Abstract
During human ontogeny, testosterone has powerful organizational and activational effects on the male organism. This has led to the hypothesis that the prenatal environment (as studied through the second-to-fourth digit ratio, 2D : 4D) is not only associated with robust adult male faces that are perceived as dominant and masculine, but also that there is an activational step during puberty. To test the latter, we collected digit ratios and frontal photographs of right-handed Caucasian boys (aged 4–11 years) along with age, body height and body weight. Using geometric morphometrics, we show a significant relationship between facial shape and 2D : 4D before the onset of puberty (explaining 14.5% of shape variation; p = 0.014 after 10 000 permutations, n = 17). Regression analyses depict the same shape patterns as in adults, namely that the lower the 2D : 4D, the smaller and shorter the forehead, the thicker the eyebrows, the wider and shorter the nose, and the larger the lower face. Our findings add to previous evidence that certain adult male facial characteristics that elicit attributions of masculinity and dominance are determined very early in ontogeny. This has implications for future studies in various fields ranging from social perception to life-history strategies.
Keywords: children, digit ratio, dominance, facial shape, geometric morphometrics, testosterone
1. Introduction
Human second-to-fourth digit ratio (2D : 4D)—the relative length of index to ring finger—and facial shape are covered separately in a rapidly increasing number of research articles (the 2D : 4D ratio alone was the topic of at least 306 publications until early 2009 [1], and is now covered in more than 60 articles per year [2]). This popularity might reflect their association with manifold traits, ranging from sex and gender to physical qualities, appearance and behaviour. Yet their direct link has so far been studied in adults only [3–5]. This is the first study to expand the evidence towards children.
(a). Second-to-fourth digit ratio: a proxy for prenatal testosterone
The Homeobox genes Hox a and d play an important role in the differentiation of the vertebrate urogenital system and also control digit development [6]. The postnatal 2D : 4D ratio correlates negatively with high levels of foetal testosterone (in relation to foetal oestradiol levels) in the earlier measured amniotic fluid [7,8]. Accordingly, the ratio serves as a retrospective marker of the level of circulating androgens in utero, along with the individual's sensitivity to these hormones [9]. Further lines of evidence, such as sexual dimorphism and specific diseases, support a negative association of 2D : 4D with prenatal testosterone exposure (summarized by Hönekopp & Thierfelder [10] and McIntyre [11]; but see Berenbaum et al. [12]). Even though this ratio increases with age, the rank order of 2D : 4D remains relatively stable until early adulthood [13,14].
(b). Prenatal and postnatal testosterone and adult facial shape
Male postnatal testosterone levels decrease until they have the same range as female plasma concentrations at two to three weeks of age [15]. Despite a secretory peak at one to three months in male infants, testosterone levels then remain equally low—actually lowest in life—in boys and girls until puberty. After the onset of puberty (at 8.5–13 years in girls [16]; at 9.5–13.5 years in boys [17]), the sex difference increases rapidly owing to a steep rise of testosterone levels in male adolescents. This rise contributes to the extended body and facial growth in males [18] and adult sexual dimorphism [19,20]. Yet adult circulating and prenatal testosterone seem to operate differently on male facial shape [5], and both of them differ from adult sexual dimorphism in faces [3,4].
Early androgen action (as estimated by 2D : 4D) seems to increase the contrast between upper and lower face in adults (with a prominent jaw, ‘robusticity’ [4,21]), whereas high adult circulating testosterone is associated with a uniform elongation of the face [5,21]. The lack of correlation between 2D : 4D and adult salivary testosterone was recently published for men and women [22,23]. The relationship between 2D : 4D and testosterone, however, is mediated by individual androgen sensitivity (cf. [24] for androgen receptor gene variation in relation to 2D : 4D).
Although the digit ratio is correlated with adult facial shape of both sexes by similar patterns, the effect was found to be about three times greater in men than in women, and non-significant for the latter [4]. Using a morphometrically more limited approach, however, Burriss et al. [3] found no association between male 2D : 4D and four facial measures, but confirmed the negative correlation of the ratio with nose width in females, as reported by Fink et al. [4].
(c). Second-to-fourth digit ratio and facial shape in boys: hypotheses and predictions
Neave et al. [25] hypothesized that high intra-uterine testosterone levels have an ‘organizational’ effect on male facial features: this effect is activated during puberty and results in masculine and dominant characteristics in adulthood. In the current work, we tested this hypothesis by studying the faces of male children. No association of their facial shape with 2D : 4D (as a proxy for prenatal testosterone) would support the notion of this activation at puberty at the earliest. Conversely, a significant link between 2D : 4D and facial shape would weaken the argument of a puberty impact and modify the hypothesis. Then, the same shape patterns as in adults (described by Fink et al. [4]) would be expected.
2. Material and methods
We measured the length of the second and the fourth digit of the right hand of boys, took standardized frontal photographs and recorded age, body height and weight.
(a). Participants
The final dataset comprised Caucasian boys aged 4–11 years (7.8 ± 2.2) from Upper Austria. The sample was distributed equally over this age range (age groups: 3–5, 6–8 and 9–11 years; χ² = 2.235, d.f. = 2, p = 0.327, n = 17). All 17 boys were right-handed and their frontal photographs met the standardization criteria listed below. Only participants without previous injuries to the fingers of their right hand were included. All children joined the study voluntarily and their parents signed a declaration of consent.
(b). Second-to-fourth digit ratio
The length of the second and fourth digit of the right hand was measured directly with a vernier calliper, from the ventral-most proximal crease to the tip of the gently stretched finger. Each length was measured three times. The ratio between the mean lengths of the second and the fourth digit was then calculated for each person.
(c). Facial photographs and measurement points
Frontal photographs were taken with a digital reflex camera (Canon EOS 300D) and a 116 mm lens. To standardize the photographs, all children were advised to look straight into the camera with neutral facial expression, to remove their glasses or any facial adornment and to tie their hair back. The camera was positioned at eye height 3.5 m away from the face. Studio lights helped standardize the light conditions. The children's heads were adjusted according to the Frankfort Horizontal Plane [26]. Photographs not meeting the standardization criteria (e.g. with laterally turned or smiling faces) were excluded.
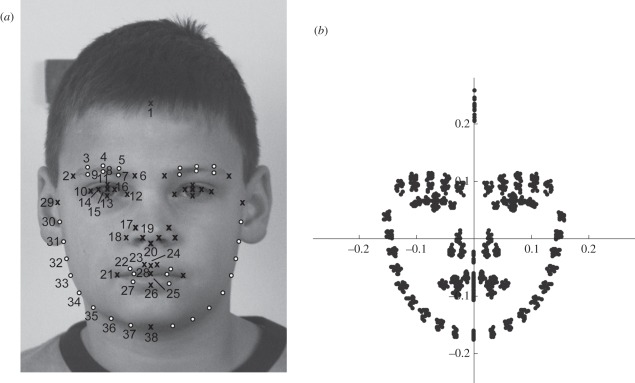
A set of 70 soft tissue measurement points (somatometric landmarks and semilandmarks) were manually digitized as two-dimensional coordinates in tpsDig2 [27] to capture facial shape (figure 1a). We used the landmark scheme by Windhager et al. [28] except for one landmark: the so-called lower lip point was not fixed in the present study, but allowed to slide between cheilon andl labrale inferius.
Figure 1.
(a) Facial measurement points and (b) resulting shape coordinates. (a) The set of 70 predefined landmarks (digitized in tpsDig2 [27]) on two-dimensional photographs of frontal faces. All X symbolize fixed somatometric landmarks, while white circles mark sliding semilandmarks on curves. (b) Landmark configurations after Procrustes superimposition, used for statistical shape analyses.
Semilandmarks are measurement points that have no morphologically defined exact position (white circles in figure 1a). They were digitized equally spaced along curves that themselves are homologous among individuals (e.g. the jaw line). Semilandmarks are allowed to slide between the adjacent landmarks so as to minimize overall bending energy before being projected back to the curve [29,30]. After sliding, they are treated like fixed somatometric landmarks in subsequent analyses.
(d). Shape analysis
As a slight turning of the face (to the left or right) is sometimes inevitable when working with young children, we first ‘symmetrized’ the faces to minimize the effect of head turning on face shape. Symmetrically slid semilandmarks were computed by sliding each face against the average symmetric shape [31]: the digitized landmark configurations were aligned in a generalized Procrustes superimposition [32], then the average shape was computed (by averaging all x and y coordinates landmark by landmark) and ‘symmetrized’ [30]. A landmark configuration is symmetrized by reflecting it, relabelling the landmarks of the reflection (so that, for example, the left alae origin in the reflection gets the same landmark number as in the original) and computing the average between original and reflection after another Procrustes superimposition. After sliding the semilandmarks to this average symmetric shape, we also symmetrized the individual faces.
The resulting shape coordinates (figure 1b) were then subjected to principal component analysis (PCA; termed relative warp analysis for shape coordinates) to assess the shape variation in the sample (electronic supplementary material, figure S1). A shape regression (a multivariate regression of shape coordinates onto an independent variable) was used to test the association of facial shape and digit ratio. A Monte Carlo permutation test [33] was used as the test statistic. The results of significant (p ≤ 0.05) shape regressions were visualized by thin-plate spline (TPS) deformation grids [34]. These depict shape changes from the average configuration to faces that are predicted for higher and lower digit ratios. Outline landmarks were connected with cubic splines to ease interpretation. The specific facial configurations were also visualized using the original photographs. For this, the photograph of each boy was ‘unwarped’ to the predicted configuration, so that the originally digitized landmarks then coincided with those of the target configuration. These unwarped pictures were averaged pixel by pixel (in tpsSuper v. 1.14 [35]), yielding a single picture for each configuration. Finally, a separate figure shows the mean and predicted facial configurations superimposed to render shape differences more visible. Outline landmarks were again connected with cubic splines.
Analyses and visualizations were carried out in Mathematica v. 6 (symmetrizing, TPS grids, superimposed configurations), S+ (symmetric sliding), tpsRelw v. 1.46 [36] (relative warps analysis), tpsRegr v. 1.36 [37] (shape regressions), tpsSuper v. 1.14 [35] (image unwarping and averaging) and SPSS v. 15 (bivariate correlations, relative warp plots). Figures were edited with Adobe Illustrator CS3.
3. Results
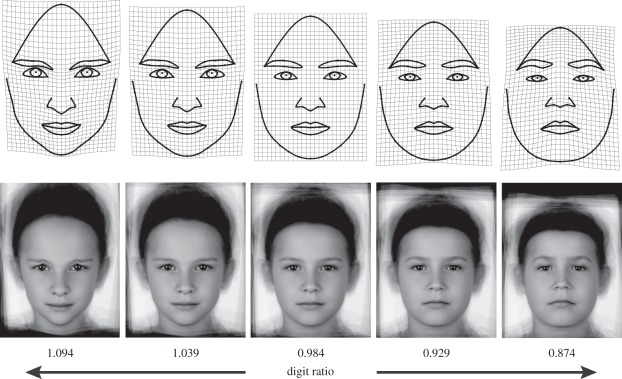
The second-to-fourth digit ratios of the 17 boys ranged from 0.91 to 1.01 (0.98 ± 0.03). The shape regression (i.e. the association of facial shape with the digit ratio) explained 14.5 per cent of shape variation in our sample and is statistically significant (p = 0.0139 after 10 000 permutations). Facial shapes associated with high and low digit ratios are visualized as shape changes from the sample average (figure 2). Size differences in the local deformations are relative to other regions. This is because absolute size (i.e. centroid size) has been standardized during Procrustes superimposition. Facial shapes associated with high digit ratios (figure 2, left panels) are characterized by a relatively large forehead, long and slim eyebrows, comparably large eyes with a round shape of the visible iris, and a relatively long distance of the eyes to the alae origins. The deformation grid in the nose area indicates a vertical stretching of the nose as well as a relatively short distance to the mouth. The cheeks are narrower, the jaw outline is less broad and the chin more pointed than predicted for faces corresponding with boys with lower digit ratios (figure 2 right panels). These faces, in contrast, have a relatively smaller and shorter forehead. The eyebrows are thicker and further apart from the smaller eyes. The distance between the eyes is comparably large. The flaring parts of the nostrils are relatively wide and short. The grids point to a prominent lower jaw that is laterally and ventrally extended compared with boys with higher digit ratios. The shape differences become especially clear when all five configurations of figure 2 (±4 s.d. of digit ratio, ±2 s.d. and average) are plotted superimposed (i.e. in the same coordinate system; figure 3). The forehead and the lower face apparently bear the largest signal associated with 2D : 4D. Procrustes superimposed raw coordinates (with semilandmarks slid towards the non-symmetrized sample average) yielded the same results for the shape regression upon 2D : 4D.
Figure 2.
Visualization of the shape regression upon 2D : 4D ratio in boys' faces. While the upper panels show thin-plate spline deformation grids from the sample average to predicted facial shapes for several digit ratios, the lower panels visualize the same facial shapes through image unwarping and image averaging. The middle column (with the undeformed grid in upper row) corresponds with the average landmark configuration and the average digit ratio for boys. The faces immediately left and right of the central face show +2 s.d. and −2 s.d., respectively, and the faces at far left and far right show +4 s.d. and −4 s.d., respectively, compared with the average 2D : 4D ratio. Ratios higher than the average 1.09 (+4 s.d.) and 1.04 (+2 s.d.), the faces on the right to lower ones 0.93 (−2 s.d.) and 0.87 (−4 s.d.). Digit ratio accounted for 14.5% of the shape variation. Note that values ± 4 s.d. are outside the observed range.
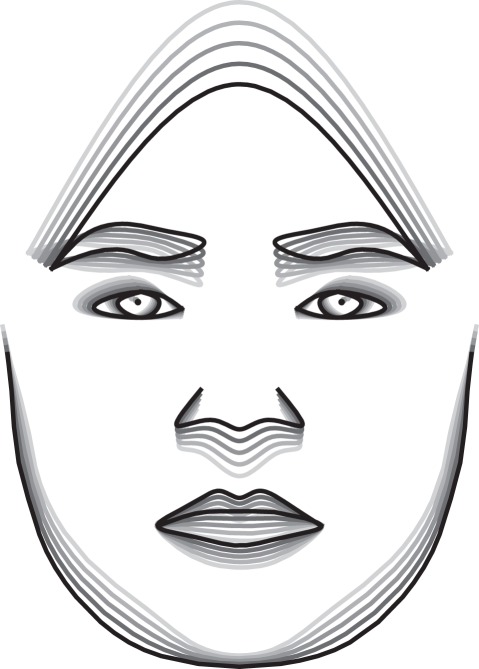
Figure 3.
Superimposed facial shape estimates for various digit ratios (−4 s.d., −2 s.d., average, +2 s.d., +4 s.d.) in boys (n = 17). The darker the line, the smaller the corresponding digit ratio.
To test if body height, body weight or age mediated the observed relationship between anatomical traits and digit ratio, we correlated these measures with the 2D : 4D ratio. There was no significant correlation between 2D : 4D ratio and age (r = −0.068, p = 0.795, n = 17), body mass index (r = 0.277, p = 0.281, n = 17), body height (r = −0.295, p = 0.251, n = 17) or body weight (r = −0.329, p = 0.198, n = 17). Also, the depicted association between facial shape and digit ratio (figure 2) was found to persist when regression vectors for body mass index, body height and body weight were projected out [38–40] individually.
4. Discussion
Our results show a clear association between the second-to-fourth digit ratio and facial shape in boys as young as 4–11 years old. The observed shape pattern mirrors previous results for adult men [4,5].
It cannot be definitively ruled out that any of the boys had entered puberty. Nonetheless, as the digit ratio was uncorrelated with age in our sample, this does not alter the validity of our main conclusion: the well-reported facial robustness associated with low 2D : 4D (i.e. high prenatal testosterone exposure) can be observed years before puberty. This supports the speculation of Neave et al. [25] that there is an ‘organizational’ relationship between (i) facial dominance and masculinity, and (ii) the prenatal hormonal environment, but our finding also shows a prepubertal onset of its activation. Another hint at the more direct pathway is that the facial shape pattern of adult salivary testosterone differs from the one of 2D : 4D in men with the shape pattern for perceived masculinity/dominance closely resembling the latter [5]. Moreover, Koehler et al. [41] could not identify any significant relationship between 2D : 4D and perceived body and facial masculinity or testes volume in adult men. The lack of correlation between prenatal and actual testosterone levels was true both for facial shape and, for example, display behaviour [42]. Finally, Bulygina et al. [43] concluded, from their analysis of the Denver Growth Study's radiographs, that the cranial shape of three-year-olds is highly correlated with the individual adult shape. Accordingly, the adult pattern of interindividual difference might already be established within the first few years of life.
Of course, this does not preclude that much facial shape change results from male adolescent hormone secretion and longer growth in men. Note that castration before or after puberty also has different effects on the male organism [44]. Despite the considerable increase in sexual dimorphism after puberty (including secondary sexual characteristics, such as beards and brows [45,46]), our results show that intermale facial shape variation owing to differences in prenatal testosterone exposure is already present in childhood. One description might be that ‘the effects of [postnatal or adult] circulating hormones are superimposed on changes induced prenatally’ ([47], p. 49). Future studies might, however, compare the magnitude of shape difference associated with 2D : 4D before and after puberty to clarify the role of sexual maturity. Likewise, dissecting causation and mediation of variables associated with the relationship between facial shape and 2D : 4D would be an important direction for future research. Such an approach might provide valuable clues to the mechanism behind prenatal testosterone's effects on face shape in children and in adults.
Males are more variable than females in a variety of traits, presumably because the former exhibit a greater range of context-dependent reproductive strategies [48]. This greater variability includes 2D : 4D [49], determined in a very narrow window during ontogeny [8]. The foetal testosterone level, in turn, is positively correlated with foetal cortisol, maternal cortisol and maternal testosterone [50]. This relates to the ‘maternal dominance hypothesis’ and empirical work of Valerie Grant and colleagues, who suggested that dominant female primates (including humans) produce more male offspring: the high testosterone levels of these females apparently play a role [51,52]. Taken together, the variation in male digit ratio might thus reflect a preparation for different life-history strategies depending on social status and environmental context (e.g. chronic stress). Specifically, we hypothesize dominant and/or stressed mothers to have children with higher prenatal testosterone exposure. Such children might behave more competitively from childhood onwards. Y chromosome-linked factors, however, might also contribute to family resemblance in digit ratio [53].
Consequently, we would expect that not only the facial correlates of prenatal testosterone (as approximated through 2D : 4D) are highly similar between children and adults, but also physical correlates, trait attribution and social interactions. The number of studies explicitly testing these associations in children is rare, but boys with lower digit ratios sprint somewhat faster when other factors, such as age, body mass index and maturity, are controlled (r = 0.15) [54]. Another helpful source is the research on the social consequences of babyish facial features. Our facial shape pattern associated with low prenatal testosterone exposure (high 2D : 4D values) closely resembles baby schema simulations in infant faces (as in Glocker et al. [55]). Follow-up studies will show whether boys with higher digit ratios are also perceived as cuter, less strong, less alert and less intelligent than less ‘baby-faced’ children [56]. Thus, they might also receive more help and more babyish talk [57,58], of course mediated by the age of the recipient. Children with low digit ratios, in contrast, might be regarded as more masculine and dominant. Furthermore, physical correlates with 2D : 4D found in adult men (such as physical fitness and competitive ability [59]) might also already be present in children and help to acquire resources in a peer or sibling context. High dispositional dominance in the form of a low 2D : 4D seems ultimately positively related to male reproductive success [60]. It remains to be investigated how hormonal predisposition interacts with differential social treatment in shaping children's behaviour. A dominant mother might, for example, be more interactive with and tougher on her offspring [61]. Altogether, the link between children's facial features and 2D : 4D raises a novel set of questions: are boys with high prenatal testosterone exposure already perceived as more dominant and masculine by children and adults? Do they acquire more resources in competition with peers, and what are their strategies? To what extent does this model apply to girls?
Acknowledgements
We thank Fred L. Bookstein, Philipp Gunz and Philipp Mitteroecker for computations and Mathematica code, Michael Stachowitsch and two anonymous reviewers for valuable comments, as well as all the children, parents and teachers for their participation.
References
- 1.Voracek M., Loibl L. M. 2009. Scientometric analysis and bibliography of digit ratio (2D : 4D) research, 1998–2008. Psychol. Rep. 104, 922–956 10.2466/PR0.104.3.922-956 (doi:10.2466/PR0.104.3.922-956) [DOI] [PubMed] [Google Scholar]
- 2.Manning J. T. 2011. Resolving the role of prenatal sex steroids in the development of digit ratio. Proc. Natl Acad. Sci. USA 108, 16 143–16 144 10.1073/pnas.1113312108 (doi:10.1073/pnas.1113312108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burriss R. P., Little A. C., Nelson E. C. 2007. 2D : 4D and sexually dimorphic facial characteristics . Arch. Sex. Behav. 36, 377–384 10.1007/s10508-006-9136-1 (doi:10.1007/s10508-006-9136-1) [DOI] [PubMed] [Google Scholar]
- 4.Fink B., Grammer K., Mitteroecker P., Gunz P., Schaefer K., Bookstein F. L., Manning J. T. 2005. Second to fourth digit ratio and face shape. Proc. R. Soc. B 272, 1995–2001 10.1098/rspb.2005.3179 (doi:10.1098/rspb.2005.3179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaefer K., Mitteroecker P., Fink B., Bookstein F. L. 2009. Psychomorphospace—from biology to perception, and back: towards an integrated quantification of facial form variation. Biol. Theory 4, 98–106 10.1162/biot.2009.4.1.98 (doi:10.1162/biot.2009.4.1.98) [DOI] [Google Scholar]
- 6.Kondo T., Zakany J., Innis J., Duboule D. 1997. Of fingers, toes and penises. Nature 390, 29. 10.1038/36234 (doi:10.1038/36234) [DOI] [PubMed] [Google Scholar]
- 7.Lutchmaya S., Baron-Cohen S., Raggatt P., Knickmeyer R., Manning J. T. 2004. 2nd to 4th digit ratios, fetal testosterone and estradiol. Early Hum. Dev. 77, 23–28 10.1016/j.earlhumdev.2003.12.002 (doi:10.1016/j.earlhumdev.2003.12.002) [DOI] [PubMed] [Google Scholar]
- 8.Zheng Z., Cohn M. J. 2011. Developmental basis of sexually dimorphic digit ratios. Proc. Natl Acad. Sci. USA 108, 16 289–16 294 10.1073/pnas.1108312108 (doi:10.1073/pnas.1108312108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breedlove S. M. 2010. Minireview: organizational hypothesis: instances of the fingerpost. Endocrinology 151, 4116–4122 10.1210/en.2010-0041 (doi:10.1210/en.2010-0041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hönekopp J., Thierfelder C. 2009. Relationships between digit ratio (2D : 4D) and sex-typed play behavior in pre-school children. Pers. Individ. Differ. 47, 706–710 10.1016/j.paid.2009.06.007 (doi:10.1016/j.paid.2009.06.007) [DOI] [Google Scholar]
- 11.McIntyre M. H. 2006. The use of digit ratios as markers for perinatal androgen action. Reprod. Biol. Endocrinol. 4, 10. 10.1186/1477-7827-4-10 (doi:10.1186/1477-7827-4-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berenbaum S. A., Bryk K. K., Nowak N., Quigley C. A., Moffat S. 2009. Fingers as a marker of prenatal androgen exposure. Endocrinology 150, 5119–5124 10.1210/en.2009-0774 (doi:10.1210/en.2009-0774) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trivers R., Manning J., Jacobson A. 2006. A longitudinal study of digit ratio (2D : 4D) and other finger ratios in Jamaican children. Horm. Behav. 49, 150–156 10.1016/j.yhbeh.2005.05.023 (doi:10.1016/j.yhbeh.2005.05.023) [DOI] [PubMed] [Google Scholar]
- 14.McIntyre M. H., Ellison P. T., Lieberman D. E., Demerarth E., Towne B. 2005. The development of sex differences in digital formula from infancy in the Fels Longitudinal Study. Proc. R. Soc. B 272, 1473–1479 10.1098/rspb.2005.3100 (doi:10.1098/rspb.2005.3100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forest M. G. 1979. Plasma androgens (testosterone and 4-androstenedione) and 17-hydroxyprogesterone in the neonatal, prepubertal and peripubertal periods in the human and the rat: differences between species. J. Steroid. Biochem. 11, 543–548 10.1016/0022-4731(79)90080-3 (doi:10.1016/0022-4731(79)90080-3) [DOI] [PubMed] [Google Scholar]
- 16.Marshall W. A., Tanner J. M. 1969. Variations in the pattern of pubertal changes in girls. Arch. Dis. Child. 44, 291–303 10.1136/adc.44.235.291 (doi:10.1136/adc.44.235.291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall W. A., Tanner J. M. 1970. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 45, 13–23 10.1136/adc.45.239.13 (doi:10.1136/adc.45.239.13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verdonck A., Gaethofs M., Carels C., de Zegher F. 1999. Effect of low-dose testosterone treatment on craniofacial growth in boys with delayed puberty. Eur. J. Orthod. 21, 137–143 10.1093/ejo/21.2.137 (doi:10.1093/ejo/21.2.137) [DOI] [PubMed] [Google Scholar]
- 19.Swaddle J. P., Reierson G. W. 2002. Testosterone increases perceived dominance but not attractiveness in human males. Proc. R. Soc. Lond. B 269, 2285–2289 10.1098/rspb.2002.2165 (doi:10.1098/rspb.2002.2165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thornhill R., Gangestad S. W. 2006. Facial sexual dimorphism, developmental stability, and susceptibility to disease in men and women. Evol. Hum. Behav. 27, 131–144 10.1016/j.evolhumbehav.2005.06.001 (doi:10.1016/j.evolhumbehav.2005.06.001) [DOI] [Google Scholar]
- 21.Schaefer K., Fink B., Mitteroecker P., Neave N., Bookstein F. L. 2005. Visualizing facial shape regression upon 2nd to 4th digit ratio and testosterone. Coll. Antropol. 29, 415–419 [PubMed] [Google Scholar]
- 22.McIntyre M. H., Li A. Y., Chapman J. F., Lipson S. F., Ellison P. T. 2011. Social status, masculinity, and testosterone in young men. Pers. Individ. Differ. 51, 392–396 10.1016/j.paid.2010.03.015 (doi:10.1016/j.paid.2010.03.015) [DOI] [Google Scholar]
- 23.Beaton A. A., Rudling N., Kissling C., Taurines R., Thome J. 2011. Digit ratio (2D : 4D), salivary testosterone, and handedness. Laterality 16, 136–155 10.1080/13576500903410369 (doi:10.1080/13576500903410369) [DOI] [PubMed] [Google Scholar]
- 24.Manning J. T., Bundred P. E., Newton D. J., Flanagan B. F. 2003. The second to fourth digit ratio and variation in the androgen receptor gene. Evol. Hum. Behav. 24, 399–405 10.1016/S1090-5138(03)00052-7 (doi:10.1016/S1090-5138(03)00052-7) [DOI] [Google Scholar]
- 25.Neave N., Laing S., Fink B., Manning J. T. 2003. Second to fourth digit ratio, testosterone and perceived male dominance . Proc. R. Soc. Lond. B 270, 2167–2172 10.1098/rspb.2003.2502 (doi:10.1098/rspb.2003.2502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farkas L. G. 1994. Examination. In Anthropometry of the head and face (ed. Farkas L. G.), pp. 3–56 New York, NY: Raven Press [Google Scholar]
- 27.Rohlf F. J. 2006. tpsDig2, v. 2.10. New York, NY: State University of New York at Stony Brook, Department of Ecology and Evolution [Google Scholar]
- 28.Windhager S., Schaefer K., Fink B. 2011. Geometric morphometrics of male facial shape in relation to physical strength and perceived attractiveness, dominance, and masculinity. Am. J. Hum. Biol. 23, 805–814 10.1002/ajhb.21219 (doi:10.1002/ajhb.21219) [DOI] [PubMed] [Google Scholar]
- 29.Bookstein F. L. 1997. Landmark methods for forms without landmarks: morphometrics of group differences in outline shape. Med. Image Anal. 1, 225–243 10.1016/S1361-8415(97)85012-8 (doi:10.1016/S1361-8415(97)85012-8) [DOI] [PubMed] [Google Scholar]
- 30.Mitteroecker P., Gunz P. 2009. Advances in geometric morphometrics. Evol. Biol. 36, 235–247 10.1007/s11692-009-9055-x (doi:10.1007/s11692-009-9055-x) [DOI] [Google Scholar]
- 31.Bookstein F. L. 2008. Semilandmarks and asymmetry. In Three handouts for the semilandmark track. Materials prepared for EVAN Morphometrics Summer School 2008, July Vienna, Austria: University of Vienna, Department of Anthropology [Google Scholar]
- 32.Rohlf F. J., Slice D. E. 1990. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst. Zool. 39, 40–59 10.2307/2992207 (doi:10.2307/2992207) [DOI] [Google Scholar]
- 33.Good P. I. 2000. Permutation tests: a practical guide to resampling methods for testing hypotheses. New York, NY: Springer [Google Scholar]
- 34.Bookstein F. L. 1991. Morphometric tools for landmark data: geometry and biology. Cambridge, UK: Cambridge University Press [Google Scholar]
- 35.Rohlf F. J. 2004. tpsSuper, superimposition and image averaging, v. 1.14. New York, NY: State University of New York at Stony Brook, Department of Ecology and Evolution [Google Scholar]
- 36.Rohlf F. J. 2008. tpsRelw, relative warps analysis, v. 1.46. New York, NY: State University of New York at Stony Brook, Department of Ecology and Evolution [Google Scholar]
- 37.Rohlf F. J. 2009. tpsRegr, shape regression, v. 1.36. New York, NY: State University of New York at Stony Brook, Department of Ecology and Evolution [Google Scholar]
- 38.Burnaby T. P. 1966. Growth-invariant discrimination functions and generalized distances. Biometrics 22, 96–110 10.2307/2528217 (doi:10.2307/2528217) [DOI] [Google Scholar]
- 39.Mitteroecker P., Gunz P., Bernhard M., Schaefer K., Bookstein F. L. 2004. Comparison of cranial ontogenetic trajectories among great apes and humans. J. Hum. Evol. 46, 679–698 10.1016/j.jhevol.2004.03.006 (doi:10.1016/j.jhevol.2004.03.006) [DOI] [PubMed] [Google Scholar]
- 40.Schaefer K., Fink B., Grammer K., Mitteroecker P., Gunz P., Bookstein F. L. 2006. Female appearance: facial and bodily attractiveness as shape. Psychology Science 48, 187–204 [Google Scholar]
- 41.Koehler N., Simmons L. W., Rhodes G. 2004. How well does second-to-fourth-digit ratio in hands correlate with other indications of masculinity in males? Proc. R. Soc. Lond. B 271, S296–S298 10.1098/rsbl.2004.0163 (doi:10.1098/rsbl.2004.0163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roney J., Maestripieri D. 2004. Relative digit lengths predict men's behavior and attractiveness during social interactions with women. Hum. Nat. 15, 271–282 10.1007/s12110-004-1009-5 (doi:10.1007/s12110-004-1009-5) [DOI] [PubMed] [Google Scholar]
- 43.Bulygina E., Mitteroecker P., Aiello L. 2006. Ontogeny of facial dimorphism and patterns of individual development within one human population. Am. J. Phys. Anthropol. 131, 432–443 10.1002/ajpa.20317 (doi:10.1002/ajpa.20317) [DOI] [PubMed] [Google Scholar]
- 44.Nelson R. J. 2005. An introduction to behavioral endocrinology. Sunderland, MA: Sinauer [Google Scholar]
- 45.Brook C. 1981. Endocrinological control of growth at puberty. Br. Med. Bull. 37, 281–285 [DOI] [PubMed] [Google Scholar]
- 46.Mazur A., Booth A. 1998. Testosterone and dominance in men. Behav. Brain Sci. 21, 353–397 [PubMed] [Google Scholar]
- 47.Baron-Cohn S., Lutchmaya S., Knickmeyer R. 2004. Prenatal testosterone in mind: amniotic fluid studies. Cambridge, MA: MIT Press [Google Scholar]
- 48.Lehre A.-C., Lehre K. P., Laake P., Danbolt N. C. 2009. Greater intrasex phenotype variability in males than in females is a fundamental aspect of the gender differences in humans. Dev. Psychobiol. 51, 198–206 10.1002/dev.20358 (doi:10.1002/dev.20358) [DOI] [PubMed] [Google Scholar]
- 49.Auyeung B., Baron-Cohen S., Ashwin E., Knickmeyer R., Taylor K., Hackett G., Hines M. 2009. Fetal testosterone predicts sexually differentiated childhood behavior in girls and in boys. Psychol. Sci. 20, 144–148 10.1111/j.1467-9280.2009.02279.x (doi:10.1111/j.1467-9280.2009.02279.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gitau R., Adams D., Fisk N. M., Glover V. 2005. Fetal plasma testosterone correlates positively with cortisol. Arch. Dis. Child. Fetal Neonatal Ed. 90, F166–F169 10.1136/adc.2004.049320 (doi:10.1136/adc.2004.049320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grant V. J., France J. T. 2001. Dominance and testosterone in women. Biol. Psychol. 58, 41–47 10.1016/S0301-0511(01)00100-4 (doi:10.1016/S0301-0511(01)00100-4) [DOI] [PubMed] [Google Scholar]
- 52.Grant V. J., Konecá M., Sonnweber R.-S., Irwin R. J., Wallner B. 2011. Macaque mothers’ preconception testosterone levels relate to dominance and to sex of offspring. Anim. Behav. 82, 893–899 10.1016/j.anbehav.2011.07.029 (doi:10.1016/j.anbehav.2011.07.029) [DOI] [Google Scholar]
- 53.Voracek M., Dressler S. 2009. Brief communication: familial resemblance in digit ratio (2D : 4D). Am. J. Phys. Anthropol. 140, 376–380 10.1002/ajpa.21105 (doi:10.1002/ajpa.21105) [DOI] [PubMed] [Google Scholar]
- 54.Manning J. T., Hill M. R. 2009. Digit ratio (2D : 4D) and sprinting speed in boys. Am. J. Hum. Biol. 21, 210–213 10.1002/ajhb.20855 (doi:10.1002/ajhb.20855) [DOI] [PubMed] [Google Scholar]
- 55.Glocker M. L., Langleben D. D., Ruparel K., Loughead J. W., Gur R. C., Sachser N. 2009. Baby schema in infant faces induces cuteness perception and motivation for caretaking in adults. Ethology 115, 257–263 10.1111/j.1439-0310.2008.01603.x (doi:10.1111/j.1439-0310.2008.01603.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montepare J. M., Zebrowitz L. A. 1998. Person perception comes of age: the salience and significance of age in social judgments. In Advances in experimental social psychology (ed. Zanna M. P.), pp. 93–163 New York, NY: Academic Press [Google Scholar]
- 57.Alley T. R. 1981. Head shape and the perception of cuteness. Dev. Psychol. 17, 650–654 10.1037/0012-1649.17.5.650 (doi:10.1037/0012-1649.17.5.650) [DOI] [Google Scholar]
- 58.Zebrowitz L. A., Brownlow S., Olson K. 1992. Baby talk to the babyfaced. J. Nonverbal Behav. 16, 143–158 10.1007/BF00988031 (doi:10.1007/BF00988031) [DOI] [Google Scholar]
- 59.Longman D., Stock J. T., Wells J. C. K. 2011. Digit ratio (2D : 4D) and rowing ergometer performance in males and females. Am. J. Phys. Anthropol. 144, 337–341 10.1002/ajpa.21407 (doi:10.1002/ajpa.21407) [DOI] [PubMed] [Google Scholar]
- 60.Manning J.T., Henzi P., Venkatramana P., Martin S., Singh D. 2003. Second to fourth digit ratio: ethnic differences and family size in English, Indian and South African populations. Ann. Hum. Biol. 30, 579–588 10.1080/0301446032000112689 (doi:10.1080/0301446032000112689) [DOI] [PubMed] [Google Scholar]
- 61.Grant V. J. 1998. Maternal personality, evolution and the sex ratio: do mothers control the sex of the infant? London, UK: Routledge [Google Scholar]