Abstract
The Ediacaran Doushantuo biota has yielded fossils that include the oldest widely accepted record of the animal evolutionary lineage, as well as specimens with alleged bilaterian affinity. However, these systematic interpretations are contingent on the presence of key biological structures that have been reinterpreted by some workers as artefacts of diagenetic mineralization. On the basis of chemistry and crystallographic fabric, we characterize and discriminate phases of mineralization that reflect: (i) replication of original biological structure, and (ii) void-filling diagenetic mineralization. The results indicate that all fossils from the Doushantuo assemblage preserve a complex mélange of mineral phases, even where subcellular anatomy appears to be preserved. The findings allow these phases to be distinguished in more controversial fossils, facilitating a critical re-evaluation of the Doushantuo fossil assemblage and its implications as an archive of Ediacaran animal diversity. We find that putative subcellular structures exhibit fabrics consistent with preservation of original morphology. Cells in later developmental stages are not in original configuration and are therefore uninformative concerning gastrulation. Key structures used to identify Doushantuo bilaterians can be dismissed as late diagenetic artefacts. Therefore, when diagenetic mineralization is considered, there is no convincing evidence for bilaterians in the Doushantuo assemblage.
Keywords: Ediacaran, Doushantuo, Bilateria, fossilization, diagenesis
1. Introduction
The Doushantuo Formation from south China has yielded a fossil assemblage that provides a rare window on multicellular life before the Cambrian. The biota contains fossils of uncontested nature, such as acritarchs [1,2] and algae [2,3] in addition to more enigmatic forms [4–6] and fossils that have been interpreted as the remains of stem- [7] or crown-metazoans [3,8–11] and even bilaterians [10,12–14]. However, many of these interpretations of Doushantuo fossils have been contested, leading to conflicting views on the timing of the divergence of the crown-metazoan clades.
If the reports of bilaterians [10,12–14] are correct, the Doushantuo assemblage attests to the divergence of bilaterian clades tens of millions of years before the base of the Cambrian. Importantly, however, several of these identifications have been contested because they rely on the presence of key biological structures that may be more readily interpreted as geological artefacts resulting from diagenetic mineralization long after biological tissues rotted away [7,9,15–19]. With evidence for bilaterians in the Doushantuo biota lacking, crown-Bilateria may well have evolved later [7]. For example, it has been suggested that because it is a well-sampled Lagerstätte that contains no bilaterians and only earlier branching lineages, the top of the Doushantuo Formation can be used as a maximum constraint on the divergence of the crown-Bilateria in molecular clock studies [20].
Much of the debate surrounding these contentious fossils has hinged on whether what is purported to be preserved biological tissue has been interpreted correctly. Here, we attempt to discriminate the phases of mineralization that reflect replication of original biological structure versus diagenetic void filling. To achieve this end, we have used a combination of back-scattered electron imaging (BSE), electron probe microanalysis (EPMA) and synchrotron X-ray tomographic microscopy (SRXTM). Using Doushantuo algal and acritarch fossils—which are uncontroversial in terms of the distribution of preserved biological tissue and void-filling mineralization—the chemistry (using BSE and EPMA) and crystallographic fabric (using BSE and SRXTM) were characterized for: (i) preserved biological structures, and (ii) void-filling diagenetic mineralization. The findings allow these two phases of mineralization to be distinguished in fossils whose interpretation has proved contentious. We critically re-evaluate taxa reported from the Doushantuo Formation in the light of these new data.
2. Material and methods
The specimens studied derive from the Upper Phosphorites of the Datang Quarry, Weng'an, Guizhou Province, China [21]. Specimens were extracted by dissolution of the host carbonate rock in 6–10% acetic acid and sorted manually with the aid of a binocular microscope. SRXTM experiments were conducted at the X02DA (TOMCAT) beamline [22] of the Swiss Light Source at the Paul Scherrer Institute, Villigen, Switzerland using methods outlined previously [19]. For BSE and EPMA analysis, specimens were embedded in epoxy resin, ground to the level of interest using silicon carbide paper before being polished with 6 µm and then 1 µm diamond paste. The specimens were carbon-coated prior to analysis. BSE analyses were carried out on a Hitachi S-3500N SEM. EPMA analyses were carried out on a Cameca SX 100 microprobe with a typical operating voltage of 15–20 kV, a dwell time of 200 ms and a beam current of 100 nA. For quantitative elemental analyses, the instrument was calibrated using a Durango apatite standard of known composition.
3. Results
(a). Algae
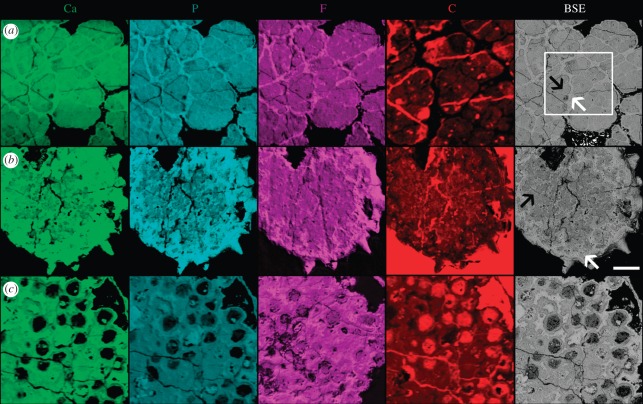
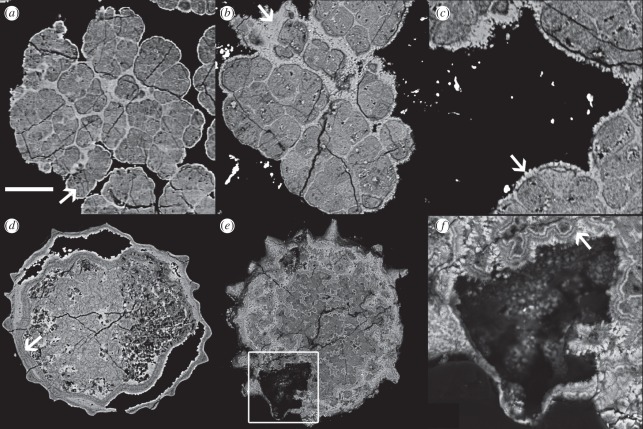
There are two distinct phases of mineralization in the specimens of the alga Sarcinophycus that we have examined. The first of these (figure 1a, black arrow), a carbon-rich fluorapatite, has lower X-ray attenuation (appearing less bright in the figured SRXTM images; figure 2a) and lower atomic number (appearing less bright in BSE images; figure 2b,c). This phase defines the outline of the cells and is composed of very fine crystals that are difficult to distinguish in BSE analysis and show no evidence for preferred orientation. When elemental abundances were mapped using EPMA, this phase was characterized as having relatively higher abundances of C, possibly reflecting kerogen produced by polymerization of labile organics in the cell or else carbon in the apatite crystal lattice, and lower abundances of Ca, P and F (figure 1a). The second phase (figure 1a, white arrow), a relatively carbon-poor fluorapatite, forms rims around the first phase with relatively coarse (greater than 1 µm in length) crystals that are approximately surface-normal (figure 2c, arrow) as well as filling spaces in the structure of the first phase. This second phase has higher X-ray attenuation, higher atomic number, higher Ca, P and F and lower C than the first phase. There is no evidence that this second phase preserves the cell walls. The distinct regions surrounding the cells that comprise this phase infill spaces and form rims, and it is a diagenetic void filling and encrusting phase. The low attenuation and low atomic number carbon-rich fluorapatite phase define the original morphology of the cells in their entirety; there is no evidence for the preservation of organic cell walls in the specimens studied.
Figure 1.
Elemental maps of (a) an alga, (b) an acritarch and (c) ‘Megaclonophycus’-stage cleavage specimens from the Doushantuo biota. Each map shows the relative abundance of the element, with brighter tones representing greater abundance. The right-hand column shows a BSE image of the same region for comparison. In (a) a carbon map of an enlarged region of part of the specimen with little resin is shown to prevent the signal within the fossil being ‘swamped’ by the very high carbon in the resin; the box in the BSE image shows the position of this map. Regions of epoxy resin (e.g. in (a) surrounding the alga and in resin filled cracks that are especially visible in the C map) have very low abundances of Ca, P and F, and very high abundance of C. Scale bar, 50 µm, except for in the enlarged region in the carbon map in (a).
Figure 2.
SRXTM and BSE images of (a–c) Doushantuo algae and (d–f) acritarchs. (a) SRXTM image; arrow indicates carbon-rich fluorapatite replicating algal morphology. (b–c) BSE images of the same specimen; arrow in (b) indicates encrusting carbon-poor fluorapatite; arrow in (c) indicates coarse, surface-normal carbon-poor fluorapatite crystals. (d) SRXTM image; arrow indicates a region where laminae are absent owing to lack of space where structures abut. (e–f) BSE images; box in (e) indicates region shown in (f); arrow in (f) indicates geoidal apatite growth. Scale bar, (a) 39 µm; (b) 36 µm; (c) 20 µm; (d) 75 µm; (e) 67 µm and (f) 22 µm.
(b). Acritarchs
As with algae, there are two phases of mineralization. The regions with high X-ray attenuation (figure 2d) in acritarchs correspond to regions of high atomic number (figure 2e,f) and high relative abundances of Ca, P and F and relatively low abundances of C (figure 1b, white arrow). Quantitative elemental compositions for these two phases in acritarchs are presented in the electronic supplementary material, table S1. The acritarchs studied have irregular layered texture in the interior, with variable X-ray attenuation and atomic number (figure 2d–f). In some instances, these layers, which are composed principally of surface-normal crystals, run concentrically around the margin of voids that still exist (figure 2f). The outer portion of the specimen has a thick region that preserves the acanthomorphic surface morphology of the acritarch cyst; there is evidence of layering in this region of some specimens (figure 2d). A similar layered region surrounds the shrunken internal body, with a phase of high attenuation and high atomic number filling the intervening space (figure 2d).
The sequence of laminations in these two regions is indistinguishable in terms of their attenuation levels. This strongly suggests that the layered structures are not biological: there is no reason that the ultrastructure of two different biological structures would be so similar. Moreover, layers are locally absent where the two structures abut (figure 2d, arrow), consistent with void-filling cementation. This observation is further evidence that the layered structures which replicate the acanthomorphic morphology are diagenetic in origin. The organic wall itself is usually absent in Doushantuo acritachs from the light-grey facies, and this is the case in these specimens. The layered texture of the interior region (figure 2e,f) also represents void-filling geoidal crystal growth. The concentric layering in surface-normal crystals, which indicate growth from a pre-existing surface, around a remaining void, provides a strong demonstration of this (figure 2f). There is no evidence for preservation of original biological structure in the interior of these specimens.
(c). General patterns identified in algae and acritarchs
Doushantuo algae and acritarchs, discussed above, are uncontroversial in terms of the distribution of preserved biological tissue and void-filling mineralization. Hence, we have identified textural and chemical patterns in these relatively well-understood taxa with the aim of establishing criteria that can be used to interpret the more contentious specimens in the biota. The patterns identified are:
— regions with higher X-ray attenuation (i.e. that appear bright in SRXTM images) consistently correspond to regions of higher atomic number (i.e. appear bright in BSE images). Regions with high X-ray attenuation and high atomic number have high relative elemental abundances of Ca, P and F, but low abundances of C. The inverse pattern is observed in low-attenuation regions (figure 2; electronic supplementary material, table S1; [23,24]);
— preserved soft tissue in these specimens is replicated in a low-attenuation cryptocrystalline carbon-rich fluorapatite phase (figure 2a, arrow). However, carbon-rich fluorapatite alone does not necessarily indicate soft tissue preservation: it can occasionally be void filling, in which case it can be identified as such on the basis of its texture;
— geoidal textures and relatively large (greater than 1 µm) crystals with long axes perpendicular to the surface are associated with diagenetic crystal growth; these regions are associated with variation in attenuation that results in a distinctly laminated texture; this presumably reflects alternation between carbon-rich and carbon-poor fluorapatite (figures 1b and 2f, arrow). They are indicative of this phase of mineralization and can be used to identify void-filling diagenetic crystal growth in other specimens from the Doushantuo biota. While it is possible for void fills to replicate the gross morphology of biological features, structures within them cannot be interpreted as biological; and
— the latest void filling and encrusting mineralization are typically preserved in a high-attenuation carbon-poor fluorapatite phase (figure 2b,c, arrows).
(d). ‘Parapandorina’-stage cleavage specimens
These fossils are assigned to the genus Tianzhushania, which has been interpreted as a senior synonym of Megasphaera (single-celled specimens), Parapandorina (multiple polyhedral cells) and Megaclonophycus (large numbers of spheroidal cells) [25–27]. Tianzhushania is interpreted here as preserving a single developmental pathway and includes all known Doushantuo cleavage specimens, except for those assigned to Spiralicellula (specimens with helicospirally coiled cells [28,29]). ‘Parapandorina’-stage fossils have been most commonly interpreted as early cleavage embryos of stem- [7] or crown-metazoans [3,14,26,30,31]; and have recently been proposed to represent cyst-forming protists [29]. They have also been interpreted alternatively as giant sulphur bacteria [32] (though see [26,27,29] and [33] for critical views).
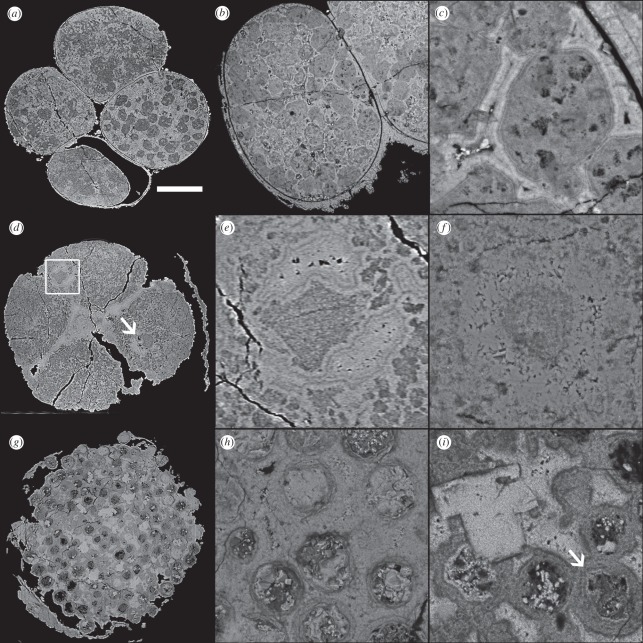
Some cleavage stage specimens contain structures (up to approx. 60 µm in diameter) that have been interpreted as lipid vesicles or yolk granules similar to those observed in modern animal embryos [7,34]. These subcellular structures are preserved in a cryptocrystalline carbon-rich fluorapatite phase (electronic supplementary material, table S1) and they are enclosed by regions of carbon-poor fluorapatite (figure 3a–c). In many specimens, there is a range in the quality of the preservation of these structures between cells. There is a continuum from discrete droplets to a clotted fabric, which can be seen clearly in figure 3a. In less clear examples, differences in the degree of preservation can give the impression of different densities of inclusions. The surrounding region consistently shows evidence of fine concentric layering, sometimes surrounding voids; these broadly isopachous layers are defined by their having different attenuation and composition. On the whole, these layers tend to be brighter in BSE images than are the lipid vesicles/yolk granules (figure 3c).
Figure 3.
SRXTM and BSE images of (a–f) ‘Parapandorina’-stage and (g–i)‘Megaclonophycus’-stage cleavage specimens from the Doushantuo biota. (a) SRXTM image of specimen with putatative lipid droplets; note differences in X-ray attenuation between cells owing to preservation quality. (b,c) BSE images. (d,e) SRXTM images of a specimen showing putative nuclei; the square in (d) indicates the region shown in detail in (e). (f) BSE image showing putative nucleus. (g) SRXTM image of ‘Megaclonophycus’ specimen. (h) BSE image of ‘Megaclonophycus’ specimen. (i) BSE image of ‘Megaclonophycus’ specimen, arrow indicates centrifugal growth rings. Scale bar, (a) 123 µm; (b) 92 µm; (c) 17 µm; (d) 133 µm; (e) 21 µm (f) 24 µm; (g) 129 µm; and (h, i) 23 µm.
The droplets are interpreted as preserved biological structures, as they have the chemistry and texture identified as being characteristic of this type of preservation. It is difficult to distinguish between the proposed alternative interpretations of lipid vesicles and yolk granules. However, lipid droplets coalesce during decay [34] and it is possible that the range of sizes in Doushantuo specimens reflects increasing coalescence of lipids as decay progresses. The geoidal textures in the regions surrounding these structures provide strong evidence that the mineralization of these regions is the product of multiple diagenetic void-filling phases, consistent with SEM textural observations [35,36]. This demonstrates that the diagenetic history of these specimens is more complex than previously supposed [7] and that even the most remarkably preserved specimens cannot be compared with freshly killed organisms without consideration of diagenetic processes.
Other cleavage specimens have structures that have been interpreted as nuclei [7,14,29] and some of these contain smaller structures (termed ‘inner body’ here) that have been interpreted as potential nucleoli [14]. We examined representative specimens using SRXTM (figure 3d,e) and BSE (figure 3f). The putative nuclei can be located in the centre of the cell or peripherally within the cell (figure 3d). They are characterized by two distinct styles of mineralization. The first of these, corresponding to the inner body, is composed of cryptocrystalline carbon-rich fluorapatite. The second phase, corresponding to the surrounding region, exhibits geoidal textures when examined with SRXTM (figure 3e). BSE analysis (figure 3f) shows that this phase is composed of large euhedral carbon-poor fluorapatite crystals, with evidence of euhedral crystals extending from opposing substrates into a central void. In the majority of specimens examined, the boundary between the two phases is irregular, though the outer region is typically crescent-shaped (figure 3d, arrow). The region surrounding the putative nuclei is characterized by a granular fabric containing structures similar in appearance to those interpreted as potential lipid vesicles above, but of smaller size (ca 10 µm). This contrasts with the inner body of the putative nucleus, which is predominantly carbon-rich, though heterogeneous at a finer scale (figure 3f).
Thus, the carbon-poor fluorapatite phase in the outer regions of putative nuclei has a texture and chemistry that is characteristic of void-filling mineralization and is interpreted as such (see also [36]). The carbon-rich fluorapatite inner body has the characteristics of preserved tissue and differs from the material outside the potential nucleus in lacking the granular fabric. It is possible that the inner body could represent decayed and collapsed nuclear material, but the surrounding region undoubtedly represents later mineralization that filled the void left by the collapsed nucleus. This requires that the surrounding cytoplasm was preserved first (either by mineralization or a biofilm [37]) allowing the space to remain open as the nucleus collapsed. This space would have subsequently been infilled by diagenetic mineralization.
An alternative interpretation that the putative nuclei result from degradational collapse of the entire cell [38] is not consistent with this distribution of mineralization. Furthermore, it has been argued that the structures have consistent size, shape, occurrence, position and volumetric relationships [29] consistent with a biological rather than taphonomic origin. Their overall morphology resembles neither the irregular products of degradational collapse seen in experimental decay [39] and fossilization [40], nor degraded and collapsed organic material in the Doushantuo biota [9].
There is also no support for the interpretation that the inner body replicates the nucleolus [14] rather than the remnants of the decayed nucleus. Given the observed distribution of mineralization, this hypothesis requires that the nucleolus had special preservation potential, remaining when the rest of the nucleus has decayed. The putative nucleolus has an irregular morphology and an outer region that frequently appears crescent-shaped in section, which is a shape associated with degradational shrinkage within an uncollapsed sphere in other Doushantuo specimens [9]. Together these facts indicate that this class of structures is more readily interpreted as the remains of the degraded nucleus than as a preserved nucleolus.
(e). ‘Megaclonophycus’-stage specimens
Fossils assigned to Megaclonophycus have been interpreted as a subsequent stage in the life cycle of Tianzhushania [30]. They are composed of hundreds to thousands of spheroidal structures interpreted as cells by previous workers (figure 3g–i). Each of these spheroidal structures contains a central spheroid that is of broadly consistent size across ‘Megaclonophycus’ specimens, typically around 30 µm in diameter (figure 3h,i). This central spheroid is hollow in some instances. In other cases, it is filled with large euhedral carbon-poor fluorapatite crystals or with irregular material that possibly reflects preservation of strongly degraded organic material.
The central spheroid is surrounded by a relatively low-attenuation rim. The thickness of this rim varies between specimens, so that these rims are in contact with one another in some specimens (figure 3i), but not in others (figure 3h). Some rings are composed of concentric layers (figure 3i). The regions of the specimens between the spheroids and rims are filled with carbon-poor fluorapatite. There are also carbon-poor fluorapatite crystals which cross-cut all the other styles of mineralization (figure 3i). The last indicates the presence of a further phase of mineralization in the Doushantuo biota (in addition to replication of biological tissue and void filling) namely later overprinting and obliteration of the two earlier mineral phases.
The interpretation of ‘Megaclonophycus’-stage specimens has important implications for understanding the phylogenetic position of Tianzhushania. Hagadorn et al. [7] noted that there was no evidence of gastrulation—the reorganization of the embryo into tissue layers—by the time the specimen had developed to ca 1000 cells. Because gastrulation would be expected by this stage of development in the embryology of living animals, its absence in the Doushantuo embryos was taken to indicate that they are more primitive than any living animal. However, this argument relies on the assumption that the cells in ‘Megaclonophycus’-stage specimens are in their original configuration. Experimental studies have shown that cell adhesion can be lost after death leading to a stereoblastula-like appearance [34]. If the cells in ‘Megaclonophycus’-stage specimens are in their original configuration, they should be in contact with one another, forming facetted Y-junctions where they abut adjacent cells [41]. In order to assess whether or not this is the case, it is important to determine which parts of the fossil correspond to the original cell boundaries of the organism.
We interpret the concentric rings (of variable thickness between specimens) around the central spheroids (of consistent diameter) to have grown outwards centrifugally to different degrees in different specimens. This interpretation of secondary centrifugal growth is supported by the fact that the rims of neighbouring cells frequently coalesce in some specimens (figure 3i). The crystals within the central spheroids are interpreted to be centripetal infills of the voids because they extend inwards from the margin into the void, which in some cases remains a void. The original cell boundary must, therefore, lie at the interface between the centrifugal and centripetal mineral growth, i.e. at the outside margin of the central spheroid and the interior margin of the surrounding concentric ring. In the specimens studied, each cell is always fully enclosed around its entire circumference by at least some thickness of concentric ring. There was therefore space beyond its external surface into which minerals could grow. Moreover, the cell boundaries are non-circular in section (figure 3i), indicating that they may represent the irregular margins of collapsed cell material on which secondary cements grew centrifugally. We therefore conclude that the cells are not in contact and, therefore, not preserved in their original configuration. Thus, ‘Megaclonophycus’-stage specimens are not informative about gastrulation in the Doushantuo biota and, therefore, absence of gastrulation cannot be used to constrain the systematic position of these fossil organisms.
4. Discussion
The findings presented here have clear implications for the interpretation of other fossils in the Doushantuo biota. First, the fact that even fossils that appear to preserve pristine cells have complex multi-phase diagenetic histories means that it is important to consider the processes of decay and mineralization when interpreting these specimens: they cannot simply be compared with candidate living counterparts [42]. Second, the identification of textural and chemical indicators of diagenetic mineralization provides a key to identifying this style of mineralization in other material from the biota.
Of particular interest are the Doushantuo specimens that have been interpreted as bilaterians. These include a suite of specimens interpreted as embryonic bilaterians on the basis of endodermal cords [14], polar lobes [43], embryonic polarity [14,31] and duet cleavage [31]. In each case, the specimens in question are more plausibly interpreted as cleavage specimens that have undergone taphonomic and diagenetic processes.
Endodermal cords of a distinct yolk-rich cell type [31] are more likely to reflect differences in the quality of preservation, which are often seen between cells of individual Doushantuo specimens. For example, the ‘Parapandorina’-stage specimen in figure 3a shows differences in X-ray attenuation, but these can be seen to result from differences in the quality of preservation of the putative lipid droplets as discussed above. Indeed, following the authors’ criteria, cells not associated with the putative endodermal cord provide almost as compelling a case for being yolk-rich.
Polar lobe embryos have been identified in Doushantuo fossils on the basis of external morphology [44], but Yin et al. [43] argue that this is unreliable owing to the connection of the neck being hard to detect and there being a dynamic change in lobe volume during development. Yin et al. assert that the key feature that is required for identification of polar lobes (uniquely bilaterian features) is the presence of a connection neck between the polar lobe and blastomere. However, the connecting neck is better interpreted as an artefact of taphonomy and diagenetic mineralization. This is caused partly by decay making cell boundaries indistinct, but diagenetic cements coalescing neighbouring cells (figure 3i) can also result in structures similar to a connection neck. In the light of our data, high-attenuation rims around cells are better interpreted as diagenetic crusts rather than cell membranes, meaning that their absence between some cells simply indicates the absence of a void rather than the absence of a membrane.
In the case of specimens proposed to exhibit polarity [14,31], the cells interpreted as micromeres and macromeres are equally well interpreted as belonging to a continuum of cell sizes and their distributions do not provide convincing evidence for cell polarity. In the specimens described as having duet cleavage, there is little difference in size between the proposed macromeres and micromeres and they are hard to distinguish from, for example, the rotational cleavage found in some sponges [45].
In addition to these supposed embryonic bilaterians, Vernanimalcula was described from specimens in thin section as a miniature bilaterian adult, preserved through mineral replication of cellular tissue layers at a subcellular level of resolution [12,13], or through diagenetic void-filling cements templating from an anatomical substrate [46]. Bengtson & Budd [17] argued against the cellular preservation of bilaterian anatomical features, interpreting them instead as artefacts of diagenetic mineralization; our findings bear out their inferences. Mineral fabrics in the figured specimens of Vernanimalcula [12,46] form layers of surface-normal crystals that exhibit botryoidal textures (see also [17]). These are indistinguishable from mineral fabrics that are demonstrably void filling in other taxa in the same biota (e.g. acritarchs, figure 2e,f). This strengthens the case that the published thin sections of Vernanimalcula represent organic substrates that contain heavily decayed tissue with several phases of diagenetic cements [9,17] and not bilaterian animals preserved to a subcellular level [12,13]. Indeed, the specimens that have been attributed to Vernanimalcula comprise part of a broader spectrum of material in the Doushantuo assemblage that exhibits multiple phases of diagenetic cement growing on strongly degraded organic substrates that do not otherwise replicate original biological structure.
5. Conclusions
Even the most remarkably preserved fossils in the Doushantuo biota preserve a complex mélange of mineral phases, which must be discriminated for accurate interpretation of the fossils. This study demonstrates that crystallography and, to a lesser extent, chemistry can be used to distinguish between preserved biological tissue and diagenetic void fill in the biota. As such they also have the potential to distinguish geology from biology within other fossil Lagerstätten and perhaps even between different biotas. Within the Doushantuo biota, critical reappraisal of the fossils in the light of these findings has important implications for our understanding of this assemblage as an archive of Ediacaran animal diversity. When diagenetic mineralization is taken into account, there is no convincing evidence for bilaterians in the assemblage.
Acknowledgements
We thank M. Rücklin and D. Murdock for assistance at the beamline. P. Orr and three anonymous reviewers provided constructive comments on the manuscript. J.A.C. and P.C.J.D. were supported by NERC standard grant NE/F00348X/1 to P.C.J.D.; C.W.T. was supported by a NERC studentship. Requests for materials should be addressed to john.cunningham@bristol.ac.uk or phil.donoghue@bristol. ac.uk.
References
- 1.Zhou C. M., Brasier M. D., Xue Y. S. 2001. Three-dimensional phosphatic preservation of giant acritarchs from the Terminal Proterozoic Doushantuo Formation in Guizhou and Hubei provinces, south China. Palaeontology 44, 1157–1178 10.1111/1475-4983.00219 (doi:10.1111/1475-4983.00219) [DOI] [Google Scholar]
- 2.Xiao S. H. 2004. New multicellular algal fossils and acritarchs in Doushantuo chert nodules (Neoproterozoic; Yangtze Gorges, south China). J. Paleontol. 78, 393–401 10.1666/0022-3360(2004)078%3C0393:NMAFAA%3E2.0.CO;2 (doi:10.1666/0022-3360(2004)078<0393:NMAFAA> 2.0.CO;2) [DOI] [Google Scholar]
- 3.Xiao S. H., Zhang Y., Knoll A. H. 1998. Three-dimensional preservation of algae and animal embryos in a Neoproterozoic phosphorite. Nature 391, 553–558 10.1038/35318 (doi:10.1038/35318) [DOI] [Google Scholar]
- 4.Xiao S. H., Hagadorn J. W., Zhou C. M., Yuan X. L. 2007. Rare helical spheroidal fossils from the Doushantuo Lagerstatte: Ediacaran animal embryos come of age. Geology 35, 115–118 10.1130/G23277A.1 (doi:10.1130/G23277A.1) [DOI] [Google Scholar]
- 5.Tang F., Chongyu Y., Bengtson S., Pengju L., Ziqiang W., Linzhi G. 2008. Octoradiate spiral organisms in the Ediacaran of South China. Acta Geol. Sin.-Engl. 82, 27–34 10.1111/j.1755-6724.2008.tb00321.x (doi:10.1111/j.1755-6724.2008.tb00321.x) [DOI] [Google Scholar]
- 6.Zhu M. Y., Gehling J. G., Xia S. H., Zhao Y. L., Droser M. L. 2008. Eight-armed Ediacara fossil preserved in contrasting taphonomic windows from China and Australia. Geology 36, 867–870 10.1130/G25203A.1 (doi:10.1130/G25203A.1) [DOI] [Google Scholar]
- 7.Hagadorn J. W., et al. 2006. Cellular and subcellular structure of Neoproterozoic animal embryos. Science 314, 291–294 10.1126/science.1133129 (doi:10.1126/science.1133129) [DOI] [PubMed] [Google Scholar]
- 8.Li C. W., Chen J. Y., Hua T. E. 1998. Precambrian sponges with cellular structures. Science 279, 879–882 10.1126/science.279.5352.879 (doi:10.1126/science.279.5352.879) [DOI] [PubMed] [Google Scholar]
- 9.Xiao S. H., Yuan X. L., Knoll A. H. 2000. Eumetazoan fossils in terminal Proterozoic phosphorites. Proc. Natl Acad. Sci. USA 97, 13 684–13 689 10.1073/pnas.250491697 (doi:10.1073/pnas.250491697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J. Y., Oliveri P., Li C. W., Zhou G. Q., Gao F., Hagadorn J. W., Peterson K. J., Davidson E. H. 2000. Precambrian animal diversity: putative phosphatized embryos from the Doushantuo formation of China. Proc. Natl Acad. Sci. USA 97, 4457–4462 10.1073/pnas.97.9.4457 (doi:10.1073/pnas.97.9.4457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J. Y., Oliveri P., Gao F., Dornbos S. Q., Li C. W., Bottjer D. J., Davidson E. H. 2002. Precambrian animal life: probable developmental and adult cnidarian forms from southwest China. Dev. Biol. 248, 182–196 10.1006/dbio.2002.0714 (doi:10.1006/dbio.2002.0714) [DOI] [PubMed] [Google Scholar]
- 12.Chen J. Y., Bottjer D. J., Oliveri P., Dornbos S. Q., Gao F., Ruffins S., Chi H., Li C. W., Davidson E. H. 2004. Small bilaterian fossils from 40 to 55 million years before the Cambrian. Science 305, 218–222 10.1126/science.10992131099213 (doi:10.1126/science.10992131099213) [DOI] [PubMed] [Google Scholar]
- 13.Chen J. Y., Oliveri P., Davidson E., Bottjer D. J. 2004. Response to comment on ‘Small bilaterian fossils from 40 to 55 million years before the Cambrian’. Science 306, 1291. 10.1126/science.1102328 (doi:10.1126/science.1102328) [DOI] [PubMed] [Google Scholar]
- 14.Chen J. Y., et al. 2009. Complex embryos displaying bilaterian characters from Precambrian Doushantuo phosphate deposits, Weng'an, Guizhou, China. Proc. Natl Acad. Sci. USA 106, 19 056–19 060 10.1073/pnas.0904805106 (doi:10.1073/pnas.0904805106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conway Morris S. 2003. The Cambrian ‘explosion’ of metazoans and molecular biology: would Darwin be satisified? Int. J. Dev. Biol. 47, 505–515 [PubMed] [Google Scholar]
- 16.Bengtson S. 2003. Tracing metazoan roots in the fossil record. In The new panorama of animal evolution (eds Sfenthourakis S., Polymeni R., Thessalou-Legaki M.), pp. 289–300 Moscow, Russia: Pensoft [Google Scholar]
- 17.Bengtson S., Budd G. 2004. Comment on ‘Small bilaterian fossils from 40 to 55 million years before the Cambrian’. Science 306, 1291. 10.1126/science.1101338 (doi:10.1126/science.1101338) [DOI] [PubMed] [Google Scholar]
- 18.Conway Morris S. 2006. Darwin's dilemma: the realities of the Cambrian ‘explosion’. Phil. Trans. R. Soc. B 361, 1069–1083 10.1098/rstb.2006.1846 (doi:10.1098/rstb.2006.1846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donoghue P. C. J., et al. 2006. Synchrotron X-ray tomographic microscopy of fossil embryos. Nature 442, 680–683 10.1038/nature04890 (doi:10.1038/nature04890) [DOI] [PubMed] [Google Scholar]
- 20.Benton M. J., Donoghue P. C. J., Asher R. J. 2009. Calibrating and constraining the molecular clock. In Dating the tree of life (ed. Hedges B., Kumar S.), pp. 35–86 Oxford, UK: Oxford University Press [Google Scholar]
- 21.Xiao S. H., Knoll A. H. 1999. Fossil preservation in the Neoproterozoic Doushantuo phosphorite Lagerstätte, South China. Lethaia 32, 219–240 10.1111/j.1502-3931.1999.tb00541.x (doi:10.1111/j.1502-3931.1999.tb00541.x) [DOI] [PubMed] [Google Scholar]
- 22.Stampanoni M., et al. 2006. Trends in synchrotron-based tomographic imaging: the SLS experience. Dev. X-Ray Tomogr. V 6318, 63180M. 10.1117/12.679497 (doi:10.1117/12.679497) [DOI] [Google Scholar]
- 23.Hubert B., Alvaro J. J., Chen J. Y. 2005. Microbially mediated phosphatization in the Neoproterozoic Doushantuo Lagerstätte, South China. Bull. Soc. Geol. France 176, 355–361 10.2113/176.4.355 (doi:10.2113/176.4.355) [DOI] [Google Scholar]
- 24.Komiya T., Suga A., Ohno T., Han J., Guo J. F., Yamamoto S., Hirata T., Li Y. 2008. Ca isotopic compositions of dolomite, phosphorite and the oldest animal embryo fossils from the Neoproterozoic in Weng'an, South China. Gondwana Res. 14, 209–218 10.1016/j.gr.2007.10.004 (doi:10.1016/j.gr.2007.10.004) [DOI] [Google Scholar]
- 25.Yin C. Y., Bengtson S., Yue Z. 2004. Silicified and phosphatized Tianzhushania, spheroidal microfossils of possible animal origin from the Neoproterozoic of South China. Acta Palaeontol. Pol. 49, 1–12 [Google Scholar]
- 26.Yin L. M., Zhu M. Y., Knoll A. H., Yuan X. L., Zhang J. M., Hu J. 2007. Doushantuo embryos preserved inside diapause egg cysts. Nature 446, 661–663 10.1038/nature05682 (doi:10.1038/nature05682) [DOI] [PubMed] [Google Scholar]
- 27.Xiao S. H., Zhou C. M., Yuan X. L. 2007. Undressing and redressing Ediacaran embryos. Nature 446, E9–E10 10.1038/nature05753 (doi:10.1038/nature05753) [DOI] [PubMed] [Google Scholar]
- 28.Xue Y.-S., Tang T.-F., Yu C.-l., Zhou C.-M. 1995. Large spheroidal Chlorophyta fossils from Doushantuo Formation phosphoritic sequence (Late Sinian), central Guizhou, South China. Acta Palaeontologica Sinica 34, 688–706 In Chinese. [Google Scholar]
- 29.Huldtgren T., Donoghue P. C. J., Cunningham J. A., Yin C., Stampanoni M., Marone F., Bengtson S. 2011. Fossilized nuclei and germination structures identify Ediacaran ‘animal embryos’ as encysting protists. Science 334, 1696–1699 10.1126/science.1209537 (doi:10.1126/science.1209537) [DOI] [PubMed] [Google Scholar]
- 30.Xiao S. H., Knoll A. H. 2000. Phosphatized animal embryos from the Neoproterozoic Doushantuo Formation at Weng'an, Guizhou, South China. J. Paleontol. 74, 767–788 10.1666/0022-3360(2000)074%3C0767:PAEFTN%3E2.0.CO;2 (doi:10.1666/0022-3360 (2000)074<0767:PAEFTN>2.0.CO;2) [DOI] [Google Scholar]
- 31.Chen J. Y., et al. 2009. Phase contrast synchrotron X-ray microtomography of Ediacaran (Doushantuo) metazoan microfossils: phylogenetic diversity and evolutionary implications. Precambrian Res. 173, 191–200 10.1016/j.precamres.2009.04.004 (doi:10.1016/j.precamres.2009.04.004) [DOI] [Google Scholar]
- 32.Bailey J. V., Joye S. B., Kalanetra K. M., Flood B. E., Corsetti F. A. 2007. Evidence of giant sulphur bacteria in Neoproterozoic phosphorites. Nature 445, 198–201 10.1038/nature05457 (doi:10.1038/nature05457) [DOI] [PubMed] [Google Scholar]
- 33.Cunningham J. A., et al. 2012. Experimental taphonomy of giant sulphur bacteria: implications for the interpretation of the embryo-like Ediacaran Doushantuo fossils. Proc. R. Soc. B 279, 1857–1864 10.1098/rspb.2011.2064 (doi:10.1098/rspb.2011.2064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raff E. C., Villinski J. T., Turner F. R., Donoghue P. C. J., Raff R. A. 2006. Experimental taphonomy shows the feasibility of fossil embryos. Proc. Natl Acad. Sci. USA 103, 5846–5851 10.1073/pnas.0601536103 (doi:10.1073/pnas.0601536103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao S., Schiffbauer J. D. 2009. Microfossil phosphatization and its astrobiological implications. In From fossils to astrobiology (eds Seckbach J., Walsh M.), pp. 89–117 Berlin, Germany: Springer Science [Google Scholar]
- 36.Schiffbauer J. D., Xiao S., Sharma K. S., Wang G. In press The origin of intracellular structures in Ediacaran metazoan embryos. Geology (doi:10.1130/G32546.1) [Google Scholar]
- 37.Raff E. C., et al. 2008. Embryo fossilization is a biological process mediated by microbial biofilms. Proc. Natl Acad. Sci. USA 105, 19 360–19 365 10.1073/pnas.0810106105 (doi:10.1073/pnas.0810106105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brasier M. D. 2009. Darwin's lost world: the hidden history of animal life. Oxford, UK: Oxford University Press [Google Scholar]
- 39.Gostling N. J., et al. 2008. Deciphering the fossil record of early bilaterian embryonic development in light of experimental taphonomy. Evol. Dev. 10, 339–349 10.1111/j.1525-142X.2008.00242.x (doi:10.1111/j.1525-142X.2008.00242.x) [DOI] [PubMed] [Google Scholar]
- 40.Francis S., Margulis L., Barghoorn E. S. 1978. On the experimetal silicification of microorganisms. II. On the time of appearance of eukaryotic organisms in the fossil record. Precambrian Res. 6, 65–100 10.1016/0301-9268(78)90055-4 (doi:10.1016/0301-9268(78)90055-4) [DOI] [Google Scholar]
- 41.Xiao S. H. 2002. Mitotic topologies and mechanics of Neoproterozoic algae and animal embryos. Paleobiology 28, 244–250 (doi:10.1666/0094-8373(2002)028<0244:MTAMON>2.0.CO;2) [DOI] [Google Scholar]
- 42.Donoghue P. C. J., Purnell M. A. 2009. Distinguishing heat from light in debate over controversial fossils. BioEssays 31, 178–189 10.1002/bies.200800128 (doi:10.1002/bies.200800128) [DOI] [PubMed] [Google Scholar]
- 43.Yin Z., Zhu M., Tafforeau P., Chen J.-Y., Liu P., Li G. In press Early embryogenesis of potential bilaterian animals with polar lobe formation from the Ediacaran Weng'an Biota, South China. Precambrian Res. (doi:10.1016/j.precamres.2011.08.011) [Google Scholar]
- 44.Chen J. Y., et al. 2006. Phosphatized polar lobe-forming embryos from the Precambrian of southwest China. Science 312, 1644–1646 10.1126/science.1125964 (doi:10.1126/science.1125964) [DOI] [PubMed] [Google Scholar]
- 45.Leys S. P., Cheung E., Boury-Esnault N. 2006. Embryogenesis in the glass sponge Oopsacas minuta: formation of syncytia by fusion of blastomeres. Integr. Comp. Biol. 46, 104–117 10.1093/icb/icj016 (doi:10.1093/icb/icj016) [DOI] [PubMed] [Google Scholar]
- 46.Petryshyn V. A., Bottjer D. J., Chen J.-Y., Gao F. In press Petrographic analysis of new specimens of the putative microfossil Vernanimalcula guizhouena (Doushantuo Formation, South China). Precambrian Res. 10.1016/j.precamres.2011.08.003 (doi:10.1016/j.precamres.2011.08.003) [DOI] [Google Scholar]