Abstract
Baleen whales (Mysticeti) communicate using low-frequency acoustic signals. These long-wavelength sounds can be detected over hundreds of kilometres, potentially allowing contact over large distances. Low-frequency noise from large ships (20–200 Hz) overlaps acoustic signals used by baleen whales, and increased levels of underwater noise have been documented in areas with high shipping traffic. Reported responses of whales to increased noise include: habitat displacement, behavioural changes and alterations in the intensity, frequency and intervals of calls. However, it has been unclear whether exposure to noise results in physiological responses that may lead to significant consequences for individuals or populations. Here, we show that reduced ship traffic in the Bay of Fundy, Canada, following the events of 11 September 2001, resulted in a 6 dB decrease in underwater noise with a significant reduction below 150 Hz. This noise reduction was associated with decreased baseline levels of stress-related faecal hormone metabolites (glucocorticoids) in North Atlantic right whales (Eubalaena glacialis). This is the first evidence that exposure to low-frequency ship noise may be associated with chronic stress in whales, and has implications for all baleen whales in heavy ship traffic areas, and for recovery of this endangered right whale population.
Keywords: right whales, glucocorticoids, stress, underwater noise, ship traffic
1. Introduction
Underwater ocean noise from anthropogenic sources has increased over the past 50 years [1,2]. This acoustic pollution is a by-product of a rising tide of human maritime activities including seismic exploration by the oil and gas industries, military and commercial use of sonar, recreational boating and shipping traffic. In many ocean areas, the dominant source of human-generated low-frequency noise (20–200 Hz) is from the propellers and engines of commercial shipping vessels, and noise levels have been increasing [2–4]. These sound frequencies can propagate efficiently over long distances in the deep-water marine environment. Studies monitoring trends of underwater noise in the Northeast Pacific found that since the 1960s, low-frequency ambient noise (less than 80 Hz) has increased by 10–12 decibels (dB), coinciding with a doubling of the global shipping fleet [5,6]. This rising level of noise has raised concerns about effects on marine mammals that rely on acoustic signalling [7–9]. In particular, shipping noise directly overlaps the frequency band of acoustic communication signals used by the largest of cetaceans, the baleen whales (Mysticeti) [10,11].
Living in an environment where sound propagates far better than light, many marine animals, particularly cetaceans, evolved to rely primarily upon acoustic signalling to communicate, locate prey and navigate [12]. The acoustic repertoire of baleen whales consists of low-frequency, long-wavelength sounds that propagate efficiently underwater, potentially allowing communication over large distances in the open ocean [10–12]. For example, data from the U. S. Navy's SOund SUrveillance System (SOSUS) has shown that blue whale (Balaenoptera musculus) calls can be detected offshore at ranges of hundreds of kilometres [13]. However, the range at which baleen whales actually communicate with each other remains unknown. Elevated low-frequency underwater noise levels near busy shipping routes and ports have the potential to interfere significantly with whale calls used to maintain contact, aggregate to feed and locate potential mates (‘acoustic masking’), potentially affecting critical life-history events [1,7–11]. Reported whale responses to increases in background noise have included: habitat displacement, behavioural changes and alterations in vocalization patterns such as shifting the frequency band or energy level of calls, making signals longer or more repetitive, or waiting to signal until the noise is reduced [8,9]. Owing to the challenges of studying free-swimming large whales, it is unknown whether these responses to background noise translate into biologically significant effects that may have long-term consequences for individuals or populations [7].
The tragic events of 11 September 2001 (9/11 hereafter) resulted in an unplanned experiment on the effects of underwater noise on western North Atlantic right whales (Eubalaena glacialis). These baleen whales congregate during late summer in the Bay of Fundy, Canada, to feed and nurse their calves. Since 1980, the New England Aquarium (Boston, MA, USA) has conducted longitudinal population surveys annually in this critical right whale habitat. In the immediate aftermath of 9/11, we noted a marked decrease in ship traffic in the Bay of Fundy, Canada, and acoustic recordings revealed a noticeable decrease in low-frequency background noise levels. A study of stress-related faecal hormone metabolites was also underway throughout the 2001 field season and over the four subsequent years. We analysed acoustic recordings and ship traffic data along with faecal glucocorticoid (fGC) measures of physiological stress before and after 9/11. Here, we show that a post-9/11 decrease in background underwater noise from reduced large ship traffic corresponded to a decrease in stress-related fGC hormone levels in right whales.
2. Material and methods
(a). Acoustic data
Acoustic data were collected in the Bay of Fundy in August and September 2001 for a project related to right whale social behaviour. Data were collected with a single factory-calibrated HTI-94-SSQ hydrophone with a built-in preamplifier recorded into a Sony TCD-D8 Digital Audio Tape (DAT) recorder with constant recording gain setting (system frequency response 50 Hz–20 kHz ± 1 dB). The hydrophone was deployed from the side of a small vessel (with the engine shut off) using a spar buoy to minimize vertical displacement.
We analysed 93 min of recordings collected from 2 days before 9/11 (25 and 29 August 2001) and 90 min of recordings from 2 days immediately following 9/11 (12 and 13 September 2001) all with the same sea-state conditions (Beaufort 1-2). Recordings collected during several individual recording sessions from each day were compiled and converted to .wav files with a sampling rate of 48 kHz. The records were then bandpass filtered to 50 Hz–20 kHz. Extraneous noises (e.g. splashing sounds and whale calls) were removed to select the quietest section of background noise in each recording. The DAT recorder gain was measured in the laboratory by recording known voltage signals directly into the recorder. The sound pressure level (SPL) at the hydrophone was calculated using the known sensitivity of the hydrophone, the hydrophone pre-amplification gain and the measured gain from the recorder to obtain the overall gain for the system. A custom Matlab program was used to calculate SPL in dBRMS re 1 µPa for the full band (50 Hz–20 kHz) and power spectrum density level (PSL) in μPa2 Hz−1 for the range 50–500 Hz.
(b). Ship traffic data
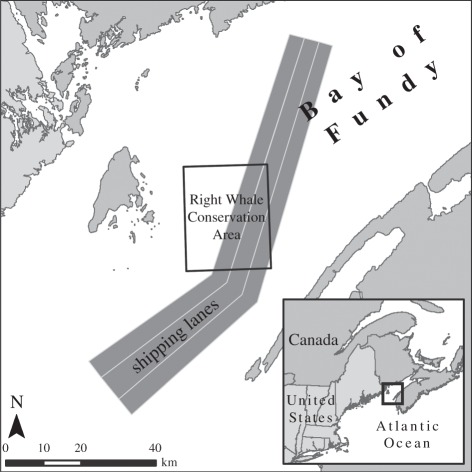
The Bay of Fundy (BOF) has a Traffic Separation Scheme (i.e. shipping lanes) that is mandatory for all vessels of or over 20 m in length, and vessel-tracking radar data are collected. Ship traffic data on the same days as the acoustic recordings were extracted from the Kongsberg Norcontrol IT Vessel Traffic Management and Information System computerized log-files stored with the Marine Communication and Traffic Services, Saint John, New Brunswick, Canada [14]. Figure 1 shows the Grand Manan Basin Right Whale Conservation Area in the Bay of Fundy where the study was conducted, and the location of the shipping lanes (in 2001).
Figure 1.
The study site in the Bay of Fundy, Canada. The Canadian Right Whale Conservation Area and the location of the designated shipping lanes (in 2001) are shown.
(c). Faecal sample collection and hormone analysis
In a second study (conducted from 2001–2005), we collected faecal samples from right whales and measured metabolites of steroid reproductive hormones (oestrogens, androgens and progestins) and adrenal glucocorticoids (GCs) [15,16]. Circulating steroid hormones are metabolized in the liver and excreted in bile (and urine), and the resulting metabolites are measurable in faeces [17,18]. The pattern of faecal metabolites reflects the average level of circulating parent hormone with a lag time of hours to days, depending on hormone turnover rates and gastrointestinal passage time for the species [18,19]. Based on data from other species, the lag time for right whales was estimated as 1 day for this study [20]. Previous work has demonstrated that concentrations of fGCs reflect adrenal activation and relative physiological stress levels in a wide variety of animals [19], including North Atlantic right whales [15].
Faecal samples were collected near right whales in the Bay of Fundy from late July to early October. Samples were found opportunistically and using detection dogs trained to find right whale faeces [21]. Floating faeces were scooped from the water using a 300 µm dipnet (Sea-Gear Corp., Melbourne, FL, USA), temporarily stored at −20°C, and shipped overnight on dry ice to the laboratory. Species of origin was confirmed photographically and by amplification of mitochondrial control region DNA [21].
Samples were lyophilized (−20°C) to remove variation owing to water content and diet, then sifted and mixed to homogenize hormone metabolites. Steroid metabolites were extracted from weighed faecal powder with a methanol vortex method [19]. Radioimmunoassays for faecal oestrogens, progestins, androgens and GCs have been previously validated for right whales, and detailed methods have been described [15,16]. Briefly, faecal extracts were diluted in the appropriate amount of assay buffer, and GC and oestrogen metabolites were assayed using double-antibody 125I radioimmunoassay kits (MP Biomedicals, Costa Mesa, CA, USA) and counted with a Packard Crystal II gamma counter. Progestin and androgen assays were in-house 3H radioimmunoassays counted with a Beckman LS6500 liquid scintillation counter. All samples were assayed in duplicate, with a full standard curve and two controls (low and high) in every assay. Any samples with percent-bound outside of the standard curve, or with greater than 10 per cent coefficient of variation between duplicates, were re-assayed. Results are expressed as nanograms of immunoreactive hormone metabolites per gram of freeze-dried faeces (abbreviated as ng g−1).
(d). Statistical methods
We compared levels of fGC metabolites before (and including) 11 September and after 11 September for the years 2001–2005 using a two-way, unbalanced Kruskall–Wallis test. The main effects were year (2001–2005) and period (before and after 11 September for all years). The null hypothesis was no effect of period, year or interaction between period and year. We were interested in assessing whether there was a pre-9/11 versus post-9/11 effect on fGCs in 2001 that differed from other years. Samples with faecal metabolites of testosterone greater than 5000 ng g−1 and progesterone greater than 6000 ng g−1 were removed from the analyses to control for physiologically normal elevations of fGC levels in adult males (mean androgens ± standard error of the mean = 10 192 ± 986 ng g−1) and pregnant females (mean progestins = 201 240 ± 27 025 ng g−1) [15,16]. The hormone cut-off values were derived from conditional inference trees used to classify identified whales with known reproductive states based on faecal hormone levels (P. J. Corkeron, unpublished data).
3. Results
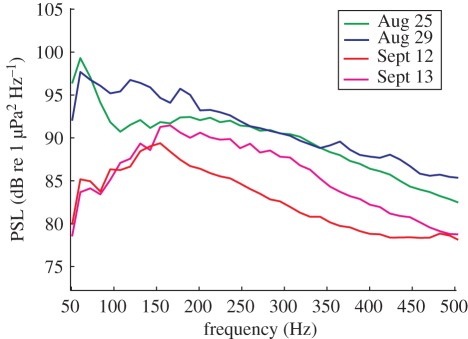
The acoustic analyses showed a 6 dB decrease in the overall background noise (50 Hz–20 kHz) in recordings made after 9/11. More importantly, the noise spectrum changed dramatically, with a significant reduction of noise below 150 Hz (figure 2). Records from the ship traffic monitoring programme in the Bay of Fundy (Fundy Traffic) confirmed a decrease in large vessel traffic following 9/11. Ship traffic (within 16 km of the Right Whale Conservation Area, figure 1) on the same dates in 2001 as the acoustic recordings, decreased from nine large ships on 25 and 29 August (five and four ships, respectively) to three ships on 12 and 13 September (one and two ships, respectively).
Figure 2.
Power spectrum density level (PSL) of background noise in the range 50–500 Hz from 2 days before (25 and 29 August 2011) and 2 days after 11 September 2001 (12 and 13 September 2011) with identical sea-state conditions. Overall noise levels (PSL in dB re 1 µPa2 Hz−1 are lower and the peak frequency (Hz) of the noise shifted to a higher frequency post-9/11.
Faecal GC levels from a total of 144 samples were used in the analyses (n = 114 before 11 September; n = 30 after 11 September, for all years). Sample sizes before/after 11 September by year were as follows: 2001 n = 14/9; 2002 n = 14/3; 2003 n = 37/4; 2004 n = 3/9; 2005 n = 46/5. Samples were collected approximately proportionally on weekdays and weekends, 96 per cent were evenly split between August and September, and 4 per cent were collected in late July and early October.
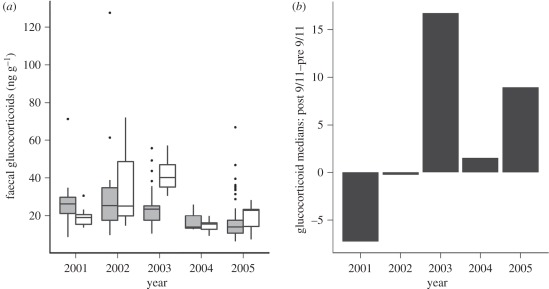
There was a significant effect of year and period on fGC levels (Kruskall–Wallis χ2 = 29.6889, d.f. = 4, p = 0.000005663; figure 3a). The only year in which there was a significant decrease in fGCs after 11 September was 2001 (figure 3b). While the data show annual variability in fGC levels, the dominant trend was for higher fGCs after 11 September in three of four control (non-2001) years (figure 3b). This trend was particularly pronounced in 2003, a year in which 75 per cent of post-11 September samples were collected after 20 September. This is in contrast to all other study years in which only 19 per cent of (post-11 September) samples were collected after 20 September. A possible explanation for higher fGC levels in later September (as in 2003) is the observation of an increase in whale participation in surface-active (courtship) groups as September progresses (New England Aquarium, unpublished data). Increased courtship activity could be a significant social stressor elevating fGC levels.
Figure 3.
(a) Levels of faecal GC metabolites (ng g−1) in North Atlantic right whales before (grey boxes) and after (white boxes.) 11 September for the years 2001–2005. Boxes show the interquartile range, the black line inside the box is the median, whiskers represent the adjacent values (most extreme observations that are not more than 1.5 times the height of the box), and outliers are represented by dots. (b) Yearly difference in median faecal GC levels (2001–2005) post 9/11–before 9/11. Significantly lower faecal GC levels after 11 September were only seen in 2001, and were associated with decreased underwater low-frequency noise resulting from a reduction in large vessel traffic.
4. Discussion
Acoustic studies have shown that right whales alter their vocalization behaviour in noisy habitats by increasing both the amplitude and frequency of their stereotyped upcalls [22,23], which are the main contact sounds used by these whales. A comparison of three right whale habitats along the east coast of the USA and Canada found that the Bay of Fundy had the highest levels of background low-frequency noise associated with heavy shipping traffic, and that the frequencies of right whale upcalls were significantly higher in this habitat [24]. While right whales alter their vocalizations in response to low-frequency underwater noise, it has been previously unclear whether these responses are accompanied by quantifiable physiological effects that could potentially lead to biologically significant impacts on individuals or populations.
Here, we show a decrease in baseline concentrations of fGCs in right whales in association with decreased overall noise levels (6 dB) and significant reductions in noise at all frequencies between 50 and 150 Hz as a consequence of reduced large vessel traffic in the Bay of Fundy following the events of 9/11. Even with relatively small sample sizes after 11 September in 2001, the decrease in fGCs after 9/11 was highly significant compared with other years. To our knowledge, there were no other factors affecting the population that could explain this difference besides the decrease in ship traffic and concomitantly reduced underwater noise disturbance after 9/11.
GCs are secreted in response to a large variety of natural stressors, such as social aggression, predators, starvation and drought, as well as anthropogenic disturbances [25,26]. Studies of terrestrial species have demonstrated increases in fGCs in response to noise-related anthropogenic stressors, such as snowmobiles [27], tourism traffic [28] and road noise [29]. Release of GCs from the adrenal cortex is mediated by the hypothalamic–pituitary–adrenal axis within minutes to an hour of experiencing (or even perceiving) a stressor [30]. This short-term stress response is beneficial to the individual by mobilizing energy reserves and initiating behaviours to respond to the threat. However, chronic elevations of GCs secondary to repeated or continuous stressors become maladaptive, suppressing growth, immune system function and reproduction [26,30], with implications for individual and population fitness. For example, circulating corticosterone levels predicted population-level survival probability in Galapagos marine iguanas (Amblyrhynchus cristatus) during an El Niño-induced famine [31], and high fGCs were predictive of individual mortality in ring-tailed lemurs (Lemur catta) [32]. Definitively linking chronic stress responses to detrimental health effects in large whales is extremely difficult because of the logistics of studying free-swimming whales and the inability to conduct a controlled study. However, a large body of literature has demonstrated that chronic stress, assessed by persistently elevated GCs, can lead to detrimental effects on health and reproduction across a variety of vertebrate taxa [26,30–32].
While the results presented here provide compelling evidence of a stress response in right whales exposed to higher levels of low-frequency underwater noise from ship traffic, this is a retrospective analysis based on a non-repeatable event, with all of the inherent limitations. Because the study was unplanned, there are no comparable acoustic recordings from the Bay of Fundy in years other than 2001 for comparison. Additionally, sample sizes after 11 September were relatively small in all years because deteriorating weather conditions in later September are much less conducive to faecal sample collection. In the absence of planned cessation of shipping traffic, future work is needed to characterize and compare underwater noise and fGC levels in right whales occupying habitats with varying levels of low-frequency noise from large ships to see if an enhanced stress response to higher noise levels is detectable given natural variability in the hormone data.
Because of their use of near-shore habitats along eastern North America, recovery of the critically endangered North Atlantic right whale population has been seriously impaired by mortalities from ship collisions and fishing gear entanglements [33]. Acoustic pollution from anthropogenic sources presents a less visible but pervasive disturbance to these coastal-dwelling whales that may have negative consequences for population viability. Exposure to potentially significant underwater noise from ships is not unique to the Bay of Fundy. For example, data modelling and analytical approaches estimated that the acoustic communication space of calling right whales in a second east coast habitat (Stellwagen Bank National Marine Sanctuary) was reduced 84 per cent by the passage of only two commercial ships during a 13.2 h period [10]. The Stellwagen area averaged six ships per day [34], suggesting that acoustic masking was occurring for the majority of the time that right whales were feeding there. The communication space of singing fin (Balaenoptera physalus) and humpback (Megaptera novaeangliae) whales was also diminished, but to a far lesser extent because of species-specific differences in acoustic signalling [10]. While increases in low-frequency ocean noise must be considered a potential anthropogenic stressor for all baleen whales in coastal areas with high levels of ship traffic, depleted populations experiencing the cumulative impact of multiple stressors and those with particular acoustic characteristics may be at heightened risk [7].
Acknowledgements
This work was supported by grants from the Office of Naval Research (S.K.), NOAA Fisheries (R.R. and S.P.), and the Northeast Consortium (R.R.). Our special thanks to the New England Aquarium right whale team and the other researchers who collected samples for the stress hormone study; to Philip Hamilton for right whale data discussions; to Jackie Ciano, Stephanie Martin and Cynthia Thomas for assistance with acoustic recordings; to Angelia Vanderlaan and Chris Taggert for supplying ship traffic data for the Bay of Fundy; and to Brooke Wikgren for graphics. This research was conducted under permits from Fisheries and Oceans, Canada and Scientific Research Permits under the Canadian Species at Risk Act.
References
- 1.National Research Council 2003. Ocean noise and marine mammals. Washington, DC: National Academies Press; [PubMed] [Google Scholar]
- 2.Hildebrand J. A. 2009. Anthropogenic and natural sources of ambient noise in the ocean. Mar. Ecol. Prog. Ser. 395, 5–20 10.3354/meps08353 (doi:10.3354/meps08353) [DOI] [Google Scholar]
- 3.Ross D. 1993. On ocean underwater ambient noise. Acoustic. Bull. 18, 5–8 [Google Scholar]
- 4.Ross D. 2005. Ship sources of ambient noise. IEEE J. Oceanic Eng. 30, 257–261 10.1109/JOE.2005.850879 (doi:10.1109/JOE.2005.850879) [DOI] [Google Scholar]
- 5.Andrew R. K., Howe B. M., Mercer J. A. 2002. Ocean ambient sound: comparing the 1960s with the 1990s for a receiver off the California coast. ARLO 3, 65–70 10.1121/1.1461915 (doi:10.1121/1.1461915) [DOI] [Google Scholar]
- 6.McDonald M. A., Hildebrand J. A., Wiggins S. M. 2006. Increases in deep ocean ambient noise in the Northeast Pacific west of San Nicolas Island, California. J. Acoust. Soc. Am. 120, 711–718 10.1121/1.2216565 (doi:10.1121/1.2216565) [DOI] [PubMed] [Google Scholar]
- 7.National Research Council 2005. Marine mammal populations and ocean noise: determining when noise causes biologically significant effects. Washington, DC: National Academies Press [Google Scholar]
- 8.Nowacek D. P., Thorne L. H., Johnston D. W., Tyack P. L. 2007. Responses of cetaceans to anthropogenic noise. Mammal Rev. 37, 81–115 10.1111/j.1365-2907.2007.00104.x (doi:10.1111/j.1365-2907.2007.00104.x) [DOI] [Google Scholar]
- 9.Weilgart L. S. 2007. The impacts of anthropogenic ocean noise on cetaceans and implications for management. Can. J. Zool. 85, 1091–1116 10.1139/Z07-101 (doi:10.1139/Z07-101) [DOI] [Google Scholar]
- 10.Clark C. W., Ellison T. E., Southall B. L., Hatch L., Van Parijs S. M., Frankel A., Ponirakis D. 2009. Acoustic masking in marine ecosystems: intuitions, analysis, and implication. Mar. Ecol. Prog. Ser. 395, 201–222 10.3354/meps08402 (doi:10.3354/meps08402) [DOI] [Google Scholar]
- 11.Tyack P. L. 2008. Implications for marine mammals of large-scale changes in the marine acoustic environment. J. Mammal. 89, 549–558 10.1644/07-mamm-s-307r.1 (doi:10.1644/07-mamm-s-307r.1) [DOI] [Google Scholar]
- 12.Tyack P. L., Clark C. W. 2000. Communication and acoustic behavior of dolphins and whales. In Hearing by whales and dolphins (eds Au W. W. L., Popper A. N., Fay R. R.), pp. 156–224 New York, NY: Springer; 10.1007/978-1-4612-1150-1_4 (doi:10.1007/978-1-4612-1150-1_4) [DOI] [Google Scholar]
- 13.Stafford K. M., Fox C. G., Clark D. S. 1998. Long-range acoustic detection and localization of blue whale calls in the northeast Pacific Ocean. J. Acoust. Soc. Am. 104, 3616–3625 10.1121/1.423944 (doi:10.1121/1.423944) [DOI] [PubMed] [Google Scholar]
- 14.Vanderlaan A. S. M., Taggart C. T., Serdynska A. R., Kenney R. D., Brown M. W. 2008. Reducing the risk of lethal encounters: vessels and right whales in the Bay of Fundy and on the Scotian Shelf. Endang. Species Res. 4, 283–297 10.3354/esr000830 (doi:10.3354/esr000830) [DOI] [Google Scholar]
- 15.Hunt K. E., Rolland R. M., Kraus S. D., Wasser S. K. 2006. Analysis of fecal glucocorticoids in the North Atlantic right whale (Eubalaena glacialis). Gen. Comp. Endocrinol. 148, 260–272 10.1016/j.ygcen.2006.03.012 (doi:10.1016/j.ygcen.2006.03.012) [DOI] [PubMed] [Google Scholar]
- 16.Rolland R. M., Hunt K. E., Kraus S. D., Wasser S. K. 2005. Assessing reproductive status of right whales (Eubalaena glacialis) using fecal hormone metabolites. Gen. Comp. Endocrinol. 142, 308–317 10.1016/j.ygcen.2005.02.002 (doi:10.1016/j.ygcen.2005.02.002) [DOI] [PubMed] [Google Scholar]
- 17.Adlercreutz H., Martin F., Pulkkinen M., Dencker H., Rimér U., Sjoberg N.- O., Tikkanen M. J. 1976. Intestinal metabolism of estrogens. J. Clin. Endocrinol. Metab. 43, 497–505 10.1210/jcem-43-3-497 (doi:10.1210/jcem-43-3-497) [DOI] [PubMed] [Google Scholar]
- 18.Schwarzenberger F. 2007. The many uses of non invasive faecal steroid monitoring in zoo and wildlife species. Int. Zoo Yearbk. 41, 52–74 10.1111/j.1748-1090.2007.00017.x (doi:10.1111/j.1748-1090.2007.00017.x) [DOI] [Google Scholar]
- 19.Wasser S. K., Hunt K. E., Brown J. L., Cooper K., Crockett C. M., Bechert U., Millspaugh J. J., Larson S., Monfort S. L. 2000. A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen. Comp. Endocrinol. 120, 260–275 10.1006/gcen.2000.7557 (doi:10.1006/gcen.2000.7557) [DOI] [PubMed] [Google Scholar]
- 20.Rolland R. M., Hunt K. E., Doucette G. J., Rickard L. G., Wasser S. K. 2007. The inner whale: hormones, biotoxins and parasites. In The urban whale: North Atlantic right whales at the crossroads. (eds Kraus S. D., Rolland R. M.), pp. 232–272 Cambridge, MA: Harvard University Press [Google Scholar]
- 21.Rolland R. M., Hamilton P. K., Kraus S. D., Davenport B., Gillett R. M., Wasser S. K. 2006. Faecal sampling using detection dogs to study reproduction and health in North Atlantic right whales (Eubalaena glacialis). J. Cetacean Res. Manage. 8, 121–125 [Google Scholar]
- 22.Parks S. E., Clark C., Tyack P. 2007. Short- and long-term changes in right whale calling behavior: the potential effects of noise on acoustic communication. J. Acoust. Soc. Am. 122, 3725–3731 10.1121/1.2799904 (doi:10.1121/1.2799904) [DOI] [PubMed] [Google Scholar]
- 23.Parks S. E., Johnson M., Nowacek D., Tyack P. L. 2010. Individual right whales call louder in increased environmental noise. Biol. Lett. 7, 33–35 10.1098/rsbl.2010.0451 (doi:10.1098/rsbl.2010.0451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parks S. E., Urazghildiiev I., Clark C. W. 2009. Variability in ambient noise levels and call parameters of North Atlantic right whales in three habitat areas. J. Acoust. Soc. Am. 125, 1230–1239 10.1121/1.3050282 (doi:10.1121/1.3050282) [DOI] [PubMed] [Google Scholar]
- 25.Wingfield J. C., Romero L. M. 2000. Adrenocortical responses to stress and their modulation in free-living vertebrates. In Handbook of physiology (sect. 7), the endocrine system (vol. 4), coping with the environment: neural and endocrine mechanisms (ed. McEwen B. S.), pp. 211–236 Oxford, UK: Oxford University Press [Google Scholar]
- 26.Sapolsky R. M., Romero L. M., Munck A. U. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89 10.1210/er.21.1.55 (doi:10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- 27.Creel S., Fox J. E., Hardy A., Sands J., Garrott B., Peterson R. O. 2002. Snowmobile activity and glucocorticoid stress responses in wolves and elk. Conserv. Biol. 16, 809–814 10.1046/j.1523-1739.2002.00554.x (doi:10.1046/j.1523-1739.2002.00554.x) [DOI] [Google Scholar]
- 28.Millspaugh J. J., Woods J. R., Hunt K. E., Raedeke K. J., Brundige G. C., Washburn B. E., Wasser S. K. 2001. Fecal glucocorticoid assays and the physiological stress response in elk. Wildl. Soc. Bull. 29, 899–907 [Google Scholar]
- 29.Wasser S. K., Bevis K., King G., Hanson E. 1997. Noninvasive physiological measures of disturbance in the northern spotted owl. Conserv. Biol. 11, 1019–1022 10.1046/j.1523-1739.1997.96240.x (doi:10.1046/j.1523-1739.1997.96240.x) [DOI] [Google Scholar]
- 30.Romero M. L., Butler L. K. 2007. Endocrinology of stress. Int. J. Comp. Psychol. 20, 89–95 [Google Scholar]
- 31.Romero L. M., Wikelski M. 2001. Corticosterone levels predict survival probabilities of Galápagos marine iguanas during El Niño events. Proc. Natl Acad. Sci. USA 98, 7366–7370 10.1073/pnas.131091498 (doi:10.1073/pnas.131091498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pride E. 2005. High faecal glucocorticoid levels predict mortality in ring-tailed lemurs (Lemur catta). Biol. Lett. 1, 60–63 10.1098/rsbl.2004.0245 (doi:10.1098/rsbl.2004.0245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraus S. D., et al. 2005. North Atlantic right whales in crisis. Science 309, 561–562 10.1126/science.1111200 (doi:10.1126/science.1111200) [DOI] [PubMed] [Google Scholar]
- 34.Hatch L., Clark C., Merrick R., Van Parijs S., Ponirakis D., Schwehr K., Thompson M., Wiley D. 2008. Characterizing the relative contributions of large vessels to total ocean noise fields: a case study using the Gerry E. Studds Stellwagen Bank National Marine Sanctuary. Environ. Manage. 42, 735–752 10.1007/s00267-008-9169-4 (doi:10.1007/s00267-008-9169-4) [DOI] [PubMed] [Google Scholar]