Abstract
Infectious diseases are intimately associated with the dynamics of biodiversity. However, the role that infectious disease plays within ecological communities is complex. The complex effects of infectious disease at the scale of communities and ecosystems are driven by the interaction between host and pathogen. Whether or not a given host–pathogen interaction results in progression from infection to disease is largely dependent on the physiological characteristics of the host within the context of the external environment. Here, we highlight the importance of understanding the outcome of infection and disease in the context of host ecophysiology using amphibians as a model system. Amphibians are ideal for such a discussion because many of their populations are experiencing declines and extinctions, with disease as an important factor implicated in many declines and extinctions. Exposure to pathogens and the host's responses to infection can be influenced by many factors related to physiology such as host life history, immunology, endocrinology, resource acquisition, behaviour and changing climates. In our review, we discuss the relationship between disease and biodiversity. We highlight the dynamics of three amphibian host–pathogen systems that induce different effects on hosts and life stages and illustrate the complexity of amphibian–host–parasite systems. We then review links between environmental stress, endocrine–immune interactions, disease and climate change.
Keywords: pathogens, biodiversity, amphibians, Batrachochytrium, trematodes, ranavirus
1. Introduction
Infectious diseases are increasing at an unprecedented rate [1] and are intimately associated with the dynamics of biodiversity [2–7]. However, the role that infectious disease plays within ecological communities is complex. For example, infectious disease may cause population declines and species extinctions [6,8] and alter the structure and function of ecological communities [9,10]. Concurrently, however, parasites may also play a role in promoting biodiversity and function as indicators of ecosystem productivity and resilience [11–13]. The complex effects of infectious disease at the scale of communities and ecosystems are fundamentally driven by the interaction between individual hosts and pathogens. Whether or not a given host–pathogen interaction results in progression from infection to disease is largely dependent on the physiological characteristics of the host within the context of the external environment. Here, we highlight the importance of understanding the outcome of infection and disease in the context of host ecophysiology using amphibians as a model system. Amphibians are ideal for such a discussion because, as we discuss below, amphibian populations are experiencing declines throughout the world and disease is an important factor implicated in declines and extinctions. Furthermore, exposure to pathogens as well as the host's responses to infection can be influenced by a wide range of factors related to physiology, including host life history, immunology, endocrinology, resource acquisition, behaviour and changing climates [14].
In our review, we first briefly discuss the relationship between disease and biodiversity. Throughout, we use the term ‘parasites’ inclusively to encompass both microparasites (e.g. viruses, bacteria and fungi) and macroparasites (e.g. helminths (worms), arthropods). ‘Pathogens’ are types of parasites that tend to cause pathology or disease in hosts under particular conditions. We highlight specific dynamics of three important amphibian host–pathogen systems that include viruses, trematodes and a chytrid fungus. These pathogens induce a variety of different effects on amphibian host species and life stages and serve to illustrate the complexity of amphibian–host–parasite systems. We then review links between environmental stress, endocrine–immune interactions and disease. Finally, as an applied problem linking host–pathogen physiology across multiple spatial and temporal scales, we discuss how climate change may influence patterns of disease and amphibian conservation.
2. The ‘biodiversity crisis’ and amphibian populations
A so-called ‘biodiversity crisis’ is exemplified by population declines, range reductions and extinctions of amphibian species around the world [15–18]. One estimate suggests that the current extinction rates of amphibians may be 211 times the background rate of extinction [19]. According to criteria of the International Union for Conservation of Nature, a higher percentage of amphibians are threatened than birds or mammals, with many amphibian populations and species on the brink of extinction [16]. From an evolutionary historical perspective, amphibians may be part of a sixth major extinction event [20].
There appears to be no single cause for amphibian population declines. The causes for the population decline or extinction of a given species may be different from region to region and even in different populations of the same species [14]. Amphibians, as other organisms, are continuously exposed to numerous stressors throughout their life cycle [21]. Natural stress associated with competition, predation, resource availability, reproduction and disease may be compounded by human-induced stresses such as habitat destruction, environmental contamination, invasive species and changes in the climate and atmosphere. These stressors affect amphibians at the molecular, physiological, individual, population and community levels. Moreover, there may be non-additive interactions between more than one factor, interspecific differences and even differences between life stages in how amphibians react to stressors.
Amphibians are hosts for a wide range of infectious organisms, including viruses, bacteria and fungi—collectively known as the microparasites—as well as trematodes, nematodes, cestodes, acanthocephalans, mites and copepods—collectively known as the macroparasites [22–24]. While many such infections often cause relatively little damage, some pathogens are particularly detrimental to amphibian viability, and infectious diseases have been implicated in numerous population declines [18,25–27]. Under certain conditions, amphibian pathogens can induce a variety of sublethal effects, including malformations, reduced growth and development, and reduced foraging and competitive abilities [21,28]. Moreover, pathogens can kill individual amphibians. At certain levels, mortality may eventually lead to local population extinctions and even extinctions of entire species [25,27–30]. In the sections that follow, we explore disease as an ecophysiological process before applying these concepts to three pathogens that cause damage to individual amphibians or entire populations.
3. Disease as an ecophysiological process
At its core, disease is both an ecological and physiological process. From an ecological standpoint, infectious diseases represent the product of interactions between at least two species, a host and a pathogen, and frequently many other co-occurring species in the community. For instance, parasite transmission can be influenced by the presence of competitors, predators and even other parasites [31–34]. From a physiological perspective, processes such as infection and pathology are strongly influenced by host immunity, endocrine regulation, pathogen replication rate and body condition that in turn are influenced by environmental conditions. This suggests that broader integration between ecological and physiological research (i.e. ecophysiology, [35]) may be particularly useful for understanding and forecasting infectious diseases in human and wildlife populations [36].
Disease is a function of host response—and is marked by disruption of physiological homeostasis. Pathology, or damage to host cells and tissues caused by progression from infection to a diseased state, may cause a modification of host behaviour and physiology. The range of physiological responses employed by diseased hosts broadly fall into two categories: tolerance and resistance [37–39]. Resistance strategies, or those that directly limit pathogen burden, include immunity and other processes whereby the host actively ‘fights’ infection. Tolerance strategies limit the damage caused by infectious disease. Tolerance mechanisms include processes for tissue repair [38], but in general are less well-understood than those related to resistance. Hosts may employ a combination of tolerance and resistance strategies against pathogens that include behaviour, immunity and other physiological responses [40–42]. The strategy employed to cope with and fight pathogens plays an important role in driving pathogen evolution and infection outcome in individual hosts, populations and ecological communities [38,39,43,44]. Furthermore, host responses to infection and disease must be considered within the context of the biotic and abiotic environments because environmental factors drive pathogen-specific traits as well as host responses.
Understanding how hosts physiologically respond to infectious pathogens provides a mechanistic explanation for disease outcome at the level of the individual, which can also cascade up to higher levels of organization. Changes in host physiology can be quantified and establish a cause-and-effect link between host response and infection outcome. However, identifying the relative importance of pathogen exposure versus host defences may not be straightforward; in many cases, disease variation is probably due to differences in exposure and host defences, highlighting the value of experimental research controlling for exposure in evaluating the role of defences among host species (although these factors clearly influence one another over evolutionary time scales) [45–47]. Moreover, pathology of amphibian infections functions within a backdrop of additional anthropogenic stressors. Factors including atmospheric change, habitat destruction, invasive species and environmental contaminants are likely to interact in complex ways with infectious disease [48]. For example, a number of studies have shown that contaminants may reduce immunocompetence in amphibians, and increase susceptibility to disease [49–51]. Similarly, ultraviolet B radiation may interact synergistically with disease to influence susceptibility [52]. Invasive species often contribute new pathogens to native amphibian assemblages and promote disease [53].
While temperature is an important factor mediating pathogen growth and reproduction [54], it also interacts with host physiology to affect disease susceptibility.
4. Three host–pathogen systems
(a). Amphibian ranaviruses
(i). Overview
Ranaviruses are large double-stranded DNA viruses (ca 105 kbp, 150 nm diameter [55]) that infect ectothermic vertebrates [56]. Currently, three amphibian ranaviruses have been identified: frog virus 3 (FV3), Bohle iridovirus (BIV) and Ambystoma tigrinum virus (ATV) [57]. Mortality events and/or infections involving viruses have been detected in amphibian population across the globe [58–68]. While ranavirus infections have been detected in both adult and larval amphibians, the virus appears to be most lethal in larvae [69]. Transmission of the virus is horizontal, and can occur by exposure to infected water or soil or via cannibalism of infected individuals [70]. Ranaviral disease is characterized by systemic haemorrhage and tissue necrosis, ultimately resulting in organ failure (usually the liver or kidneys) within less than a week of exposure [56,62].
(ii). Factors that influence pathogen abundance and transmission
Disease dynamics in this system are a function of both host and virus processes together with environmental factors. Given that the transmission of ranaviruses largely occurs within aquatic habitats, we focus on disease dynamics within the larval population of amphibian hosts. The likelihood of exposure to ranaviruses is influenced by multiple factors including virus persistence outside of hosts. Many amphibians use pond habitats as breeding sites. These sites are characterized by variation in hydroperiod (i.e. proportion of days with water). Importantly, amphibian species differ in their preference for permanent versus ephemeral water bodies (e.g. wood frogs (Rana sylvatica) breed in temporary ponds while American bullfrogs (Rana catesbeiana) breed in permanent ponds). Although contaminated water and sediment are effective media for virus transmission within natural ponds, the virus is inactivated following pond drying [71]. Thus, species that breed in temporary ponds that dry each year may be exposed to ranaviruses less frequently, because viability of the virus among seasons outside the host is unlikely [72]. Without frequent exposure to the virus, the selective pressure on such species may not be strong enough to favour the evolution of resistance. In contrast, species that breed in permanent water bodies may be exposed to ranaviruses more frequently because the pathogen can persist in water, sediment or other reservoir species [56]. Such frequent exposure may have selected for resistance to ranavirus infection.
The presence of intra- and interspecific reservoirs in the environment also can play an important role in virus transmission. For example, adult and juvenile tiger salamanders (Ambystoma tigrinum) function as intraspecific reservoirs for ATV transmission by introducing the virus into the larval population [73]. Although pond drying may eliminate the virus from the system, intraspecific reservoirs can act to maintain the pathogen in the system between years. In permanent water bodies, potential reservoirs include amphibian species with larvae that develop over more than one season (e.g. R. catesbeiana), that exhibit paedomorphosis (e.g. A. tigrinum), or with aquatic adults (e.g. red spotted newts (Notophthalmus viridescens); [56]. However, little is currently known regarding interspecific transmission of ranaviruses within natural amphibian habitats.
(iii). Factors that influence host responses to infection
Once introduced into a community, a number of factors may contribute to host pathology including host traits [74] as well as environmental stressors. Owing to similarities in innate and adaptive immune responses to pathogens, species within the same family are expected to show similar levels of susceptibility to infection. Recently, one of us demonstrated that host phylogeny was an important factor driving patterns in species susceptibility to ranavirus infection in the laboratory [47]: species in the family Ranidae were more susceptible to infection compared with species within the families Ambystomatidae and Hylidae. These results support field patterns of frequently reported die-off events in the family Ranidae [63]. However, the mechanisms underlying such family-level variation in susceptibility have yet to be examined.
In wild amphibian populations, mass mortality events associated with ranaviruses have frequently been associated with individuals undergoing metamorphosis. Given that metamorphosis is a natural period of immune suppression in amphibians, it has been hypothesized that susceptibility to ranaviruses (i.e. infection and mortality rates) is highest during this stage of development [56]. While this hypothesis was supported for several species within the genus Rana, species tested from other amphibian families were equally susceptible across developmental stages [75,76].
(iv). Consequences of infection for amphibian populations and ecosystems
Natural and anthropogenic stressors are common in aquatic communities. Research over the last decade has shown that environmental stressors could be important contributors to ranaviral disease dynamics within amphibian habitats. Water temperature is one environmental stressor that could affect host susceptibility to ranavirus infection. For example, Rojas et al. [77] reported an increase in ATV virulence at lower temperatures, which appeared to be related to a decrease in host immune function at colder temperatures. In support, Maniero & Carey [78] reported a decrease in T-lymphocyte proliferation and serum complement activity at low temperatures in northern leopard frogs. They hypothesized that a decrease in T-lymphocyte production would result in decreased signalling of B-lymphocytes, which would reduce antibody production and, in turn, compromise immunity during periods of lower temperature. Thus, it is likely that prevalence of ranaviruses in amphibian populations is influenced by water temperature [79].
(b) Trematodes. and amphibian disease
(i). Overview
Digenetic trematodes are flatworm parasites (or flukes) with complex life cycles involving sequential transmission from a molluscan first intermediate host (e.g. a snail) to an invertebrate or vertebrate second intermediate host (e.g. a frog) to a vertebrate definitive host (e.g. a bird) (although there is considerable variation around this basic template) [80]. Owing to the biphasic life cycle of amphibians, which generally includes an aquatic larval stage followed by a more terrestrial adult stage, they function as both second intermediate or definitive hosts to hundreds of different trematode species [22,81–83]. Infection is often acquired in one of two ways. For some trematodes, larval amphibians get colonized and invaded by free-swimming infectious stages called cercariae, which emerge from infected snails and actively penetrate second intermediate host tadpoles. These parasites will encyst within the amphibian tissue as metacercariae, ultimately awaiting the frog host's consumption by a suitable definitive host [22,84,85]. Alternatively or additionally, amphibians can become infected by consuming an infected host, such as an insect, a mollusc, or another amphibian. In this instance, the amphibian will function as a definitive host (i.e. the host in which the parasite reproduces sexually). Thus, parasite transmission is horizontal but depends on the convergence of and interactions among multiple host species within a community.
(ii). Factors that influence host responses to infection
Historically, it was often assumed that trematodes caused little to no pathology in amphibian hosts [22]. While often true, important exceptions have emerged that have the potential to influence host survival and population viability. For instance, the trematode Ribeiroia ondatrae has gained recent attention for its role in causing limb deformities in frogs and toads [86–89]. This parasite, which uses rams horn snails, larval amphibians and birds as its primary hosts, causes increased mortality and grotesque malformations in a wide range of North American amphibian species [90,91]. These malformations, which can affect greater than 50 per cent of emerging frogs in a population, primarily affect the limbs, including extra limbs or limb elements, missing limbs, abnormal skin webbings and misshapen limb bones [92–97]. Intriguingly, malformations—while detrimental to the amphibian—may be adaptive for the parasite by increasing the likelihood an infected frog is consumed by a suitable definitive host such as a bird [87,89,98]. Other trematodes, such as the broadly distributed echinostome group that infect amphibian kidneys, have also been shown to influence amphibian survival and morphological development under certain conditions [99–101]. For instance, exposure to cercariae of Echinstoma trivolvis can cause renal impairment in early-stage tadpoles leading to whole-body oedema or mortality.
Unlike infections by microparasites, macroparasites do not generally reproduce within a single host. Thus, the numbers of parasites observed within a host represent separate exposure events, rather than a single infection followed by intrahost replication (as seen for microparasites). Because the risk of pathology depends on the number of parasites within a host (intensity-dependent pathology), factors that influence host exposure play a central role in driving disease risk for macroparasitic infections. With Ribeiroia, for example, the likelihood of a tadpole dying or become malformed increases monotonically with the number of cercariae to which it is exposed [91,93]. Correspondingly, the mean Ribeiroia infection in an amphibian population can be a strong predictor of the malformation frequency among metamorphosing frogs [95].
After accounting for parasite exposure, we can also explore the factors that determine whether a host exhibits pathology, which will be influenced by both host resistance and host tolerance [38]. Experimental studies involving trematode parasites and larval amphibians have provided clear evidence that hosts vary in their defences against infection. For instance, in a comparative study involving standardized exposures of 13 amphibian species to Ribeiroia cercariae, Johnson et al. [91] found that resistance, or one minus the proportion of parasites recovered following exposure, differed by two orders of magnitude among species. While some species had low resistance (approx. 40% of parasites recovered), others had near complete resistance (less than 1% recovery), which probably reflects differences in behaviour [102,103], development time and especially immunity [49,51,104].
Variation in pathology can also be driven by differences in host tolerance, such that the risk of disease differs among hosts even after accounting for infection differences [43]. For instance, early-stage amphibian larvae with smaller body sizes often exhibit lower tolerance to infection and a higher likelihood of mortality or other pathologies relative to late-stage larvae [43,44,97,101]. The mechanism for this pattern is not well understood, but may stem in part from the proportional increase in tissue damage caused by parasites in smaller host individuals (or smaller species, i.e. each parasite impacts a greater fraction of the host's total body volume). Finally, features of the parasite under consideration will also influence pathology. Larger trematode cercariae that use chemical proteases to penetrate amphibian tissue typically cause more damage than species with small cercariae that enter existing host body cavities [89]. Taken together, these examples illustrate the multitude of both host and parasite factors that jointly determine patterns of disease in ecological communities.
(iii). Factors that influence pathogen abundance and transmission
In natural systems, several ecological factors are likely to influence trematode parasite exposure in larval amphibians. Chief among these is habitat use and whether an amphibian develops in the type of aquatic environment used by the snail intermediate host for a given trematode. Ribeiroia depends on rams horn snails (Helisoma spp.) that are most common in lentic habitats such as ponds and lakes; amphibians developing in streams are therefore unlikely to be exposed or to develop parasite-induced malformations. Thus, one of the greatest predictors of amphibian exposure will be the density of infected snails in a system multiplied by the number of cercariae released per snail (parasite production). Second, the timing of host development will affect the number of parasites to which a host is exposed. Some amphibians, such as wood frogs (Lithobates sylvaticus), develop very early in the season and can sometimes complete metamorphosis before pond temperatures warm enough for snails to release large numbers of cercariae. Moreoever, species that develop quickly (e.g. western toads, 45 days to metamorphosis) will probably be exposed to far fewer parasites than those with extended larval development (e.g. bullfrogs, 2 years to metamorphosis). Indeed, Todd [105] hypothesized that rapid development and a more terrestrial life style in amphibians represent evolutionary responses to decrease water-borne infection risk.
Habitat usage can also have important effects on parasite abundance and infection success in amphibian hosts. The snail hosts used by Ribeiroia, for instance, tend to be most common in lentic habitats that are highly productive or eutrophic (see [106,107]). Experimental studies by Johnson et al. [108] showed that increases in nutrient enrichment, as might occur in association with livestock grazing or fertilizer application, can increase both the density of infected snails and the number of cercariae produced per infected snail. This led to a two- to fourfold increase in infection within amphibian hosts [108]. Similar links between nutrient enrichment and infections have also been reported for other pathogens of both humans and wildlife [109,110]. Rohr et al. [51] also found that, alongside a positive effect of nutrients, herbicides such as atrazine could further increase infections in amphibians by reducing their resistance to infection (although this pathway was not shown for Ribeiroia specifically). These findings indicate that agricultural habitats can enhance trematode infections in amphibian hosts both by increasing parasite abundance and by increasing infection success. It is worth noting, however, that agricultural practices can also cause a decrease in the activity of definitive hosts, such as birds, highlighting the complexity of interactions between land-use change, infection patterns and amphibian pathology [102,111,112].
Finally, other species in the environment can influence tadpole exposure by reducing the survival or transmission success of parasite cercariae. In experimental studies, for instance, aquatic insects and small fishes actively consume Ribeiroia cercariae, ultimately reducing infection in amphibian hosts by up to 48 per cent [113]. Predation on parasites is considered to be one important mechanism through which community diversity can influence disease risk [34,114]. Alternate hosts represent another mechanism through which community composition affects parasite success. While Ribeiroia can infect a wide range of amphibian species, these species differ considerably in their ability to support an infection. As a result, the specific composition of the larval amphibian assemblage has the potential to influence the ability of the parasite to spread from snail to amphibian hosts and the resulting availability of infection for potential bird hosts. For instance, the presence of hosts with low infection rates (such as gray treefrogs) can significantly reduce the amount of parasites that colonize more sensitive hosts (such as American toads), causing an increase in amphibian survival, a reduction in pathology, and a decrease in the total number of parasites in the system [115]. While further work is needed to understand the importance of such mechanisms under natural conditions, these experimental results suggest that the composition of aquatic communities have the potential to strongly affect parasite transmission and patterns of amphibian disease.
(iv). Consequences of infection for amphibian populations and ecosystems
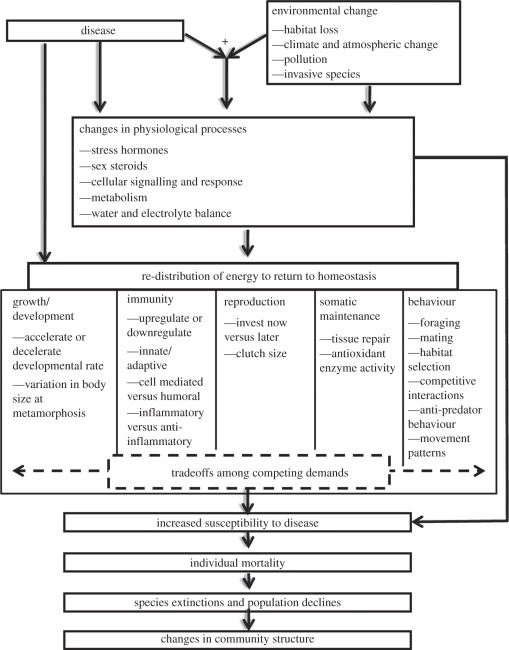
Little is known about the consequences of trematode infections for amphibian populations and aquatic ecosystems as a whole. What is clear is that infections by trematodes such as Ribeiroia can strongly reduce individual host performance and survival. For instance, while parasite-induced malformations can affect a large fraction (e.g. 20–100%) of the recently metamorphosed frog population, few malformations are ever observed in adult frogs returning to breed, suggesting that such abnormalities are detrimental to frog survival [94,98]. Furthermore, experimental studies have demonstrated that malformed frogs have reduced jumping distances, poorer foraging success, decreased endurance and slower swimming speeds [98]. In natural environments, malformed frogs were more likely to occupy suboptimal habitats and allowed simulated predators to approach more closely than did their normal counterparts [98]. Perhaps as a result, metamorphic frogs with one or more malformations exhibited a 22 per cent lower biweekly survival rate relative to normal individuals [98]. Considering that malformations have been observed in a wide range of amphibian species, including those known to be in decline, there is significant potential for infections and malformations to influence amphibian survival (figure 1).
Figure 1.
Disease can impose direct or indirect effects on individuals, species and populations via modification of physiological processes and subsequent changes in growth, development, reproduction, somatic maintenance, immunity and behaviour. Because energy is finite, there are tradeoffs among competing homeostatic and physiological demands that operate in the context of the ecological environment. Synergistic interactions between disease and other anthropogenic sources of environmental change result in complex and often unpredictable changes to disease susceptibility, mediated through physiological interactions. Energetic and metabolic tradeoffs are particularly important in ectotherms, including amphibians, and may play an important role in putting amphibians at particular risk for tradeoffs with infection and physiology. Physiology-based approaches to understand variation in and changes to disease susceptibility allow for a more thorough understanding of the mechanisms mediating species extinctions and declines. These approaches provide cause-and-effect relationships between infection and mortality events at the individual, species and population level.
Collectively, these observations suggest that infections by trematodes such as Ribeiroia have the potential to affect amphibian population dynamics and, by extension, community interactions and ecosystem processes. Nonetheless, the long-term data necessary to evaluate this hypothesis are not yet available, precluding a quantitative assessment of the influence of parasite infection. Thus, while infection and malformations likely cause high mortality in larval and metamorphic amphibians, survival of these life stages already tend to be low and highly variable through time, such that the mortality caused by parasites could be compensatory. Moreover, given that the distribution of parasites such as Ribeiroia is variable across space and requires the presence of other species such as rams horn snails, parasite-induced impacts in one wetland could be alleviated by migration from surrounding, parasite-free sites (i.e. a ‘rescue effect’).
(c). The chytrid fungus, Batrachochytrium dendrobatidis
(i). Overview
The emerging fungal pathogen Batrachochytrium dendrobatidis (Bd) has been detected on every continent where amphibians exist [116] and is implicated in numerous amphibian population declines and extinctions globally [26,30,116]. A broad range of species are susceptible to Bd; as of 2009, 350 amphibian species have tested positively for Bd infection [116].
Bd undergoes a complex lifecycle, consisting of an infective, substrate-independent, aquatic zoospore stage and a non-motile, substrate-dependent zoosporangia stage. A thermal optimum between 17 and 25°C exists for Bd growth and development [117]. Exposure to temperatures exceeding 30°C can kill Bd cultures [117,118] and cold temperatures (less than 17°C) slow growth rates and lengthen zoospores maturation time [117]. Woodhams et al. [119] showed that the pathogen might compensate for these temperature effects by increasing the total number of zoospores produced in each zoosporangia at non-optimal (cooler) temperatures, when development rate is slowed. Because temperature plays an important role in Bd persistence and growth, seasonal variation in risk of exposure to Bd is an important factor in mediating disease epidemics. For example, Bd-associated mortality tends to be correlated with cooler seasons [120,121]. Bd also appears to have an optimal pH of 6–7.5 and growth is slower at more acidic and more alkaline conditions [122]. Some evidence suggests that Bd is not killed by ultraviolet light (270–320 nm). However, recent field data found a negative association of Bd with ultraviolet radiation in Europe [123]. Bd is easily killed by several disinfectants [124,125]. Bd is highly sensitive to desiccation. Complete drying at room temperature kills Bd within 3 h [124]. It has been suggested that Bd may be capable of persisting in a ‘resting’ stage or in a saprobic form within the abiotic environment during unfavourable conditions [118,126] and studies have shown that zoospores can survive for up to seven weeks in lake water, three to four weeks in deionized water [124], and up to three months in sterile moist river sand without nutrients [122]. While abiotic optima exist for Bd and are likely to drive risk of exposure, Bd can grow and reproduce outside of these optima, allowing the pathogen to persist in both temperate and tropical areas.
(ii). Factors that influence host responses to infection
Infection outcome, specifically manifestation of the disease chytridiomycosis, varies considerably among amphibian species [127–131]. Some species appear highly susceptible to Bd [128], others may possess resistance to the pathogen [127], and some species vary more subtly within the middle of the ‘susceptibility continuum’ [128,131].
Exposure to Bd is probably influenced in complex ways by a number of species-specific life-history characteristics operating within the context of the biotic and abiotic environments [27,30,116,132]. For example, Bancroft et al. [74] found that body size at maturity is a good predictor of documented infection for amphibian species in the continental United States. As would be predicted in the context of an aquatic pathogen, several studies have found that the degree of reliance on aquatic habitat (versus terrestrial habitat) is an important correlate of infection [74,133]. Other host-specific traits, including social behaviour, may predispose certain species to greater risk of exposure to and infection with Bd [74,128].
Bd is chemically attracted to and infects keratinized cells of amphibians [134]. Infection occurs in both larval and post-metamorphic stages of amphibian development; larval infection is restricted to keratinized tooth rows and jaw sheaths, while infection in post-metamorphic amphibians may be spread over the entire surface of the body since the epidermis is completely keratinized. Larval infection may lead to impaired foraging efficiency and therefore reduced growth and slower development [135,136]. In post-metamorphic amphibians, infection of keratinized epidermal layers may lead to a disruption of electrolyte transport across the skin and impairment of osmoregulation, which can be fatal [137,138]. Pathogenicity of Bd in amphibians may also be driven by damage to host tissue caused by enzymes secreted by the fungal zoospores and zoosporangia [139].
The metamorphic stage has been suggested as the most vulnerable life-history stage in amphibians [140,141]. During metamorphosis, amphibians undergo a temporary period of hormonally induced immunosuppression [141]. This may lead to greater susceptibility to disease at the metamorphic stage, or greater pathology of infection at this developmental transition. In a field survey, Russell et al. [142] observed the highest frequency of infection and highest infection load in metamorphic amphibians, when compared with larval and adult amphibians of the same species, surveyed at the same location, at the same time.
Infection intensity, specifically the accumulation of infection load above a specific threshold value, may determine Bd dynamics within species [143] and populations [140,144]. For the mountain yellow-legged frog (Rana muscosa), Vredenburg et al. [144] and Briggs et al. [140] found that across surveyed populations of various sizes, declines in frog numbers were not evident until average infection intensity reached a threshold level of zoospore equivalents. Above this threshold, mass mortalities and population declines were consistently observed. In a laboratory setting, mortality is greatest in individuals with the highest infection loads [138,143].
Responses to infection may include tolerance and resistance strategies. The physiological mechanisms employed through these strategies may determine whether infection progresses to a disease state and whether disease results in reduced fitness. Resistance strategies to Bd are likely to include defensive immune responses that may drive Bd pathogenesis. It is unknown how Bd is recognized by the immune system [145]. Innate responses have been the most thoroughly studied aspects of immunity against Bd [146–149] although the range of innate responses studied has been limited [147,145]. For example, antimicrobial peptides (AMPs) secreted from the glands of amphibian skin have been well-studied aspects of immunity against Bd [146,147]. AMPS constitute an important and relevant defence against Bd by helping to prevent initial colonization of the skin by the fungal pathogen, and some studies have shown that AMPs may correlate strongly with susceptibility to Bd [148]. The full picture of pathogen-induced immune regulation is likely to include other innate, adaptive and signalling (e.g. cytokine) responses to Bd. There is evidence of cellular and humoral responses to Bd, with some evidence of cellular responses at the innate and adaptive levels, including skin inflammation [150,151] and little support for a memory response to the pathogen [149,152]. It is possible that pathology of infection is caused not only by Bd infection itself, but also from the inflammatory responses triggered by hosts themselves. Immunopathology is a little understood phenomenon in the amphibian–Bd system.
Fever may be a part of the behavioural–physiological repertoire in response to a pathogen and is the most outstanding component of the acute phase immune response [153]. Thus, Richards-Zawacki [154] discovered that during a Bd epidemic, increases in the body temperature of Panamanian golden frogs (Atelopus zeteki) reduced the odds of infection. Geiger et al. [155] showed that elevated temperature regimes cleared Bd from Midwife toad tadpoles (Alytes obstetricans), while Weinstein [156] and Retallick & Miera [157] showed that housing infected adults at high temperatures cleared experimental Bd infection. Han et al. [158] found no evidence of behavioural fever in larvae of four frog species exposed to Bd.
Tolerance mechanisms employed by amphibians against Bd are relatively unknown. However, it is possible that some species naturally possess more effective barriers to infection (i.e. increased skin thickness, greater constitutive levels of protective mucus or anti-fungal secretions from skin). The presence of certain strains of bacterial microflora on amphibian skin may be important mechanisms of tolerance of Bd infection (as they are not an active resistance strategy employed by the host). Certain bacterial strains found on amphibian skin produce toxins that inhibit and also kill Bd [159–161].
In addition to amphibian-produced skin peptides, recent evidence shows that the skin of healthy amphibians is host to a diverse symbiotic bacterial community [162–167]. Not surprisingly, in vitro some of these bacteria inhibit Bd growth [159] by producing metabolites that are likely to inhibit Bd zoospore development or colonization [160,168]. Recent in vivo experiments suggest that supplementing the skin microbiota with these anti-Bd bacteria can actually reduce morbidity and mortality associated with Bd infection in amphibians [169,170] and thus reducing the natural microbiota can increase morbidity following Bd exposure [171]. There is potential for using beneficial bacteria for prevention and treatment of Bd in threatened populations. However, many basic ecological and evolutionary questions relating to these symbiotic communities are also being discussed [7]. For instance, does natural selection act on the structure or function of these communities more strongly? And is richness per se of the community important for disease resistance or is the presence of single key mutualists driving Bd resistance? Rapid advances in molecular sequencing technologies are allowing for much more complete quantitative assessments of these symbiotic communities and will allow us to address many of these questions. For example, McKenzie et al. [172] have recently completed the first analysis of frog skin microbial communities and have demonstrated clear species-specific communities across sites.
In reality, innate immunity on amphibian skin may be a function of the balance of antimicrobial peptides and symbiotic bacteria [173,174]. Antimicrobial peptides might serve as a filter for selecting the symbiotic bacterial community, or may fill in gaps in immune defence where symbiotic bacteria fall short. The challenge will be to design integrative studies that are able to examine the numerous defence mechanisms of frogs, both host and bacterially driven, that all function to influence disease resistance in natural populations of amphibians.
Genetic and phenotypic strain differences in Bd may lead to differences in virulence, pathogen growth and development [116,143], as well as infectivity [143,157,175,176]. Further, the type and the magnitude of host physiological responses may ultimately determine if an individual becomes infected with a pathogen as well as the outcome of infection after host and pathogen make contact [177].
(iii). Factors that influence pathogen abundance and transmission
Both external (environmental) and internal (host-specific) factors may drive patterns of susceptibility in the amphibian–Bd system. At a global scale, climatic factors—most notably patterns of temperature and precipitation—have been correlated with disease susceptibility [18,27,178]. Environmental factors may also alter pathogen growth and development [117,119] as well as behavioural, ecological and physiological responses of hosts [30,116].
Exposure to Bd is determined by persistence and transmission of Bd in the biotic and abiotic environments. Similarly, traits associated with certain amphibian species, namely those that put them at more or less risk of coming into contact with Bd, drive patterns of disease across geographical regions [74,133]. These traits may include affinity for certain temperatures or social tendencies [74,128,133,158]. Once exposure to Bd occurs, host responses as well as pathogen-specific traits play an integral role in driving the pathology of infection.
Persistence of Bd in the environment and, therefore, the chance of exposure to the pathogen may be driven by the presence or absence and abundance of other biota, especially certain reservoir hosts (i.e. hosts that support maintenance and growth of the pathogen) may change pathogen dynamics from density dependent to density independent [179]. Several species have been suggested to be reservoir hosts for Bd. Most notably, the American bullfrog (Lithobates catesbeianus) can persist with extremely high infection loads without signs of infection or manifestation of chytridiomycosis [53,127,128] and may be highly competent disease reservoirs in some amphibian assemblages [180]. Because larval amphibians can support sublethal infection between larval and post-metamorphic amphibians [181], it has been suggested that the larval stage of amphibians may similarly serve as a pathogen reservoir in the Bd system [141]. Moreover, the invasion of exotic reservoir hosts could dramatically increase the Bd exposure in native hosts whose susceptibility to infection is unknown [53]. Because substantial variation in interspecific and intraspecific susceptibility to Bd exists, it is possible that exposure to Bd is dependent on species diversity and identity in amphibian assemblages.
(iv). Consequences of infection for amphibian populations and ecosystems
Because Bd has a global distribution and is associated with numerous population declines and extinctions throughout the world, one can expect changes in populations of sympatric species and within ecosystems as Bd emerges. However, because different species, life stages and populations may display different effects after exposure to Bd, it is difficult to predict how the specific changes will be manifested in natural systems. Recent evidence suggests that Bd can alter community structure. For example, the presence of competitors and predators can affect growth, susceptibility and survival of larval amphibians exposed to Bd [182,183]. Sub-lethal consequences of Bd infection can alter host behaviours with consequences for disease dynamics. For example, Bd can cause changes in schooling larvae [158] and natural contact patterns between hosts [184] may increase Bd transmission probability.
As Bd infection and amphibian declines occur in some of the world's most species-diverse communities, examining Bd-induced changes in community structure is of considerable importance. For example, high amphibian species richness could underlie long-term, low-level persistence of chytridiomycosis through reservoir species [32].
Despite the rapid and continued spread of Bd, the influence of host diversity on Bd dynamics is not well known. However, recent experimental evidence by Searle et al. [185] showed a dilution effect where increased species richness reduced disease risk, even when accounting for changes in density. The influence of host species diversity on infection dynamics remains an important topic in disease ecology for which the amphibian–chytridiomycosis system may provide a tractable test.
Recent studies have shown that chytridiomycosis also has consequences at the ecosystem level. For example, through experimental exclusions of tadpoles from pre- and post-decline streams, Connelly et al. [186] found dramatic increases in inorganic sediments, increases in stream algae biomass and changes in algal community composition immediately following larval amphibian extirpations. Ecosystem-level consequences of such extensive biodiversity loss are likely to be complex and long-lasting, encompassing concurrent changes in primary productivity, energy transfer between aquatic and terrestrial habitats and the composition and interactions among organisms found at multiple trophic levels.
5. Summary of host–pathogen systems
A comparison of the three host–pathogen systems presented here reveals common trends and provides insight into the larger phenomenon of ecophysiology of amphibian disease. For example, sensitivity and susceptibility to different pathogens varies between amphibian life-history stages. In addition, susceptibility to pathogens and/or the host's resulting pathology may be exacerbated during the transition between the larval and post-metamorphic stage. Hormonally induced immunological restructuring and suppression occurring naturally at metamorphosis may predispose amphibians to opportunistic infections and pathogen-induced mortality [141,187]. In many instances, including those outlined here, infection load and pathology of infection show a positive relationship; as hosts acquire more trematodes, virus particles or fungal zoospores, they tend to suffer greater morbidity and mortality as a function of infection [91,93,138,144]. In addition, species or pathogen-specific ‘infection thresholds’ may exist, above which mortality occurs [144]. The phenomenon of infection thresholds has yet to be tested in different pathogen taxa, but may be particularly relevant when elevated infection load causes greater impairment of vital organ function. The observations that microparasites (e.g. viruses, bacteria and fungi) and macroparasites (e.g. worms and arthropods) can both cause intensity-dependent pathology owing to differential exposure rates are intriguing.
An important theme emerging from and extending beyond the case studies provided is the importance of environmental reservoirs and cofactors in driving disease dynamics in host–pathogen systems. Biotic and abiotic sources for maintenance of the pathogen maintain disease even when highly susceptible hosts decline [179,188]. In our case studies, substantial interspecific variation in susceptibility to the pathogens exists [47,56,90,91,131] and may drive patterns of pathogen transmission and disease emergence and re-emergence over time and space. Generally, in terms of host responses to pathogens, there is variation in interspecific and intraspecific tolerance and resistance to infection [43,44,132]. Mechanisms for tolerance and resistance may differ among life-history stages and may change based on tradeoffs with competing physiological demands such as growth and reproduction. Finally, ecological stressors may compound the effects of disease and may act synergistically to promote mortality events associated with disease [21,28].
6. Links between environmental stress, endocrine–immune interactions and disease
In the host–pathogen systems discussed above, and presumably in other systems, infections are more likely to cause host damage under particular conditions. Therefore, a more in-depth examination of how environmental stress can influence host defences against infections is warranted. One primary physiological response of vertebrates to stressful environmental stimuli, including stress tied with disease, is an increase in circulating glucocorticoids (cortisol or corticosterone, depending on the vertebrate species). These hormones are produced from the adrenal gland (interrenal in amphibians) via the hypothalamic–pituitary–adrenal axis (HPA; HPI in amphibians) and help animals survive environmental perturbations by mobilizing energy stores, modulating immune responses and suppressing non-vital physiological responses, such as reproduction [189].
In amphibians, diverse environmental stressors ranging from pond-drying [190,191] to predator presence [192,193], to environmental pollutants [194–197] to habitat fragmentation [198] can alter the HPA (HPI in amphibians) axis activity and influence circulating corticosterone levels. The direction and the magnitude of response vary based on numerous factors, including the specific ‘stressor’ being examined, the amphibian species and the life stage. A key point with all of these studies though is that typically negative feedback within the system prevents long-term elevation of these steroids in free-living animals, although there are certainly cases where chronic alteration of the stress response can occur in wildlife [199,200].
Longer term elevation of glucocorticoids is known, based primarily on human and captive animal studies, to have increasing negative consequences, including eventual muscle catabolism and immunosuppression. Inhibition of the immune response by chronic elevation of glucocorticoids occurs at multiple levels. Glucocorticoids can alter cytokine production [201], suppress T-cell proliferation and antibody production [201,202] and inhibit inflammatory responses [203]. Some studies have demonstrated these types of responses specifically in amphibians as well [204]. However, much of what we know about these responses is based on treatment of animals in the laboratory with pharmacological doses of synthetic steroids (e.g. dexamethasone); less work has examined effects of glucocorticoids with realistic, endogenous levels of hormone [205]. Based on these more realistic studies with endogenous hormone, McEwen et al. [206] suggested that glucocorticoids should be viewed more as modulators of the immune response instead of as immunosuppressive hormones. There are clearly very complex interactions between the endocrine and immune systems of vertebrates. Work in mammalian laboratory models is advancing our mechanistic understanding of these interactions [207]. However, understanding how these systems interact in an organismal context to impact survival and fitness in free-living wildlife, including amphibians, remains an elusive goal, although new research in the field of ecological immunology is starting to consider this question [208].
Given the potential for immunosuppression, increases in glucocorticoids in response to environmental stressors have often been suggested as a possible mechanism explaining increased infection in natural populations of animals. This is a difficult link to establish in free-living amphibians. For example, while exogenous treatment with glucocorticoids in the laboratory can alter the immune response and increase parasite infection in tadpoles [104], extrapolating this response to physiological increases in hormone levels experienced by free-living animals has not been done. Indeed, many factors such as density that may elevate glucocorticoid levels in the laboratory [209,210], may not result in longer term elevation of glucocorticoids in more natural field conditions [211], which might make these factors less likely to lead to the immunsuppresive states demonstrated in laboratory models of chronic stress. One recent approach has been to examine haematological profiles (defined as the ratio of neutrophils to lymphocytes) as an indicator of stress, as these measures have often been associated with increased glucocorticoids in vertebrates [212]. Several studies have examined these profiles in amphibians [213,214], but again, interpretation in light of physiological function is not entirely clear, and future studies will probably need to address more clearly the implications of variation in these profiles for an individual's fitness.
A recent study by Warne et al. [76] provides what might be the most realistic assessment of the role of glucocorticoid hormones and the HPI axis in modulating infection dynamics, and represents perhaps the only study to address this issue directly in amphibians. They suggest that during certain developmental windows (e.g. during metamorphosis), energy might be limited and the allocation of energy to development versus the immune system that is modulated by the HPI axis could have critical impacts on infection. In their system, this conflict results in increased ranavirus mortality during the late-stages of metamorphosis. Adopting this more sophisticated model of HPI interaction with the immune response will provide future researchers with a more complete picture of the complexities of these systems for free-living amphibians.
7. Climate change as an applied challenge in disease research
Climate change can alter host–pathogen interactions and the physiology of both host and pathogen in ways that could increase or decrease levels of disease ([14] and references therein). As global temperatures increase, pathogens may experience faster growth and reproduction that could potentially increase the severity of infectious diseases [54,215]. Conversely, elevated temperatures outside the thermal optima for growth and reproduction of pathogens may reduce the severity of infection. In the case of Bd, increases in temperature of a few degrees (above the optimum for growth and reproduction of sporangia/zoospores) may limit the severity of infection and disease [117,118].
Additionally, warmer winters and night-time temperatures may reduce the cycle of pathogen die-offs that naturally occur during colder regimes [216,217]. Higher water temperatures can induce eutrophication with blooms of algae, bacteria, protozoans and small metazoans [218,219], which could enhance infections by a wide range of parasites [90,109], including the trematode Ribeiroia ondatrae [108].
Climate change could also alter host–pathogen relationships through changes in precipitation or hydrology. Because many amphibian pathogens are aquatically transmitted, increased rainfall that leads to more standing water could increase transmission rates. Conversely, reduced precipitation could result in aggregation of greater numbers of animals at fewer, smaller water bodies, promoting transmission of pathogens/disease. Also, aquatically transmitted pathogens can be sensitive to water conditions. For example, Bd dies after 3 h of desiccation [124], so loss of moisture or precipitation could affect the survival of this pathogen. High temperatures impede growth and can kill Bd in the laboratory [117,118]. Kriger [220] argues that droughts should reduce the severity of Bd epidemics, but there is also some evidence that droughts actually increase outbreaks [221,222].
Changes in climate could also shift the ranges of the pathogen, hosts or the pathogen vector. As climate change alters local habitats, new areas may appear that are suitable for the host or pathogen, while others disappear. For example, Seimon et al. [223] documented upward range expansion of both amphibian hosts and Bd in the Andes with warming. As high elevation sites experience loss of glaciers, this opens up new habitat for amphibians in the area. Bd has been detected on amphibians at these new sites, demonstrating a shift in both host and pathogen ranges. In other scenarios, the pathogen or host could potentially shift ranges without the other following.
On a local scale, an experimental field study by Kiesecker et al. [48] illustrates a complex inter-relationship among climate change, changes in the atmosphere, amphibian mortality events and disease. Kiesecker et al. [48] linked El Niño–Southern Oscillation events with decreased winter precipitation in the Oregon USA Cascade Range. They suggested that less winter snow pack resulted in lower water levels, when western toads (Bufo boreas) breed in early spring. Toad embryos developing in shallower water are exposed to higher levels of ultraviolet B radiation, which may compromise embryo disease defence mechanisms, which results in increased mortality from the pathogenic oomycete, Saprolegnia ferax.
Kupferberg et al. [224] presented another example of how changing hydrology may influence amphibian diseases on a local level. The authors found that outbreaks of a pathogenic copepod, Lernaea cyprinacea, are more severe following unusually warm summers in northern California. The authors speculated that this could be partly caused by changes in hydrology. During the warm summer of the outbreak, water levels were reduced, forcing amphibian larvae into high densities. Reduced discharge slowed river velocities, which could have allowed for easier transmission of the copepod parasite.
Climate change and spatial dynamics have both been used to explain the spread of Bd in tropical America. Bd is implicated as the proximate cause for Atelopus frog population crashes and species extinctions in tropical America. Pounds et al. [225] presented a mechanistic explanation for how climate change may influence outbreaks of Bd by modifying conditions in montane areas of Central and South America, where night-time temperatures are shifting closer to the thermal optimum for Bd, while increased daytime cloudiness prevents frogs from finding thermal refuges from the pathogen. Climate change and outbreaks of chytridiomycosis have been reported in several other studies. Bosch et al. [226] showed a significant association between rising temperatures and outbreaks of chytridiomycosis in Spain. D'Amen & Bombi [227] showed the emergence of Bd after a climatic shift or extreme weather event in Italy. Increases in chydriomycosis were correlated with low summer temperatures in Australia [228]. Lips et al.'s [229] analysis of amphibian population declines and Bd spread did not support the climate-linked epidemic hypothesis of Pounds [225]. They suggest that Bd is an introduced pathogen that has been spreading throughout the American tropics since the 1970s. This spatio-temporal hypothesis suggests that Bd spreads independently of climate shifts. Parmesan & Singer [230] suggest that both the climate and spatio-temporal hypotheses are supported by numerous studies, are not mutually exclusive and may be interactive. Rohr et al. [231] found no support for the ‘chytrid-thermal optimum hypothesis’ [225]. However, they suggest that climate change is likely to play an important role in amphibian population declines worldwide.
Long-term studies in South Carolina, USA, illustrate those populations of several amphibian species have been in decline [232]. At this site, the presence of Bd was rare and there was no evidence of chytridiomycosis. The investigators concluded that the population declines in this region were more likely owing to extreme weather such as low rainfall and shortened hydroperiod for breeding rather than a Bd epidemic.
Overall, the effects of climate change on amphibian diseases are difficult to predict. While some pathogens may increase in prevalence and severity, others may decline.
Regardless of how climate change may influence amphibian disease dynamics, there are obvious spatial and temporal considerations that may influence the physiology of disease. Thus, disease prevalence can fluctuate seasonally if there are temporal thermal refugia. However, if temperatures continually fall within or outside of the optimal growth temperature range of the pathogen, this fluctuation might not be observed. For example, in Australia, Drew et al. [228] observed that Bd prevalence was more likely at locations where the average summer maximum temperature remained below 30°C.
Even if a disease does not show seasonal fluctuations and is endemic to a geographical range, disease prevalence can vary on smaller geographical scales. In Australia, Van Sluys & Hero [233] found Bd-infected male stoney creek frogs (Litoria wilcoxii) were more likely to be found in forested rather than open habitats. While prevalence was determined to be lower in the upland-forested habitats, mean infection intensity was not different among habitat types. In this study, the authors were unable to determine if the difference in prevalence was due to host density, host ability to clear infection, ability to avoid exposure to Bd zoospores or some other behavioural or ecological explanation that was confounded with these habitat differences.
Muths et al. [234] found Bd-infected western toads (Bufo boreas) more likely at lower elevations in the Rocky Mountains of the USA than at higher elevations. Historical mean maximum temperature explained the distribution of Bd-infected frogs. However, since the Bd-infected frogs from lower elevation were found near the lower limit of the optimum growth range of the pathogen (17–25°C), an increase in elevation (and a subsequent decrease in temperature) may have limited the range of this pathogen.
Raffel et al. [235] found that Bd infection levels in newts increased in ponds with a more complex substrate consisting of either dead tree leaves or live emergent vegetation. This complex substrate and emergent vegetation allow for both cooler water temperature and a reduced diurnal temperature range. The authors suggest that whereas a complex substrate provides a set of cooler microhabitats, the canopy overhead limits the area available for a host to thermoregulate and clear Bd infection at a higher temperature. The authors propose that the around-shore canopy cover creating a complex substrate, and the emergent vegetation all limit the thermal refugia available to these newts leading to increased pathogen loads.
Alongside its effects on community composition, climate change will affect host and parasite physiology, phenology and co-evolutionary dynamics leading to complex shifts in disease dynamics [236,237]. Predicting the specific outcome of such interactions for human and wildlife diseases is remarkably challenging, in part because there is considerable uncertainty surrounding the magnitude of changes in climate variables such as precipitation, temperature variability and regional-scale warming patterns [238]. Nonetheless, focusing efforts on identifying key mechanisms likely to dominate climate-driven changes to disease severity in model host–parasite systems can provide important information about where to direct resources for mitigating climate-driven increases in disease [237].
Laboratory studies on one such model system, the complex life-cycle parasite Ribeiroia ondatrae, suggest that the net effect of climate change on host pathology will be determined by changes occurring at multiple steps in the transmission cycle. Because Ribeiroia is sequentially transmitted between birds, snails and amphibians, understanding the net effects of temperature changes on infection requires careful examination of the individual hosts and their interactions with parasites. The production of infectious stages (cercariae) by trematodes is often positively influenced by temperature, which has led some to suggest forecasted warming could exacerbate infections [239]. Higher temperatures could also cause cercariae to be released earlier in the season, which could have important implications for the Ribeiroia system. Tadpoles exposed in earlier stages of development or to greater doses of the parasite are much more likely to experience pathology (e.g. mortality or limb deformities) as a result of infection [44]. While tadpoles will probably also accelerate their growth in response to climate warming, parasites are generally expected to increase developmental rates even more quickly owing to their smaller body sizes and higher metabolic rates. Moreover, the timing of amphibian breeding is typically co-controlled by temperature and precipitation, suggesting they may be less plastic in their ability to change developmental timing. Thus, temperature-driven changes that alter the timing of amphibian exposure or the abundance of parasites to which they are exposed could affect the net pathology experienced by amphibians.
In a series of laboratory experiments, Paull & Johnson [240] found that increases in temperature accelerated the development of Ribeiroia eggs in the environment, the development of rediae within snail hosts and the time-to-parasite release by snails. Moreover, parasite cercariae were more active and more infectious to amphibians at higher temperatures. Interestingly, however, parasite-induced malformations and total Ribeiroia metacercariae were greatest at intermediate temperatures. This stemmed from two factors: first, the increase in development rate of tadpoles at warmer temperatures allowed them to ‘escape’ the vulnerable stages of limb growth more quickly; and second, while parasite infectivity increased with temperature, parasite persistence tended to decrease at the highest temperature such that these tadpoles supported lower overall infections (and therefore fewer malformations). These observations, in combination with the fact that infected snails also exhibit higher mortality at high temperatures, highlight the complexity and multi-faceted nature of host–parasite interactions in response to changing temperatures.
8. Summary
Ultimately, infection outcome in amphibians is determined by the complex interaction between environmental factors, pathogen characteristics and host traits and responses. A useful method for uncovering the most important factors that drive infection dynamics in this system involves taking a bottom-up approach. Understanding effects at higher levels of organization depends on establishing basic cause-and-effect relationships between pathogen exposure, host responses, manifestation of disease and infection outcome. Cause-and-effect relationships between host physiological responses to a pathogen and infection outcome provide the baseline for understanding more complex interactions [241,242].
A persistent challenge at the frontier of disease ecology research is to synthesize information from physiology, life-history theory and ecology to better understand and predict patterns of disease risk among hosts. Given the role of multi-host pathogens in emerging infections of both plants and animals, understanding the drivers of disease variation has immediate relevance for human health, economic growth and wildlife conservation [1,8,243]. Because amphibians serve as hosts for a tremendous diversity of micro- and macroparasites, including viruses, helminths, parasitic arthropods, fungi, protists and bacteria [22,23,85], they are ideal for studying disease ecology dynamics. Furthermore, amphibians are declining globally and infectious diseases as well as life-history characteristics are considered important factors in causing or predicting losses [74,178,244], highlighting the importance of identifying factors that predict disease patterns. Beyond amphibians, a broader integration between physiological and ecological studies of disease has applied importance for identifying species that are either at risk of disease-driven population declines or those that function as reservoir or ‘superspreading’ hosts with the potential to influence epidemics or epizootics [237,245]. Finally, physiological research must be integrated into conservation to identify species or populations that are most vulnerable to disease as well as other threats [246].
Acknowledgements
We thank Craig Franklin for inviting us to write this review and the anonymous reviewers for their comments.
References
- 1.Jones K. E., Patel N. G., Levy M. A., Storeygard A., Balk D., Gittleman J. L., Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451, 990–994 10.1038/nature06536 (doi:10.1038/nature06536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harvell C. D., et al. 1999. Emerging marine diseases—climate links and anthropogenic factors. Science 285, 1505–1510 10.1126/science.285.5433.1505 (doi:10.1126/science.285.5433.1505) [DOI] [PubMed] [Google Scholar]
- 3.Deem S. L., Karesh W. B., Weisman W. 2001. Putting theory into practice: wildlife health in conservation. Conserv. Biol. 15, 1224–1233 10.1046/j.1523-1739.2001.00336.x (doi:10.1046/j.1523-1739.2001.00336.x) [DOI] [Google Scholar]
- 4.Daszak P., Cunningham A. A. 2002. Emerging infectious diseases a key role for conservation medicine. In Conservation medicine ecological health in practice (eds Aguirre A. A., Ostfeld R. S., Tabor G. M., House C., Pearl M. C.), pp. 40–61 Oxford, UK: Oxford University Press [Google Scholar]
- 5.Anderson P. K., Cunningham A. A., Patel N. G., Morales F. J., Epstein P. R., Daszak P. 2004. Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 19, 535–544 10.1016/j.tree.2004.07.021 (doi:10.1016/j.tree.2004.07.021) [DOI] [PubMed] [Google Scholar]
- 6.Smith K. F., Acevedo-Whitehouse K., Penderson A. B. 2009. The role of infectious disease in biological conservation. Anim. Conserv. 12, 1–12 10.1111/j.1469-1795.2008.00228.x (doi:10.1111/j.1469-1795.2008.00228.x) [DOI] [Google Scholar]
- 7.Keesing F., et al. 2010. Impact of biodiversity on the emergence and transmission of infectious diseases. Nature 468, 647–652 10.1038/nature09575 (doi:10.1038/nature09575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daszak P., Cunningham A. A., Hyatt A. D. 2000. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 287, 443–449 10.1126/science.287.5452.443 (doi:10.1126/science.287.5452.443) [DOI] [PubMed] [Google Scholar]
- 9.Horwitz P., Wilcox B. A. 2005. Parasites, ecosystems and sustainability: an ecological and complex systems perspective. Inter. J. Parasitol. 35, 725–732 10.1016/j.ijpara.2005.03.002 (doi:10.1016/j.ijpara.2005.03.002) [DOI] [PubMed] [Google Scholar]
- 10.Eviner V. T., Likens G. E. 2008. Effects of pathogens on terrestrial ecosystem function. In Infectious disease ecology: effects of ecosystems on disease and of disease on ecosystems (eds Ostfeld R. S., Keesing F., Eviner V. T.), pp. 260–284 Princeton, NJ: Princeton University Press [Google Scholar]
- 11.Hudson P.J., Dobson A. P., Lafferty K. D. 2006. Is a healthy ecosystem one that is rich in parasites? Trends Ecol. Evol. 21, 381–385 10.1016/j.tree.2006.04.007 (doi:10.1016/j.tree.2006.04.007) [DOI] [PubMed] [Google Scholar]
- 12.Lafferty K. D. 2008. Effects of disease on community interactions and food webs. In Infectious disease ecology: the effects of ecosystems on disease and of disease on ecosystems (eds Ostfeld R., Keesing F., Eviner V. T.), pp. 205–222 Princeton, NJ: Princeton University Press [Google Scholar]
- 13.Hatcher M. J., Dick J. T. A., Dunn A. M. In press Diverse effects of parasites in ecosystems. Front. Ecol. Evol . [Google Scholar]
- 14.Blaustein A. R., Walls S. C., Bancroft B. A., Lawler J. J., Searle C. L., Gervasi S. S. 2010. Direct and indirect effects of climate change on amphibian populations. Diversity 2, 281–313 10.3390/d2020281 (doi:10.3390/d2020281) [DOI] [Google Scholar]
- 15.Houlahan J. E., Findlay C. S., Schmidt B. R., Meyer A. H., Kuzmin S. L. 2000. Quantitative evidence for global amphibian population declines. Nature 404, 752–755 10.1038/35008052 (doi:10.1038/35008052) [DOI] [PubMed] [Google Scholar]
- 16.Stuart S. N., Chanson J. S., Cox N. A., Young B. E., Rodrigues A. S. L., Fischmann D. L., Waller R. W. 2004. Status and trends of amphibian declines and extinctions worldwide. Science 306, 1783–1786 10.1126/science.1103538 (doi:10.1126/science.1103538) [DOI] [PubMed] [Google Scholar]
- 17.Lannoo M. 2005. Amphibian declines: the conservation status of United States species. Berkeley, CA: USA University California Press [Google Scholar]
- 18.Bielby J., Cooper N., Cunningham A. A., Garner T. W. J., Purvis A. 2008. Predicting susceptibility to rapid declines in the world's frogs. Conserv. Lett. 1, 82–90 10.1111/j.1755-263X.2008.00015.x (doi:10.1111/j.1755-263X.2008.00015.x) [DOI] [Google Scholar]
- 19.McCallum M. L. 2007. Amphibian decline or extinction? Current declines dwarf background extinction rate. J. Herpetol. 41, 483–491 10.1670/0022-1511(2007)41[483:ADOECD]2.0.CO;2 (doi:10.1670/0022-1511(2007)41[483:ADOECD]2.0.CO;2) [DOI] [Google Scholar]
- 20.Wake D. B., Vredenburg V. T. 2008. Are we in the midst of the sixth mass extinction? A review from the world of amphibians. Proc. Natl Acad. Sci. USA 105, 11 466–11 473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blaustein A. R., Han B., Relyea R. A., Johnson P. T. J., Buck J. C., Gervasi S. S., Kats L. B. 2011. The complexity of amphibian population declines: understanding the role of cofactors in driving amphibian losses. Ann. NY Acad. Sci. 1223, 108–119 10.1111/j.1749-6632.2010.05909.x (doi:10.1111/j.1749-6632.2010.05909.x) [DOI] [PubMed] [Google Scholar]
- 22.Prudhoe S., Bray R. A. 1982. Platyhelminth parasites of the Amphibia. Oxford, UK: Oxford University Press [Google Scholar]
- 23.Densmore C., Green D. 2007. Diseases of amphibians. ILAR J. 48, 235–254 [DOI] [PubMed] [Google Scholar]
- 24.Klaphake E. 2009. Bacterial and parasitic diseases of amphibians. Vet. Clin. N. Am. Exotic 12, 597–608 10.1016/j.cvex.2009.06.005 (doi:10.1016/j.cvex.2009.06.005) [DOI] [PubMed] [Google Scholar]
- 25.Daszak P. A., Cunningham A., Hyatt A. D. 2003. Infectious disease and amphibian population declines. Divers. Distrib. 9, 141–150 10.1046/j.1472-4642.2003.00016.x (doi:10.1046/j.1472-4642.2003.00016.x) [DOI] [Google Scholar]
- 26.Skerratt L. F., Berger L., Speare R., Cashins S., McDonald K. R., Phillott A. D., Hines H. B., Kenyon N. 2007. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 4, 125–134 10.1007/s10393-007-0093-5 (doi:10.1007/s10393-007-0093-5) [DOI] [Google Scholar]
- 27.Rödder D., et al. 2009. Global amphibian extinction risk assessment for the panzootic chytrid fungus. Diversity 1, 52–66 10.3390/d1010052 (doi:10.3390/d1010052) [DOI] [Google Scholar]
- 28.Blaustein A. R., Kiesecker J. M. 2002. Complexity in conservation: lessons from the global decline of amphibian populations. Ecol. Lett. 5, 597–608 10.1046/j.1461-0248.2002.00352.x (doi:10.1046/j.1461-0248.2002.00352.x) [DOI] [Google Scholar]
- 29.Mendelson J. R., III, et al. 2006. Confronting amphibian declines and extinctions. Science 313, 48. 10.1126/science.1128396 (doi:10.1126/science.1128396) [DOI] [PubMed] [Google Scholar]
- 30.Kilpatrick A. M., Briggs C. J., Daszak P. 2010. The ecology and impact of chytridiomycosis: an emerging disease of amphibians. Trends. Ecol. Evol. 25, 109–118 10.1016/j.tree.2009.07.011 (doi:10.1016/j.tree.2009.07.011) [DOI] [PubMed] [Google Scholar]
- 31.Hudson P. J., Greenman J. V. 1998. Competition mediated by parasites: biological and theoretical progress. Trends Ecol. Evol. 13, 387–390 10.1016/S0169-5347(98)01475-X (doi:10.1016/S0169-5347(98)01475-X) [DOI] [PubMed] [Google Scholar]
- 32.Keesing F., Holt R. D., Ostfeld R. S. 2006. Effects of species diversity on disease risk. Ecol. Lett. 9, 485–498 10.1111/j.1461-0248.2006.00885.x (doi:10.1111/j.1461-0248.2006.00885.x) [DOI] [PubMed] [Google Scholar]
- 33.Ostfeld R., Keesing F., Eviner V. T. 2008. Infectious disease ecology: the effects of ecosystems on disease and of disease on ecosystems. Princeton, NJ: Princeton University Press [Google Scholar]
- 34.Johnson P. T. J., Dobson A., Lafferty K. D., Marcogliese D. J., Memmott J., Orlofske S. A., Poulin R., Thieltges D. W. 2010. When parasites become prey: ecological and epidemiological significance of eating parasites. Trends Ecol. Evol. 25, 362–371 10.1016/j.tree.2010.01.005 (doi:10.1016/j.tree.2010.01.005) [DOI] [PubMed] [Google Scholar]
- 35.Tracy C. R., Turner J. S. 1982. What is physiological ecology? Bull. Ecol. Soc. Am. 63, 340–347 [Google Scholar]
- 36.Ricklefs R. E., Wikelski M. 2002. The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468 10.1016/S0169-5347(02)02578-8 (doi:10.1016/S0169-5347(02)02578-8) [DOI] [Google Scholar]
- 37.Raberg L., Sim D., Read A. F. 2007. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 318, 812–814 10.1126/science.1148526 (doi:10.1126/science.1148526) [DOI] [PubMed] [Google Scholar]
- 38.Raberg L., Graham A. L., Read A. F. 2009. Decomposing health: tolerance and resistance to parasites in animals. Phil. Trans. R. Soc. B 364, 37–49 10.1098/rstb.2008.0184 (doi:10.1098/rstb.2008.0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Read A. F., Graham A. L., Raberg L. 2008. Animal defenses against infectious agents: is damage control more important than pathogen control. PLoS Biol. 6, 2638–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hart B. L. 1994. Behavioral defense against parasites—interaction with parasite invasiveness. Parasitology 109, S139–S151 10.1017/S0031182000085140 (doi:10.1017/S0031182000085140) [DOI] [PubMed] [Google Scholar]
- 41.Schmid-Hempel P., Ebert D. 2003. On the evolutionary ecology of specific immune defence. Trends Ecol. Evol. 18, 27–32 10.1016/S0169-5347(02)00013-7 (doi:10.1016/S0169-5347(02)00013-7) [DOI] [Google Scholar]
- 42.Hasselquist D. 2007. Comparative immunoecology in birds: hypotheses and tests. J. Ornithol. 148, S571–S582 10.1007/s10336-007-0201-x (doi:10.1007/s10336-007-0201-x) [DOI] [Google Scholar]
- 43.Rohr J. R., Raffel T. R., Hall C. A. 2010. Developmental variation in resistance and tolerance in a multi-host–parasite system. Funct. Ecol. 24, 1110–1121 10.1111/j.1365-2435.2010.01709.x (doi:10.1111/j.1365-2435.2010.01709.x) [DOI] [Google Scholar]
- 44.Johnson P. T. J., Kellermanns E., Bowerman J. 2011. Critical windows of disease risk: amphibian pathology driven by developmental changes in host resistance and tolerance. Funct. Ecol. 25, 726–734 10.1111/j.1365-2435.2010.01830.x (doi:10.1111/j.1365-2435.2010.01830.x) [DOI] [Google Scholar]
- 45.Komar N., Langevin S., Hinten S., Nemeth N., Edwards E., Hettler D., Davis B., Bowen R., Bunning M. 2003. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 9, 311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin L. B., Weil Z. M., Nelson R. J. 2007. Immune defense and reproductive pace of life in Peromyscus mice. Ecology 88, 2516–2528 10.1890/07-0060.1 (doi:10.1890/07-0060.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoverman J. T., Gray M. J., Haislip N. A., Miller D. L. 2011. Phylogeny and ecology impact amphibian susceptibility to ranaviruses. EcoHealth 8, 301–319 10.1007/s10393-011-0717-7 (doi:10.1007/s10393-011-0717-7). [DOI] [PubMed] [Google Scholar]
- 48.Kiesecker J. M., Blaustein A. R., Belden L. K. 2001. Complex causes of amphibian population declines. Nature 410, 681–684 10.1038/35070552 (doi:10.1038/35070552) [DOI] [PubMed] [Google Scholar]
- 49.Kiesecker J. M. 2002. Synergism between trematode infection and pesticide exposure: a link to amphibian limb deformities in nature. Proc. Natl Acad. Sci. USA 99, 9900–9904 10.1073/pnas.152098899 (doi:10.1073/pnas.152098899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forson D. D., Storfer A. 2006. Atrazine increases ranavirus susceptibility in the tiger salamander, Ambystoma tigrinum. Ecol. Appl. 16, 2325–2332 10.1890/1051-0761(2006)016[2325:AIRSIT]2.0.CO;2 (doi:10.1890/1051-0761(2006)016[2325:AIRSIT]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 51.Rohr J. R., et al. 2008. Agrochemicals increase trematode infections in a declining amphibian species. Nature 455, 1235–1239 10.1038/nature07281 (doi:10.1038/nature07281) [DOI] [PubMed] [Google Scholar]
- 52.Bancroft B. A., Baker N. J., Blaustein A. R. 2008. A meta-analysis of the effects of ultraviolet B radiation and its synergistic interactions with pH, contaminants, and disease on amphibian survival. Conserv. Biol. 22, 987–996 10.1111/j.1523-1739.2008.00966.x (doi:10.1111/j.1523-1739.2008.00966.x) [DOI] [PubMed] [Google Scholar]
- 53.Garner T. W. J., Perkins M. W., Govindarajulu P., Segile D., Walker S., Cunningham A. A., Fisher M. C. 2006. The emerging amphibian pathogen Batrachochytrium dendrobatidis globally infects introduced populations of the North American bullfrog, Rana catesbeiana . Biol. Lett. 2, 455–459 10.1098/rsbl.2006.0494 (doi:10.1098/rsbl.2006.0494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harvell C. D., Mitchell C., Ward J., Altizer S., Dobson A., Ostfeld R., Samuel M. 2002. Climate warming and disease risks for terrestrial and marine biota. Science 296, 2158–2162 10.1126/science.1063699 (doi:10.1126/science.1063699) [DOI] [PubMed] [Google Scholar]
- 55.Williams T., Barbosa-Solomieu V., Chinchar G. D. 2005. A decade of advances in iridovirus research. In Advances in virus research, vol. 65 (eds Maramorosch K., Shatkin A.), pp. 173–248 New York, NY: Academic Press: [DOI] [PubMed] [Google Scholar]
- 56.Gray M. J., Miller D. L., Hoverman J. T. 2009. Ecology and pathology of amphibian ranaviruses. Dis. Aquat. Org. 87, 243–266 10.3354/dao02138 (doi:10.3354/dao02138) [DOI] [PubMed] [Google Scholar]
- 57.Chinchar G. D., Essbauer S., He J. G., Hyatt A., Miyazaki T., Seligy V., Williams T. 2005. Iridoviridae. In Virus taxonomy: 8th report of the Int. committee on the taxonomy of viruses (eds Fauguet C. M., Mayo M. A., Maniloff J., Desselberger U., Ball L. A.), pp. 163–175 London, UK: Elsevier [Google Scholar]
- 58.Cunningham A. A., Langton T. E. S., Bennett P. M., Lewin J. F., Drury S. E. N., Gough R. E., MacGregor S. K. 1996. Pathological and microbiological findings from incidents of unusual mortality of the common frog (Rana temporaria). Phil. Trans. R. Soc. Lond. B 351, 1539–1557 10.1098/rstb.1996.0140 (doi:10.1098/rstb.1996.0140) [DOI] [PubMed] [Google Scholar]
- 59.Bollinger T. K., Mao J. H., Schock D., Brigham R. M., Chinchar V. G. 1999. Pathology, isolation, and preliminary molecular characterization of a novel iridovirus from tiger salamanders in Saskatchewan. J. Wildl. Dis. 35, 413–429 [DOI] [PubMed] [Google Scholar]
- 60.Green D. E., Converse K. A., Schrader A. K. 2002. Epizootiology of sixty-four amphibian morbidity and mortality events in the USA, 1996–2001. Ann. NY Acad. Sci. 969, 323–339 10.1111/j.1749-6632.2002.tb04400.x (doi:10.1111/j.1749-6632.2002.tb04400.x) [DOI] [PubMed] [Google Scholar]
- 61.Carey C., Pessier A. P., Peace A. D. 2003. Pathogens, infectious disease, and immune defenses. In Amphibian conservation (ed. Semlitsch R. D.), pp. 127–136 Washington, DC: Smithsonian Institute [Google Scholar]
- 62.Converse K. A., Green D. E. 2005. Diseases of tadpoles. In Wildlife diseases: landscape epidemiology, spatial distribution and utilization of remote sensing technology (eds Majumdar S. K., Huffman J. E., Brenner F. J., Panah A. I.), pp. 72–88 Easton, PA: Penn. Acad. Science [Google Scholar]
- 63.Greer A. L., Berrill M., Wilson P. J. 2005. Five amphibian mortality events associated with ranavirus infection in south central Ontario, Canada. Dis. Aquat. Org. 67, 9–14 10.3354/dao067009 (doi:10.3354/dao067009) [DOI] [PubMed] [Google Scholar]
- 64.Jancovich J. K., Davidson E. W., Parameswaran N., Mao J., Chinchar V. G., Collins J. P., Jacobs B. L., Storfer A. 2005. Evidence for emergence of an amphibian iridoviral disease because of human-enhanced spread. Mol. Ecol. 14, 213–224 10.1111/j.1365-294X.2004.02387.x (doi:10.1111/j.1365-294X.2004.02387.x) [DOI] [PubMed] [Google Scholar]
- 65.Fox S. F., Greer A. L., Torres-Cervantes R., Collins J. P. 2006. First case of ranavirus-associated morbidity and mortality in natural populations of the South American frog Atelognathus patagonicus. Dis. Aquat. Org. 72, 87–92 10.3354/dao072087 (doi:10.3354/dao072087) [DOI] [PubMed] [Google Scholar]
- 66.Ariel E., Kielgast J., Svart H. E., Larsen K., Tapiovaara H., Jensen B. B., Holopainen R. 2009. Ranavirus in wild edible frogs Pelophylax kl. esculentus in Denmark. Dis. Aquat. Org. 85, 7–14 10.3354/dao02060 (doi:10.3354/dao02060) [DOI] [PubMed] [Google Scholar]
- 67.Balseiro A., Dalton K. P., del Cerro A., Marquez I., Cunningham A. A., Parra F., Prieto J. M., Casais R. 2009. Pathology, isolation and molecular characterisation of a ranavirus from the common midwife toad Alytes obstetricans on the Iberian Peninsula. Dis. Aquat. Org. 84, 95–104 10.3354/dao02032 (doi:10.3354/dao02032) [DOI] [PubMed] [Google Scholar]
- 68.Une Y., Nakajima K., Taharaguchi S., Ogihara K., Murakami M. 2009. Ranavirus infection outbreak in the Salamander (Hynobius Nebulosus) in Japan. J. Comp. Pathol. 141, 310–310 10.1016/j.jcpa.2009.08.133 (doi:10.1016/j.jcpa.2009.08.133) [DOI] [Google Scholar]
- 69.Gantress J., Maniero G. D., Cohen N., Robert J. 2003. Development and characterization of a model system to study amphibian immune responses to iridoviruses. Virology 311, 254–262 10.1016/S0042-6822(03)00151-X (doi:10.1016/S0042-6822(03)00151-X) [DOI] [PubMed] [Google Scholar]
- 70.Harp E. M., Petranka J. W. 2006. Ranavirus in wood frogs (Rana sylvatica): potential sources of transmission within and between ponds. J. Wildl. Dis. 42, 307–318 [DOI] [PubMed] [Google Scholar]
- 71.Brunner J. L., Schock D. M., Collins J. P. 2007. Transmission dynamics of the amphibian ranavirus Ambystoma tigrinum virus. Dis. Aquat. Org. 77, 87–95 10.3354/dao01845 (doi:10.3354/dao01845) [DOI] [PubMed] [Google Scholar]
- 72.Daszak P., Berger L., Cunningham A. A., Hyatt A. D., Green D. E., Speare R. 1999. Emerging infectious diseases and amphibian population declines. Emerg. Infect. Dis. 5, 735–748 10.3201/eid0506.990601 (doi:10.3201/eid0506.990601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brunner J. L., Schock D. M., Davidson E. W., Collins J. P. 2004. Intraspecific reservoirs: complex life history and the persistence of a lethal ranavirus. Ecology 85, 560–566 10.1890/02-0374 (doi:10.1890/02-0374) [DOI] [Google Scholar]
- 74.Bancroft B. A., Han A. A., Searle C. L., Biga L. M., Olson D. H., Kats L. B., Lawler J. J., Blaustein A. R. 2011. Species-level correlates of susceptibility to the pathogenic amphibian fungus Batrachochytrium dendrobatidis in the United States. Biodivers. Conserv. 20, 1911–1920 10.1007/s10531-011-0066-4 (doi:10.1007/s10531-011-0066-4) [DOI] [Google Scholar]
- 75.Haislip N. A., Gray M. J., Hoverman J. T., Miller D. L. 2011. Development and disease: how susceptibility to an emerging pathogen changes through anuran development. PLoS ONE 6, e22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Warne R. W., Crespi E. J., Brunner J. L. 2011. Escape from the pond: stress and developmental responses to ranavirus infection in wood frog tadpoles. Funct. Ecol. 25, 139–146 10.1111/j.1365-2435.2010.01793.x (doi:10.1111/j.1365-2435.2010.01793.x) [DOI] [Google Scholar]
- 77.Rojas S., Richards K., Jancovich J. K., Davidson E. W. 2005. Influence of temperature on Ranavirus infection in larval salamanders Ambystoma tigrinum. Dis. Aquat. Org. 63, 95–100 10.3354/dao063095 (doi:10.3354/dao063095) [DOI] [PubMed] [Google Scholar]
- 78.Maniero G. D., Carey C. 1997. Changes in selected aspects of immune function in the leopard frog, Rana pipiens, associated with exposure to cold. J. Comp. Physiol. B 167, 256–263 10.1007/s003600050072 (doi:10.1007/s003600050072) [DOI] [PubMed] [Google Scholar]
- 79.Gray M. J., Miller D. L., Schmutzer A. C., Baldwin C. A. 2007. Frog virus 3 prevalence in tadpole populations inhabiting cattle-access and non-access wetlands in Tennessee, USA. Dis. Aquat. Org. 77, 97–103 10.3354/dao01837 (doi:10.3354/dao01837) [DOI] [PubMed] [Google Scholar]
- 80.Esch G. W., Barger M. A., Fellis K. J. 2002. The transmission of digenetic trematodes: style, elegance, complexity. ICB 42, 304–312 [DOI] [PubMed] [Google Scholar]
- 81.Goater C. P., Vandenbos R. E. 1997. Effects of larval history and lungworm infection on the growth and survival of juvenile wood frogs (Rana sylvatica). Herpetologica 53, 331–338 [Google Scholar]
- 82.Yoder H. R., Coggins J. R., Reinbold J. C. 2001. Helminth parasites of the green frog (Rana clamitans) from southeastern Wisconsin, USA. Comp. Parasitol. 68, 269–272 [Google Scholar]
- 83.King K. C., McLaughlin J. D., Boily M., Marcogliese D. J. 2010. Effects of agricultural landscape and pesticides on parasitism in native bullfrogs. Biol. Conserv. 143, 302–310 10.1016/j.biocon.2009.10.011 (doi:10.1016/j.biocon.2009.10.011) [DOI] [Google Scholar]
- 84.Smyth J. D., Smyth M. M. 1980. Frogs as host–parasite systems I: an introduction to parasitology through the parasites of Rana temporaria, R. esculenta and R. pipiens. London, UK: Macmillan Press [Google Scholar]
- 85.Sutherland D. R. 2005. Parasites of North American frogs. In Amphibian declines: the conservation status of United States species (ed. Lannoo M. J.), pp. 109–123 Berkley, CA: University of California Press [Google Scholar]
- 86.Blaustein A. R., Johnson P. T. 2003. The complexity of deformed amphibians. Front. Ecol. Environ. 1, 87–94 10.1890/1540-9295(2003)001[0087:TCODA]2.0.CO;2 (doi:10.1890/1540-9295(2003)001[0087:TCODA]2.0.CO;2) [DOI] [Google Scholar]
- 87.Johnson P. T. J., Sutherland D. R., Kinsella J. M., Lunde K. B. 2004. Review of the trematode genus Ribeiroia (Psilostomidae): ecology, life history, and pathogenesis with special emphasis on the amphibian malformation problem. Adv. Parasitol. 57, 191–253 10.1016/S0065-308X(04)57003-3 (doi:10.1016/S0065-308X(04)57003-3) [DOI] [PubMed] [Google Scholar]
- 88.Johnson P. T. J., McKenzie V. J. 2008. Effects of environmental change on helminth infections in amphibians: exploring the emergence of Ribeiroia and Echinostoma infections in North America. In The Biology of echinostomes (eds Fried B., Toledo R.). New York, NY: Springer [Google Scholar]
- 89.Rohr J. R., Raffel T. R., Sessions S. K. 2009. Digenetic trematodes and their relationship to amphibian declines and deformities. In Amphibian biology, vol. 8. Amphibian decline: diseases, parasites, maladies, and pollution (eds Heatwole H., Wilkinson J. W.), pp. 3067–3088 Chipping Norton, Australia: Surrey Beatty & Sons [Google Scholar]
- 90.Johnson P. T. J., Reeves M. K., Krest S. K., Pinkney A. E. 2010. A decade of deformities: advances in our understanding of amphibian malformations and their implications. In Ecotoxicology of amphibians and reptiles, 2nd edn (eds Sparling D. W., Linder G., Bishop C., Krest S. K.), pp. 511–536 Pensacola, FL: SETAC Press [Google Scholar]
- 91.Johnson P. T. J., Rohr J. R., Hoverman J. T., Kellermanns E., Bowerman J., Lunde K. B. 2012. Living fast and dying of infection: host life history drives interspecific variation in infection and disease risk. Ecol. Lett. 15, 235–242 10.1111/j.1461-0248.2011.01730.x (doi:10.1111/j.1461-0248.2011.01730.x) [DOI] [PubMed] [Google Scholar]
- 92.Sessions S. K., Ruth S. B. 1990. Explanation of naturally-occurring supernumerary limbs in amphibians. J. Exp. Zool. 254, 38–47 10.1002/jez.1402540107 (doi:10.1002/jez.1402540107) [DOI] [PubMed] [Google Scholar]
- 93.Johnson P. T. J., Lunde K. B., Ritchie E. G., Launer A. E. 1999. The effect of trematode infection on amphibian limb development and survivorship. Science 284, 802–804 10.1126/science.284.5415.802 (doi:10.1126/science.284.5415.802) [DOI] [PubMed] [Google Scholar]
- 94.Johnson P. T. J., Lunde K. B., Ritchie E. G., Reaser J. K., Launer A. E. 2001. Morphological abnormality patterns in a California amphibian community. Herpetologica 57, 336–352 [Google Scholar]
- 95.Johnson P. T. J., et al. 2002. Parasite (Ribeiroia ondatrae) infection linked to amphibian malformations in the western United States. Ecol. Monogr. 72, 151–168 10.1890/0012-9615(2002)072[0151:PROILT]2.0.CO;2 (doi:10.1890/0012-9615(2002)072[0151:PROILT]2.0.CO;2) [DOI] [Google Scholar]
- 96.Sessions S. K., Franssen R. A., Horner V. L. 1999. Morphological clues from multilegged frogs: are retinoids to blame. Science 284, 800–802 10.1126/science.284.5415.800 (doi:10.1126/science.284.5415.800) [DOI] [PubMed] [Google Scholar]
- 97.Schotthoefer A. M., Koehler A. V., Meteyer C. U., Cole R. A. 2003. Influence of Ribeiroia ondatrae (Trematoda: Digenea) infection on limb development and survival of northern leopard frogs (Rana pipiens): effects of host stage and parasite-exposure level. Can. J. Zool. 81, 1144–1153 10.1139/z03-099 (doi:10.1139/z03-099) [DOI] [Google Scholar]
- 98.Goodman B. A., Johnson P. T. J. 2011. Disease and the extended phenotype: parasites control host performance and survival through induced changes in body plan. PLoS ONE 6, 5–10 10.1371/journal.pone.0020193 (doi:10.1371/journal.pone.0020193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fried B., Frazer B. A., Reddy A. 1997. Chemoattraction and penetration of Echinostoma trivolvis and E. caproni cercariae in the presence of Biomphalaria glabrata, Helisoma trivolvis, and Lymnaea elodes dialysate. Parasitol. Res. 83, 193–197 10.1007/s004360050231 (doi:10.1007/s004360050231) [DOI] [PubMed] [Google Scholar]
- 100.Beasley V. R., et al. 2005. Risk factors and the decline of the cricket frog, Acris crepitans: evidence for the involvement of herbicides, parasitism, and habitat modifications. In Amphibian declines: the conservation status of United States species (ed. Lannoo M. J.), pp. 75–87 Chicago, IL: University of Chicago Press [Google Scholar]
- 101.Holland M. P., Skelly D. K., Kashgarian M., Bolden S. R., Harrison L. M., Cappello M. 2007. Echinostome infection in green frogs (Rana clamitans) is stage and age dependent. J. Zool. 271, 455–462 10.1111/j.1469-7998.2006.00229.x (doi:10.1111/j.1469-7998.2006.00229.x) [DOI] [Google Scholar]
- 102.Koprivnikar J., Baker R. L., Forbes M. R. 2006. Environmental factors influencing trematode prevalence in grey tree frog (Hyla versicolor) tadpoles in southern Ontario. J. Parasitol. 92, 997–1001 10.1645/GE-771R.1 (doi:10.1645/GE-771R.1) [DOI] [PubMed] [Google Scholar]
- 103.Daly E. W., Johnson P. T. J. 2011. Beyond immunity: quantifying the effects of host anti-parasite behavior on parasite transmission. Oecologia 165, 1043–1050 10.1007/s00442-010-1778-y (doi:10.1007/s00442-010-1778-y) [DOI] [PubMed] [Google Scholar]
- 104.Belden L. K., Kiesecker J. M. 2005. Glucocorticosteroid hormone treatment of larval treefrogs increases infection by Alaria sp. trematode cercariae. J. Parasitol. 91, 686–688 10.1645/GE-397R (doi:10.1645/GE-397R) [DOI] [PubMed] [Google Scholar]
- 105.Todd B. D. 2007. Parasites lost? An overlooked hypothesis for the evolution of alternative reproductive strategies in amphibians. Am. Nat. 170, 793–799 10.1086/521958 (doi:10.1086/521958) [DOI] [PubMed] [Google Scholar]
- 106.Chase J. M. 2003. Experimental evidence for alternative stable equilibria in a benthic pond food web. Ecol. Lett. 6, 733–741 10.1046/j.1461-0248.2003.00482.x (doi:10.1046/j.1461-0248.2003.00482.x) [DOI] [Google Scholar]
- 107.Johnson P. J., Lunde K. B. 2005. Parasite infection and limb malformations: a growing problem in amphibian conservation. In Amphibian declines: the conservation status of United States species (ed. Lannoo M.), pp. 124–138 Berkeley, CA: University of California Press [Google Scholar]
- 108.Johnson P. T. J., Chase J. M., Dosch K. L., Hartson R. B., Gross J. A., Larson D. J., Sutherland D. R., Carpenter S. R. 2007. Aquatic eutrophication promotes pathogenic infection in amphibians. Proc. Natl Acad. Sci. USA 104, 15 781–15 786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McKenzie V. J., Townsend A. R. 2007. Parasitic and infectious disease responses to a changing nitrogen cycle. EcoHealth 4, 384–396 10.1007/s10393-007-0131-3 (doi:10.1007/s10393-007-0131-3) [DOI] [Google Scholar]
- 110.Johnson P. T. J., Carpenter S. R. 2008. Influence of eutrophication on disease in aquatic ecosystems: patterns, processes, and predictions. In Infectious disease ecology: effects of ecosystems on disease and of disease on ecosystems (eds Ostfeld R., Keesing F.), pp. 71–99 Princeton, NJ: Princeton University Press [Google Scholar]
- 111.King K. C., Mclaughlin J. D., Gendron A. D., Pauli B. D., Giroux I., Rondeau B., Boily M., Juneau P., Marcogliese D. J. 2007. Impacts of agriculture on the parasite communities of northern leopard frogs (Rana pipiens) in southern Quebec. Can. Parasitol. 134, 2063–2080 [DOI] [PubMed] [Google Scholar]
- 112.Hartson R. B., Orlofske S. A., Keller V., Dillon R. T., Jr, Johnson P. T. J. Submitted Land use and wetland spatial position jointly determine amphibian parasite communities. [DOI] [PubMed] [Google Scholar]
- 113.Schotthoefer A. M., Labak K. M., Beasley V. R. 2007. Ribeiroia ondatrae cercariae are consumed by aquatic invertebrate predators. J. Parasitol. 93, 1240–1243 10.1645/GE1129R.1 (doi:10.1645/GE1129R.1) [DOI] [PubMed] [Google Scholar]
- 114.Thieltges D. W., Jensen K. T., Poulin R. 2008. The role of biotic factors in the transmission of free-living endohelminth stages. Parasitology 135, 407–426 [DOI] [PubMed] [Google Scholar]
- 115.Johnson P. T. J., Hartson R. B., Larson D. J., Sutherland D. R. 2008. Diversity and disease: community structure drives parasite transmission and host fitness. Ecol. Lett. 11, 1017–1026 10.1111/j.1461-0248.2008.01212.x (doi:10.1111/j.1461-0248.2008.01212.x) [DOI] [PubMed] [Google Scholar]
- 116.Fisher M. C., Garner T. W. J., Walker S. F. 2009. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time and host . Annu. Rev. Microbiol. 63, 291–310 10.1146/annurev.micro.091208.073435 (doi:10.1146/annurev.micro.091208.073435) [DOI] [PubMed] [Google Scholar]
- 117.Piotrowski J. S., Annis S. L., Longcore J. E. 2004. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96, 9–15 10.2307/3761981 (doi:10.2307/3761981) [DOI] [PubMed] [Google Scholar]
- 118.Longcore J. E., Pessier A. P., Nichols D. K. 1999. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91, 219–227 10.2307/3761366 (doi:10.2307/3761366) [DOI] [Google Scholar]
- 119.Woodhams D. C., Alford R. A., Briggs C. J., Johnson M., Rollins-Smith L. A. 2008. Life-history trade-offs influence disease in changing climates: strategies of an amphibian pathogen. Ecology 89, 1627–1639 10.1890/06-1842.1 (doi:10.1890/06-1842.1) [DOI] [PubMed] [Google Scholar]
- 120.Berger L., et al. 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl Acad. Sci. USA. 95, 9031–9036 10.1073/pnas.95.15.9031 (doi:10.1073/pnas.95.15.9031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Savage A. E., Sredl M. J., Zamudio K. R. 2011. Disease dynamics vary spatially and temporally in a North American amphibian. Biol. Conserv. 144, 1910–1915 10.1016/j.biocon.2011.03.018 (doi:10.1016/j.biocon.2011.03.018) [DOI] [Google Scholar]
- 122.Johnson M. L., Speare R. 2005. Possible modes of dissemination of the amphibian chytrid Batrachochytrium dendrobatidis in the environment. Dis. Aquat. Org. 65, 181–186 10.3354/dao065181 (doi:10.3354/dao065181) [DOI] [PubMed] [Google Scholar]
- 123.Ortiz-Santaliestra M. E., Fisher M. C., Fernandez-Beaskoetzea S., Fernandez-Beneitez M. J., Bosch J. 2011. Ambient ultraviolet B radiation and prevalence of infection by Batrachochytrium dendrobatidis in two amphibian species. Conserv. Biol. 25, 975–982 [DOI] [PubMed] [Google Scholar]
- 124.Johnson M. L., Berger L., Philips L., Speare R. 2003. Fungicidal effects of chemical disinfectants, UV light, dessication and heat on the amphibian chytrid Batrachochytrium dendrobatidis. Dis. Aquat. Org. 57, 255–260 10.3354/dao057255 (doi:10.3354/dao057255) [DOI] [PubMed] [Google Scholar]
- 125.Webb R., Mendez D., Berger L., Speare R. 2007. Additional disinfectants effective against the amphibian chytrid fungus Batrachochytrium dendrobatidis. Dis. Aquat. Org. 74, 13–16 10.3354/dao074013 (doi:10.3354/dao074013) [DOI] [PubMed] [Google Scholar]
- 126.Di Rosa I., Simoncelli F., Fagotti A., Pascolini R. 2007. Ecology: the proximate cause of frog declines. Nature 447, E4–E5 10.1038/nature05941 (doi:10.1038/nature05941) [DOI] [PubMed] [Google Scholar]
- 127.Daszak P., Strieby A., Cunningham A. A., Longcore J. E., Brown C. C., Porter D. 2004. Experimental evidence that the bullfrog (Rana catesbeiana) is a potential carrier of chytridiomycosis, an emerging fungal disease of amphibians. Herpetol. J. 14, 201–208 [Google Scholar]
- 128.Blaustein A. R., Romansic J. M., Scheessele E. A., Han B. A., Pessier A. P., Longcore J. E. 2005. Interspecific variation in susceptibility of frog tadpoles to the pathogenic fungus Batrachochytrium dendrobatidis. Conserv. Biol. 19, 1460–1468 10.1111/j.1523-1739.2005.00195.x (doi:10.1111/j.1523-1739.2005.00195.x) [DOI] [Google Scholar]
- 129.Carey C., Bruzgul J. E., Livo L. J., Walling M. L., Kuehl K. A., Dixon B. F., Pessier A. P., Alford R. A., Rogers K. B. 2006. Experimental exposures of boreal toads (Bufo boreas) to a pathogenic chytrid fungus (Batrachochytrium dendrobatidis). EcoHealth 3, 5–21 10.1007/s10393-005-0006-4 (doi:10.1007/s10393-005-0006-4) [DOI] [Google Scholar]
- 130.Garcia T. S., Romansic J. M., Blaustein A. R. 2006. Survival of three species of anuran metamorphs exposed to UV-B radiation and the pathogenic fungus Batrachochytrium dendrobatidis. Dis. Aquat. Org. 72, 163–169 10.3354/dao072163 (doi:10.3354/dao072163) [DOI] [PubMed] [Google Scholar]
- 131.Searle C. L., Gervasi S.S., Hua J., Hammond J. I., Relyea R. A., Olson D. H., Blaustein A. R. 2011. Differential host susceptibility to Batrachochytrium dendrobatidis, an emerging amphibian pathogen. Conserv. Biol. 25, 965–974 10.1111/j.1523-1739.2011.01708.x (doi:10.1111/j.1523-1739.2011.01708.x) [DOI] [PubMed] [Google Scholar]
- 132.Ron S. R. 2005. Predicting the distribution of the amphibian pathogen Batrachochytrium dendrobatidis in the New World. Biotropic 37, 209–221 10.1111/j.1744-7429.2005.00028.x (doi:10.1111/j.1744-7429.2005.00028.x) [DOI] [Google Scholar]
- 133.Lips K. R., Reeve J. D., Witters L. R. 2003. Ecological traits predicting amphibian population declines in Central America. Conserv. Biol. 17, 1078–1088 10.1046/j.1523-1739.2003.01623.x (doi:10.1046/j.1523-1739.2003.01623.x) [DOI] [Google Scholar]
- 134.Moss A. S., Reddy N. S., Dortaj I. M., Francisco M. J. 2008. Chemotaxis of the amphibian pathogen Batrachochytrium dendrobatidis and its response to a variety of attractants. Mycologia 100, 1–5 10.3852/mycologia.100.1.1 (doi:10.3852/mycologia.100.1.1) [DOI] [PubMed] [Google Scholar]
- 135.Venesky M. D., Parris M. J. 2009. Effects of Batrachochytrium dendrobatidis infection on larval foraging performance. ICB 49, E176–E176 [DOI] [PubMed] [Google Scholar]
- 136.Venesky M. D., Parris M. J., Storfer A. 2010. Impacts of Batrachochytrium dendrobatidis infection on tadpole foraging performance . Ecohealth 6, 565–575 10.1007/s10393-009-0272-7 (doi:10.1007/s10393-009-0272-7) [DOI] [PubMed] [Google Scholar]
- 137.Voyles J., Berger L., Young S., Speare R., Webb R., Warner J., Rudd D., Campbell R., Skerratt L. F. 2007. Electrolyte depletion and osmotic imbalance in amphibians with chytridiomycosis . Dis. Aquat. Org. 77, 113–118 10.3354/dao01838 (doi:10.3354/dao01838) [DOI] [PubMed] [Google Scholar]
- 138.Voyles J., et al. 2009. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science 326, 582–585 10.1126/science.1176765 (doi:10.1126/science.1176765) [DOI] [PubMed] [Google Scholar]
- 139.Rosenblum E. B., Stajich J. E., Maddox N., Eisen M. B. 2008. Global gene expression profiles for life stages of the deadly amphibian pathogen Batrachochytrium dendrobatidis. Proc. Natl Acad. Sci. USA 105, 17 034–17 039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Briggs C. J., Knapp R. A., Vredenburg V. T. 2010. Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proc. Natl Acad. Sci. USA 107, 9695–9700 10.1073/pnas.0912886107 (doi:10.1073/pnas.0912886107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rollins-Smith L. A. 1998. Metamorphosis and the amphibian immune system. Immunol. Rev. 166, 221–230 10.1111/j.1600-065X.1998.tb01265.x (doi:10.1111/j.1600-065X.1998.tb01265.x) [DOI] [PubMed] [Google Scholar]
- 142.Russell D. M., Goldberg C. S., Waits L. P., Rosenblum E. B. 2010. Batrachochytrium dendrobatidis infection dynamics in the Columbia spotted frog Rana luteiventris in north Idaho, USA. Dis. Aquat. Org. 92, 223–230 10.3354/dao02286 (doi:10.3354/dao02286) [DOI] [PubMed] [Google Scholar]
- 143.Voyles J., Rosenblum E.B., Berger L. 2011. Interactions between Batrachochytrium dendrobatidis and its amphibian hosts: a review of pathogenesis and immunity. Microbes Infect. 13, 25–32 10.1016/j.micinf.2010.09.015 (doi:10.1016/j.micinf.2010.09.015) [DOI] [PubMed] [Google Scholar]
- 144.Vredenburg V. T., Knapp R. A., Tunstall T. S., Briggs C. J. 2010. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc. Natl Acad. Sci. USA 107, 9689–9694 10.1073/pnas.0914111107 (doi:10.1073/pnas.0914111107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Richmond J. Q., Savage A. E., Zamudio K. R., Rosenblum E. B. 2009. Toward immunogenetic studies of amphibian chytridiomycosis: linking innate and acquired immunity. Bioscience 54, 311–320 10.1525/bio.2009.59.4.9 (doi:10.1525/bio.2009.59.4.9) [DOI] [Google Scholar]
- 146.Rollins-Smith L. A., Carey C., Longcore J., Doersam J. K., Boutte A., Bruzgal J. E., Conlon J. M. 2002. Activity of antimicrobial skin peptides from ranid frogs against Batrachochytrium dendrobatidis, the chytrid fungus associated with global amphibian declines. Dev. Comp. Immunol. 26, 471–479 10.1016/S0145-305X(01)00088-X (doi:10.1016/S0145-305X(01)00088-X) [DOI] [PubMed] [Google Scholar]
- 147.Rollins-Smith L. A., Conlon J. M. 2005. Antimicrobial peptide defenses against chytridiomycosis, an emerging infectious disease of amphibian populations. Dev. Comp. Immunol. 29, 589–598 10.1016/j.dci.2004.11.004 (doi:10.1016/j.dci.2004.11.004) [DOI] [PubMed] [Google Scholar]
- 148.Woodhams D. C., Rollins-Smith L. A., Carey C., Reinert L., Tyler M. J., Alford R. A. 2006. Population trends associated with skin peptide defenses against chytridiomycosis in Australian frogs. Oecologia 146, 531–540 10.1007/s00442-005-0228-8 (doi:10.1007/s00442-005-0228-8) [DOI] [PubMed] [Google Scholar]
- 149.Rollins-Smith L. A., Ramsey J. P., Reinert J. K., Woodhams D. C., Livo L. J., Carey C. 2009. Immune defenses of Xenopus laevis against Batrachochytrium dendrobatidis. Front. Biosci. S1, 68–91 [DOI] [PubMed] [Google Scholar]
- 150.Nichols D. K., Lamirande E. W., Pessier A. P., Longcore J. E. 2001. Experimental transmission of cutaneous chytridiomycosis in dendrobatid frogs. J. Wildl. Dis. 37, 1–11 [DOI] [PubMed] [Google Scholar]
- 151.Berger L., Speare R., Skerratt L. F. 2005. Distribution of Batrachochytrium dendrobatidis and pathology in the skin of green tree frogs Litorria caerulea with severe chytridiomycosis. Dis. Aquat. Org. 68, 65–70 10.3354/dao068065 (doi:10.3354/dao068065) [DOI] [PubMed] [Google Scholar]
- 152.Ribas L., et al. 2009. Expression profiling the temperature-dependent amphibian response to infection by Batrachochytrium dendrobatidis . PLoS ONE 4, e8408. 10.1371/journal.pone.0008408 (doi:10.1371/journal.pone.0008408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Blatteis C. M., Sehic E. 1997. Fever: how may circulating pyrogens signal the brain? News Physiol. Sci. 12, 1–9 [Google Scholar]
- 154.Richards-Zawacki C. L. 2009. Thermoregulatory behaviour affects prevalence of chytrid fungal infection in a wild population of Panamanian golden frogs . Proc. R. Soc. B 277, 519–528 10.1098/rspb.2009.1656 (doi:10.1098/rspb.2009.1656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Geiger C. C., Küpfer E., Schär S., Wolf S., Schmidt B. R. 2011. Elevated temperature clears chytrid fungus infections from tadpoles of the midwife toad, Alytes obstetricans. Amphibia-Reptilia 32, 276–280 10.1163/017353711X556970 (doi:10.1163/017353711X556970) [DOI] [Google Scholar]
- 156.Weinstein S. 2009. An aquatic disease on a terrestrial salamander: individual and population level effects of the amphibian chytrid fungus, Batrachochytrium dendrobatidis, on Batrachoseps attenuatus (Plethodontidae). Copeia 4, 653–660 10.1643/CH-08-180 (doi:10.1643/CH-08-180) [DOI] [Google Scholar]
- 157.Retallick R. W. R., Miera V. 2007. Strain differences in the amphibian chytrid Batrachochytrium dendrobatidis and non-permanent, sub-lethal effects of infection. Dis. Aquat. Org. 75, 201–207 10.3354/dao075201 (doi:10.3354/dao075201) [DOI] [PubMed] [Google Scholar]
- 158.Han B. A., Bradley P. W., Blaustein A. R. 2008. Ancient behaviors of larval amphibians in response to an emergent fungal pathogen, Batrochochytrium dendrobatidis. Behav. Ecol. Sociobiol. 63, 241–250 10.1007/s00265-008-0655-8 (doi:10.1007/s00265-008-0655-8) [DOI] [Google Scholar]
- 159.Harris R. N., James T. Y., Lauer A., Simon M. A., Patel A. 2006. Amphibian pathogen Batrachochytrium dendrobatidis is inhibited by the cutaneous bacteria of amphibian species. EcoHealth 3, 53–56 10.1007/s10393-005-0009-1 (doi:10.1007/s10393-005-0009-1) [DOI] [Google Scholar]
- 160.Brucker R. M., Harris R. N., Schwantes C. R., Gallaher T. N., Flaherty D. C., Lam B. A., Minbiole K. P. C. 2008. Amphibian chemical defense: antifungal metabolites of the microsymbiont Janthinobacterium lividum on the salamander Plethodon cinereus. J Chem. Ecol. 34, 1422–1429 10.1007/s10886-008-9555-7 (doi:10.1007/s10886-008-9555-7) [DOI] [PubMed] [Google Scholar]
- 161.Lam B. A., Walton D. B., Harris R. N. 2011. Motile zoospores of Batrachochytrium dendrobatidis move away from antifungal metabolites produced by amphibian skin bacteria. Ecohealth 8, 36–45 10.1007/s10393-011-0689-7 (doi:10.1007/s10393-011-0689-7) [DOI] [PubMed] [Google Scholar]
- 162.Bettin C., Greven H. 1986. Bacteria on the skin of Salamandra salamandra (L) (Amphibia, Urodela) with notes on their possible significance. Zool . Anz. 216, 267–270 [Google Scholar]
- 163.Barra D., Simmaco M., Boman H. G. 1998. Gene-encoded peptide antibiotics and innate immunity. Do ‘animalcules’ have defence budgets. FEBS Lett. 430, 130–134 [DOI] [PubMed] [Google Scholar]
- 164.Austin R. M., Jr 2000. Cutaneous microbial flora and antibiosis in Plethodon ventralis. In The biology of plethodontid salamanders (eds Bruce R. C., Jaegar R. G., Houck L. D.), pp. 451–462 New York, NY: Kluwer Academic/Plenum Publishers [Google Scholar]
- 165.Culp C. E., Falkinham J. O., Belden L. K. 2007. Identification of the natural bacterial microflora on the skin of eastern newts, bullfrog tadpoles and redback salamanders. Herpetologica 63, 66–71 10.1655/0018-0831(2007)63[66:IOTNBM]2.0.CO;2 (doi:10.1655/0018-0831(2007)63[66:IOTNBM]2.0.CO;2) [DOI] [Google Scholar]
- 166.Lauer A., Simon M. A., Banning J. L., Andre E., Duncan K., Harris R. N. 2007. Common cutaneous bacteria from the eastern red-backed salamander can inhibit pathogenic fungi. Copeia 2007, 630–640 10.1643/0045-8511(2007)2007[630:CCBFTE]2.0.CO;2 (doi:10.1643/0045-8511(2007)2007[630:CCBFTE]2.0.CO;2) [DOI] [Google Scholar]
- 167.Lauer A., Simon M. A., Banning J. L., Lam B. A., Harris R. N. 2008. Diversity of cutaneous bacteria with antifungal activity isolated from female four-toed salamanders. ISME J. 2, 145–157 10.1038/ismej.2007.110 (doi:10.1038/ismej.2007.110) [DOI] [PubMed] [Google Scholar]
- 168.Brucker R. M., Baylor C. M., Walters R. L., Lauer A., Harris R. N., Minbiole K.P.C. 2008. The identification of 2,4-diacetylphloroglucinol as an antifungal metabolite produced by cutaneous bacteria of the salamander Plethodon cinereus. J. Chem. Ecol. 34, 39–43 10.1007/s10886-007-9352-8 (doi:10.1007/s10886-007-9352-8) [DOI] [PubMed] [Google Scholar]
- 169.Harris R. N., et al. 2009. Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J. 3, 818–824 10.1038/ismej.2009.27 (doi:10.1038/ismej.2009.27) [DOI] [PubMed] [Google Scholar]
- 170.Harris R. N., Lauer A., Simon M. A., Banning J. L., Alford R. A. 2009. Addition of antifungal skin bacteria to salamanders ameliorates the effects of chytridiomycosis. Dis. Aquat. Org. 83, 11–16 10.3354/dao02004 (doi:10.3354/dao02004) [DOI] [PubMed] [Google Scholar]
- 171.Becker M. H., Harris R. N. 2010. Cutaneous bacteria of the redback salamander prevent morbidity associated with a lethal disease. PLoS ONE 5, e10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.McKenzie V. J., Bowers R. M., Fierer N., Knight R., Lauber C. L. 2012. Co-habiting amphibian species harbor unique skin bacterial communities in wild populations. ISME J. 6, 588–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Boman H. G. 2000. Innate immunity and the normal microflora. Immunol. Rev. 173, 5–16 10.1034/j.1600-065X.2000.917301.x (doi:10.1034/j.1600-065X.2000.917301.x) [DOI] [PubMed] [Google Scholar]
- 174.Conlon J. M. 2011. The contribution of skin antimicrobial peptides to the system of innate immunity in anurans. Cell Tissue Res. 343, 201–212 10.1007/s00441-010-1014-4 (doi:10.1007/s00441-010-1014-4) [DOI] [PubMed] [Google Scholar]
- 175.Rosenblum E. B., Stajich J. E., Maddox N., Eisen M. B. 2008. Global gene expression profile for life stages of the deadly amphibian pathogen Batcahochytrium dendrobatidis. Proc. Natl Acad. Sci. USA 105, 17 034–17 039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rosenblum E. B., Fisher M. C., James T. Y., Stajich J. E., Longcore J. E., Gentry L. R., Poorten T. J. 2009. A molecular perspective: biology of the emerging pathogen Batrachochytrium dendrobatidis. Dis. Aquat. Org. 92, 131–147 10.3354/dao02179 (doi:10.3354/dao02179) [DOI] [PubMed] [Google Scholar]
- 177.Beldomenico P.M., Begon M. 2009. Disease spread, susceptibility and infection intensity: vicious circles. Trends Ecol. Evol. 25, 21–27 10.1016/j.tree.2009.06.015 (doi:10.1016/j.tree.2009.06.015) [DOI] [PubMed] [Google Scholar]
- 178.Sodhi N. S., Bickford D., Diesmos A. C., Lee T. M., Koh L. P., Brook B. W., Sekercioglu C. H., Bradshaw C. J. A. 2008. Measuring the meltdown: drivers of global amphibian extinction and decline. PLoS ONE 3, e1636. 10.1371/journal.pone.0001636 (doi:10.1371/journal.pone.0001636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Haydon D. T., Cleaveland S., Taylor L. H., Laurenson M. K. 2002. Identifying reservoirs of infection: a conceptual and practical challenge. Emerg. Infect. Dis. 8, 1468–1473 10.3201/eid0812.010317 (doi:10.3201/eid0812.010317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hanselmann R., Rodrigues A., Lampo M., Fajardo-Ramos L., Aguirre A. A., Kilpatrick A. M., Rodriguez J. P., Daszak P. 2004. Presence of an emerging pathogen of amphibians in introduced bullfrogs Rana catesbeiana in Venezuela. Biol. Conserv. 120, 115–119 10.1016/j.biocon.2004.02.013 (doi:10.1016/j.biocon.2004.02.013) [DOI] [Google Scholar]
- 181.Rachowicz L. J., Vredenburg V. T. 2004. Transmission of Batrachochytrium dendrobatidis within and between amphibian life stages. Dis. Aquat. Org. 61, 75–83 10.3354/dao061075 (doi:10.3354/dao061075) [DOI] [PubMed] [Google Scholar]
- 182.Parris M. J., Beaudoin J. G. 2004. Chytridiomycosis impacts predator–prey interactions in larval amphibian communities. Oecologia 140, 626–632 10.1007/s00442-004-1631-2 (doi:10.1007/s00442-004-1631-2) [DOI] [PubMed] [Google Scholar]
- 183.Parris M. J., Cornelius T. O. 2004. Fungal pathogen causes competitive and developmental stress in larval amphibian communities. Ecology 85, 3385–3395 10.1890/04-0383 (doi:10.1890/04-0383) [DOI] [Google Scholar]
- 184.Rowley J. J. L., Alford R. A. 2007. Behaviour of Australian rainforest stream frogs may affect the transmission of chytridiomycosis. Dis. Aquat. Org. 77, 1–9 10.3354/dao01830 (doi:10.3354/dao01830) [DOI] [PubMed] [Google Scholar]
- 185.Searle C., Biga L., Spatafora J., Blaustein A. R. 2011. A dilution effect in the emerging amphibian pathogen, Batrachochytrium dendrobatidis. Proc. Natl Acad. Sci USA 16 322–16 326 10.1073/pnas.1108490108 (doi:10.1073/pnas.1108490108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Connelly S., Pringle M. C., Bixby R. J., Brenes R., Whiles M. R., Lips M. R., Kilham S., Huryn A. D. 2008. Changes in stream primary producer communities resulting from large-scale catastrophic amphibian declines: can small-scale experiments predict effects of tadpole loss? Ecosystems 11, 1262–1276 10.1007/s10021-008-9191-7 (doi:10.1007/s10021-008-9191-7) [DOI] [Google Scholar]
- 187.Carey C., Cohen N., Rollins-Smith L. 1999. Amphibian declines: an immunological perspective. Dev. Comp. Immunol. 23, 459–472 10.1016/S0145-305X(99)00028-2 (doi:10.1016/S0145-305X(99)00028-2) [DOI] [PubMed] [Google Scholar]
- 188.Power A. G., Mitchell C. E. 2004. Pathogen spillover in disease epidemics. Am. Nat. 164, S79–S89 10.1086/424610 (doi:10.1086/424610) [DOI] [PubMed] [Google Scholar]
- 189.Sapolsky R. M. 1993. Neuroendocrinology of the stress-response. In Behavioral endocrinology (eds Becker J. B., Breedlove S. M., Crews D.), pp. 287–324 Cambridge, MA: MIT Press [Google Scholar]
- 190.Denver R. J. 1998. Hormonal correlates of environmentally induced metamorphosis in the western spadefoot toad, Scaphiopus hammondii. Gen. Comp. Endorcr. 110, 326–336 10.1006/gcen.1998.7082 (doi:10.1006/gcen.1998.7082) [DOI] [PubMed] [Google Scholar]
- 191.Denver R. J., Mirhadi N., Phillips M. 1998. Adaptive plasticity in amphibian metamorphosis: response of Scaphiopus hammondii tadpoles to habitat desiccation. Ecology 79, 1859–1872 [Google Scholar]
- 192.Fraker M. E., et al. 2009. Characterization of an alarm pheromone secreted by amphibian tadpoles that induces behavioral inhibition and suppression of the neuroendocrine stress axis. Horm. Behav. 55, 520–529 10.1016/j.yhbeh.2009.01.007 (doi:10.1016/j.yhbeh.2009.01.007) [DOI] [PubMed] [Google Scholar]
- 193.Hossie T. J., Ferland-Raymond B., Burness G., Murray D. L. 2010. Morphological and behavioural responses of frog tadpoles to perceived predation risk: a possible role for corticosterone mediation. Ecoscience 17, 100–108 10.2980/17-1-3312 (doi:10.2980/17-1-3312) [DOI] [Google Scholar]
- 194.Gendron A. D., Bishop C. A., Fortin R., Hontela A. 1997. In vivo testing of the functional integrity of the corticosterone-producing axis in mudpuppy (Amphibia) exposed to chlorinated hydrocarbons in the wild. Environ. Toxicol. Chem. 16, 194–170 [Google Scholar]
- 195.Hopkins W. A., Mendonca M. T., Congdon J. D. 1997. Increased circulating levels of testosterone and corticosterone in southern toads, Bufo terrestris, exposed to coal combustion waste. Gen. Comp. Endocr. 108, 237–246 10.1006/gcen.1997.6969 (doi:10.1006/gcen.1997.6969) [DOI] [PubMed] [Google Scholar]
- 196.Larson D. L., McDonald S., Fivizzani A. J., Newton W. E., Hamilton S. J. 1998. Effects of the herbicide atrazine on Ambystoma tigrinum metamorphosis: duration, larval growth, and hormonal response. Physiol. Zool. 71, 671–679 [DOI] [PubMed] [Google Scholar]
- 197.Peterson J. D., Peterson V. A., Mendonca M. T. 2009. Exposure to coal combustion residues during metamorphosis elevates corticosterone content and adversely affects oral morphology, growth, and development in Rana sphenocephala. Comp. Biochem. Phys. C 149, 36–39 [DOI] [PubMed] [Google Scholar]
- 198.Janin A., Lena J. P., Joly P. 2011. Beyond occurrence: body condition and stress hormone as integrative indicators of habitat availability and fragmentation in the common toad. Biol. Conserv. 144, 1008–1016 10.1016/j.biocon.2010.12.009 (doi:10.1016/j.biocon.2010.12.009) [DOI] [Google Scholar]
- 199.Norris D. O., Donahue S., Dores R. M., Lee J. K., Maldonado T. A., Ruth T., Woodling J. D. 1999. Impaired adrenocorical response to stress in brown trout, Salmo trutta, living in metal-contaminated waters of the Eagle River, Colorado. Gen. Comp. Endocr. 113, 1–8 10.1006/gcen.1998.7177 (doi:10.1006/gcen.1998.7177) [DOI] [PubMed] [Google Scholar]
- 200.Cyr N. E., Romero L. M. 2007. Chronic stress in free-living European starlings reduces corticosterone concentrations and reproductive success. Gen. Comp. Endocr. 151, 82–89 10.1016/j.ygcen.2006.12.003 (doi:10.1016/j.ygcen.2006.12.003) [DOI] [PubMed] [Google Scholar]
- 201.Maule A. G., VanderKooi S. P. 1999. Stress-induced immune–endocrine interactions. In Stress physiology in animals (ed. Balm P. H. M.), pp. 205–245 Sheffield, UK: Sheffield Academic Press [Google Scholar]
- 202.Ottaviani E., Franceschi C. 1996. The neuroimmunology of stress from invertebrates to man. Prog. Neurobiol. 48, 421–440 10.1016/0301-0082(95)00049-6 (doi:10.1016/0301-0082(95)00049-6) [DOI] [PubMed] [Google Scholar]
- 203.Mastorakos G., Bamberger C., Chrousos G. P. 1999. Neuroendocrine regulation of the immune process. In Cytokines: stress and immunity (eds Plotnikoff N. P., Faith R. E., Murgo A. J., Good R. A.), pp. 17–37 Boca Raton, CA: CRC Press [Google Scholar]
- 204.Tournefier A. 1982. Corticosteroid action on lymphocyte subpopulations and humoral immune responses of axolotl (urodele amphibian). Immunology 46, 155–162 [PMC free article] [PubMed] [Google Scholar]
- 205.Wilckens T., De Rijk R. 1997. Glucocorticoids and immune function: unknown dimensions and new frontiers. Immunol. Today 18, 418–424 10.1016/S0167-5699(97)01111-0 (doi:10.1016/S0167-5699(97)01111-0) [DOI] [PubMed] [Google Scholar]
- 206.McEwen B. S., et al. 1997. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res. Rev. 23, 79–133 10.1016/S0165-0173(96)00012-4 (doi:10.1016/S0165-0173(96)00012-4) [DOI] [PubMed] [Google Scholar]
- 207.Coutinho A. E., Chapman K. E. 2011. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 335, 2–13 10.1016/j.mce.2010.04.005 (doi:10.1016/j.mce.2010.04.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Pederson A. B., Babayan S. A. 2011. Wild immunology. Mol. Ecol. 20, 872–880 10.1111/j.1365-294X.2010.04938.x (doi:10.1111/j.1365-294X.2010.04938.x) [DOI] [PubMed] [Google Scholar]
- 209.Hayes T. B. 1997. Steroids as potential modulators of thyroid hormone activity in anuran metamorphosis. Am. Zool. 37, 185–194 [Google Scholar]
- 210.Glennemeier K. A., Denver R. J. 2002. Role for corticoids in mediating the response of Rana pipiens tadpoles to intraspecific competition. J. Exp. Zool. 292, 32–40 10.1002/jez.1140 (doi:10.1002/jez.1140) [DOI] [PubMed] [Google Scholar]
- 211.Belden L. K., Rubbo M. J., Wingfield J. C., Kiesecker J. M. 2007. Searching for the physiological mechanism of density-dependence: does corticosterone regulate tadpole responses to density? Physiol. Biochem. Zool. 80, 444–451 10.1086/518375 (doi:10.1086/518375) [DOI] [PubMed] [Google Scholar]
- 212.Davis A. K., Maney D. L., Maerz J. C. 2008. The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct. Ecol. 22, 760–772 10.1111/j.1365-2435.2008.01467.x (doi:10.1111/j.1365-2435.2008.01467.x) [DOI] [Google Scholar]
- 213.Davis A. K., Maerz J. C. 2008. Comparison of hematological stress indicators in recently captured and captive paedomorphic Mole Salamanders, Ambystoma talpoideum. Copeia 2008, 613–617 10.1643/CP-07-133 (doi:10.1643/CP-07-133) [DOI] [Google Scholar]
- 214.Davis A. K., Maerz J. C. 2009. Effects of larval density on hematological stress indices in Salamanders. J. Exp. Zool. A 311A, 697–704 10.1002/jez.557 (doi:10.1002/jez.557) [DOI] [PubMed] [Google Scholar]
- 215.McMenamin S. K., Hadley E. A., Wright C. K. 2008. Climatic change and wetland desiccation cause amphibian decline in Yellowstone National Park. Proc. Natl Acad. Sci. USA 105, 16 988–16 993 10.1073/pnas.0809090105 (doi:10.1073/pnas.0809090105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Burdon J., Elmqvist T. 1996. Selective sieves in the epidemiology of Melampsora lini. Plant Pathol. 45, 933–943 10.1111/j.1365-3059.1996.tb02904.x (doi:10.1111/j.1365-3059.1996.tb02904.x) [DOI] [Google Scholar]
- 217.Dwyer G., Elkinton J. S. 1993. Using simple models to predict virus epizootics in gypsy moth populations. J. Anim. Ecol. 62, 1–11 10.2307/5477 (doi:10.2307/5477) [DOI] [Google Scholar]
- 218.Schindler D. W. 1997. Widespread effects of climatic warming on freshwater ecosystems in North America. Hydrol. Process. 11, 1043–1067 10.1002/(SICI)1099-1085(19970630)11:8%3C1043::AID-HYP517%3E3.0.CO;2-5 (doi:10.1002/(SICI)1099-1085(19970630)11:8<1043::AID-HYP517>3.0.CO;2-5) [DOI] [Google Scholar]
- 219.IPCC 2008. Climate change and water. In Technical Paper of the Intergovernmental Panel on Climate Change (eds Bates B. C., Kundzewicz Z. W., Wu S., Palutikof J. P.), Geneva, Switzerland: Secretariat [Google Scholar]
- 220.Kriger K. M. 2009. Lack of evidence for the drought-linked chytridiomycosis hypothesis. J. Wildl. Dis. 45, 537–541 [DOI] [PubMed] [Google Scholar]
- 221.Burrowes P. A., Joglar R. L., Green D. L. 2004. Potential causes for amphibian declines in Puerto Rico. Herpetologica 60, 141–154 10.1655/03-50 (doi:10.1655/03-50) [DOI] [Google Scholar]
- 222.Lampo M., Rodríguez-Contreras A., La Marca E., Daszak P. 2006. A chytridiomycosis epidemic and a severe dry season precede the disappearance of Atelopus species from the Venezuelan Andes. Herpetol. J. 16, 395–402 [Google Scholar]
- 223.Seimon T. A., et al. 2007. Upward range extension of Andean anurans and chytridiomycosis to extreme elevations in response to tropical deglaciation. Glob. Change Biol. 13, 288–299 10.1111/j.1365-2486.2006.01278.x (doi:10.1111/j.1365-2486.2006.01278.x) [DOI] [Google Scholar]
- 224.Kupferberg S. J., Catenazzi A., Lunde K., Lind A. J., Palen W. J. 2009. Parasitic Copepod (Lernaea cyprinacea) outbreaks in foothill yellow-legged Frogs (Rana boylii) linked to unusually warm summers and amphibian malformations in Northern California. Copeia 2009, 529–537 10.1643/CH-08-011 (doi:10.1643/CH-08-011) [DOI] [Google Scholar]
- 225.Pounds A., et al. 2006. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439, 161–167 10.1038/nature04246 (doi:10.1038/nature04246) [DOI] [PubMed] [Google Scholar]
- 226.Bosch J., Carrascal L. M., Duran L., Walker S., Fisher M. C. 2006. Climate change and outbreaks of amphibian chytridiomycosis in a montane area of Central Spain; is there a link? Proc. R. Soc. B 274, 253–260 10.1098/rspb.2006.3713 (doi:10.1098/rspb.2006.3713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.D'Amen M., Bombi P. 2009. Global warming and biodiversity: evidence of climate-linked amphibian declines in Italy. Biol. Conserv. 142, 3060–3067 10.1016/j.biocon.2009.08.004 (doi:10.1016/j.biocon.2009.08.004) [DOI] [Google Scholar]
- 228.Drew A., Allen E. J., Allen L. J. S. 2006. Analysis of climatic and geographic factors affecting the presence of chytridiomycosis in Australia. Dis. Aquat. Org. 68, 245–250 10.3354/dao068245 (doi:10.3354/dao068245) [DOI] [PubMed] [Google Scholar]
- 229.Lips K. R., Diffendorfer J., Mendelson J. R., III, Sears M. W. 2008. Riding the wave: reconciling the roles of disease and climate change in amphibian declines. Plos Biol. 6, 441–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Parmesan C., Singer M. C. 2008. Amphibian extinctions: disease not the whole story. Plos Biol. 28 March 2008. (doi:10.1371/journal.pbio.0060072#r2213) [Google Scholar]
- 231.Rohr J. R., Raffel T. R., Romansic J. M., McCallum H., Hudson P. J. 2008. Evaluating the links between climate, disease spread, and amphibian declines. Proc. Natl Acad. Sci. USA 105, 17 436–17 441 10.1073/pnas.0806368105 (doi:10.1073/pnas.0806368105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Daszak P., Scott D. E., Kilpatrick A. M., Faggioni C., Gibbons J. W., Porter D. 2005. Amphibian population declines at Savannah River Site are linked to climate, not chytridiomycosis. Ecology 86, 3232–3237 10.1890/05-0598 (doi:10.1890/05-0598) [DOI] [Google Scholar]
- 233.Van Sluys M., Hero J.-M. 2009. How does Chytrid infection vary among habitats? The case of Litoria wilcoxii (Anura, Hylidae) in SE Queensland, Australia. EcoHealth 6, 576–583 10.1007/s10393-010-0278-1 (doi:10.1007/s10393-010-0278-1) [DOI] [PubMed] [Google Scholar]
- 234.Muths E., Pilliod D. S., Livo L. J. 2008. Distribution and environmental limitations of an amphibian pathogen in the Rocky Mountains, USA. Biol. Conserv. 141, 1484–1492 10.1016/j.biocon.2008.03.011 (doi:10.1016/j.biocon.2008.03.011) [DOI] [Google Scholar]
- 235.Raffel T. R., Michel P. J., Sites E. W., Rohr J. R. 2010. What drives Chytrid infections in newt populations? Associations with substrate, temperature, and shade. EcoHealth 7, 526–536 10.1007/s10393-010-0358-2 (doi:10.1007/s10393-010-0358-2) [DOI] [PubMed] [Google Scholar]
- 236.Rohr J. R., Halstead N. T., Raffel T. R. 2011. Modelling the future distribution of the amphibian chytrid fungus: the influence of climate and human-associated factors. J. Appl. Ecol. 48, 174–176 10.1111/j.1365-2664.2010.01891.x (doi:10.1111/j.1365-2664.2010.01891.x) [DOI] [Google Scholar]
- 237.Paull S. H., Johnson P. T. J. In press Predicting the synergies between climate change and infectious disease. In Conserving wildlife populations in a changing climate (eds Brodie J., Post E., Doak D.). Chicago, IL: University of Chicago Press [Google Scholar]
- 238.Lawler J. J., White D., Neilson R. P., Blaustein A. R. 2006. Predicting climate-induced range shifts: model differences and model reliability. Global Change Biol. 12, 1568–1584 10.1111/j.1365-2486.2006.01191.x (doi:10.1111/j.1365-2486.2006.01191.x) [DOI] [Google Scholar]
- 239.Poulin R. 2006. Global warming and temperature-mediated increases in cercarial emergence in trematode parasites. Parisitology 132, 143–151 10.1017/S0031182005008693 (doi:10.1017/S0031182005008693) [DOI] [PubMed] [Google Scholar]
- 240.Paull S. H., Johnson P. T. J. 2011. High temperature enhances host pathology in a snail–trematode system: possible consequences of climate change for the emergence of disease. Freshw. Biol. 56, 767–778 10.1111/j.1365-2427.2010.02547.x (doi:10.1111/j.1365-2427.2010.02547.x) [DOI] [Google Scholar]
- 241.Tracy C. R., et al. 2006. The importance of physiological ecology in conservation biology. Integr. Compar. Biol. 46, 1191–1205 10.1093/icb/icl054 (doi:10.1093/icb/icl054) [DOI] [PubMed] [Google Scholar]
- 242.Carey C. 2005. How physiological methods and concepts can be useful in conservation biology. Integr comp. Biol. 45, 4–11 10.1093/icb/45.1.4 (doi:10.1093/icb/45.1.4) [DOI] [PubMed] [Google Scholar]
- 243.Cronin J. P., Welsh M. E., Dekkers M. G., Abercrombie S. T., Mitchell C. E. 2010. Host physiological phenotype explains pathogen reservoir potential. Ecol. Lett. 13, 1221–1232 10.1111/j.1461-0248.2010.01513.x (doi:10.1111/j.1461-0248.2010.01513.x) [DOI] [PubMed] [Google Scholar]
- 244.Zuk M., Stoehr A. M. 2002. Immune defense and host life history. Am. Nat. 160, S9–S22 10.1086/342131 (doi:10.1086/342131) [DOI] [PubMed] [Google Scholar]
- 245.Lloyd-Smith J. O., Schreiber S. J., Kopp P. E., Getz W. M. 2005. Superspreading and the effect of individual variation on disease emergence. Nature 438, 355–359 10.1038/nature04153 (doi:10.1038/nature04153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Seebacher F., Franklin C. E. 2012. Determining environmental causes of biological effects: the need for a mechanistic physiological dimension in conservation biology. Phil. Trans. R. Soc. B 367, 1607–1614 10.1098/rstb.2012.0036 (doi:10.1098/rstb.2012.0036) [DOI] [PMC free article] [PubMed] [Google Scholar]