mitchell K. A., Hoffmann A. A. 2011. Thermal tolerance in widespread and tropical Drosophila species: does phenotypic plasticity increase with latitude? Am. Nat. 178, S80–S96 10.1086/661780 (doi:10.1086/661780) [DOI] [PubMed] [Google Scholar]
- 86.Campbell H. A., Dwyer R. G., Gordos M., Franklin C. E. 2010. Diving through the thermal window: implications for a warming world. Proc. R. Soc. B 277, 3837–3844 10.1098/rspb.2010.0902 (doi:10.1098/rspb.2010.0902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Donelson J. M., Munday P. L., McCormick M. I., Pitcher C. R. 2012. Rapid transgenerational acclimation of a tropical reef fish to climate change. Nat. Clim. Change 2, 30–32 10.1038/nclimate1323 (doi:10.1038/nclimate1323) [DOI] [Google Scholar]
- 88.Hart J. S. 1957. Climatic and temperature induces changes in the energetics of homeotherms. Rev. Can. Biol. 16, 133–174 [PubMed] [Google Scholar]
- 89.Dawson W. R., Carey C. 1976. Seasonal acclimatization to temperature in Cardueline finches I. Insulative and metabolic adjustments. J. Comp. Physiol. 112, 317–333 [Google Scholar]
- 90.Stevenson R. D., Peterson C. R., Tsuji J. S. 1985. The thermal dependence of locomotion, tongue flicking, digestion and oxygen consumption in the wandering garter snake. Physiol. Zool. 58, 46–57 [Google Scholar]
- 91.Angilletta M. J., Jr, Hill T., Robson M. A. 2002. Is physiological performance optimized by thermoregulatory behavior? A case study of the eastern fence lizard, Sceloporus undulatus. J. Therm. Biol. 27, 199–204 10.1016/S0306-4565(01)00084-5 (doi:10.1016/S0306-4565(01)00084-5) [DOI] [Google Scholar]
- 92.Arnold S. J. 1983. Morphology, performance and fitness. Am. Zool. 23, 347–361 [Google Scholar]
- 93.Bennett A. F., Huey R. B. 1990. Studying the evolution of physiological performance. In Oxford surveys in evolutionary biology, vol 7 (eds Futuyma D. J., Antonovics J.), pp. 251–284 Oxford, UK: Oxford University Press [Google Scholar]
- 94.Jayne B. C., Bennett A. F. 1990. Selection on locomotor performance capacity in a natural population of garter snakes. Evolution 44, 1204–1229 10.2307/2409283 (doi:10.2307/2409283) [DOI] [PubMed] [Google Scholar]
- 95.Calsbeek R., Irschick D. J. 2007. The quick and the dead: correlational selection on morphology, performance, and habitat use in island lizards. Evolution 61, 2493–2503 10.1111/j.1558-5646.2007.00206.x (doi:10.1111/j.1558-5646.2007.00206.x) [DOI] [PubMed] [Google Scholar]
- 96.Irschick D. J., Garland T., Jr 2001. Integrating function and ecology in studies of adaptation: investigations of locomotor capacity as a model system. Annu. Rev. Ecol. Syst. 32, 367–396 10.1146/annurev.ecolsys.32.081501.114048 (doi:10.1146/annurev.ecolsys.32.081501.114048) [DOI] [Google Scholar]
- 97.Kingsolver J. G., Massie K. R., Shlichta J. G., Smith M. H., Ragland G. J., Gomulkiewicz R. 2007. Relating environmental variation to selection on reaction norms: an experimental test. Am. Nat. 169, 163–174 10.1086/510631 (doi:10.1086/510631) [DOI] [PubMed] [Google Scholar]
- 98.Carey J. R. 1993. Applied demography for biologists. Oxford, UK: Oxford University Press [Google Scholar]
- 99.Bennett A. F., Lenski R. E., Mittler J. E. 1992. Evolutionary adaptation to temperature. I. Fitness responses of Escherichia coli to changes in its thermal environment. Evolution 46, 16–30 10.2307/2409801 (doi:10.2307/2409801) [DOI] [PubMed] [Google Scholar]
- 100.Frazier M. R., Huey R. B., Berrigan D. 2006. Thermodynamics constrains the evolution of insect population growth rates: ‘warmer is better’. Am. Nat. 168, 512–520 10.1086/506977 (doi:10.1086/506977) [DOI] [PubMed] [Google Scholar]
- 101.Knies J. L., Kingsolver J. G., Burch C. L. 2009. Hotter is higher and broader: adaptation to temperature in a population of bacteriophages. Am. Nat. 173, 419–430 10.1086/597224 (doi:10.1086/597224) [DOI] [PubMed] [Google Scholar]
- 102.Anderson J. L., Albergotti L., Ellebaracht B., Huey R. B., Phillips P. C. 2011. Does thermoregulatory behavior maximize reproductive fitness of natural isolates of Caenorhabditis elegans? BMC Evol. Biol. 22, 257. 10.1186/1471-2148-11-157 (doi:10.1186/1471-2148-11-157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Terblanche J. S., Deere J. A., Clusella Trullas S., Janion C., Chown S. L. 2007. Critical thermal limits depend on methodological context. Proc. R. Soc. B 274, 2935–2942 10.1098/rspb.2007.0985 (doi:10.1098/rspb.2007.0985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mitchell K. A., Hoffmann A. A. 2010. Thermal ramping rate influences evolutionary potential and species differences for upper thermal limits in Drosophila. Funct. Ecol. 24, 694–700 10.1111/j.1365-2435.2009.01666.x (doi:10.1111/j.1365-2435.2009.01666.x) [DOI] [Google Scholar]
- 105.Rezende E. L., Tejedo M., Santos M. 2011. Estimating the adaptive potential of critical thermal limits: methodological problems and evolutionary implications. Funct. Ecol. 25, 111–121 10.1111/j.1365-2435.2010.01778.x (doi:10.1111/j.1365-2435.2010.01778.x) [DOI] [Google Scholar]
- 106.Berrigan D. 2000. Correlations between measures of thermal stress resistance within and between species. Oikos 89, 301–304 10.1034/j.1600-0706.2000.890211.x (doi:10.1034/j.1600-0706.2000.890211.x) [DOI] [Google Scholar]
- 107.Folk D. G., Hoekstra L. A., Gilchrist G. W. 2007. Critical thermal maxima in knockdown-selected Drosophila: are thermal endpoints correlated? J. Exp. Biol. 219, 2649–2656 10.1242/jeb.003350 (doi:10.1242/jeb.003350) [DOI] [PubMed] [Google Scholar]
- 108.Chown S. L., Jumbam K. R., Sørensen J. G., Terblanche J. S. 2009. Phenotypic variance, plasticity and heritability estimates of critical thermal limits depend on methodological context. Funct. Ecol. 23, 133–140 10.1111/j.1365-2435.2008.01481.x (doi:10.1111/j.1365-2435.2008.01481.x) [DOI] [Google Scholar]
- 109.Terblanche J. S., Hoffmann A. A., Mitchell K. A., Rako L., le Roux P. C., Chown S. L. 2011. Ecologically relevant measures of tolerance to potentially lethal temperatures. J. Exp. Biol. 214, 3613–3725 10.1242/jeb.061283 (doi:10.1242/jeb.061283) [DOI] [PubMed] [Google Scholar]
- 110.Chown S. L., Addo-Bediako A., Gaston K. J. 2003. Physiological diversity: listening to the large-scale signal. Funct. Ecol. 17, 562–572 10.1046/j.1365-2435.2003.07431.x (doi:10.1046/j.1365-2435.2003.07431.x) [DOI] [Google Scholar]
- 111.Huey R. B. 1987. Phylogeny, history, and the comparative method. In New directions in ecological physiology (eds Feder M. E., Bennett A. F., Burggren W. W., Huey R. B.), pp. 76–98 Cambridge, UK: Cambridge University; Press. [Google Scholar]
- 112.Chown S. L., Terblanche J. S. 2007. Physiological diversity in insects: ecological and evolutionary contexts. Adv. Insect Physiol. 33, 50–152 10.1016/S0065-2806(06)33002-0 (doi:10.1016/S0065-2806(06)33002-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pörtner H. O. 2001. Climate change and temperature dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88, 137–146 10.1007/s001140100216 (doi:10.1007/s001140100216) [DOI] [PubMed] [Google Scholar]
- 114.Lighton J. R. B., Turner R. J. 2004. Thermolimit respirometry: an objective assessment of critical thermal maxima in two sympatric desert harvester ants, Pogonomyrmex rugosus and P. californicus. J. Exp. Biol. 207, 1903–1913 10.1242/jeb.00970 (doi:10.1242/jeb.00970) [DOI] [PubMed] [Google Scholar]
- 115.Bernardo J., Spotila J. R. 2006. Physiological constraints on organismal response to global warming: mechanistic insights from clinally varying populations and implications for assessing endangerment. Biol. Lett. 2, 135–139 10.1098/rsbl.2005.0417 (doi:10.1098/rsbl.2005.0417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Welbergen J. A., Klose S. M., Marcus N., Eby P. 2008. Climate change and the effects of temperature extremes on Australian flying-foxes. Proc. R. Soc. B 275, 419–425 10.1098/rspb.2007.1385 (doi:10.1098/rspb.2007.1385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McKecknie A. E., Wolf B. O. 2010. Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol. Lett. 6, 253–256 10.1098/rsbl.2009.0702 (doi:10.1098/rsbl.2009.0702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Meehl G. A., Tebaldi C. 2004. More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305, 994–997 10.1126/science.1098704 (doi:10.1126/science.1098704) [DOI] [PubMed] [Google Scholar]
- 119.Bennett A. F., Huey R. B., John-Alder H., Nagy K. A. 1984. The parasol tail and thermoregulatory behavior of the Cape Ground Squirrel Xerus inauris. Physiol. Zool. 57, 57–62 [Google Scholar]
- 120.Chappell M. A., Bartholomew G. A. 1981. Activity and thermoregulation of the antelope ground squirrel Ammospermophilus leucurus in winter and summer. Physiol. Zool. 54, 215–223 [Google Scholar]
- 121.Porter W. P., Mitchell J. W., Beckman W. A., DeWitt C. B. 1973. Behavioral implications of mechanistic ecology. Thermal and behavioral modeling of desert ectotherms and their microenvironment. Oecologia 13, 1–54 10.1007/BF00379617 (doi:10.1007/BF00379617) [DOI] [PubMed] [Google Scholar]
- 122.Savage V. M. 2004. Improved approximations to scaling relationships for species, populations, and ecosystems across latitudinal and elevational gradients. J. Theor. Biol. 227, 525–534 10.1016/j.jtbi.2003.11.030 (doi:10.1016/j.jtbi.2003.11.030) [DOI] [PubMed] [Google Scholar]
- 123.Dillon M. E., Wang G., Huey R. B. 2010. Global metabolic impacts of recent climate warming. Nature 467, 704–706 10.1038/nature09407 (doi:10.1038/nature09407) [DOI] [PubMed] [Google Scholar]
- 124.Chown S., Hoffmann A. A., Kristensen T. N., Angilletta M. J., Jr, Stenseth N. C., Pertoldi C. 2010. Adapting to climate change: a perspective from evolutionary physiology. Clim. Res. 43, 3–15 10.3354/cr00879 (doi:10.3354/cr00879) [DOI] [Google Scholar]
- 125.Kingsolver J. G., Woods H. A., Buckley L. B., Potter K. A., MacLean H. J., Higgins J. K. 2011. Complex life cycles and the responses of insects to climate change. Integr. Comp. Biol. 51, 719–732 10.1093/icb/icr015 (doi:10.1093/icb/icr015) [DOI] [PubMed] [Google Scholar]
- 126.Bakken G. S. 1992. Measurement and application of operative and standard operative temperatures in ecology. Am. Zool. 32, 194–216 10.1093/icb/32.2.194 (doi:10.1093/icb/32.2.194) [DOI] [Google Scholar]
- 127.Mitchell N. J., Kearney M. R., Nelson N. J., Porter W. P. 2009. Predicting the fate of a living fossil: how will global warming affect sex determination and hatching phenology in tuatara? Proc. R. Soc. B 275, 2185–2193 10.1098/rspb.2008.0438 (doi:10.1098/rspb.2008.0438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hertz P. E. 1992. Temperature regulation in Puerto Rican Anolis lizards: a field test using null hypotheses. Ecology 73, 1405–1417 10.2307/1940686 (doi:10.2307/1940686) [DOI] [Google Scholar]
- 129.Dzialowski E. M. 2005. Use of operative temperature and standard operative temperature models in thermal biology. J. Therm. Biol. 30, 317–334 10.1016/j.jtherbio.2005.01.005 (doi:10.1016/j.jtherbio.2005.01.005) [DOI] [Google Scholar]
- 130.O'Connor M. P. 2000. Extracting operative temperatures from temperatures of physical models with thermal inertia. J. Therm. Biol. 25, 329–343 10.1016/S0306-4565(99)00102-3 (doi:10.1016/S0306-4565(99)00102-3) [DOI] [PubMed] [Google Scholar]
- 131.Seebacher F., Elsey R. M., Trosclair P. L. I. 2003. Body temperature null distributions in reptiles with nonzero heat capacity: seasonal thermoregulation in the American Alligator (Alligator mississippiensis). Physiol. Biochem. Zool. 76, 348–359 10.1086/375426 (doi:10.1086/375426) [DOI] [PubMed] [Google Scholar]
- 132.Christian K. A., Tracy C. R., Tracy C. R. 2006. Evaluating thermoregulation in reptiles: an appropriate null model. Am. Nat. 168, 421–430 10.1086/506528 (doi:10.1086/506528) [DOI] [PubMed] [Google Scholar]
- 133.Tracy C. R., Betts G., Tracy C. R., Christian K. A. 2007. Plaster models to measure operative temperature and evaporative water loss of amphibians. J. Herpetol. 41, 597–604 10.1670/07-006.1 (doi:10.1670/07-006.1) [DOI] [Google Scholar]
- 134.Porter W. P., Munger J. C., Stewart W. E., Budaraju S., Jaeger J. 1994. Endotherm energetics: from a scalable individual-based model to ecological applications. Aust. J. Zool. 42, 125–162 10.1071/ZO9940125 (doi:10.1071/ZO9940125) [DOI] [Google Scholar]
- 135.Grant B. W., Dunham A. E. 1988. Thermally imposed time constraints on the activity of the desert lizard Sceloporus merriami. Ecology 69, 167–176 10.2307/1943171 (doi:10.2307/1943171) [DOI] [Google Scholar]
- 136.Bakken G. S., Erksine D. J., Santee W. R. 1983. Construction and operation of heated taxidermic mounts used to measure standard operative temperature. Ecology 64, 1658–1662 10.2307/1937520 (doi:10.2307/1937520) [DOI] [Google Scholar]
- 137.Chappell M. A. 1980. Insulation, radiation, and convection in small arctic mammals. J. Mammal. 61, 268–277 10.2307/1380048 (doi:10.2307/1380048) [DOI] [Google Scholar]
- 138.Bennett A. F., Huey R. B., John-Alder H. B., Nagy K. 1984. The parasol tail and thermoregulatory behavior of the Cape Ground Squirrel (Xerus inauris). Physiol. Zool. 57, 57–62 [Google Scholar]
- 139.Kearney M. R., Ferguson E., Fumei S., Gallacher A., Mitchell P., Woodford R., Handasyde K. 2011. A cost-effective method of assessing thermal habitat quality for endotherms. Austral. Ecol. 36, 297–302 10.1111/j.1442-9993.2010.02150.x (doi:10.1111/j.1442-9993.2010.02150.x) [DOI] [Google Scholar]
- 140.Havera S. P. 1979. Temperature variation in a fox squirrel nest box. J. Wildl. Manage. 43, 251–253 10.2307/3800666 (doi:10.2307/3800666) [DOI] [Google Scholar]
- 141.Cowles R. B., Bogert C. M. 1944. A preliminary study of the thermal requirements of desert reptiles. Bull. Am. Mus. Nat. Hist. 83, 261–296 [Google Scholar]
- 142.Stevenson R. D. 1985. The relative importance of behavioral and physiological adjustments controlling body temperature in terrestrial ectotherms. Am. Nat. 126, 362–386 10.1086/284423 (doi:10.1086/284423) [DOI] [Google Scholar]
- 143.Heath J. E. 1965. Temperature regulation and diurnal activity in horned lizards. Univ. Calif. Publ. Zool. 64, 97–136 [Google Scholar]
- 144.Huey R. B., Pianka E. R., Hoffmann J. 1977. Seasonal variation in thermoregulatory behavior and body temperatures of diurnal Kalahari lizards. Ecology 58, 1066–1075 10.2307/1936926 (doi:10.2307/1936926) [DOI] [Google Scholar]
- 145.Huey R. B., Peterson C. R., Arnold S. J., Porter W. P. 1989. Hot rocks and not-so-hot rocks: retreat-site selection by garter snakes and its thermal consequences. Ecology 70, 931–944 10.2307/1941360 (doi:10.2307/1941360) [DOI] [Google Scholar]
- 146.Huey R. B., Hertz P. E., Sinervo B. 2003. Behavioral drive versus behavioral inertia: a null model approach. Am. Nat. 161, 357–366 10.1086/346135 (doi:10.1086/346135) [DOI] [PubMed] [Google Scholar]
- 147.Bartholomew G. A. 2005. Integrative biology: an organismic biologist's point of view. Integr. Comp. Biol. 45, 330–332 10.1093/icb/45.2.330 (doi:10.1093/icb/45.2.330) [DOI] [PubMed] [Google Scholar]
- 148.Hertz P. E., Huey R. B., Stevenson R. D. 1993. Evaluating temperature regulation by field-active ectotherms: the fallacy of the inappropriate question. Am. Nat. 142, 796–818 10.1086/285573 (doi:10.1086/285573) [DOI] [PubMed] [Google Scholar]
- 149.Clusella-Trullas S., Chown S. L. 2011. Comment on ‘Erosion of lizard diversity by climate change and altered thermal niches’. Science (Wash.) 332, 537. 10.1126/science.1195193 (doi:10.1126/science.1195193) [DOI] [PubMed] [Google Scholar]
- 150.Kearney M., Porter W. P. 2004. Mapping the fundamental niche: physiology, climate, and the distribution of a nocturnal lizard. Ecology 85, 3119–3131 10.1890/03-0820 (doi:10.1890/03-0820) [DOI] [Google Scholar]
- 151.Marais E., Chown S. L. 2008. Beneficial acclimation and the Bogert effect. Ecol. Lett. 11, 1027–1036 10.1111/j.1461-0248.2008.01213.x (doi:10.1111/j.1461-0248.2008.01213.x) [DOI] [PubMed] [Google Scholar]
- 152.Kingsolver J. G., Huey R. B. 1998. Evolutionary analyses of morphological and physiological plasticity in thermally variable environments. Am. Zool. 38, 323–336 10.1193/icb/38.3.545 (doi:10.1193/icb/38.3.545) [DOI] [Google Scholar]
- 153.Davison T. F. 1969. Changes in temperature tolerance during life cycle of Calliphora erythrocephala. J. Insect. Physiol. 15, 977–988 10.1016/0022-1910(69)90138-3 (doi:10.1016/0022-1910(69)90138-3) [DOI] [Google Scholar]
- 154.Hoffmann A. A., Sørensen J. G., Loeschcke V. 2003. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J. Therm. Biol. 26, 175–216 10.1016/S0306-4565(02)00057-8 (doi:10.1016/S0306-4565(02)00057-8) [DOI] [Google Scholar]
- 155.Pappas C., Hyde D., Bowler K., Loeschcke V., Sørensen J. G. 2007. Post-eclosion decline in ‘knock-down’ thermal resistance and reduced effect of heat hardening in Drosophila melanogaster. Comp. Biochem. Physiol.—Part A: Mol. Integr. Physiol. 146, 355–359 10.1016/j.cbpa.2006.11.010 (doi:10.1016/j.cbpa.2006.11.010) [DOI] [PubMed] [Google Scholar]
- 156.Kearney M. R., Briscoe N. J., Karoly D., Porter W. P., Norgate M., Sunnucks P. 2010. Early emergence in a butterfly causally linked to anthropogenic warming. Biol. Lett. 6, 674–677 10.1098/rsbl.2010.0053 (doi:10.1098/rsbl.2010.0053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bowler K., Terblanche J. S. 2008. Insect thermal tolerance: what is the role of ontogeny, ageing and senescence. Biol. Rev. Camb. Phil. Soc 83, 339–355 10.1111/j.1469-185X.2008.00046.x (doi:10.1111/j.1469-185X.2008.00046.x) [DOI] [PubMed] [Google Scholar]
- 158.Coyne J. A., Bundgaard J., Prout T. 1983. Geographic variation of tolerance to environmental stress in Drosophila pseudoobscura. Am. Nat. 122, 474–488 10.1086/284150 (doi:10.1086/284150) [DOI] [Google Scholar]
- 159.Du W.-G., Zhao B., Chen Y., Shine R. 2011. Behavioral thermoregulation by turtle embryos. Proc. Natl Acad. Sci. USA 108, 9413–9515 10.1073/pnas.1102965108 (doi:10.1073/pnas.1102965108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.David J., Clavel M.-F. 1969. Influence de la température sur le nombre, le pourcentage d'éclosion et la taille des oeufs fondus par Drosophila melanogaster. Ann. Soc. Ent. Fr. 5, 161–177 [Google Scholar]
- 161.Muth A. 1980. Physiological ecology of desert iguana (Dipsosaurus dorsalis) eggs: temperature and water relations. Ecology 61, 1335–1343 10.2307/1939042 (doi:10.2307/1939042) [DOI] [Google Scholar]
- 162.Porter W. P., Tracy C. R. 1983. Biophysical analyses of energetics, time-space utilization, and distributional limits. In Lizard ecology: studies of a model organism (eds Huey R. B., Pianka E. R., Schoener T. W.), pp. 55–83 Cambridge, MA: Harvard University Press [Google Scholar]
- 163.Kingsolver J. G. 1996. Experimental manipulation of wing pigment pattern and survival in western white butterflies. Am. Nat. 147, 296–306 10.1086/285852 (doi:10.1086/285852) [DOI] [Google Scholar]
- 164.Kingsolver J. G. 1979. Thermal and hydric aspects of environmental heterogeneity in the pitcher plant mosquito. Ecol. Monogr. 49, 357–376 10.2307/1942468 (doi:10.2307/1942468) [DOI] [Google Scholar]
- 165.Jones J. S., Coyne J. A., Partridge L. 1987. Estimation of the thermal niche of Drosophila melanogaster using a temperature-sensitive mutant. Am. Nat. 130, 83–90 10.1086/284699 (doi:10.1086/284699) [DOI] [Google Scholar]
- 166.Potter K., Davidowitz G., Woods H. A. 2009. Insect eggs protected from high temperatures by limited homeothermy of plant leaves. J. Exp. Biol. 212, 3448–3454 10.1242/jeb.033365 (doi:10.1242/jeb.033365) [DOI] [PubMed] [Google Scholar]
- 167.Feder M. E., Blair N., Figueras H. 1997. Oviposition site selection: unresponsiveness of Drosophila to cues of potential thermal stress. Anim. Behav. 53, 585–588 10.1006/anbe.1996.0333 (doi:10.1006/anbe.1996.0333) [DOI] [Google Scholar]
- 168.Kolbe J. J., Janzen F. J. 2002. Impact of nest-site selection on nest success and nest temperature in natural and disturbed habitats. Ecology 83, 269–281 10.1890/0012-9658(2002)083[0269:IONSSO]2.0.CO;2 (doi:10.1890/0012-9658(2002)083[0269:IONSSO]2.0.CO;2) [DOI] [Google Scholar]
- 169.Doody J. S., Guarion F., Georges A., Corey B., Murray G., Ewert M. W. 2006. Nest site choice compensates for climate effects on sex ratios in a lizard with environmental sex determination. Evol. Ecol. 20, 307–330 10.1007/s10682-006-0003-2 (doi:10.1007/s10682-006-0003-2) [DOI] [Google Scholar]
- 170.Bradshaw W. E., Holzapfel C. M. 2001. Genetic shift in photoperiodic response correlated with global warming. Proc. Natl Acad. Sci. USA 98, 14 509–14 511 10.1073/pnas.241391498 (doi:10.1073/pnas.241391498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schwanz L. E., Janzen F. J. 2008. Climate change and temperature-dependent sex determination: can individual plasticity in nesting phenology prevent extreme sex ratios? Physiol. Biochem. Zool. 81, 826–834 10.1086/590220 (doi:10.1086/590220) [DOI] [PubMed] [Google Scholar]
- 172.Cowles R. B. 1930. The life history of Varanus niloticus (Linnaeus) as observed in Natal South Africa. J. Entomol. Zool. 22, 1–32 [Google Scholar]
- 173.Shine R. 1985. The evolution of viviparity in reptiles: an ecological analysis. In Biology of the Reptilia (eds Gans C., Billet F.), pp. 605–694 New York, NY: Wiley-Interscience [Google Scholar]
- 174.Bennett A. F., Dao K. M., Lenski R. E. 1990. Rapid evolution in response to high temperature selection. Nature 346, 79–81 10.1038/346079a0 (doi:10.1038/346079a0) [DOI] [PubMed] [Google Scholar]
- 175.Angilletta M. J., Jr, Bennett A. F., Guderley H., Navas C. A., Seebacher F., Wilson R. S. 2006. Coadapation: a unifying principle in evolutionary thermal biology. Physiol. Biochem. Zool. 79, 282–294 10.1086/499990 (doi:10.1086/499990) [DOI] [PubMed] [Google Scholar]
- 176.Huey R. B., Bennett A. F. 1987. Phylogenetic studies of coadaptation: preferred temperatures versus optimal performance temperatures of lizards. Evolution 41, 1098–1115 10.2307/2409194 (doi:10.2307/2409194) [DOI] [PubMed] [Google Scholar]
- 177.Lynch M., Lande R. 1993. Evolution and extinction in response to environmental change. In Biotic interactions and global change (eds Kareiva P. M., Kingsolver J. G., Huey R. B.), pp. 234–250 Sunderland, MA: Sinauer Associates [Google Scholar]
- 178.Gomulkiewicz R., Holt R. D. 1995. When does evolution by natural selection prevent extinction? Evolution 49, 201–207 10.2307/2410305 (doi:10.2307/2410305) [DOI] [PubMed] [Google Scholar]
- 179.Hoffmann A. A., Sgro C. M. 2011. Climate change and evolutionary adaptation. Nature 470, 479–485 10.1038/nature09670 (doi:10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 180.Kingsolver J. G. 2009. ASN Presidential Address: the well-temperatured biologist. Am. Nat. 174, 755–768 10.1086/648310 (doi:10.1086/648310) [DOI] [PubMed] [Google Scholar]
- 181.Kearney M., Porter W. P., Williams C. K., Ritchie S. A., Hoffmann A. A. 2009. Integrating biophysical models and evolutionary theory to predict climatic impacts on species' ranges: the dengue mosquito Aedes aegypti in Australia. Funct. Ecol. 23, 528–538 10.1111/j.1365-2435.2008.01538.x (doi:10.1111/j.1365-2435.2008.01538.x) [DOI] [Google Scholar]
- 182.Bogert C. M. 1949. Thermoregulation in reptiles, a factor in evolution. Evolution 3, 195–211 10.2307/2405558 (doi:10.2307/2405558) [DOI] [PubMed] [Google Scholar]
- 183.Bartholomew G. A. 1964. The roles of physiology and behaviour in the maintenance of homeostasis in the desert environment. Symp. Soc. Exp. Biol. 18, 7–29 [PubMed] [Google Scholar]
- 184.Ruibal R. 1961. Thermal relations of five species of tropical lizards. Evolution 15, 98–111 10.2307/2405846 (doi:10.2307/2405846) [DOI] [Google Scholar]
- 185.Tewksbury J. J., Huey R. B., Deutsch C. A. 2008. Putting the heat on tropical animals. Science 320, 1296–1297 10.1126/science.1159328 (doi:10.1126/science.1159328) [DOI] [PubMed] [Google Scholar]
- 186.Diamond S. E., Sorger D. M., Hulcr J., Pelini S. L., Del Toro I., Hirsch C., Oberg E., Dunn R. R. 2011. Who likes it hot? A global analysis of the climatic, ecological, and evolutionary determinants of warming tolerance in ants. Glob. Change Biol. 18, 448–456 10.1111/j.1365-2486.2011.02542.x (doi:10.1111/j.1365-2486.2011.02542.x) [DOI] [Google Scholar]
- 187.Corlett R. T. 2011. Impacts of warming on tropical lowland rainforests. Trends Ecol. Evol. 26, 606–613 10.1016/j.tree.2011.06.015 (doi:10.1016/j.tree.2011.06.015) [DOI] [PubMed] [Google Scholar]
- 188.Mahlstein I., Knjutti R., Solomon S., Portmann R. W. 2011. Early onset of significant local warming in low latitude countries. Environ. Res. Lett. 6, 034009. 10.1088/1748-9326/6/3/034009 (doi:10.1088/1748-9326/6/3/034009) [DOI] [Google Scholar]
- 189.Davis A. J., Jenkinson L. S., Lawton J. H., Shorrocks B., Wood S. 1998. Making mistakes when predicting shifts in species range in response to global warming. Nature 391, 783–786 10.1038/35842 (doi:10.1038/35842) [DOI] [PubMed] [Google Scholar]
- 190.Gorman G. C., Hillman S. 1977. Physiological basis for climatic niche partitioning in two species of Puerto Rican Anolis (Reptilia, Lacertilia, Iguanidae). J. Herpetol. 11, 337–340 10.2307/1563246 (doi:10.2307/1563246) [DOI] [Google Scholar]
- 191.Clark D. A., Piper S. C., Keeling C. D., Clark D. B. 2003. Tropical rain forest tree growth and atmospheric carbon dynamics linked to interannual variation during 1984–2000. Proc. Natl Acad. Sci. USA 100, 5852–5857 10.1073/pnas.0935903100 (doi:10.1073/pnas.0935903100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Feeley K. J., Wright S. J., Supardi M. N. N., Kassim A. R., Davies S. J. 2007. Decelerating growth in tropical forest trees. Ecol. Lett. 10, 461–469 10.1111/j.1461-0248.2007.01033.x (doi:10.1111/j.1461-0248.2007.01033.x) [DOI] [PubMed] [Google Scholar]
- 193.Vitt L. J., Avila-Pires T. C. S., Caldwell J. P., Oliveira V. R. L. 1998. The impact of individual tree harvesting on thermal environments of lizards in Amazonian rain forest. Conserv. Biol. 12, 654–664 10.1046/j.1523-1739.1998.96407.x (doi:10.1046/j.1523-1739.1998.96407.x) [DOI] [Google Scholar]
- 194.Tracy C. R. 1976. A model of the dynamic exchanges of water and energy between a terrestrial amphibian and its environment. Ecology 46, 293–326 10.2307/1942256 (doi:10.2307/1942256) [DOI] [Google Scholar]
- 195.McJannet D., Wallace J., Reddell P. 2007. Precipitation interception in Australian tropical rainforests: II. Altitudinal gradients of cloud interception, stemflow, throughfall and interception. Hydrol. Proc. 21, 1703–1718 10.1002/hyp.6346 (doi:10.1002/hyp.6346) [DOI] [Google Scholar]
- 196.Colwell R. K., Brehm G., Cardelús C. L., Gilman A. C., Longino J. T. 2008. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 322, 258–261 10.1126/science.1162547 (doi:10.1126/science.1162547) [DOI] [PubMed] [Google Scholar]
- 197.Raxworthy C. J., Pearson R. G., Rabibisoa N., Rakotondrazafy A. M., Ramanamanjato J.-B., Raselimanana A. P., Wu S., Nussbaum R. A., Stone D. A. 2008. Extinction vulnerability of tropical montane endemism from warming and upslope displacement: a preliminary appraisal for the highest massif in Madagascar. Glob. Change Biol. 14, 1703–1720 10.1111/j.1365-2486.2008.01596.x (doi:10.1111/j.1365-2486.2008.01596.x) [DOI] [Google Scholar]
- 198.Moritz C., Patton J. L., Conroy C. J., Parra J. L., White G. C., Beissinger S. R. 2008. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 322, 261–264 10.1126/science.1163428 (doi:10.1126/science.1163428) [DOI] [PubMed] [Google Scholar]
- 199.Chen I.-C., Shiu H.-J., Benedick S., Holloway J. D., Cheye V. K., Barlow H. S., Hill J. K., Thomas C. D. 2009. Elevation increases in moth assemblages over 42 years on a tropical mountain. Proc. Natl Acad. Sci. USA 106, 1479–1483 10.1073/pnas.0809320106 (doi:10.1073/pnas.0809320106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Longino J. T., Colwell R. K. 2011. Density compensation, species compositon, and richness of ants on a neotropical elevational gradient. Ecosphere 2, art 29. 10.1890/ES10-00200.1 (doi:10.1890/ES10-00200.1) [DOI] [Google Scholar]
- 201.Sheldon K. S., Yang S., Tewksbury J. J. 2011. Climate change and community disassembly: impacts of warming on tropical and temperate montane community structure. Ecol. Lett. 14, 1191–1200 10.1111/j.1461-0248.2011.01689.x (doi:10.1111/j.1461-0248.2011.01689.x) [DOI] [PubMed] [Google Scholar]
- 202.McCain C. M., Colwell R. K. 2011. Assessing the threat to montane biodiversity from discordant shifts in temperature and precipitation in a changing climate. Ecol. Lett. 14, 1236–1245 10.1111/j.1461-0248.2011.01695.x (doi:10.1111/j.1461-0248.2011.01695.x) [DOI] [PubMed] [Google Scholar]
- 203.Crozier L., Dwyer G. 2006. Combining population-dynamic and ecophysiological models to predict climate-induced insect range shifts. Am. Nat. 167, 853–866 10.1086/504848 (doi:10.1086/504848) [DOI] [PubMed] [Google Scholar]
- 204.Helmuth B., Kingsolver J. G., Carrington E. 2005. Biophysics, physiological ecology, and climate change: does mechanism matter? Annu. Rev. Physiol. 67, 177–201 10.1146/annurev.physiol.67.040403.105027 (doi:10.1146/annurev.physiol.67.040403.105027) [DOI] [PubMed] [Google Scholar]
- 205.Holt R. D. 1990. The microevolutionary consequences of climate change. Trends Ecol. Evol. 5, 311–315 10.1016/0169-5347(90)90088-U (doi:10.1016/0169-5347(90)90088-U) [DOI] [PubMed] [Google Scholar]
- 206.Parsons P. A. 1989. Conservation and global warming: a problem in biological adaptation to stress. Ambio 18, 322–325 [Google Scholar]
- 207.Hoffmann A. A., Hallas R. J., Dean J. A., Schiffer M. 2003. Low potential for climatic stress adaptation in a rainforest Drosophila species. Science 301, 100–102 10.1126/science.1084296 (doi:10.1126/science.1084296) [DOI] [PubMed] [Google Scholar]
- 208.Kellerman V., van Heerwaarden B., Sgrò C. M., Hoffmann A. A. 2009. Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science 325, 1244–1246 10.1126/science.1175443 (doi:10.1126/science.1175443) [DOI] [PubMed] [Google Scholar]