Abstract
Tropical ectotherms are regarded as being especially threatened by global warming, but the extent to which populations vary in key thermal physiological traits is little known. In general, central and peripheral populations are most likely to differ where divergent selection pressures are un-opposed by gene flow. This leads to the prediction that persistent and long-isolated lineages in peripheral regions, as revealed by phylogeography, may differ physiologically from larger centrally located lineages. We test this prediction through comparative assays of critical thermal limits (minimum and maximum critical thermal limits, CTmin, CTmax) and optimal performance parameters (B80 and Topt) across central and peripheral lineages of three species of ground-dwelling skinks endemic to the rainforests of northeast Australia. Peripheral lineages show significantly increased optimal performance temperatures (Topt) relative to central populations as well as elevated CTmin, with the latter trait also inversely related to elevation. CTmax did not vary between central and peripheral lineages, but was higher in a forest edge species than in the forest interior species. The results suggest that long-isolated populations in peripheral rainforests harbour genotypes that confer resilience to future warming, emphasizing the need to protect these as well as larger central habitats.
Keywords: ecophysiology, niche evolution, ectotherms, climate change
1. Introduction
Physiological limits are emerging as one key predictor of species' sensitivity to rapid environmental change [1–3]. This is because limits to cold and heat tolerance are major determinants of the fundamental niche of a species [3–5]. Comparative studies suggest that tropical ectotherms have narrower thermal tolerances than their temperate counterparts [2,6,7]. Even with modest changes in environmental conditions, this could render tropical taxa very sensitive to future climatic change ([8], but see [9]), especially when combined with higher metabolic rates [10]. The extent to which these potential impacts are realized depends on capacity both to buffer via migration, microhabitat choice and acclimatization, and to respond via evolution of physiological tolerances [11–13].
Whether evolution of physiological limits can ameliorate impacts of rapid climate change on species has attracted considerable attention from theoreticians [14] and empiricists [13], and potential adaptive responses have been incorporated into predictive modelling of future distributions [15]. Many species show latitudinal or altitudinal clines in key traits (reviewed in Angilletta [16]) and in some cases these have been demonstrated to be largely genetically based rather than plastic in origin. The potential for varying physiological tolerances among populations within species is often overlooked in models of potential range shifts, yet, if present, such within taxon niche structure can alter predictions [17,18].
Less attention has been given to how key physiological traits might vary across independently evolving (phylogeographic) lineages within species [17]. It is well established that, subject to persistence, adaptation to challenging abiotic conditions is most likely where response to local selection is unopposed by gene flow from populations in relatively benign environments [19–21]. Phylogeographic lineages within species denote sets of populations with a long history of isolation from other such sets of populations, and so represent independent arenas for local adaptation. To the extent that such lineages occupy different abiotic environments, we should observe differences in corresponding physiological traits. Such differences could be especially evident between central and peripheral lineages, representing benign and stringent environmental conditions, respectively [22]. Peripheral populations may differ in current climatic parameters and/or have been less stable in the face of palaeoclimatic change.
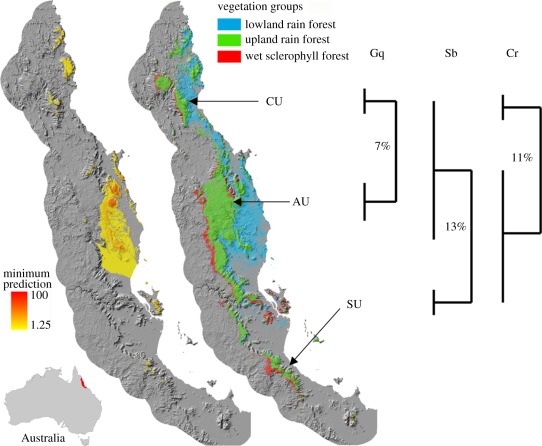
Here, we test for differences in key thermal physiological parameters among geographically central and peripheral lineages within each of three species of forest floor scincid lizards restricted to the tropical rainforests of northeast Australia. The Australian Wet Tropics (AWT) has a well-understood history of contraction of rainforests to mostly montane refugia during glacial maxima and subsequent expansion [23–25], resulting in strong phylogeographic structuring of regionally endemic rainforest specialist fauna [26]. In particular, most low-vagility species, such as those studied here, include a major, often genetically diverse lineage in the central wet tropics and one or more divergent lineages in peripheral and less stable montane areas to the north or south (figure 1). However, the species examined here vary in microhabitat use and in degree of restriction to rainforest. At one extreme, Gnypetoscincus queenslandiae is a log-dwelling, forest-interior species. At the other extreme, Carlia rubrigularis, although a rainforest species, is mostly associated with edges and clearings within the forest and extends out into the adjacent wet sclerophyll forests. Between these, Saproscincus basiliscus typically occurs in the forest interior and is shade-tolerant, often active during the early morning and evening but is also active in edge habitat. In broad terms, G. queenslandiae can be regarded as a strict thermoconformer, whereas S. basiliscus and especially C. rubrigularis actively select appropriate thermal environments (S. Williams & G. Langham 2007, unpublished data).
Figure 1.
The Australian Wet Tropics and schematic of central (AU) and peripheral (SU, CU) regions sampled in relation to phylogeographic structure within species. (Centre) Map of the pre-clearing distribution of low- and high-elevation rainforest and adjacent wet sclerophyll forest and (left) minimum area of rainforest and wet sclerophyll forest inferred from spatial modelling relative to palaeoclimates inferred for the Last Glacial Maximum, Holocene and the present. Labels indicate disjunct high-elevation regions on the Carbine (CU), Atherton (AU) and Spec (SU) uplands. (Right) Schematic of distribution of major phylogeographic lineages (with net sequence divergence of mitochondrial DNA) for each of the study species: Gq, G. queenslandiae; Sb, S. basiliscus; Cr, C. rubrigularis. See the studies of Dolman & Moritz [27], Schneider et al. [28] and Moussalli et al. [29] for detail. Maps on left and centre are modified from VanDerWal et al. [25].
Given long-term isolation and the potential for divergent selection, we expect to see differences in thermal physiological traits between central versus peripheral lineages. Although tropical thermoregulators are expected to be sensitive to high temperatures because of constraints on activity time [12,30], thermoconformers should be even more prone to effect of extreme environmental conditions [2,31]. Thus, we expect to see stronger divergence between central and peripheral lineages in G. queenslandiae than in C. rubrigularis and S. basiliscus. Using the same logic, the rainforest-dependent S. basiliscus has less access to a varied thermal environment than the edge-dwelling C. rubrigularis and thus might be expected to be more similar to the forest-interior, thermoconforming G. queenslandiae than is C. rubrigularis. In addition, the edge species have a greater capacity to behaviourally avoid temperature extremes and thus avoid selection on thermal criteria (the Bogert effect, [32]). Consequently, we expect to see the strongest divergence between central and peripheral populations in G. queenslandiae, intermediate in S. basiliscus and the least in C. rubrigularis.
2. Methods
(a). Sampling of central and peripheral lineages
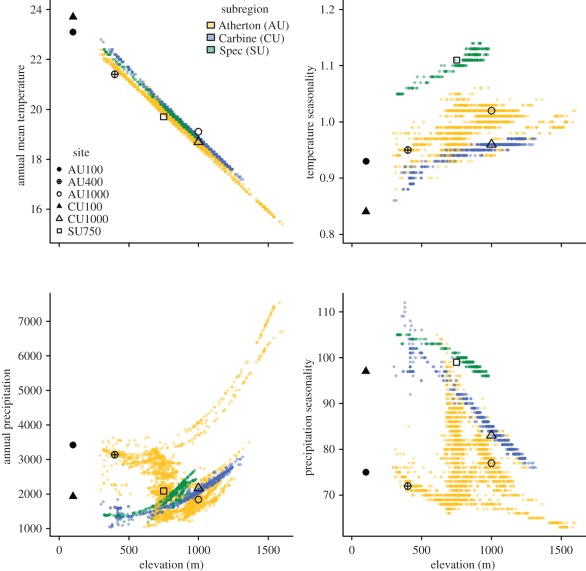
Our sampling design was limited by geographical ranges of the focal taxa and their component lineages, but allowed us to estimate divergence in physiological traits among high-elevation populations of geographically central versus peripheral lineages for each species as follows: G. queenslandiae, central (AU) versus peripheral (CU); S. basiliscus, central (AU) versus peripheral (SU) and C. rubrigularis, central (AU + SU) versus peripheral (CU; figure 1). Gnypetoscinus queenslandiae does not occur in the southern (SU) isolate, and S. basiliscus is not found at high elevations in the northern (CU) isolate [33]. We combine individuals of C. rubrigularis from SU and AU as the Central lineage as they represent a single phylogeographic lineage that is highly divergent from the northern (CU) populations [27]. Each of the peripheral regions (SU and CU) has similar annual precipitation and temperature across elevations to the large central AU region, but they differ in seasonality: the southern SU region has higher seasonality for both temperature and precipitation, whereas the northern CU region has somewhat higher seasonality for precipitation (figure 2). The peripheral regions, SU and CU, also experienced much more severe reduction in habitat area than did the central AU region under restrictive palaeoclimates, such as the cold–dry Last Glacial Maximum (figure 1). As thermal parameters often vary with latitude or elevation [16], we also sampled populations across elevations (AU, 100, 400, 1000 m; CU, 100, 1000 m; SU 750 m) to assess within-lineage trait variation in the presence of strong temperature gradients. Sampling details for each species and location are given in the electronic supplementary material, table S1.
Figure 2.
Estimates of current climate parameters, plotted against elevation, across the subregions and specific locations for which physiological data were obtained (Atherton, AU; Carbine, CU; Spec Uplands, SU). Values are interpolated at 80 m spatial resolution [25]. Peripheral areas differ primarily in having higher seasonality of precipitation (CU, SU) and temperature (SU).
(b). Experimental procedures and estimates of thermal parameters
Following capture, we transferred captured individuals to plastic containers with leaf litter. An ice cooler was used to moderate temperature fluctuation during transport. Once back at the laboratory, each lizard was measured and weighed and then returned to the container with food and water. Over the course of the experiments (9 days), animals were maintained in an animal house at a temperature of approximately 24°C with 12 L : 12 D light schedule for 1–3 days. We provided water and mealworms daily and containers were stored on a rack with a heating element running along the back end and heated daily from 9.00 to 15.00 h.
(i). Thermal tolerances
We tested for critical minimum (CTmin) and maximum (CTmax) temperatures with righting tests. When flipped onto their back, skinks will right themselves immediately, if possible, but skinks lose this ability at low and high extremes. To test for CTmin, we placed lizards in a plastic container sitting in an ice bath. We checked for sluggish behaviour, and then performed righting tests. As soon as a lizard could no longer right itself, we measured Tb with a fine-gauge, quick-read thermocouple (cloacal; TC1000 Sable Systems). Similarly, to test for CTmax, we placed skinks in plastic container with high walls and placed a 245 W bulb above the container. We observed each skink closely and then performed righting tests and temperature readings as described above. Immediately following the reading, we placed each skink in a cool water bath since high temperatures are potentially lethal. No animals died during either test.
(ii). Thermal performance
We tested thermal sensitivity of sprint performance by running each skink at one temperature per day. Each skink was run across a range of temperatures that correspond with field site temperatures (15–40°C). Five groups of skinks were run in a series of experiments across which the sequence of temperatures (15.7°C, 19.2°C, 24°C, 26°C, 28°C, 30°C, 31.6°C, 36.2°C and 40°C) were randomized. Skinks were only run between 10.00 and 15.00 h. All skinks were placed into a large incubator at the appropriate temperature for at least 60 min. We then removed a skink and placed it on the sprint track which had a darkened hiding place at the end. A video camera mounted 1 m above the track was used to record experiments. We encouraged skinks to run by gently touching the tail with a paintbrush. Skinks were returned to the incubator for an hour and run in this manner five times per day at a single temperature. To estimate optimal performance temperature (Topt) and optimal thermal range (B80), we used observations of maximum velocity (averaged across replicates) at each experimental temperature and for each lizard. To approximate the known shape of ectotherm performance curves [16,34], these observations were then plotted against temperature along with CTmin and CTmax for the same individual, and curves were fitted using a Gaussian (left side) × Gompertz (right side) function, using a Splus script (R. Huey 2008, personal communication). To calculate optimal thermal temperature ranges at 80 per cent of maximal (B80), we multiplied the maximum velocity by 0.8 and graphically solved the intersection with the fitted curve. The intersections represent the upper and lower bounds of B80, where sprint speeds are at least 80 per cent of maximum potential speed [34].
(c). Statistical analyses
We applied analyses of variance across both multi-species and single-species datasets to test for effects of central versus peripheral lineage on physiological traits, with body mass and elevation as covariates.
3. Results
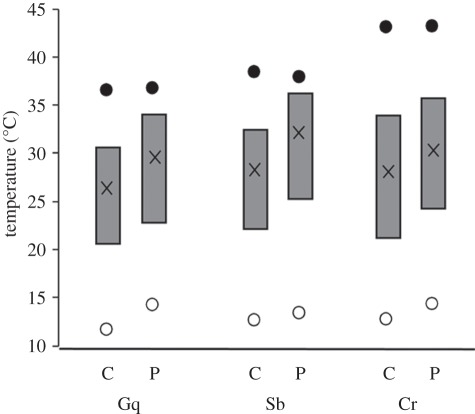
Substantial variation in thermal physiological traits was observed across species and between central and peripheral lineages within species (figure 3 and table 1). CTmax varies across species, with C. rubrigularis having markedly higher thermal tolerance than either G. queenslandiae or S. basiliscus, whereas Topt and CTmin differ mainly between central and peripheral lineages within species (figure 3). For the peripheral lineages of G. queenslandiae and S. basiliscus in particular, the evident increase in Topt (and the corresponding upper limit of high-performance range, B80), but not for CTmax, results in narrower margins between conditions for optimal performance and rapidly declining performance. No significant differences were observed for optimal performance range (B80).
Figure 3.
Comparison of key thermal traits among species (Gq, G. queenslandiae; Sb, S. basiliscus; Cr, C. rubrigularis) and between central (C) and peripheral (P) lineages of each species. Solid circles, CTmax; open circles, CTmin; filled boxes, the B80 high performance ranges; crosses within boxes, Topt. See table 1 for details.
Table 1.
Means ± s.e. and sample sizes (in parentheses) for physiological parameters by species and central versus peripheral lineages.
| species | lineage | CTmax (°C) | CTmin (°C) | Topt (°C) | B80 (°C) |
|---|---|---|---|---|---|
| Gnypetoscincus queenslandiae | |||||
| all | 36.7 ± 0.48 (13) | 12.6 ± 0.53 (22) | 27.7 ± 0.93 (18) | 10.4 ± 0.45 (15) | |
| central | 36.6 ± 0.57 (7) | 11.8 ± 0.65 (15) | 26.4 ± 0.76 (11) | 9.8 ± 0.33 (11) | |
| peripheral | 36.8 ± 0.86 (6) | 14.3 ± 0.47 (7) | 29.8 ± 1.92 (7) | 11.3 ± 0.53 (7) | |
| Saproscincus basiliscus | |||||
| all | 38.4 ± 0.33 (14) | 12.9 ± 0.44 (22) | 29.4 ± 1.1 (15) | 10.4 ± 0.33 (18) | |
| central | 38.5 ± 0.39 (10) | 12.8 ± 0.52 (18) | 28.3 ± 1.35 (11) | 10.3 ± 0.42 (11) | |
| peripheral | 38 ± 0.69 (4) | 13.5 ± 0.54 (4) | 32.3 ± 0.81 (4) | 10.9 ± 1.33 (4) | |
| Carlia rubrigularis | |||||
| all | 43.2 ± 0.25 (26) | 13.4 ± 0.45 (26) | 28.6 ± 0.96 (26) | 12.5 ± 0.35 (26) | |
| central | 43.2 ± 0.28 (22) | 12.8 ± 0.61 (16) | 28.1 ± 0.97 (20) | 12.8 ± 0.39 (20) | |
| peripheral | 43.3 ± 0.52 (4) | 14.4 ± 0.55 (10) | 30.4 ± 2.68 (6) | 11.5 ± 0.69 (6) | |
In the full (combined species) statistical analysis, optimal performance (Topt) differed significantly among lineages (F1,52 = 5.43, p = 0.024), being consistently higher in peripheral than central lineages, and with no significant effect of species or elevation. For individual species, the thermoconformer, G. queenslandiae, showed the strongest effect, with a significant difference (F1,15 = 5.85, p = 0.029) between the AU and the CU populations and no effect of elevation. As expected, the other species showed this effect less strongly; there was a difference between central and peripheral in S. basiliscus (F1,11 = 9.11, p = 0.012), but accompanied by a significant effect of elevation (F1,11 = 4.9, p = 0.049), and the effect was non-significant in C. rubrigularis.
Thermal limits, CTmax and CTmin, showed different patterns of variation. For CTmin, there was a significant difference between central and peripheral lineages across all species (F1,63 = 12.49, p = 0.0008); as for Topt, peripheral lineages had higher values (figure 3). However, there was also a significant effect of elevation (F1,63 = 12.62, p = 0.0007), with lower values of CTmin at higher elevations (data not shown). When species were analysed individually, there were significant differences between central and peripheral lineages for G. queenslandiae (F1,19 = 6.2, p = 0.022) and near significant differences for C. rubrigularis (F1,23 = 3.7, p = 0.06). However, the other thermoregulator, S. basiliscus, was not intermediate in effect; there was no effect of lineage, but elevation was significant (F1,19 = 2.5, p = 0.002). In contrast with CTmin (and Topt), CTmax differed among species (F2,44 = 54.51, p < 0.0001), as expected, with C. rubrigularis having substantially higher thermal tolerance than the intermediate S. basiliscus and lowest G. queenslandiae (figure 3), but there were no significant differences between central and peripheral lineages or across elevations.
4. Discussion
In accord with predictions, comparisons of key physiological traits revealed substantial differences between lineages, with central lineages having lower optimal performance temperatures (Topt) and lower thermal limits (CTmin) than peripheral lineages. The differences in Topt are independent of elevation, despite the very strong air temperature gradient it imposed (roughly 5°C across the elevational gradient). This lack of response to elevation suggests that Topt does not exhibit a strong plastic response, as each population is likely to be acclimatized to local conditions. By contrast, CTmin is affected by elevation, such that plasticity is a possible explanation for variation in this parameter [16]. These observations are interesting in several respects. First, they demonstrate significant differences in physiological tolerances between phylogeographic lineages within species. These differences in physiological traits are all the more remarkable in that, at least for G. queenslandiae and C. rubrigularis, there are no corresponding differences in eco-morphological traits between lineages [35,36]. Second, the central lineages differ from peripheral lineages in consistent ways; specifically, the peripheral lineages appear more warm-adapted, with upwards shifts of high-performance temperature ranges. Countering this, CTmax differs across but not within species, being higher in the edge-dwelling heliotherm (C. rubrigularis), intermediate in the more forest-dwelling thermoregulator (S. basiliscus) and lowest in the forest interior thermoconformer (G. queenslandiae); as a corollary, the margin between optimal performance temperature and CTmax is narrower for peripheral than for central populations. Third, the differences in CTmin and Topt among lineages are more marked for the thermoconformer, G. queenslandiae, than for the edge-dwelling thermoregulator, C. rubrigularis, although the forest-dependent thermoregulator, S. basiliscus, showed a mixed pattern with a large change in Topt and small change in CTmin.
As encouraging as the results are, the present analysis should be regarded as a preliminary evaluation of differences in key physiological traits between central and peripheral lineages of ectotherms in this tropical rainforest system. There is opportunity to test these initial results by expanding the comparative data to include additional, phylogeographically distinct, geographically peripheral lineages in these three species [27–29], and also to compare central and peripheral populations in a range of vertebrate and invertebrate ectotherms for which multiple phylogeographic lineages have been mapped across these rainforests [28,37–40]. As is generally the case [16], common garden and reciprocal transplant experiments are required to determine the extent to which the observed differences in thermal traits are genetic or plastic. There is also scope to directly measure selection on thermal parameters through field-based mark–recapture studies.
If substantiated through further analyses, these results have broad implications. There is an ongoing debate about whether key physiological traits, and hence the fundamental niche, are evolutionarily conserved versus labile [41,42] and at what temporal scale conservatism is prevalent [43,44]. Further, both plasticity and evolutionary response can contribute to persistence under changing climates [13,14]. Better understanding of these issues has broad implications for understanding speciation processes and evolution of range limits [7,19–21,45,46] and predicting biotic consequences of rapid climate change [1–3,11–16]. Most studies of intraspecific variation in physiological traits to date (reviewed in Hoffmann & Sgro [13] and Angilletta [16]) have examined broad-scale latitudinal variation in relatively high dispersal species (e.g. Drosophila) or altitudinal variation across continuous transects—situations where local selection is opposed by gene flow. We suggest that more attention should be paid to comparisons across independently evolving (phylogeographic) lineages, especially when they occur across different environments, as this provides the ideal setting for local adaptation. In fact, there already exists substantial comparative data within species for key thermal traits [9,30] that could be placed better into historical demographic framework. In short, it would be fruitful to integrate phylogeography with evolutionary physiology and thereby extend comparative analyses from intraspecific lineages to entire clades [47].
That the peripheral lineages examined here appear to have evolved higher thermal optima relative to centrally located lineages contrasts with the usual assumption that local adaptation of peripheral populations will be overwhelmed by gene flow from the centre of the species range [19–21] or, in the absence of immigration, will experience higher extinction rates [14,20]. Indeed, in the AWT system, many species now in peripheral regions did not persist there through repeated climate cycles of the Late Pleistocene but, rather, recolonized during the cool–wet period of the Early Holocene [24,27,28,48]. Why those that did persist as long-term isolates have also evolved towards increased optimal performance temperature (and perhaps also increased CTmin) in peripheral areas is not immediately obvious. In terms of current environment, the peripheral areas (SU and CU) do not differ markedly in mean annual temperature or precipitation from the central region (AU), especially at the higher elevations examined here (figure 2). However, they do show higher seasonality for temperature (SU) and precipitation (CU and SU), which could have bearing on these physiological traits [9]. An alternative hypothesis is that we are seeing the ghosts of selection past—i.e. that the differences reflect altered selection pressures during restrictive climates of the Late Quaternary [49]. Whereas the central region (AU) maintained a relatively large refugial area for rainforest species, the southern and northern regions were likely reduced to very small and highly fragmented forests ([25]; figure 1), such that few rainforest-specialist species survived and those that did were selected to persist in more ecologically marginal environments (e.g. more open sclerophyll forests or boulder microhabitats, see [50]). This hypothesis can be tested through more extensive comparative studies as outlined above.
If indeed peripherally isolated lineages do harbour unique adaptations, they would be especially relevant to strategies to sustain diversity under rapid climate change [22,51]. It is often assumed that large central populations have higher genetic diversity and, thus greater potential for adaptive response to environmental change. But this is not always the case, perhaps because of relaxed selection in a consistently benign environment [13]. Lineages that have persisted as isolates in peripheral areas through past climate change might well have genotypes that will confer greater resistance to future warming and which could be exploited for genetic translocation [1]. In this instance, as temperatures rise, the peripheral populations, with their elevated Topt, but similar environmental operative temperatures available to them, will experience either an increase in their fitness relative to the central population or a lesser decrement in fitness. Either way, increased attention to protection and ecological management of peripheral habitat isolates, by reducing other extrinsic pressures, may provide some level of insurance with ongoing global climate change.
Acknowledgements
This research was conducted in accordance with Animal Use and Ethics protocols approved by the James Cook University and University of California Berkeley, and collecting permits from the Queensland Department of Environment and Heritage.
We thank R. Holt for advice on analysis of thermal performance curves and C. Franklin, C. Jennings and B. Phillips for comments on the manuscript. Funding was provided from the National Science Foundation (post-doctoral Fellowship to G. Langham and DEB 0416250 to C. Moritz).
References
- 1.Chown S., Hoffmann A. A., Kristensen T. N., Angilletta M. J., Jr, Stenseth N. C., Pertoldi C. 2010. Adapting to climate change: a perspective from evolutionary physiology. Clim. Res. 43, 3–15 10.3354/cr00879 (doi:10.3354/cr00879) [DOI] [Google Scholar]
- 2.Huey R. B., Kearney M. R., Krockenberger A., Holtum J. A. M., Jess M., Williams S. E. 2012. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Phil. Trans. R. Soc. B 367, 1665–1679 10.1098/rstb.2012.0005 (doi:10.1098/rstb.2012.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seebacher F., Franklin C. E. 2012. Determining environmental causes of biological effects: the need for a mechanistic physiological dimension in conservation biology. Phil. Trans. R. Soc. B 367, 1607–1614 10.1098/rstb.2012.0036 (doi:10.1098/rstb.2012.0036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soberon J., Nakamura M. 2009. Niches and distributional areas: concepts, methods and assumptions. Proc. Natl Acad. Sci. USA 106(Suppl 2), 19 644–19 650 10.1073/pnas.0901637106 (doi:10.1073/pnas.0901637106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kearney M. R., Porter W. 2009. Mechanistic niche modeling: combining physiological and spatial data to predict species ranges. Ecol. Lett. 12, 334–350 10.1111/j.1461-0248.2008.01277.x (doi:10.1111/j.1461-0248.2008.01277.x) [DOI] [PubMed] [Google Scholar]
- 6.Sunday J. M., Bates A. E., Dulvy N. K. 2010. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823–1830 10.1098/rspb.2010.1295 (doi:10.1098/rspb.2010.1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kellerman V., van Heerwaarden B., Sgr C. M., Hoffmann A. A. 2009. Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science 325, 1244–1246 10.1126/science.1175443 (doi:10.1126/science.1175443) [DOI] [PubMed] [Google Scholar]
- 8.Deutsch C. A., Tewksbury J. J., Huey R. B., Sheldon K. S., Ghalambor C. K., Haak D. C., Martin P. R. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672 10.1073/pnas.0709472105 (doi:10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clusella-Trullas S., Blackburn T. M., Chown S. L. 2011. Climatic predictors of temperature performance curve parameters in ectotherms imply complex responses to climate change. Am. Nat. 177, 738–751 10.1086/660021 (doi:10.1086/660021) [DOI] [PubMed] [Google Scholar]
- 10.Dillon M. E., Wang G., Huey R. B. 2010. Global metabolic impacts of recent climate warming. Nature 467, 704–706 10.1038/nature09407 (doi:10.1038/nature09407) [DOI] [PubMed] [Google Scholar]
- 11.Williams S. E., Shoo L. P., Isaac J. L., Hoffmann A. A., Langham G. 2008. Towards an integrated framework for assessing the vulnerability of species to climate change. PLoS Biol. 6, 2621–2626 10.1371/journal.pbio.0060325 (doi:10.1371/journal.pbio.0060325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kearney M., Shine R., Porter W. P. 2009. The potential for behavioral thermoregulation to buffer ‘cold-blooded’ animals against climate warming. Proc. Natl Acad. Sci. USA 106, 3835–3840 10.1073/pnas.0808913106 (doi:10.1073/pnas.0808913106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann A. A., Sgro C. M. 2011. Climate change and evolutionary adaptation. Nature 470, 479–485 10.1038/nature09670 (doi:10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 14.Chevin L.-M., Lande R., Mace G. M. 2010. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357. 10.1371/journal.pbio.1000357 (doi:10.1371/journal.pbio.1000357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kearney M., Porter W. P., Williams C. K., Ritchie S. A., Hoffmann A. A. 2009. Integrating biophysical models and evolutionary theory to predict climatic impacts on species’ ranges: the dengue mosquito Aedes aegypti in Australia. Funct. Ecol. 23, 528–538 10.1111/j.1365-2435.2008.01538.x (doi:10.1111/j.1365-2435.2008.01538.x) [DOI] [Google Scholar]
- 16.Angilletta M. J., Jr 2009. Thermal adaptation: a theoretical and empirical synthesis. Oxford, UK: Oxford University Press [Google Scholar]
- 17.Pearman P. B., D'Amen M., Graham C. H., Thuiller W., Zimmerman N. E. 2010. Within-taxon niche structure: niche conservatism, divergence and predicted effects of climate change. Ecography 33, 990–1003 10.1111/j.1600-0587.2010.06443.x (doi:10.1111/j.1600-0587.2010.06443.x) [DOI] [Google Scholar]
- 18.Angert A. L., Seth S. N., Paul J. R. 2011. Incorporating population-level variation in thermal performance into predictions of geographical range shifts. Integr. Comp. Biol. 51, 733–750 10.1093/icb/icr048 (doi:10.1093/icb/icr048) [DOI] [PubMed] [Google Scholar]
- 19.Kirkpatrick M., Barton N. H. 1997. Evolution of a species’ range. Am. Nat. 150, 1–23 10.1086/286054 (doi:10.1086/286054) [DOI] [PubMed] [Google Scholar]
- 20.Holt R. D., Knight T. M., Barfield M. 2005. Allee effects, immigration and the evolution of species’ niches. Am. Nat. 163, 253–262 10.2307/3473275 (doi:10.2307/3473275) [DOI] [PubMed] [Google Scholar]
- 21.Bridle J., Vines T. H. 2007. Limits to evolution at range margins: when and why does adaptation fail? Trends Ecol. Evol. 22, 140–147 10.1016/j.tree.2006.11.002 (doi:10.1016/j.tree.2006.11.002) [DOI] [PubMed] [Google Scholar]
- 22.Hampe A., Petit R. J. 2005. Conserving biodiversity under climate change: the rear edge matters. Ecol. Lett. 8, 461–467 10.1111/j.1461-0248.2005.00739.x (doi:10.1111/j.1461-0248.2005.00739.x) [DOI] [PubMed] [Google Scholar]
- 23.Nix H. A. 1991. Biogeography: patterns and process. In Rainforest animals: atlas of vertebrates endemic to Australia's Wet Tropics (eds Nix H. A., Switzer M.), pp. 11–39 Canberra, Australia: Australian Nature Conservation Agency [Google Scholar]
- 24.Graham C. H., Moritz C., Williams S. E. 2006. Habitat history improves prediction of biodiversity in rainforest fauna. Proc. Natl Acad. Sci. USA 103, 632–636 10.1073/pnas.0505754103 (doi:10.1073/pnas.0505754103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VanDerWal J., Shoo L. P., Williams S. E. 2008. New approaches to understanding late Quaternary climate fluctuations and refugial dynamics in Australian wet tropical rain forests. J. Biogeogr. 36, 291–301 10.1111/j.1365-2699.2008.01993.x (doi:10.1111/j.1365-2699.2008.01993.x) [DOI] [Google Scholar]
- 26.Moritz C., Hoskin C. J., MacKenzie J. B., Phillips B. L., Tonione M., Silva N., VanDerWal J., Williams S. E., Graham C. H. 2009. Identification and dynamics of a cryptic suture zone in tropical rainforest. Proc. R. Soc. B 276, 1235–1244 10.1098/rspb.2008.1622 (doi:10.1098/rspb.2008.1622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dolman G., Moritz C. 2006. A multilocus perspective on refugial isolation and divergence in rainforest skinks (Carlia). Evolution 60, 573–582 10.1111/j.0014-3820.2006.tb01138.x (doi:10.1111/j.0014-3820.2006.tb01138.x) [DOI] [PubMed] [Google Scholar]
- 28.Schneider C. J. S., Cunningham M., Moritz C. 1998. Comparative phylogeography and the history of endemic vertebrates in the Wet Tropics rainforest of Australia. Mol. Ecol. 7, 487–498 10.1046/j.1365-294x.1998.00334.x (doi:10.1046/j.1365-294x.1998.00334.x) [DOI] [Google Scholar]
- 29.Moussalli A., Moritz C., Williams S. E., Carnaval A. C. 2009. Variable responses of skinks to a common history of rainforest fluctuation: concordance between phylogeography and palaeo-distribution models. Mol. Ecol. 18, 483–499 10.1111/j.1365-294X.2008.04035.x (doi:10.1111/j.1365-294X.2008.04035.x) [DOI] [PubMed] [Google Scholar]
- 30.Sinervo B., et al. 2010. Global climate change, shrinking thermal niches, limits to thermal adaptation, and the collapse of global lizard biodiversity. Science 328, 894–899 10.1126/science.1184695 (doi:10.1126/science.1184695) [DOI] [PubMed] [Google Scholar]
- 31.Huey R. B., Deutsch C. A., Tewksbury J. J., Vitt L. J., Hertz P. E., lvarez H. J. 2009. Why tropical forest lizards are vulnerable to climate warming. Proc. R. Soc. B 276, 1939–1948 10.1098/rspb.2008.1957 (doi:10.1098/rspb.2008.1957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huey R. B., Hertz P. E., Sinervo B. 2003. Behavioral drive versus behavioral inertia: a null model approach. Am. Nat. 161, 357–366 10.1086/346135 (doi:10.1086/346135) [DOI] [PubMed] [Google Scholar]
- 33.Williams S. E., et al. 2010. Distributions, life-history, specialization and phylogeny of the rainforest vertebrates in the Australian Wet Tropics. Ecology 91, 2493. 10.1890/09-1069.1 (doi:10.1890/09-1069.1) [DOI] [Google Scholar]
- 34.Huey R. B., Stevenson R. D. 1979. Integrating thermal physiology and ecology of ectotherms: discussion of approaches. Am. Zool. 19, 357–366 10.1093/icb/19.1.357 (doi:10.1093/icb/19.1.357) [DOI] [Google Scholar]
- 35.Schneider C. J. S., Moritz C. 1999. Refugial isolation and evolution in the Wet Tropics rainforests of Australia. Proc. R. Soc. Lond. B 266, 191–196 10.1098/rspb.1999.0621 (doi:10.1098/rspb.1999.0621) [DOI] [Google Scholar]
- 36.Schneider C. J. S., Smith T. B., Larison B., Moritz C. 1999. A test of alternative models of diversification in tropical rainforests: ecological gradients versus rainforest refugia. Proc. Natl Acad. Sci. USA 99, 13 869–13 873 10.1073/pnas.96.24.13869 (doi:10.1073/pnas.96.24.13869) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hugall A., Moritz C., Moussalli A., Stanisic J. 2002. Reconciling paleodistribution models and comparative phylogeography in the Wet Tropics rainforest land snail Gnarosophia bellendenkerensis (Brazier 1875). Proc. Natl Acad. Sci. USA 99, 6112–6117 10.1073/pnas.092538699 (doi:10.1073/pnas.092538699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bell K. L., Moritz C., Moussalli A., Yeates D. K. 2007. Comparative phylogeography and speciation of dung beetles from the Australian Wet Tropics rainforest. Mol. Ecol. 16, 4984–4998 10.1111/j.1365-294X.2007.03533.x (doi:10.1111/j.1365-294X.2007.03533.x) [DOI] [PubMed] [Google Scholar]
- 39.Bell R. C., Parra J. L., Tonione M., Hoskin C. J., MacKenzie J. B., Williams S. E., Moritz C. 2010. Patterns of persistence and isolation indicate resilience to climate change in montane rainforest lizards. Mol. Ecol. 19, 2531–2544 10.1111/j.1365-294X.2010.04676.x (doi:10.1111/j.1365-294X.2010.04676.x) [DOI] [PubMed] [Google Scholar]
- 40.Hoskin C., Tonione M., Higgie M., MacKenzie J. B., Williams S. E., VanDerWal J., Moritz C. 2011. Persistence in peripheral refugia promotes phenotypic divergence and speciation in a rainforest frog. Am. Nat. 178, 561–578 10.1086/662164 (doi:10.1086/662164) [DOI] [PubMed] [Google Scholar]
- 41.Wiens J. J., Graham C. H. 2005. Niche conservatism: integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst. 36, 519–539 10.1146/annurev.ecolsys.36.102803.095431 (doi:10.1146/annurev.ecolsys.36.102803.095431) [DOI] [Google Scholar]
- 42.Pearman P. B., Guisan A., Broennimann O., Randin C. F. 2008. Niche dynamics in space and time. Trends Ecol. Evol. 23, 149–158 10.1016/j.tree.2007.11.005 (doi:10.1016/j.tree.2007.11.005) [DOI] [PubMed] [Google Scholar]
- 43.Labra A., Pienaar J., Hansen T. F. 2009. Evolution of thermal physiology in Liolaemis lizards: adaptation, phylogenetic inertia and niche tracking. Am. Nat. 174, 204–220 10.1086/600088 (doi:10.1086/600088) [DOI] [PubMed] [Google Scholar]
- 44.Peterson A. T. 2011. Ecological niche conservatism: a time-structured review of evidence. J. Biogeogr. 38, 817–827 10.1111/j.1365-2699.2010.02456.x (doi:10.1111/j.1365-2699.2010.02456.x) [DOI] [Google Scholar]
- 45.Janzen D. H. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 233–249 [Google Scholar]
- 46.Ghalambor C. K., Huey R. B., Martin P. R., Tewksbury J. J., Wang G. 2006. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integr. Comp. Biol. 46, 5–17 10.1093/icb/icj003 (doi:10.1093/icb/icj003) [DOI] [PubMed] [Google Scholar]
- 47.Salamin N., Wuest R. O., Lavergne S., Thuiller W., Pearman P. B. 2011. Assessing rapid evolution in a changing environment. Trends Ecol. Evol. 25, 692–698 10.1016/j.tree.2010.09.009 (doi:10.1016/j.tree.2010.09.009) [DOI] [PubMed] [Google Scholar]
- 48.Oza A. U., Lovett K. E., Williams S. E., Moritz C. 2011. Recent speciation and limited phylogeographic structure in Mixophyes frogs in the Australian Wet Tropics. Mol. Phylogenet. Evol. 62, 407–413 10.1016/j.ympev.2011.10.010 (doi:10.1016/j.ympev.2011.10.010) [DOI] [PubMed] [Google Scholar]
- 49.Vanzolini P. E., Williams E. E. 1981. The vanishing refuge: a mechanism for ecogeographic speciation. Papeis Avulsos de Zoologia, Sao Paulo 34, 251–255 [Google Scholar]
- 50.Couper P. J., Hoskin C. J. 2008. Litho-refugia: the importance of rock landscapes for the long-term persistence of Australian rainforest fauna. Austr. Zool. 34, 554–560 [Google Scholar]
- 51.Lesica P., Allendorf F. W. 1995. When are peripheral populations valuable for conservation? Conserv. Biol. 9, 753–760 10.1046/j.1523-1739.1995.09040753.x (doi:10.1046/j.1523-1739.1995.09040753.x) [DOI] [Google Scholar]