Abstract
Physiological studies focus on the responses of cells, tissues and individuals to stressors, usually in laboratory situations. Conservation and management, on the other hand, focus on populations. The field of conservation physiology addresses the question of how abiotic drivers of physiological responses at the level of the individual alter requirements for successful conservation and management of populations. To achieve this, impacts of physiological effects at the individual level need to be scaled to impacts on population dynamics, which requires consideration of ecology. Successfully realizing the potential of conservation physiology requires interdisciplinary studies incorporating physiology and ecology, and requires that a constructive dialogue develops between these traditionally disparate fields. To encourage this dialogue, we consider the increasingly explicit incorporation of physiology into ecological models applied to marine fish conservation and management. Conservation physiology is further challenged as the physiology of an individual revealed under laboratory conditions is unlikely to reflect realized responses to the complex variable stressors to which it is exposed in the wild. Telemetry technology offers the capability to record an animal's behaviour while simultaneously recording environmental variables to which it is exposed. We consider how the emerging insights from telemetry can strengthen the incorporation of physiology into ecology.
Keywords: telemetry, behaviour, habitat use, ecosystem modelling, physiology
1. Introduction
The development of conservation physiology as a discipline has been driven, in part, by the long-term recognition that: (i) the abiotic environment can shape an organism's physiological development; (ii) human activities are altering the abiotic environment on a global scale; and (iii) abiotic impacts on an organism's physiologies can affect individual fitness with implications for population-level processes or, in other words, physiologists have knowledge and techniques that are useful for addressing contemporary applied conservation issues at the scale of populations.
Conservation physiology was originally defined as ‘the study of physiological responses of organisms to human alteration of the environment that might cause or contribute to population declines' by Wikelski & Cooke [1]. However, for this discussion, we consider conservation physiology to be an applied subdiscipline within ecophysiology and define conservation physiology as ‘the study of physiological responses of organisms to environmental changes and human-induced impacts, and their implications for population and ecosystem dynamics’. Our refined definition: (i) takes account of the fact that natural variations in the environment may alter the response of organisms to human impacts; (ii) does not solely focus on population declines; and (iii) places emphasis on the critical issue of scaling from physiological effects to population, community and ecosystem level processes. While considering definitions, it should be noted that in this discussion, ‘conservation’ is taken to refer to management of exploited living resources as well as conservation of biodiversity.
There are three mechanisms by which changes in the abiotic environment can effect a species' population dynamics: (i) through direct impacts on the physiology of the focal species; (ii) through changes in physical transport of planktonic stages of the focal species (e.g. larval transport and dispersal); and (iii) indirectly through changes in ecological relationships, such as predator or prey abundance, where the abiotic changes impact ecologically related species rather than the focal species itself. As conservation physiology addresses the physiological responses of organisms to changes in the environment, we focus this discussion on the first mechanism, although passing mention is made to other mechanisms where appropriate. In addition, because current telemetry methods cannot yet be applied to small individuals, our discussion is restricted to macro-metazoans and not plankton or fish larvae.
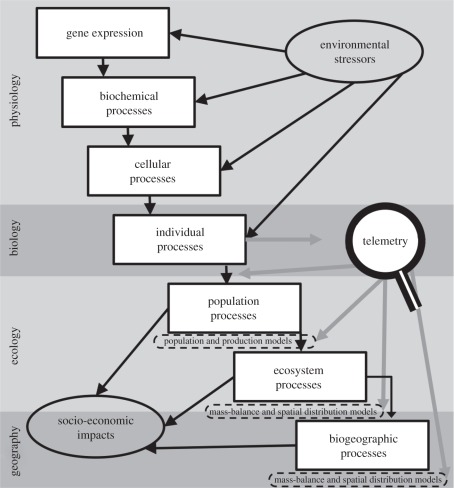
Although physiology operates at the level of individual organisms and below (e.g. cellular or molecular levels), societal concern for the marine environment operates at the scale of populations, communities and ecosystems. Therefore, the application of conservation physiology requires scaling from physiology to ecology (figure 1). This is a complex process which requires more than direct scaling of individual physiological effects to societal impacts because many biotic and abiotic factors modulate population-level responses to environmental changes. Owing to the spatial and temporal scale at which ecological processes operate, many of the key questions related to applied conservation, such as the possible outcomes of different management interventions, are inaccessible to traditional manipulative experiments, and therefore require qualitative assessments or predictive modelling studies. A key part of the development of conservation physiology will therefore be the incorporation of physiology in ecological modelling frameworks.
Figure 1.
The hierarchical levels of biological complexity, and their incorporation into existing resource management models. Processes are identified as interlinked boxes overlaid on a background (light and dark grey) of increasing biogeographic scale. The impacts of environmental stressors on, and socio-economic outputs from, these processes are indicated by black arrows. The contribution of telemetry to understanding individual processes and to the different types of modelling (described in the dashed text boxes under the relevant process box) is indicated by grey arrows. Adapted from Le Quesne & Pinnegar [2].
Animals in the wild only experience the microenvironment in their immediate vicinity, yet environmental variability occurs over a range of temporal and spatial scales. Controlled laboratory experiments may tell us how an animal's physiology or behaviour may alter in response to a change in one or a limited set of stressors. But laboratory experiments are rarely structured in a way that takes account of: (i) the full range of stressors that occur and interact in the natural environment; (ii) changes in the animal's state through space and time that affects the relative importance of these stressors to its biology; and (iii) the behavioural response of the animal that may modulate the impact of the stressor on the animal.
Telemetry, using electronic devices attached to or implanted into fish, offers the ability to record an animal's movements and behaviour while simultaneously recording at least some of the environmental variables to which it is exposed [3]. Furthermore, recent advances in telemetry now enable measurements to be recorded from marine fish over seasonal, and even multi-annual, timescales. Therefore, information gained from telemetry studies can enhance conservation physiology by providing insights into the realized behaviour and physiology of fish in the wild to validate or modify understanding developed from laboratory studies.
The successful realization of conservation physiology requires truly interdisciplinary studies that incorporate physiological knowledge and ecological understanding to develop advice on conservation management strategies. Achieving this requires development of a working dialogue between the traditionally disparate fields of physiology, and ecology and ecological modelling. To develop this dialogue, we firstly provide a review of fisheries population and ecosystem modelling methods, and how knowledge of fish physiology and responses to abiotic changes has been incorporated into them. Then, using cod and tuna as examples, we review case studies where telemetry has provided information about movements and behaviour in relation to environmental factors that has both validated and challenged our understanding of expected responses. Finally, we consider two examples that provide a framework for integrating knowledge on relationships between abiotic drivers and physiology directly into the processes that underpin applied modelling studies that can be used to provide advice in support of conservation objectives.
2. Modelling
The fundamental focus of marine fisheries and conservation science over the past century has been to understand the factors controlling population dynamics and to predict the impact, in terms of population abundances and/or yields, of different human activities, particularly fishing. Although it has long been recognized that population dynamics can be influenced by the environment [4–6], the tendency over most of the past century has been to consider environmental factors as stochastic noise that cannot be further resolved [7]. However, in recent years, advances in climate forecasting, improved environmental timeseries and increases in computing power have allowed environmental variability to be explicitly incorporated into ecological modelling studies [7]. The increasing capacity for models explicitly to handle environmental variation provides an opportunity to incorporate knowledge of the relationships between physiology and abiotic drivers into population and system modelling frameworks and provides a mechanism to scale up effects observed at the individual level to potential population- and system-level impacts. However, the manner in which physiological information can be incorporated into models varies greatly depending on the model structure and the physiological process being considered [2].
Both empirical and mechanistic models are used in predictive modelling studies and each has its strengths and weaknesses. Typically, empirical models describe processes with empirical or statistical relationships that are developed based on observations [8] and often represent complex processes in simple equations with relatively few parameters. However, a major limitation is that previously observed empirical relationships may break down in situations when conditions change substantially. Empirical models therefore offer only limited power for extrapolation. By contrast, mechanistic (i.e. ‘process-based’) models start from a theoretical consideration of what processes need to be modelled and only then consider what data are required [9]. Mechanistic models are considered more robust when extrapolating beyond previously observed conditions. However, mechanistic physiological models frequently require data that are not always readily available and are often difficult to collect. Improved process description can therefore come at the cost of increased measurement error in the model owing to increased data requirements [10].
Although empirical models do not explicitly represent the fundamental processes driving the dynamics of a system, the empirical relationships used within a model supposedly capture the net or emergent effects of the underlying processes. Consequently, it is conceptually possible to emulate alterations in the underlying processes by modifying model relationships or parameter values in a manner that captures the emergent effects of the underlying change. There are many examples of modelling studies that have used such an approach of fusing process-based modifiers to empirical models, such as forcing (the application of independent data in a model that influences the outcome) stock–recruit relationships [11] or growth parameters [12] in response to environmental variability within fisheries population models. However, the clear distinction between empirical and mechanistic models implied in the above discussion is, for convenience, a simplification. In reality, there is a spectrum of model types from very simplistic empirical models though to highly complex mechanistic models, with a host of intermediate forms.
A wide variety of model types have been applied to issues of living marine resource management which can crudely be grouped into four main classes: population models, individual-based models (IBMs), species distribution models (SDMs) and mass- or energy-balanced models. Although the output of different models and different model types varies considerably and is based on model requirements, their components, either implicitly or explicitly, represent processes at a range of different biological and ecological scales (figure 1). Model types differ in their principal modelling units, which may be individuals, populations or even ecosystems, and the types and influence of modifiers (which themselves may be model outcomes that feedback into successive cycles of a time-based model), environmental stressors, or both. To understand how information on the relationship between physiology and the environment may be incorporated into these models, we briefly describe each model type (below) along with examples of how environmental variability has been incorporated into them. This is not intended as an exhaustive review, or as a definitive description of the model types, but rather an overview to give a flavour of the range of model types available and approaches used to introduce environmental influences on physiology.
(a). Individual-based models
IBMs track individuals on the basis of rules governing the behaviour and development of individuals, and population dynamics emerge as the sum of the individuals that comprise the population. IBMs are particularly amenable to incorporating physiological and behavioural data as the algorithms controlling development of organisms can incorporate detailed physiological representation of processes controlling development which then drive the emergent properties of the population [13]. For example, Daewel et al. [14] applied a fish larval IBM coupled with a three-dimensional hydrodynamic and lower trophic-level model to examine the impact of environmental variability on larval development of cod (Gadus morhua) and sprat (Sprattus sprattus). In this model, temperature directly modified larval metabolic rates and developmental times, whereas the hydrodynamics contributed to environmental forcing of the lower trophic-level model.
(b). Population or production models
Surplus production models consider populations as a single biomass pool and simulate population biomass dynamics only with two parameters: the intrinsic rate of population growth (r) and the system carrying capacity (K) [15,16]. Within this framework, the emergent outcome of all the underlying processes is expressed solely via their effect on either r or K. In a 500 year retrospective analysis of the Newfoundland cod population, Rose [17] introduced environmental forcing by modifying r with a tree-ring temperature proxy and found the inclusion of environmental forcing improved the fit with historical data compared with runs without environmental forcing.
Stage-structured models evolved from surplus production models and take a variety of forms. They are now one of the main tools applied to marine living resource management. Rather than considering populations as a single biomass pool, populations are divided into age- or size-based stages, and numbers tracked from one stage to the next allowing for natural and anthropogenic mortality. Stage-structured models can assume constant recruitment in the form of biomass or yields per recruit models, or can incorporate a stock–recruit relationship to provide a description of a ‘whole’ population [18]. Stage-structured models are used in single-species assessments, or multi-species analyses where individual populations are linked through technical [19] and/or trophic interactions [20]. Stage-structured models typically use growth–mortality relationships, and these are key factors that can be modified on the basis of physiological information. For example, Cheung et al. [12] modelled the implications of climate change and ocean acidification on growth potential of fish by relating asymptotic body size and the K parameter of the von Bertalanffy growth equation to abiotic variables incorporated in the model.
(c). Mass-balance models
Biomass- or energy mass-balanced models are based around a model ‘currency’ (e.g. biomass, energy, carbon or nitrogen) and treat groups of organisms as a single pool of the model currency. The biomass pools can comprise multiple populations of different species, or may just represent a single age class of a specific population. The pools are linked within the model to create a trophic web, often extending from primary producers to terminal predators. Although the model currency is not always explicitly defined as energy, in many cases the currency chosen essentially acts as a proxy for energy flow through a system. Thus, mass-balance models can be amenable to representing physiologically mediated changes in energy uptake, partitioning and use at the level of the individual animal. Ainsworth et al. [21] examined five different biophysical impacts of climate change in Ecopath with Ecosim ecosystem models [22] by emulating changes in bioenergetics of different biological groups in response to different climate impacts. The use of energy as a model currency in ecosystem models makes them particularly amenable to incorporating physiological knowledge on bioenergetics. Furthermore, energy-based ecosystem models can represent a range of different physiological impacts with varying consequences for bioenergetics [2].
(d). Species distribution models
SDMs have been widely applied to predict past, current and future distributions of organisms under different environmental conditions [23,24]. This class of models generally determines empirical relationships between environmental variables and species distributions based on observed distributional and environmental data. A range of approaches has been used with the choice of approach partly dependent on the type of distributional data available [8,25]. Most existing SDMs are purely empirical and do not consider process descriptions of physiology or population dynamics, and their predictive power has been questioned when applied to novel environmental conditions [26]. However, some approaches have started to incorporate more mechanistic considerations by coupling SDMs with population dynamic models. For example, Cheung et al. [12] explicitly modelled the influences of temperature, oxygen and alkalinity changes on population dynamics and distribution of marine fish via physiological processes description (see later).
As different models structure the world in fundamentally different ways, it is useful to have different model types when incorporating physiological data into population- and system-level modelling frameworks. The manner in which physiological information is incorporated into these models, as well as the resolution at which physiological processes are resolved, varies significantly between different model types. There is no consistent correct choice of what type of model to apply. The appropriate model framework and level of complexity to apply depend on the physiological process being emulated, the question being asked and the data available [27].
3. Telemetry and behaviour
Incorporating physiology and behaviour into predictive models requires an understanding of how fish respond to the ever changing complex of environmental stressors in their natural environment. Because telemetry offers the capability to record an animal's behaviour while simultaneously recording the environmental variables to which it is exposed, this technology provides the means to validate or challenge assumptions based on laboratory experiments which, on their own, are unlikely to be able truly to reflect the realized responses of individuals to the dynamic environment they experience in the wild.
The advent of micro-electronics in the 1950s paved the way for electronic devices that could be made small enough to be attached externally to or implant into fish (see review by Arnold & Dewar [28]). Early electronic tags allowed individual fish to be tracked, usually from a boat or research vessel or, in association with static listening stations, to record the arrival and departure of individuals at particular locations [29]. Tracking experiments have provided detailed information about the spatial (both vertical and geographical) movements of individuals, and if environmental data (temperature, water currents, etc.) are gathered simultaneously by the tracking vessel it is also possible to relate fish behaviour to the local environment [30]. However, tracking studies are limited because usually only one fish can be followed at a time, and tag life is limited, usually to no more than a week or two. These limitations restricted the extent to which tracking studies can reveal how a fish's behaviour alters through time, either owing to changes in its state or to changes in environmental conditions.
More recently, integrated circuit technology has allowed the development of a new form of telemetry, often referred to as ‘biologging’, in which ‘data storage’ or ‘archival’ tags intermittently record and store information from on-board sensors that measure environmental variables such as pressure (to give depth), temperature and ambient daylight. Once recovered, either through acoustic, radio or satellite links, or through physical recovery of the tag itself, the data not only provide fine-scale information about behaviour (e.g. vertical movements derived from pressure readings) but can also be used to determine geographical movement based on geolocations determined from recoded data on many occasions while the fish is at liberty [3]. This behavioural and movement information can then be integrated with environmental data that are either recorded by the tag at the same time (e.g. water temperature), that are collected independently (e.g. satellite data of sea surface temperature or biological productivity) or are derived through other means (e.g. dissolved oxygen in the open oceans) for the same geographical area [31–34] to provide an understanding of how fish behave in different environmental conditions and at different times. Further developments in sensor and telemetry technology continue to provide the capability directly to record physiological variables such as heart rate and muscle activity (see review by Cooke et al. [35]).
Because data can be gathered from the same individual over months and even years, data storage tags also offer the prospect of observing how behaviour changes with the fish's state (e.g. feeding, migrating, spawning, etc.) and over longer term (e.g. seasonal) changes in the environment. As such, and given the ongoing development of more advanced sensors for measuring organismal state (see later), telemetry can provide a bridge between the physiological processes that are ‘upstream’ of the realized performance of individuals and the ecological processes that are the integrated (‘downstream’) outcome of collective performance.
Having discussed the need for physiological and behavioural data to parametrize biologically realistic mechanistic models, together with the potential for telemetry to refine our thinking about how physiological and behavioural data should be implemented in such models, we now consider examples of where telemetry studies have either validated or challenged physiological assumptions within models.
(a). Temperature and cod
The response to temperature is a commonly occurring topic in laboratory-based physiological studies of marine fish. This is predominantly because temperature can be easily controlled and because it is a key factor affecting fish physiology. It acts directly through the regulation of vital (i.e. feeding, metabolism, transformation, etc.) and non-vital (i.e. vitellogenesis) processes. Responses to temperature should also be one of the easiest to corroborate in the field because temperature data can be easily and accurately collected over a wide spatial extent, with a range of sampling and telemetry methods available to measure physiological and behavioural responses. Cod is a widely distributed and historically important commercial species across its range. Because of this, both its biology and physiology have been fairly extensively described from both laboratory and field studies, providing an ideal foundation for the application of conservation physiology principles.
Cod inhabit a wide range of water temperatures and display a variety of different life-history characteristics associated with living in particular temperature regimes [36–39]. Numerous studies demonstrate that cod physiology is profoundly affected by environmental temperature [40–46]. Over 90 per cent of the variation in growth in cod is attributed to temperature [41], with growth being reduced at lower temperatures and, as a result, individuals achieving maturity at an older age [41,43]. Estimates of the ‘optimum’ temperature for growth of cod range from 7°C to 17°C [40,47,48 and references therein]. Other experiments suggest that cod behave in ways that reduce thermal stress. For example, tank studies indicate that cod's thermal preferendum is in the range 11–14°C [49,50], and their metabolic scope is maximized in the temperature range 13–15°C [42]. In maturation studies, cod held at higher temperatures produce more oocytes and devote a greater proportion of their resources to egg production [51]. Cod of differing stock origin held under the same environmental conditions, however, will invest more in reproduction if they come from a stock that lives at a higher average temperature [52].
Perhaps because of the plasticity of cod behaviour, and the innate tolerance of this species to a range of temperatures, studies of the response of cod to different thermal drivers in their natural environment are not quite so clear cut, and the results of different field studies commonly disagree. For example, growth estimates of wild cod have been analysed, e.g. Brander [41], who suggested that, in contrast to laboratory studies, the relationship between temperature and growth is linear. However, because the data were collected from biological sampling studies over a limited range of temperatures, and across a range of cod sub-stocks, the results have been criticized as unrealistic [53]. However, recent data collected using data storage tags have also shown that the long-term temperature that cod experience correlates linearly with growth [32] and that, while the underlying relationship is the same, the growth rate of cod of different stocks does not increase at the same rate with temperature, thus corroborating Brander [41] and emphasizing the fact that regional and ontogenetic factors are likely to be important in determining physiological responses to different environments.
Responses to thermal gradients at timescales that are too short to have a measurable effect on growth have also been investigated. While cod show a relatively wide physiological tolerance for different thermal environments, experiments show that cod are responsive to even small changes in temperature. Adaptations within their thermal envelope appear to be linked to metabolic adjustments and trade-offs depending on the particular conditions experienced. Evidence for short-term responses to thermal gradients under natural conditions is, however, relatively uncommon. The concept that movements of wild cod populations are related to changes in water temperature (and depth) on a seasonal scale has long been based on timeseries of cod abundance at a range of spatial scales, or evidence of changes in cod abundance or occurrence at particular times of year [54–58]. However, through data storage tagging experiments, Neat & Righton [59] showed that cod in the North Sea did not occupy the more ‘optimal’ thermal environments, or indeed migrate away from areas where sea temperature was very high. Instead, individuals in both the southern and northern North Sea appeared to prefer the warmest proportion of the environment. This result was further supported in a subsequent study [32], where broad thermal tolerances of cod were shown to both short-term thermal stress resulting from movement across a 5°C thermocline and to long-term thermal stress resulting from occupancy of very warm (18°C) or very cold (less than 0°C) environments. Growth in these environments was lower than in more favourable environments, probably as a consequence of the reduced aerobic scope in such conditions [45].
It is clear that different populations of cod respond differently to temperature and thermal gradients [60–62] and, as a population-rich species [63], this population complexity is a key driver in modulating the way that cod populations will respond to environmental change. Integrating this degree of complexity into resource management and climate change models is likely to be a considerable challenge and it may be that simpler but valid generalizations will need to be developed and tested in future field studies.
(b). Oxygen and tuna
Similar to temperature, oxygen availability is another abiotic variable that determines habitat suitability for marine fish. It is well established that fish growth is reduced if they are kept in water with reduced (below air saturation) oxygen concentrations (hypoxia) [64], with reduced growth being a consequence of reduced feeding and food assimilation (see Pauly [65]). Hypoxia-induced reductions in growth have knock-on effects, reducing maximum size, age at first maturity and, therefore, potential reproductive success. It is therefore to be expected that fish would seek to maximize reproductive potential by avoiding oxygen-poor (hypoxic) environments, or may ‘choose’ to inhabit or visit oxygen-poor environments because the advantages of other habitat features outweigh the negative impact of hypoxia [66,67].
Pauly [65] has argued that a physiological approach, integrating biotic (metabolic rate and fish growth dynamics) and abiotic (depth, oxygen and temperature) parameters can explain how fish, both in the course of their ontogeny and seasonally, migrate vertically and geographically so as to remain in the species-specific temperature/oxygen regime that is appropriate for their size, suggesting that this therefore determines their overall geographical distributions. However, there are other environmental variables that, it might be argued, could also affect movements and distribution—food availability and the presence of predators come readily to mind—but very little field data exist to confirm the extent to which individuals remain in their predicted temperature/oxygen regime.
Hypoxia is a common phenomenon in a range of sea areas such as the Baltic Sea and the Gulf of St Lawrence [68], as well as large parts of the eastern Tropical Pacific (ETP) and eastern Tropical Atlantic (ETA) at depths below about 100 m [33]. The oxygen-minimum zones in the tropical Pacific and Atlantic are among the largest areas of naturally occurring hypoxia in the world oceans and are predicted to expand with climate change [33]. The impact that hypoxia, along with temperature, may have on the movements and distribution of fish populations is being recognized, and increasingly habitat preferences and physiological limitations are being used to try and standardize fish population assessment methods [69]. However, Brill [70]—who has reviewed the development of our understanding of the temperature and oxygen tolerances of various tuna species based largely on laboratory studies—concludes that widely cited estimates of limiting oxygen levels are inaccurate because of underestimates of oxygen demand at low swimming speeds. Brill [70] suggests that the high metabolic rates observed in tunas at slow swimming speeds are a consequence of the high osmoregulatory costs, or other adaptations for achieving very high maximum metabolic rates. So, while it is clear that tunas are highly aerobic fish, the complex nature of their physiology suggests that their movements and distribution will also depend on other factors such as temperature, feeding, maturity, etc. However, until recently, very few data were available to characterize how such pelagic species actually use the water column in different oceanic habitats.
In an attempt to resolve such issues, Prince & Goodyear [71] and Prince et al. [33] recently used depth recording pop-off satellite transmitting archival tags to gain a fuller understanding of the vertical movements of tunas and billfishes in relation to oxygen availability in the Atlantic and Pacific oceans. Their results show that, in the ETP and ETA, respectively, sailfish and marlin are subject to hypoxia-based habitat compression, being largely restricted to the narrow surface layer where oxygen levels are above approximately 3.5 ml l−1 (greater than approximately 50% saturation) and that extends down to a variable boundary defined by a shallow thermocline, often at 25–50 m, which separates it from deeper, colder, hypoxic water. However, this is not the situation in the western North Atlantic (WNA) where oxygen availability is not limiting and may remain above 4.0 ml l−1 down to depths of 300 m. Interestingly, Atlantic sailfish in the ETP and ETA are larger than those in the WNA [33,71], and these authors suggest the larger sizes may reflect enhanced foraging opportunities afforded by the closer proximity of predator and prey in the compressed habitat, as well as by the higher productivity in these areas. They also point out that being confined to a shallow band of acceptable habitat makes these fish more vulnerable to over-exploitation by surface fishing gears and that, not surprisingly, the long-term landings of tropical pelagic tunas from areas of habitat compression have been far greater than those from surrounding areas. Such understanding therefore not only provides better basis for standardizing commercial catch per unit effort (CPUE) data to improve population assessments, but also helps to identify habitats where conservation measures may need to reflect the higher vulnerability of fish to exploitation.
(c). Oxygen and cod
Using acoustic telemetry to monitor the movement and behaviour of cod in a 125 m3 tower tank, Claireaux et al. [66] showed that while cod generally avoid hypoxic (less than approximately 50% saturation) water, they will, in certain conditions, enter water with oxygen saturation levels as low as approximately 14% for short periods, particularly if food is offered. Similarly, studies conducted using archival tags showed that cod in the Bornholm Basin of the Baltic occasionally experienced oxygen saturations as low as 10% and spent a third of the total time at liberty in water having an oxygen saturation below 50% [72]. These authors suggest that cod move into deeper, hypoxic water for short periods to feed on sprat and other prey species that are generally more tolerant to longer term exposure to low oxygen, but then move back into the relatively well-oxygenated surface waters that can support the higher oxygen demand needed to digest the food they have consumed. Thus, in the Baltic, moving into prey-rich hypoxic water to feed and then returning to normoxic environments to digest their food may not result in any reduced growth despite that fact that they experience hypoxic water for a relatively large part of the time. These authors point out that although their telemetry data did not reveal any particularly new insights into the threshold nature of hypoxia for determining the distribution of cod in the Baltic, their results allowed individual residence periods to be monitored continuously over the full range of available oxygen conditions, thereby enabling the identification of more complex behaviours than simple avoidance.
4. Scaling physiology to population dynamics
The above discussion has identified the opportunities for taking account of physiological data in considering resource management, and highlighted some examples of how a growing body of telemetry-derived data can help advance existing management tools. Currently, these advances help to shape model assumptions and thereby influence the development of new management policies. The next step towards a true conservation physiology approach to fisheries management will integrate knowledge on relationships between abiotic drivers and physiology directly into the processes that underpin applied modelling studies. We now focus on two existing models that provide a framework to realize this, the dynamic bioclimatic envelope model (DBEM) [12,25,73] and the spatial ecosystem and population dynamics model (SEAPODYM) [74,75].
The DBEM was developed to assess the effects of global ocean and climate changes on marine biodiversity and fisheries [12,25,76]. The model was applied to study more than 1000 species of fishes and invertebrates globally to project rates of species range shifts and changes in catch potential by the year 2050 under different scenarios of CO2 emissions, outputs which are directly relevant to conservation and management. Specifically, the model outputs show many marine fishes and invertebrates would shift their distribution pole-ward and to deeper water, at an average rate of approximately 40 km decade−1 towards higher latitude and approximately 3.3 m decade−1 into deeper water (for demersal species). These would result in high rates of species invasion in higher latitude regions and local extinction along the tropics and in semi-enclosed seas.
The model which explicitly links ecophysiology with spatial population dynamics is based on three key axioms. Firstly, growth is dependent on aerobic scope, described by a set of equations determining oxygen demand and supply for growth, derived from the von Bertalanffy growth function [12]. Schematically, it can be represented by a ‘p-diagram’ [65,77,78] (figure 2). In the model, growth parameters are expressed as a function of temperature and other biogeochemical conditions of the environment that are known to affect growth, such as dissolved oxygen concentration. Secondly, life-history characteristics are closely correlated with each other. Thus, the model determines other life-history characteristics, such as mortality rate and length-at-maturity, from growth parameters using known empirical models [79,80]. Thirdly, movement, growth and carrying capacity of the population are dependent on the environmental conditions and the physiological preferences and limits of the population. The model infers the physiological and ecological preferences of organisms to environmental conditions such as temperature, salinity and habitat types by overlaying predicted distribution maps with gridded oceanographic data predicted by earth system models [73]. These parameters then determine the population dynamics and carrying capacity of the modelled population in each 0.5° latitude × 0.5° longitude cell. Changes in physiology and population dynamics are driven by projected changes in ocean conditions. The initial conditions of the model are based on predicted current distributions of the modelled populations, von Bertalanffy growth parameters, coefficients for metabolic scaling, movement rate of adults and species' degree of association to major habitat types.
Figure 2.
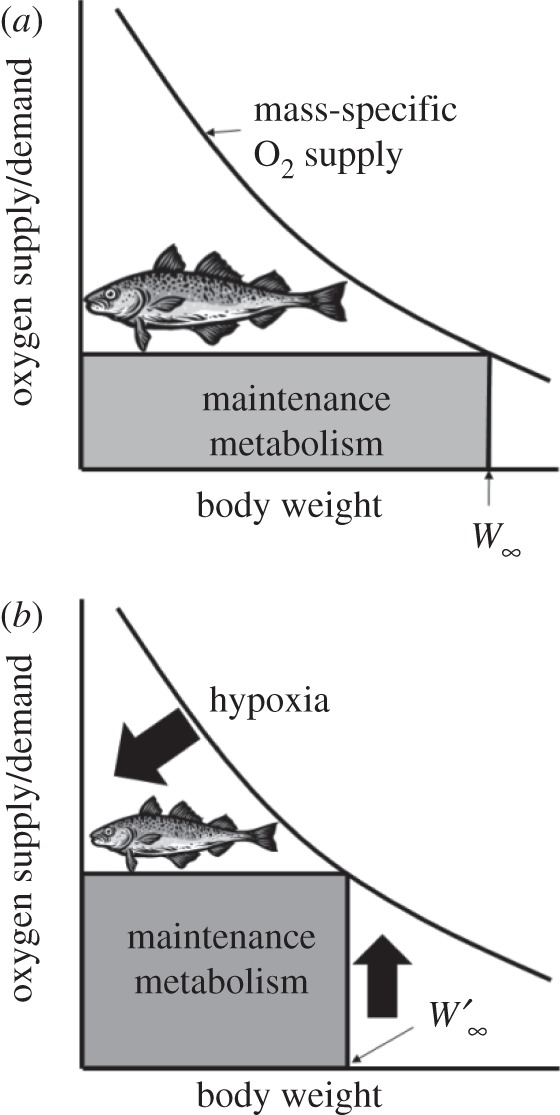
Two p-diagrams illustrating how (a) growth and maximum (i.e. asymptotic) size of fish depend on the balance between oxygen supply and demand; (b) factors that increase maintenance metabolism (e.g. increased temperature, physiological stress) or reduced oxygen supply (e.g. hypoxia) will reduce growth and asymptotic weight (from W∞ to W′∞). Oxygen supply scales allometrically with body mass, whereas oxygen demand (for maintenance metabolism) is directly proportional to body mass. Growth depends on the aerobic scope, i.e. on the difference between oxygen supply and demand curves. Asymptotic size is reached when oxygen supply is just enough to meet oxygen demand for basic body maintenance. Hence, increase in oxygen demand or decrease in oxygen supply will affect growth and asymptotic size. Adapted from Cheung et al. [12].
The SEAPODYM is specifically designed to provide mechanistic predictions on spatial population dynamics of tunas and other ocean top-predators under global change [81]. Similar to DEMB, SEAPODYM simulates changes in biomass of each population cohort based on population dynamics and advection–diffusion–reaction equations. Specifically, the model is forced by environmental conditions (temperature, currents, primary production, habitat suitability and dissolved oxygen concentration), prey abundance and fishing [81]. The model uses a simplified multi-layer ocean ecosystem with a coarse description of mid-trophic levels but detailed size-/age-structured spatial population dynamics of high-trophic-level species of interest (e.g. bigeye tuna, Thunnus obesus). The model includes a rigorous parameter optimization and the habitat preferences of the species are estimated based on historical environmental forcing and fishing data. The estimated habitat index implicitly represents physiological responses of the animals to changing environmental conditions (e.g. temperature and oxygen). The model is linked to an earth system model that provides projections of abiotic and biogeochemical conditions.
SEAPODYM can inform fisheries management and conservation of large ocean predators in response to climate change. For example, using SEAPODYM, Lehodey et al. [81] examined potential changes in bigeye tuna populations in the twentieth-first century. The model suggests an expansion of bigeye spawning habitat and improvement in adult feeding habitat in the eastern tropical Pacific, whereas bigeye in the western tropical Pacific may decrease because of warming and declines in sub-surface oxygen and food availability.
In these two modelling approaches, data from physiological studies have been useful in improving the projections from the models. In DBEM, the three key axioms, i.e. relationship between oxygen, temperature and growth, correlation between life-history characteristics and linkages between habitat preference and spatial distribution, are largely based on physiological data of the type we have described in our case studies on the physiology and behaviour of cod and tuna. However, these models focus on dynamics at relatively coarse spatial and temporal scales and do not represent the fine-scale influences of environment and their trade-offs. These models also assume the organisms are in a steady state and do not explicitly incorporate scope for a wide range of potential adaptive responses. However, as such issues are increasingly becoming amenable to telemetry studies, future developments of these and similar models should take advantage of the increased resolution that this new technology offers.
5. Conclusions
We have shown how knowledge from physiological and telemetry studies can directly inform the structural development and parametrization of population models. Physiological studies on thermal tolerance, aerobic scope and growth provide useful information to develop the ecophysiology components. Data obtained from telemetry studies inform the parametrization of animals’ movement and dispersal rates and their responses to environmental changes in the model. Comparisons between estimated parameters from the models and experimental findings, for example thermal tolerance and response functions to water chemistry, can also be used to calibrate the models. These models thus allow the up-scaling of experimental findings to address larger scale ecological questions that are more relevant to conservation and management. However, existing models do not generally have the capacity to represent the fine-scale cellular or molecular details that most physiological studies focus on, nor the short-term (e.g. hourly or daily) behavioural responses of animal movement.
How then does one deal with the fact that the responses of fish in different environments are complex? Further, what are the consequences of multiple trade-offs between different stimuli, energy acquisition, energy conservation, stress management and physiological compensation when applying conservation physiology to inform management decisions? Telemetry, laboratory studies, large-scale sampling or modelling cannot, each on their own, provide a final answer but, drawn together, they can provide increasingly robust management advice (cf. Cooke et al. [82]). Telemetry is particularly valuable for providing insights into short-term behavioural decisions that, we assume, maximize life-time fitness over longer timescales, and adds important context to laboratory studies. However, to date, telemetry has been hampered by the problem that it is very difficult to measure parameters that directly relate to whole organism life-time fitness, such as growth or energy acquisition. However, recent development of telemetry devices with tri-axial accelerometers may offer fresh insights into the potential to estimate metabolic rate in the field from dynamic body acceleration [83,84].
Continuing advances in physiology and telemetry will provide new insights into the multitude of factors that influence the realized responses of organisms to environmental challenges in the wild. However, a key challenge for applied conservation physiology is to see through the complexity and to develop realistic generalizations that capture the critical physiological mechanisms and behaviour responses that can meaningfully be integrated into the ecological models and understanding that are ultimately used to advise on conservation and management interventions.
References
- 1.Wikelski M., Cooke S. J. 2006. Conservation physiology. Trends Ecol. Evol. 21, 38–46 10.1016/j.tree.2005.10.018 (doi:10.1016/j.tree.2005.10.018) [DOI] [PubMed] [Google Scholar]
- 2.Le Quesne W. J. F., Pinnegar J. K. In press. The potential impacts of ocean acidification: scaling from physiology to fisheries. Fish Fish. (doi:10.1111/j.1467-2979.2011.00423.x) [Google Scholar]
- 3.Metcalfe J. D., Righton D. A., Hunter E., Eastwood P. 2008. Migration and habitat choice in marine fish. In Fish behaviour (eds Magnhagen C., Braithwaite V. A., Forsgren E., Kapoor B. G.), pp. 187–233 Enfield, NH: Science Publishers Inc. [Google Scholar]
- 4.Hjort J. 1914. Fluctuations in the great fisheries of northern Europe, viewed in the light of biological research. Rap. Process 20, 1–228 [Google Scholar]
- 5.Johansen A. C. 1925. On the influence of currents upon frequency of the mackerel in the Kattegat and adjacent parts of the Skagerak. Meddelelser fra Kommissionen for Havundersogelser 7, 26 [Google Scholar]
- 6.Carruthers J. H. 1938. Fluctuations in the herrings of the east Anglian autumn fishery, the yield of the Ostend spent herring fishery and the Haddock of the North Sea in the light of relevant wind conditions. Rap. Process 107, 1–15 [Google Scholar]
- 7.Keyl F., Wolff M. 2008. Environmental variability and fisheries: What can models do? Rev. Fish Biol. Fisher. 18, 273–299 10.1007/s11160-007-9075-5 (doi:10.1007/s11160-007-9075-5) [DOI] [Google Scholar]
- 8.Ready J., Kaschner K., South A. B., Eastwood P. D., Rees T., Rius J., Agbayani E., Kullander S., Froese R. 2010. Predicting the distributions of marine organisms at the global scale. Ecol. Model. 221, 467–478 10.1016/j.ecolmodel.2009.10.025 (doi:10.1016/j.ecolmodel.2009.10.025) [DOI] [Google Scholar]
- 9.van der Meer J. 2006. An introduction to dynamic energy budget (DEB) models with special emphasis on parameter estimation. J. Sea Res. 56, 85–102 10.1016/j.seares.2006.03.001 (doi:10.1016/j.seares.2006.03.001) [DOI] [Google Scholar]
- 10.Walters C. J., Martell S. J. D. 2004. Fisheries ecology and management. Princeton, NJ: Princeton University Press [Google Scholar]
- 11.Kell L., Pilling G. M., O'Brien C. M. 2005. Implications of climate change for the management of North Sea cod (Gadus morhua). ICES J. Mar. Sci. 62, 1483–1491 10.1016/j.icesjms.2005.05.006 (doi:10.1016/j.icesjms.2005.05.006) [DOI] [Google Scholar]
- 12.Cheung W. W. L., Dunne J., Sarmiento J. L., Pauly D. 2011. Integrating ecophysiology and plankton dynamics into projected maximum fisheries catch potential under climate change in the Northeast Atlantic. ICES J. Mar. Sci. 68, 1008–1018 10.1093/icesjms/fsr012 (doi:10.1093/icesjms/fsr012) [DOI] [Google Scholar]
- 13.Grimm V., et al. 2005. Pattern-oriented modelling of agent-based complex systems: lessons from ecology. Science 310, 987–991 10.1126/science.1116681 (doi:10.1126/science.1116681) [DOI] [PubMed] [Google Scholar]
- 14.Daewel U., Peck M. A., Schrum C. 2011. Life history strategy and impacts of environmental variability on the early life stages of two marine fishes in the North Sea: an individual-based modelling approach. Can. J. Fish. Aquat. Sci. 68, 426–443 10.1139/F10-164 (doi:10.1139/F10-164) [DOI] [Google Scholar]
- 15.Schaefer M. B. 1954. Some aspects of the dynamics of populations important to the management of commercial marine fisheries. Inter-Am. Trop. Tuna Comm. Bull. 1, 23–56 [Google Scholar]
- 16.Schaefer M. B. 1957. A study of the dynamics of the fishery for yellowfish tuna in the eastern tropical Pacific Ocean. Inter-Am. Trop. Tuna Comm. Bull. 2, 247–268 [Google Scholar]
- 17.Rose G. A. 2004. Reconciling overfishing and climate change with stock dynamics of Atlantic cod (Gadus morhua) over 500 years. Can. J. Fish. Aquat. Sci. 61, 1553–1557 10.1139/f04-173 (doi:10.1139/f04-173) [DOI] [Google Scholar]
- 18.Beverton R. J. H., Holt S. J. 1957. On the dynami cs of exploited fish populations. Ministry of Agriculture, Fisheries and Food. Fishery Investigations Series 2, No. 19. London, UK: Her Majesty's Stationery Office [Google Scholar]
- 19.Murawski S. 1984. Mixed-species yield-per-recruit analyses accounting for technological interactions. Can. J. Fish. Aquat. Sci. 41, 897–916 10.1139/f84-106 (doi:10.1139/f84-106) [DOI] [Google Scholar]
- 20.Pope J. G., Knights B. J. 1982. Simple models of predation in multi-age multispecies fisheries for considering the estimation of fishing mortality and its effects. Can. Spec. Publ. Fish. Aquat. Sci. 59, 64–69 [Google Scholar]
- 21.Ainsworth C. H., Samhouri J. F., Busch D. S., Cheung W. W. L., Dunne J., Okey T. A. 2011. Potential impacts of climate change on Northeast Pacific marine foodwebs and fisheries. ICES J. Mar. Sci. 68, 1217–1229 10.1093/icesjms/fsr043 (doi:10.1093/icesjms/fsr043) [DOI] [Google Scholar]
- 22.Christensen V., Pauly D. 1992. Ecopath ii: a software for balancing steady-state ecosystem models and calculating network characteristics. Ecol. Model. 61, 169–185 10.1016/0304-3800(92)90016-8 (doi:10.1016/0304-3800(92)90016-8) [DOI] [Google Scholar]
- 23.Peterson A. T. 2006. Uses and requirements of ecological niche models and related distributional models. Bioinformatics 3, 59–72 [Google Scholar]
- 24.Elith J., Leathwick J. R. 2009. Species distribution models: ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 40, 677–697 10.1146/annurev.ecolsys.110308.120159 (doi:10.1146/annurev.ecolsys.110308.120159) [DOI] [Google Scholar]
- 25.Cheung W. W. L., Close C., Kearney K., Lam V., Sarmiento J., Watson R., Pauly D. 2009. Projections of global marine biodiversity impacts under climate change scenarios. Fish Fish. 10, 235–251 10.1111/j.1467-2979.2008.00315.x (doi:10.1111/j.1467-2979.2008.00315.x) [DOI] [Google Scholar]
- 26.Lo Y. -H., Blanco J. A., Kimmins J. P. 2010. A word of caution when projecting future shifts of tree species ranges. Forest. Chron. 86, 312–316 [Google Scholar]
- 27.Fulton E. A., Smith A. D. M., Johnson C. R. 2003. Effect of complexity on marine ecosystem models. Mar. Ecol. Prog. Ser. 253, 1–16 10.3354/meps253001 (doi:10.3354/meps253001) [DOI] [Google Scholar]
- 28.Arnold G. P., Dewar H. 2001. Electronic tags in marine fisheries research: a 30 year perspective. In Electronic tagging and tracking in marine fisheries (eds Sibert J. R., Nielsen J. L.), pp. 7–64 Dordrecht, The Netherlands: Kluwer Academic Press [Google Scholar]
- 29.Righton D. R., Metcalfe J. D., Connolly P. 2001. Electronic tags reveal behavioural differences between North Sea and Irish Sea cod. Nature 411, 156. 10.1038/35075667 (doi:10.1038/35075667) [DOI] [PubMed] [Google Scholar]
- 30.Buckley A. A., Arnold G. P. 2001. Orientation and swimming speed of plaice migrating by selective tidal stream transport. In Electronic tagging and tracking in marine fisheries (eds Sibert J. R., Nielsen J. L.), pp. 263–277 Dordrecht, The Netherlands: Kluwer Academic Press [Google Scholar]
- 31.Sims D. W., Southall E. J., Richardson A. J., Reid P. C., Metcalfe J. D. 2003. Foraging and migratory behaviour of basking sharks over seasonal scales: no evidence for winter hibernation. Mar. Ecol. Prog. Ser. 248, 187–196 10.3354/meps248187 (doi:10.3354/meps248187) [DOI] [Google Scholar]
- 32.Righton D. A., et al. 2010. Thermal niche of Atlantic cod Gadus morhua: limits, tolerance and optima. Mar. Ecol. Prog. Ser. 420, 1–13 10.3354/meps08889 (doi:10.3354/meps08889) [DOI] [Google Scholar]
- 33.Prince E. D., Luo J., Goodyear C. P., Hoolihan J. P., Snodgrass D., Orbesen E. S., Serafy J. E., Ortizi M., Schirripa M. J. 2010. Ocean scale hypoxia-based habitat compression of Atlantic istiophorid billfishes. Fish. Oceanogr. 19, 448–462 10.1111/j.1365-2419.2010.00556.x (doi:10.1111/j.1365-2419.2010.00556.x) [DOI] [Google Scholar]
- 34.Block B. A., et al. 2011. Tracking apex marine predator movements in a dynamic ocean. Nature 475, 86–90 10.1038/nature10082 (doi:10.1038/nature10082) [DOI] [PubMed] [Google Scholar]
- 35.Cooke S. J., Hinch S. G., Wikelski M., Andrews R. D., Wolcott T. G., Butler P. J. 2004. Biotelemetry: a mechanistic approach to ecology. Trends Ecol. Evol. 19, 334–343 10.1016/j.tree.2004.04.003 (doi:10.1016/j.tree.2004.04.003) [DOI] [PubMed] [Google Scholar]
- 36.Colosimo A., Giuliani A., Maranghi F., Brix O., Thorkildsen S., Fischer T., Knust R., Pörtner H. O. 2003. Physiological and genetical adaptation to temperature in fish populations. Cont. Shelf Res. 23, 1919–1928 10.1016/j.csr.2003.06.012 (doi:10.1016/j.csr.2003.06.012) [DOI] [Google Scholar]
- 37.Goddard S. V., Morgan M. J., Fletcher G. L. 1997. Influence of plasma antifreeze glycoproteins on temperature selection by Atlantic cod (Gadus morhua) in a thermal gradient. Can. J. Fish. Aquat. Sci. 54, 88–93 10.1139/f96-165 (doi:10.1139/f96-165) [DOI] [Google Scholar]
- 38.Lannig G., Eckerle L. G., Serendero I., Sartoris F. J., Fischer T., Knust R., Johansen T., Pörtner H. O. 2003. Temperature adaption in eurythermal cod (Gadus morhua): a comparison of mitochondrial enzyme capacities in boreal and Arctic populations. Mar. Biol. 142, 589–599 10.1007/s00227-002-0967-6 (doi:10.1007/s00227-002-0967-6) [DOI] [Google Scholar]
- 39.Michalsen K., Ottersen G., Nakken O. 1998. Growth of north-east Arctic cod (Gadus morhua L.) in relation to ambient temperature. ICES J. Mar. Sci. 55, 863–877 10.1006/jmsc.1998.0364 (doi:10.1006/jmsc.1998.0364) [DOI] [Google Scholar]
- 40.Jobling M. 1988. A review of the physiological and nutritional energetics of cod, Gadus morhua L., with particular reference to growth under farmed conditions. Aquaculture 70, 1–19 10.1016/0044-8486(88)90002-6 (doi:10.1016/0044-8486(88)90002-6) [DOI] [Google Scholar]
- 41.Brander K. M. 1995. The effect of temperature on growth of Atlantic cod (Gadus morhua L.). ICES J. Mar. Sci. 52, 1–10 10.1016/1054-3139(95)80010-7 (doi:10.1016/1054-3139(95)80010-7) [DOI] [Google Scholar]
- 42.Claireaux G., Webber D. M., Lagardère J.-P., Kerr S. R. 2000. Influence of water temperature and oxygenation on the aerobic metabolic scope of Atlantic cod (Gadus morhua). J. Sea Res. 44, 257–265 10.1016/S1385-1101(00)00053-8 (doi:10.1016/S1385-1101(00)00053-8) [DOI] [Google Scholar]
- 43.Pörtner H. O., et al. 2001. Climate induced temperature effects on growth performance, fecundity and recruitment in marine fish: developing a hypothesis for cause and effect relationships in Atlantic cod (Gadus morhua) and common eelpout (Zoarces viviparus). Cont. Shelf Res. 21, 1975–1997 10.1016/S0278-4343(01)00038-3 (doi:10.1016/S0278-4343(01)00038-3) [DOI] [Google Scholar]
- 44.Björnsson B., Steinarsson A. 2002. The food-unlimited growth rate of Atlantic cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 59, 494–502 10.1139/f02-028 (doi:10.1139/f02-028) [DOI] [Google Scholar]
- 45.Lannig G., Bock C., Sartorius F. J., Pörtner H. O. 2004. Oxygen limitation of thermal tolerance in cod, Gadus morhua L., studied by magnetic resonance imaging and on-line venous oxygen monitoring. Am. J. Physiol. 287, 902–910 10.1152/ajpregu.00700.2003 (doi:10.1152/ajpregu.00700.2003) [DOI] [PubMed] [Google Scholar]
- 46.Yoneda M., Wright P. J. 2005. Effects of varying temperature and food availability on growth and reproduction in first-time spawning female Atlantic cod. ICES J. Mar. Sci. 62, 1387–1393 10.1016/j.icesjms.2005.04.018 (doi:10.1016/j.icesjms.2005.04.018) [DOI] [Google Scholar]
- 47.Boutilier R. G. 1998. Physiological ecology in cold ocean fisheries: a case study in Atlantic cod. In Cold ocean physiology (eds Pörtner H. O., Playle R. C.), pp. 463–469 Cambridge, UK: Cambridge University Press [Google Scholar]
- 48.Björnsson B., Steinarsson A., Oddgeirsson M. 2001. Optimal temperature for growth and feed conversion of immature cod (Gadus morhua L.). ICES J. Mar. Sci. 58, 29–38 10.1006/jmsc.2000.0986 (doi:10.1006/jmsc.2000.0986) [DOI] [Google Scholar]
- 49.Clark D. S., Green J. M. 1991. Seasonal variation in temperature preference of juvenile Atlantic cod (Gadus morhua), with evidence supporting an energetic basis for their diel vertical migration. Can. J. Zool. 69, 1302–1307 10.1139/z91-183 (doi:10.1139/z91-183) [DOI] [Google Scholar]
- 50.Schurmann H., Steffensen J. F. 1992. Lethal oxygen levels at different temperatures and the preferred temperature during hypoxia of the Atlantic cod, Gadus morhua. J. Fish. Biol. 41, 927–934 10.1111/j.1095-8649.1992.tb02720.x (doi:10.1111/j.1095-8649.1992.tb02720.x) [DOI] [Google Scholar]
- 51.Kjesbu O. S., Righton D., Kruger-Johnson M., Thorsen A., Michalsen K., Fonn M., Witthames P. M. 2010. Thermal dynamics of ovarian maturation in Atlantic cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 67, 605–625 10.1139/F10-011 (doi:10.1139/F10-011) [DOI] [Google Scholar]
- 52.Harrald M., Wright P. J., Neat F. C. 2010. Substock variation in reproductive traits in North Sea cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 67, 866–876 10.1139/F10-030 (doi:10.1139/F10-030) [DOI] [Google Scholar]
- 53.Clark R. A., Fox C. J., Viner D., Livermore M. 2003. North Sea cod and climate change - modelling the effects of temperature on population dynamics. Glob. Change Biol. 9, 1669–1680 10.1046/j.1365-2486.2003.00685.x (doi:10.1046/j.1365-2486.2003.00685.x) [DOI] [Google Scholar]
- 54.Aro E. 1989. A review of fish migration patterns in the Baltic. Rap. Process 190, 72–96 [Google Scholar]
- 55.Heesen H., Daan N. 1993. Cod distribution and temperature in the North Sea. In Cod and Climate Change: A symposium held in Reykjavik, 23–27 August 1993, pp. 244–253 ICES Marine Science Symposia Series, vol. 198; Copenhagen, Denmark: International Council for the Exploration of the Sea. [Google Scholar]
- 56.Ottersen G., Michalsen K., Nakken O. 1998. Ambient temperature and distribution of Northeast Arctic cod. ICES J. Mar. Sci. 55, 67–85 10.1006/jmsc.1997.0232 (doi:10.1006/jmsc.1997.0232) [DOI] [Google Scholar]
- 57.Page F. H., Losier R. J., Smith S. J., Hatt K. 1994. Associations between cod, and temperature, salinity and depth within the Canadian groundfish bottom trawl surveys (1970–1993) conducted in NAFO Divisions 4VWX and 5Z. Canadian Technical Report of Fisheries and Aquatic Sciences, No. 1958, 167 pp
- 58.Stensholt B. K. 2001. Cod migration patterns in relation to temperature: analysis of storage tag data. ICES J. Mar. Sci. 58, 770–793 10.1006/jsmc.2001.1067 (doi:10.1006/jsmc.2001.1067) [DOI] [Google Scholar]
- 59.Neat F., Righton D. 2006. Warm water occupancy by North Sea cod. Proc. R. Soc. B 274, 789–798 10.1098/rspb.2006.0212 (doi:10.1098/rspb.2006.0212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nielsen E. E., Hansen M. M., Ruzzante D. E., Meldrup D., Grønkjær P. 2003. Evidence of a hybrid-zone in Atlantic cod (Gadus morhua) in the Baltic and the Danish Belt Sea revealed by individual admixture analysis. Mol. Ecol. 12, 1497–1508 10.1046/j.1365-294X.2003.01819.x (doi:10.1046/j.1365-294X.2003.01819.x) [DOI] [PubMed] [Google Scholar]
- 61.Petersen M. F., Steffansen J. F. 2003. Preferred temperature of juvenile Atlantic cod Gadus morhua with different haemoglobin genotypes at normoxia and moderate hypoxia. J. Exp. Biol. 206, 359–364 10.1242/jeb.00111 (doi:10.1242/jeb.00111) [DOI] [PubMed] [Google Scholar]
- 62.Salvanes A. G. V., Skjæraasen J. E., Nilsen T. 2004. Sub-populations of coastal cod with different behaviour and life-history strategies. Mar. Ecol. Prog. Ser. 267, 241–251 10.3354/meps267241 (doi:10.3354/meps267241) [DOI] [Google Scholar]
- 63.Sinclair M. 1988. Marine populations. An essay on population regulation and speciation, 252 pp Seattle, WA: Washington Sea Grant [Google Scholar]
- 64.Chabot D., Dutil J. D. 1999. Reduced growth of Atlantic cod in non-lethal hypoxic conditions. J. Fish Biol. 55, 472–491 10.1111/j.1095-8649.1999.tb00693.x (doi:10.1111/j.1095-8649.1999.tb00693.x) [DOI] [Google Scholar]
- 65.Pauly D. 2010. Gasping fish and panting squids: oxygen, temperature and the growth of water-breathing animals. Excellence in Ecology 22 Oldendorf/Luhe, Germany: International Ecology Institute [Google Scholar]
- 66.Claireaux G., Webber D. M., Kerr S. R., Boutilier R. G. 1995. Physiology and behaviour of free-swimming Atlantic cod (Gadus morhua) facing fluctuating salinity and oxygenation conditions. J. Exp. Biol. 198, 61–69 [DOI] [PubMed] [Google Scholar]
- 67.Nilsson G. E., Randall D. J. 2010. Adaptations to hypoxia in fishes. In Respiratory physiology of vertebrates life with and without oxygen (ed. Nilsson G. E.). Cambridge, UK: Cambridge University Press [Google Scholar]
- 68.D'Amours D. 1993. The distribution of cod (Gadus morhua) in relation to temperature and oxygen level in the Gulf of St Lawrence. Fish. Oceanogr. 198, 61–60 [Google Scholar]
- 69.Hinton M. G., Nakano H. 1996. Standardizing catch and effort statistics using physiological, ecological, or behavioural constraints and environmental data, with an application to blue marlin (Makaira nigricans) catch and effort data from the Japanese longline fisheries in the Pacific. Bull. IATTC 21, 171–200 [Google Scholar]
- 70.Brill R. W. 1994. A review of temperature and oxygen tolerance studies of tunas pertinent to fisheries oceanography, movement models and stock assessments. Fish. Oceanogr. 3, 204–216 10.1111/j.1365-2419.1994.tb00098.x (doi:10.1111/j.1365-2419.1994.tb00098.x) [DOI] [Google Scholar]
- 71.Prince E. D., Goodyear C. P. 2006. Hypoxia-based habitat compression of tropical pelagic fishes. Fish. Oceanogr. 15, 451–464 10.1111/j.1365-2419.2005.00393.x (doi:10.1111/j.1365-2419.2005.00393.x) [DOI] [Google Scholar]
- 72.Neuenfeldt S., Andersen K. H., Hinrichsen H.-H. 2009. Some Atlantic cod Gadus morhua in the Baltic visit hypoxic water briefly but often. J. Fish Biol. 75, 290–294 10.1111/j.1095-8649.2009.02281.x (doi:10.1111/j.1095-8649.2009.02281.x) [DOI] [PubMed] [Google Scholar]
- 73.Cheung W. W. L., Lam V. M. Y., Pauly D. 2008. Modelling present and climate shifted distributions of marine fishes and invertebrates. Fish. Centre Res. Rep. 16, 51–62 University of British Columbia, Canada [Google Scholar]
- 74.Lehodey P., Senina I., Murtugudde R. 2008. A spatial ecosystem and populations dynamics model (SEAPODYM): modelling of tuna and tuna-like populations. Prog. Oceanogr. 78, 304–318 10.1016/j.pocean.2008.06.004 (doi:10.1016/j.pocean.2008.06.004) [DOI] [Google Scholar]
- 75.Senina I., Sibert J., Lehodey P. 2008. Parameter estimation for basin-scale ecosystem-linked population models of large pelagic predators: application to skipjack tuna. Prog. Oceanogr. 78, 319–335 10.1016/j.pocean.2008.06.003 (doi:10.1016/j.pocean.2008.06.003) [DOI] [Google Scholar]
- 76.Cheung W .W. L., Lam V. W. Y., Sarmiento J. L., Kearney K., Watson R., Zeller D., Pauly D. 2010. Large-scale redistribution of maximum fisheries catch potential in the global ocean under climate change. Glob. Change Biol. 16, 24–35 10.1111/j.1365-2486.2009.01995.x (doi:10.1111/j.1365-2486.2009.01995.x) [DOI] [Google Scholar]
- 77.Pauly D. 1981. The relationship between gill surface area and growth performance in fish: a generalization of von Bertalanffy's theory of growth. Ber. Deut. Wiss. Komm. 28, 251–282 [Google Scholar]
- 78.Kolding J., Haug L., Stefansson S. 2008. Effect of ambient oxygen on growth and reproduction in Nile tilapia (Oreochromis niloticus). Can. J. Fish Aquat. Sci. 65, 1413–1424 10.1139/F08-059 (doi:10.1139/F08-059) [DOI] [Google Scholar]
- 79.Pauly D. 1980. On the interrelationships between natural mortality, growth parameters and mean environmental temperature in 175 fish stocks. J. Conseil 39, 175–192 10.1093/icesjms/39.2.175 (doi:10.1093/icesjms/39.2.175) [DOI] [Google Scholar]
- 80.Pauly D. 1984. A mechanism for the juvenile-to-adult transition in fishes. J. Conseil 41, 280–284 10.1093/icesjms/41.3.280 (doi:10.1093/icesjms/41.3.280) [DOI] [Google Scholar]
- 81.Lehodey P., Senina I., Sibert J., Bopp L., Calmetter B., Hampton J., Murtugudde R. 2010. Preliminary forecasts of Pacific bigeye tuna population trends under the A2 IPCC scenario. Prog. Oceanogr. 86, 302–315 10.1016/j.pocean.2010.04.021 (doi:10.1016/j.pocean.2010.04.021) [DOI] [Google Scholar]
- 82.Cooke S. J., et al. 2008. Developing a mechanistic understanding of fish migrations by linking telemetry with physiology, behaviour, genomics and experimental biology: an interdisciplinary case study on adult Fraser River sockeye salmon. Fisheries 33, 321–338 10.1577/1548-8446-33.7.321 (doi:10.1577/1548-8446-33.7.321) [DOI] [Google Scholar]
- 83.Clark T. D., Sandblom E., Hinch S. G., Patterson D. A., Frappell P. B., Farrell A. P. 2010. Simultaneous biologging of heart rate and acceleration, and their relationships with energy expenditure in free-swimming sockeye salmon (Oncorhynchus nerka). J. Comp. Physiol. B 180, 673–684 10.1007/s00360-009-0442-5 (doi:10.1007/s00360-009-0442-5) [DOI] [PubMed] [Google Scholar]
- 84.Gleiss A. C., Dale J. J., Holland K. N., Wilson R. P. 2010. Accelerating estimates of activity-specific metabolic rate in fish: testing the applicability of acceleration data-loggers. J. Exp. Mar. Biol. Ecol. 385, 85–91 10.1016/j.embe.2010.01.012 (doi:10.1016/j.embe.2010.01.012) [DOI] [Google Scholar]