Abstract
The risk of global extinction of reef-building coral species is increasing. We evaluated extinction risk using a biological trait-based resiliency index that was compared with Caribbean extinction during the Plio-Pleistocene, and with extinction risk determined by the International Union for Conservation of Nature (IUCN). Through the Plio-Pleistocene, the Caribbean supported more diverse coral assemblages than today and shared considerable overlap with contemporary Indo-Pacific reefs. A clear association was found between extant Plio-Pleistocene coral genera and our positive resilience scores. Regional extinction in the past and vulnerability in the present suggests that Pocillopora, Stylophora and foliose Pavona are among the most susceptible taxa to local and regional isolation. These same taxa were among the most abundant corals in the Caribbean Pliocene. Therefore, a widespread distribution did not equate with immunity to regional extinction. The strong relationship between past and present vulnerability suggests that regional extinction events are trait-based and not merely random episodes. We found several inconsistencies between our data and the IUCN scores, which suggest a need to critically re-examine what constitutes coral vulnerability.
Keywords: biological trait, coral, extinction risk, Plio-Pleistocene, resilience, vulnerability
1. Introduction
Climate change is increasing the severity and the intensity of thermal stress events on modern coral reefs [1–4]. One of the primary concerns of conservation biologists and ecosystem managers is assessing the vulnerability of ecologically important taxa and mitigating their risk. The risk of global extinction of coral species is currently assigned by the International Union for Conservation of Nature (IUCN) using population reduction thresholds. For the vast majority of coral species, IUCN conservation status is evaluated from a weighted average calculated by multiplying the area of reef, within a species' distribution range, by the per cent of total coral cover loss in 17 different geographical regions. Using this method, the IUCN has documented an increase in the elevated extinction risk status for coral species from 2 per cent of 704 species during pre-1998 to 33 per cent by 2008 [5]. This approach is currently the only comprehensive assessment of coral extinction risk, but it is calculated with limited data on species-specific trends, and lacks a historical validation process [6,7].
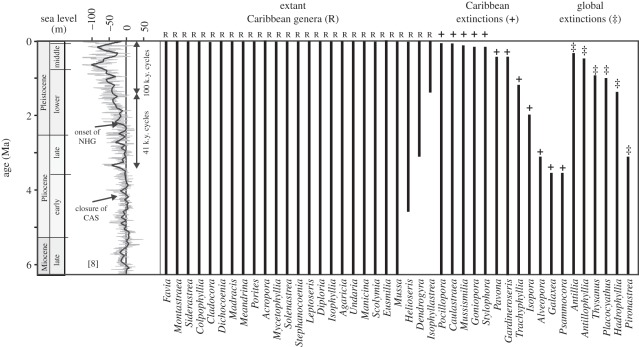
The regional extinctions in the Plio-Pleistocene may shed light on which coral genera may be vulnerable or resistant to stress during contemporary ocean warming. During the Plio-Pleistocene turnover, 25 coral genera persisted, while 18 were lost from the Caribbean region (figure 1). During this period, some corals went regionally extinct with persisting congenerics in the present-day Indo-Pacific (Alveopora, Caulastraea, Galaxea, Gardinoseris, Goniopora, Isopora, Pavona, Pocillopora, Psammocora, Stylophora and Trachyphyllia), whereas others went globally extinct (Antillia, Antillophyllia, Hadrophyllia, Pironastrea, Placocyathus and Thysanus) [9]. The rates of change towards regional and global extinction in the Caribbean varied considerably; some genera gradually declined, whereas others declined rapidly [10].
Figure 1.
Stratigraphic ranges of recorded reef coral genera (total = 43) within the Caribbean region from 6.8 Ma to Recent. Of the 18 genera that went regionally extinct in the Caribbean, 12 persist in the Indo-Pacific today. Key palaeo-environmental events, including closure of the Central American Seaway (CAS) and the onset of Northern Hemisphere glaciation (NHG), are highlighted next to the global sea-level curve of Miller et al. [8].
In the early Pliocene, large colonies of Pocillopora dominated the shallow reefs along with Stylophora [11,12]. Stylophora was highly vulnerable to the climate shifts of the Plio-Pleistocene, and went regionally extinct approximately 1.8 Ma from the Caribbean. By contrast, Pocillopora gradually diminished over millions of years, shifting from a ubiquitous distribution of predominantly large colonies, to a patchy distribution of small colonies, and eventually to regional extinction during the last interglacial. The foliose Pavona that went extinct in the Caribbean through the Plio-Pleistocene was never dominant but diminished over several million years through repeated isolation [10]. The two Caribbean Acropora species (Acropora palmata and Acropora cervicornis), increased in relative abundance through the Pliocene and Pleistocene. The Montastraea annularis complex, Siderastrea and branched Porites changed little over this 5 Myr period [10].
If indeed biological traits lead to differences in fitness, then the march to regional extinction during the Plio-Pleistocene in the Caribbean should be reflected in the genealogy of contemporary species in the warming Indo-Pacific. By contrast, if individuals on coral reefs are responding to changing conditions equally and independently of species traits [13], species may march through time showing random episodes of extinction. Here, we test an alternative to the concept of random episodes of regional extinctions, and propose that biological traits could play a larger role than suggested by neutral theory [13]. We test these ideas by comparing modern corals with coral populations that lived on ancient Plio-Pleistocene reefs of the Caribbean that experienced a protracted period of complex environmental change and extinction [9,14,15]. Our objective was to evaluate whether regional extinction events of the Plio-Pleistocene Caribbean region lend insight into future events in the Indo-Pacific. Our central hypothesis was that trends through the Plio-Pleistocene will reflect contemporary trends on Indo-Pacific reefs. A mismatch between past and present trends would lend support to neutral theory, which suggests that species extinctions are a random process. By contrast, a match between past and present trends would suggest a non-random, biological trait-based process.
2. Material and methods
(a). Modern genera resilience scores
We examined whether biological traits play a large role in the outcome of coral persistence through environmental change by first categorizing modern corals based on a resilience score. To determine the resilience score of modern corals, a group of experts compiled a suite of biological traits and processes that were considered as potentially subjected to selective pressure. These characteristics encompassed morphological, physiological and reproductive traits and processes of coral genera. Quorum responses and consensus decision-making [16] constituted that the traits and scoring were purposefully decided a priori (table 1), and that all traits were maintained throughout the analysis to avoid tautology. A consensus was reached among 10 coral-reef scientists on the general principle underlying each trait and the methodology of the scoring protocol (table 1) [16]. For analysis, we selected 14 dominant (modern) coral genera for the Indo-Pacific Ocean and 15 for the Caribbean Sea. Two genera were subdivided based on morphology with Pavona classified as either foliose or encrusting/submassive and Porites as branching or massive. Montastraea in the Caribbean were evaluated as either Montastraea annularis complex or Montastraea cavernosa. Although both genera and subgenera groups were examined, we refer to all corals at the genus level. Each coral genus was then evaluated on their tolerance to a hypothetical two-month + 3°C thermal stress anomaly, during the solar insolation maximum. Similarly, four key process variables were scored for a suite of coral taxa, to address whether each process would affect recovery from the temperature anomaly (table 1). The scores for the traits and processes were summed for each coral genus and classified as a resilience score. Although contemporary species within each genus differs in terms of their geographical distribution—some are widespread and others are restricted—classic taxonomic texts [17] and recent molecular techniques show evidence that many genera (e.g. Acropora, Porites, Stylophora, Pocillopora and foliose Pavona) are distinct, and coherent monophyletic entities are closely related and often difficult to distinguish [18–22]. Therefore, for this investigation, we considered it appropriate to lump species within genera.
Table 1.
Biological traits and processes of coral taxa with the rationale for their tolerance to and recovery from a thermal stress.
| physical trait | rationale | tolerant | intolerant |
|---|---|---|---|
| morphology | influences thermal and light exposure | massive | branching |
| tissue thickness | provides energy storage | thick | thin tissues |
| distance between corallites | promotes partial mortality | large | small: no coenosteum |
| calcification rate | increases demand for energy | low | high rate |
| colony size | increases available energy | large | small |
| corallite size | promotes energy storage | large | small |
| association with Symbiodinium Clade D | gives thermal tolerance | greater than10% D by numbers reported | ≤10% D by numbers |
| skeletal structure | creates refuge for tissue | porous | non-porous |
| biological process | rationale | high recovery | low recovery |
| mode of sexual reproduction | low investment and high numbers good in disturbed environments | broadcasting | brooding |
| recruitment density | high numbers good in disturbed environments | high | low |
| colony regrowth | allows recovery from partial mortality | regrowth from remaining tissue | no regrowth |
| colony growth | allows recovery after disturbance | high | low |
(b). Plio-Pleistocene reef data
The fossil data were based on samples extracted from outcrop exposures at 70 localities through four Plio-Pleistocene sequences: Curacao [11], Costa Rica [23], the Dominican Republic [12,24] and Jamaica [11]. The collections comprised approximately 6528 specimens and 154 species, and were deposited at the US National Museum of Natural History (USNM), the University of Iowa (SUI) and the Natural History Museum in Basel, Switzerland (NMB). All of the specimens were identified to species using a standard set of morphological characters and character states, established in part by comparing morphological and molecular data and detailed in the Neogene Marine Biota of Tropical America (NMITA) taxonomic database [25]. Localities were grouped into faunules, which were defined as a set of lithologically similar localities from a small geographical area (usually less than 1 km or approximately the size of a large rock quarry) and restricted stratigraphic interval (usually less than 20 m). Age dates for the faunules were obtained by integrating data using high-resolution chronostratigraphic methods, including nannofossils and planktonic foraminiferal biostratigraphy, palaeomagnetics and strontium isotope analyses [26,27], and generally range in accuracy from 0.5 to 2 Myr. The dataset consisted of counts of specimens belonging to species within each faunule, and is available on the NMITA website.
(c). Data analysis
We sought to answer the question: what was the probability that a coral taxon that went regionally extinct during the Plio-Pleistocene, in the Caribbean, was also vulnerable on modern reefs in the Indian and Pacific Oceans? The probability that a coral went extinct in the Plio-Pleistocene, Pp(x), was determined from the fossil dataset for the Caribbean region (see §2b). For the modern corals, we defined contemporary vulnerability as a coral that was among the three lowest-ranked corals in the experts' resilience list (outlined above). This contemporary vulnerability was defined as Mp(x). The product of the probabilities (Pp(x).Mp(x)) defined the chance that a coral would have gone extinct through the Plio-Pleistocene in the Caribbean, and also ranked among the three lowest (modern) resilience scores. Using Bayes's theorem, we iteratively calculated posterior probabilities for extinction using Markov chain Monte Carlo simulations and Gibbs sampling. We used a binomial distribution θ for extinction probability and a beta prior (1, 1) to calculate the posterior probabilities, p(θ|y) given the data y. All models were implemented using OpenBugs [28].
Using a penalized maximum-likelihood logistic regression [29,30], we estimated how the probability of persistence of coral genera in the Caribbean region, through the Plio-Pleistocene, varied with the resilience scores applied to the same genera in the modern Indo-Pacific. Included in the analysis were the eight coral genera that were present in the Plio-Pleistocene Caribbean region and were also present in the modern Indo-Pacific Ocean, which included Acropora, Indo-Pacific Montastraea, Pavona (branching), Pavona (foliose), Pocillopora, Porites (branching), Porites (massive) and Stylophora. The binary-dependent variable in the analysis described the status of each genera at the end of the Pliocene as either extant (with a value of 1) or extinct (0) with the resilience scores for the modern Indo-Pacific as the independent predictor variable.
We compared the resilience scores with the conservation status of reef-building corals determined by the IUCN Red List criteria. The IUCN has classified coral species using a set of criteria incorporating observed or anticipated population trends and levels of geographical isolation into the following categories: data-deficient (dd), least concern (lc), vulnerable (vu), endangered (en) or critically endangered (cr) [5]. Following these methods, we categorized as ‘Threatened’, the coral species identified as vu, en or cr within the Indo-Pacific region (table 2). For the Indo-Pacific coral genera, we compared the rank correlation (Kendall's tau) of the percentage of threatened species within each genera and morphological type, where described, using classifications from Veron & Stafford-Smith [17] and their resilience scores. Statistical models were constructed in the R statistical software (http://www.r-project.org).
Table 2.
Number of species in IUCN 2008 Red List categories for modern reef-building coral genera of the Indo-Pacific region. (IUCN categories are data-deficient: (dd), least concern (lc), near-threatened (nt), vulnerable (vu), endangered (en) and critically endangered (cr) with the sum of the number of species within each taxa (spp). Percentage of threatened species within a taxa (thr) includes all non-data-deficient species listed as vu, en or cr. IP indicates Indo-Pacific.)
| Indo-Pacific taxa | dd | lc | nt | vu | en | cr | spp | thr (%) |
|---|---|---|---|---|---|---|---|---|
| Acropora | 67 | 27 | 22 | 48 | 3 | 0 | 167 | 51 |
| Cyphastrea/Leptastrea | 0 | 8 | 3 | 4 | 0 | 0 | 15 | 27 |
| Echinopora | 3 | 3 | 6 | 2 | 0 | 0 | 14 | 18 |
| Favia | 1 | 6 | 13 | 1 | 0 | 0 | 21 | 5 |
| Favites | 0 | 1 | 12 | 1 | 0 | 0 | 14 | 7 |
| Goniastrea | 0 | 5 | 6 | 2 | 0 | 0 | 13 | 15 |
| IP Montastraea | 0 | 1 | 4 | 3 | 0 | 0 | 8 | 38 |
| Montipora | 8 | 19 | 17 | 28 | 2 | 0 | 74 | 46 |
| Pavona encrusting/massive | 0 | 5 | 1 | 4 | 0 | 0 | 10 | 40 |
| Pavona foliose | 1 | 3 | 0 | 2 | 0 | 0 | 6 | 40 |
| Platygyra | 0 | 4 | 6 | 1 | 0 | 0 | 11 | 9 |
| Pocillopora | 2 | 9 | 1 | 5 | 1 | 0 | 18 | 38 |
| Porites branching | 6 | 14 | 5 | 12 | 3 | 1 | 41 | 46 |
| Porites massive | 0 | 7 | 6 | 0 | 0 | 0 | 13 | 0 |
| Seriatopora | 0 | 2 | 2 | 2 | 0 | 0 | 6 | 33 |
| Stylophora | 0 | 4 | 2 | 0 | 1 | 0 | 7 | 14 |
| total | 88 | 118 | 106 | 115 | 10 | 1 | 438 |
3. Results
(a). Modern genera resilience scores
The capacity of coral taxa to persist regionally was defined as a resilience score, which was a combination of their biological trait and ecological processes. Three Indo-Pacific corals were ranked low and most vulnerable (less than or equal to −3; Stylophora, Pocillopora and foliose Pavona), and four were ranked high and least vulnerable (greater than or equal to 4; Goniastrea, Favia, massive Porites and Indo-Pacific Montastraea; tables 3 and 4). In the Caribbean, only Madracis and branching Porites ranked low and vulnerable (less than or equal to −3) and three ranked high and tolerant (greater than or equal to 4; Siderastrea, Montastraea annularis complex and Diploria; tables 3 and 4).
Table 3.
Biological trait scores for modern coral taxa in the Indo-Pacific and Caribbean regions. (Scores were given for traits as tolerant (1) or intolerant (−1) to a thermal stress event (with zero as no effect). Traits are morphology (mor), tissue thickness (tis), distance between corallites (dis), calcification rate (cal), colony size (col), corallite size (cor), association with Symbiodinium Clade D (sym) and skeletal structure (ske). IP indicates Indo-Pacific.)
| traits |
||||||||
|---|---|---|---|---|---|---|---|---|
| mor | tis | dis | cal | col | cor | sym | ske | |
| Indo-Pacific taxa | ||||||||
| Acropora | −1 | −1 | 1 | −1 | −1 | −1 | 1 | 1 |
| Cyphastrea/Leptastrea | 1 | 1 | 1 | 1 | 0 | −1 | −1 | −1 |
| Echinopora | −1 | 1 | 1 | 1 | 0 | 1 | 1 | −1 |
| Favia | 1 | 1 | 1 | −1 | 0 | 1 | −1 | −1 |
| Favites | 1 | 1 | −1 | −1 | 0 | 1 | −1 | −1 |
| Goniastrea | 1 | 1 | 1 | −1 | 0 | 1 | 1 | −1 |
| IP Montastraea | 1 | 1 | 1 | −1 | 0 | 1 | 1 | −1 |
| Montipora | −1 | −1 | 1 | −1 | 0 | −1 | 0 | 1 |
| Pavona, encrusting/massive | 0 | −1 | −1 | 1 | 0 | −1 | 1 | −1 |
| Pavona, foliose | 0 | −1 | −1 | −1 | 0 | −1 | 1 | −1 |
| Platygyra | 1 | 1 | −1 | −1 | 0 | 1 | −1 | −1 |
| Pocillopora | −1 | −1 | 1 | −1 | 0 | −1 | 1 | −1 |
| Porites, branching | −1 | 1 | −1 | 1 | 0 | −1 | −1 | 1 |
| Porites, massive | 1 | 1 | −1 | 1 | 1 | −1 | −1 | 1 |
| Seriatopora | −1 | −1 | 1 | −1 | 0 | −1 | 1 | −1 |
| Stylophora | −1 | −1 | 1 | −1 | 0 | −1 | −1 | −1 |
| Caribbean taxa | ||||||||
| Acropora | −1 | −1 | 1 | −1 | 1 | −1 | 1 | 1 |
| Agaricia | 0 | −1 | −1 | −1 | 0 | −1 | 1 | −1 |
| Colpophyllia | 1 | 1 | −1 | 1 | 1 | 1 | −1 | −1 |
| Dendrogyra | 1 | 1 | 1 | 1 | 1 | 0 | −1 | −1 |
| Dichocoenia | 1 | 0 | 1 | 1 | −1 | 0 | −1 | −1 |
| Diploria | 1 | 1 | 1 | 1 | 1 | 1 | −1 | −1 |
| Eusmilia | −1 | 1 | −1 | 1 | −1 | 1 | −1 | −1 |
| Favia | 1 | −1 | 1 | 1 | 1 | 1 | −1 | 0 |
| Madracis | −1 | −1 | 1 | −1 | −1 | −1 | −1 | −1 |
| Manicina | 1 | 1 | −1 | 1 | −1 | 1 | −1 | −1 |
| Meandrina | 1 | 1 | −1 | 1 | −1 | 1 | −1 | −1 |
| Montastraea ann. com. | 1 | 1 | 1 | 1 | 1 | −1 | 1 | −1 |
| Montastraea cavernosa | 1 | 1 | 1 | 1 | 0 | 1 | 1 | −1 |
| Porites, branching | −1 | 1 | −1 | −1 | −1 | −1 | −1 | 1 |
| Porites, encrusting | 1 | 1 | −1 | 1 | −1 | −1 | −1 | 1 |
| Siderastrea | 1 | −1 | −1 | 1 | 0 | 0 | 1 | 1 |
| Stephanocoenia | 1 | −1 | −1 | −1 | 0 | −1 | −1 | 1 |
Table 4.
Biological process scores and resilience scores for modern coral taxa in the Indo-Pacific and Caribbean regions. (Scores were given for processes that enhance recovery (1) or inhibit recovery (−1) from a thermal stress event (with zero as no effect). Processes are sexual recruitment (sex), density of recruitment (rec), colony regrowth (reg) and colony growth (gro). Resilience scores are the sum of biological process scores and trait scores (that were summed from data in table 3). IP indicates Indo-Pacific.)
| process |
|||||||
|---|---|---|---|---|---|---|---|
| sex | rec | reg | gro | process score | trait score | resilience score | |
| Indo-Pacific taxa | |||||||
| Acropora | 1 | 1 | 0 | 1 | 3 | −2 | 1 |
| Cyphastrea/Leptastrea | 1 | 1 | 1 | −1 | 2 | 1 | 3 |
| Echinopora | 1 | −1 | 1 | −1 | 0 | 3 | 3 |
| Favia | 1 | 1 | 1 | 1 | 4 | 1 | 5 |
| Favites | 1 | 1 | 1 | 1 | 4 | −1 | 3 |
| Goniastrea | 1 | 1 | 1 | 1 | 4 | 3 | 7 |
| IP Montastraea | 1 | 0 | 1 | 1 | 3 | 3 | 6 |
| Montipora | 1 | 1 | 1 | 1 | 4 | −2 | 2 |
| Pavona, encrusting/massive | −1 | 1 | 1 | −1 | 0 | −2 | −2 |
| Pavona, foliose | −1 | 1 | −1 | 1 | 0 | −4 | −4 |
| Platygyra | 1 | 0 | 1 | 1 | 3 | −1 | 2 |
| Pocillopora | −1 | 1 | −1 | 1 | 0 | −3 | −3 |
| Porites, branching | 1 | 1 | 1 | −1 | 2 | −1 | 1 |
| Porites, massive | 1 | 1 | 1 | −1 | 2 | 2 | 4 |
| Seriatopora | −1 | 1 | 1 | 1 | 2 | −3 | −1 |
| Stylophora | −1 | 1 | −1 | 1 | 0 | −5 | −5 |
| Caribbean taxa | |||||||
| Acropora | 1 | −1 | 1 | 1 | 2 | 0 | 2 |
| Agaricia | −1 | 1 | 1 | 1 | 2 | −4 | −2 |
| Colpophyllia | 1 | −1 | 1 | −1 | 0 | 2 | 2 |
| Dendrogyra | −1 | 1 | −1 | 0 | 3 | 3 | |
| Dichocoenia | 1 | −1 | 1 | −1 | 0 | 0 | 0 |
| Diploria | 1 | 0 | 1 | −1 | 1 | 4 | 5 |
| Eusmilia | 1 | −1 | 1 | 1 | 2 | −2 | 0 |
| Favia | −1 | 1 | 1 | −1 | 0 | 3 | 3 |
| Madracis | −1 | −1 | 1 | 1 | 0 | −6 | −6 |
| Manicina | −1 | −1 | 1 | −1 | −2 | 0 | −2 |
| Meandrina | 0 | −1 | 1 | −1 | −1 | 0 | −1 |
| Montastraea ann. com. | 1 | −1 | 1 | −1 | 0 | 4 | 4 |
| Montastraea cavernosa | 1 | −1 | −1 | −1 | −2 | 5 | 3 |
| Porites, branching | −1 | 1 | 1 | −1 | 0 | −4 | −4 |
| Porites, encrusting | −1 | 1 | 0 | 1 | 1 | 0 | 1 |
| Siderastrea | 0 | 1 | 1 | 1 | 3 | 2 | 5 |
| Stephanocoenia | 1 | −1 | 1 | 1 | 2 | −3 | −1 |
(b). Plio-Pleistocene and modern coral vulnerability
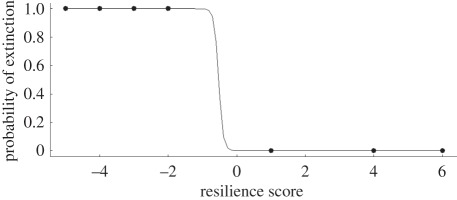
If extinction was a chance event, and biological traits and ecological processes were irrelevant to extinction probability, then the probability of a coral going extinct in the Plio-Pleistocene, Pp(x), was 0.42. The probability that a modern species was listed among the three least resilient corals in the Indo-Pacific, Mp(x), was 0.21. Therefore, there was approximately a 9 per cent chance (95% Bayesian credible intervals 2–16%), Pp(x).Mp(x), that a coral would have gone regionally extinct through the Plio-Pleistocene and that it was also at the bottom of the resilience list. Against the odds, this happened for three taxa: Stylophora, foliose Pavona and Pocillopora. With three vulnerable genera at risk today having also experienced Plio-Pleistocene extinction, this result strongly rejects a non-selective, random mechanism for extinction events and supports the proposition that biological traits play a critical role in determining vulnerability. Indeed, we identified a strong relationship between the fate of specific coral genera during the Plio-Pleistocene and their contemporary resilience score for the modern Indo-Pacific (figure 2). For the eight coral genera examined, the four that went regionally extinct during the Plio-Pleistocene all possessed negative resilience scores, while the four extant taxa all had positive scores. The significant association between extant Plio-Pleistocene coral genera and positive resilience scores (penalized logistic regression-likelihood ratio χ2, p = 0.01) supports the use of a biological trait-based framework for evaluating coral extinction risks. These associations also support the notion that coral extinction are trait-based, further rejecting neutral theory that suggests coral extinctions are a random process.
Figure 2.
Penalized likelihood logistic regression of the relationship between Plio-Pleistocene persistence in the Caribbean region and the modern resiliency index score for eight coral genera of the modern Indo-Pacific region.
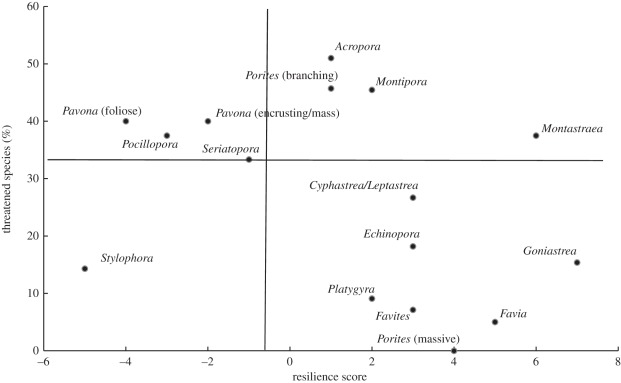
If both the IUCN risk of extinction scores [5] and our trait-based approach accurately predict the risk of extinction, we would expect a direct negative relationship between our trait-based resilient scores, where positive numbers suggest resilience, and positive IUCN scores suggest high extinction risk. We found this general relationship (Kendall's tau = −0.32, p = 0.09) among the rank correlation of the IUCN status and resilience scores, but it was not statistically significant. Most genera exhibited a concurrence between the trait-based score and IUCN status but Stylophora, and the Montastraea annularis complex were prominent outliers (figure 3). We suggest that Stylophora is more threatened than is predicted by the IUCN scores, whereas the Montastraea annularis complex is more resilient than is predicted by the IUCN scores (figure 3).
Figure 3.
The relationship between the resilience score and the percentage of threatened species within coral genera using the IUCN Red List criteria for Indo-Pacific coral taxa [5]. The majority of genera display good correspondence of conservation status between the methods (e.g. the 11 genera present in the upper left and lower right areas). IUCN conservation status for corals is low relative to the resilience score in the lower left area and high relative to the resilience score in the upper right area. The vertical line represents the threshold value for extinct coral genera from the Plio-Pleistocene fossil record analysis and the horizontal line provides the percentage of all threatened coral species as identified by the IUCN Red List criteria.
4. Discussion
Understanding the vulnerability of corals to thermal stress is critical to management efforts in rapidly warming oceans. We compared contemporary vulnerability of modern corals with fossil data on extinctions from the Plio-Pleistocene, which experienced dynamic climate fluctuations. The strong relationship between past trajectories and modern vulnerability shows that inherent genealogies can guide our predictions of the possible future state of coral communities. Furthermore, our trait and historical approach may offer another method to evaluate vulnerability of coral taxa and augment existing methods.
Biological trait-based approaches have been recently applied to the evaluation of extinction risk among a variety of taxa including terrestrial plants [7], geometrid moths [31], desert fishes [32] and amphibians [33]. Our study used a similar strategy but also benefited from, and was limited by, the inclusion of fossil data for historical validation of extinction vulnerabilities of corals. The primary caveats of working with fossil data were the limited taxonomic scope available [9–12] and implicit assumption of trait conservation for extant lineages. Furthermore, our expert-based approach, which qualified biological traits of coral species and processes, is in need of further quantification for all extant coral species. Nonetheless, general patterns observed across the breadth of taxa included in the analysis support the use of biological trait-based frameworks for the determination of extinction risk in corals.
The past episodes of regional extinction were strongly related to the vulnerability of modern corals. On modern reefs, Stylophora, Pocillopora and foliose Pavona are all highly sensitive to temperature anomalies [34,35], salinity changes [36] and irradiance extremes [37]. Yet, encrusting and submassive Pavona (including Pavona varians, Pavona decussata and Pavona venosa) were not sensitive to warm temperate events in the 1998 warm anomaly [35]. The percentage cover of Pavona and Pocillopora has increased on Kenyan reefs over the last 20 years [38]. Nevertheless, in the unusually warm and variable regions of the Arabian Gulf, these taxa are either not present, or patchy and their numbers are too low to be measured by standard ecological transects [39–41]. If the Arabian Gulf is a good contemporary analogue for future reefs, then the patchy success of Pavona and Pocillopora on some Kenyan reefs may be short-lived in the warmer and more variable conditions predicted for the future. The consistent trends between the past and the present suggest that regional extinctions were not random episodes [13], but were instead a consequence of genealogies and associated traits that were vulnerable to climate fluctuations.
Estimates of the partial pressure of carbon dioxide (pCO2) in the early Pliocene are higher than any other part of the Neogene [42], with mean sea-surface temperatures in the Caribbean estimated to be 1–2°C warmer than present [43]. Final closure of the Central American Seaway (4.2-3.4 Myr ago) isolated the Caribbean from the Indo-Pacific and corresponded with increased salinity, increased carbonate precipitation and more oligotrophic conditions in the Caribbean [44–46]. Interestingly, the main extinction peak for both molluscs and corals lags approximately 2 Myr behind final closure of the Central American Seaway [9,15,47,48]. The extinction peak occurred during a period of global cooling (glaciation) and an eustatic sea-level drop that changed oceanic circulation and increased oligotrophy [45].
Irrespective of the timing of the extinctions, and judging by the modern responses, stenothermal species, such as Stylophora and Pocillopora, were likely to be vulnerable to the highly fluctuating climate, whereas eurythermal species, such as Indo-Pacific Montastraea, Porites and Favia, may have simply tolerated the fluctuating conditions. Nevertheless, persistence can be a convergent trait, being dependent on local tolerance and regional ubiquity. Local thermal tolerance is attributed to the biological properties of the host and the symbionts, such as massive growth forms, thick tissue, low-metabolic rates and whether corals support thermally tolerant symbionts [49]. Stylophora and Pocillopora are branched, with thin tissue [34], and heat-tolerant endosymbionts seldom dominant these taxa [50]. However, regional persistence is also attributed to the life-history properties of corals, including their fecundity and growth rates. Weedy species with high fecundity and rapid growth are predisposed to regional success, especially in areas that support a variety of habitats that are frequently disturbed. Recent studies show that a number of species that were short-term losers under thermal stress, such as Acropora digitifera and Acropora gemmifera, turned out to be long-term winners, in part, because of their ubiquitous distribution [51].
The upward trajectories of Acropora through the Plio-Pleistocene do not match the declines of Acropora in the modern Caribbean. The two species of Acropora found on modern Caribbean reefs (Acropora palmata and Acropora cervicornis) are highly vulnerable to extinction, with no opportunity for redundancy. By contrast, there are over a hundred modern species of Acropora in the Indo-Pacific region, with varying levels of susceptibility to thermal events [51,52].
It could be argued that given the unprecedented rate of change in the modern climate, historical events in the Plio-Pleistocene may tell us little about future trajectories. Yet, our comparison across time shows consistent patterns, indicating that conserved genealogies allow a glimpse into near-future trends. Stylophora and Pocillopora may change from local dominance and a ubiquitous distribution in the Indo-Pacific region, to a more sparse distribution and local rarity. Isolated reef aggregations in the Pacific and Indian Oceans and those reefs with few neighbours may be particularly vulnerable to climate change, and may lose corals such as Stylophora and Pocillopora. By contrast, localities in the Pacific and Indian Oceans supporting diverse habitats and dense reef aggregations may continue to support climatically sensitive coral species.
Our capacity to successfully predict the risk of coral extinction will ideally lead to management policy that reduces such threats. Importantly, we would expect that coral species most at risk, with high IUCN scores, would show a strong negative relationship with our trait-based resilient scores. However, several inconsistencies were found when using the IUCN criteria. Therefore, predictions of extinction risk based on the IUCN criteria should be re-evaluated to incorporate biological traits and ecological processes. Progress towards this goal would be accomplished by a comprehensive accounting of the biological traits of coral species, quantifying changes in local and regional abundance patterns, and determining geographical range changes through time.
Acknowledgements
Many thanks extend to Sandra van Woesik and Peter Edmunds for editorial comments and Shirley Han for research assistance. Funding provided by the NSF (DEB-0102544, DEB-9705199, EAR-9219138, EAR-0445789 to A.F.B.; EF-0553768 to NCEAS for ‘Tropical coral reefs of the future: modelling ecological outcomes from the analyses of current and historical trends’ Working Group, awarded to Ruth Gates and Pete Edmunds; OCE-1041673 to M.J.D. for E.C.F., NOAA NMSP (MOA 2005-008/66882 for E.C.F.), the US EPA (FP917096 to E.C.F.). This is HIMB contribution no. 1483 and SOEST contribution no. 8550. This is contribution 61 from the Institute for Research on Global Climate Change at the Florida Institute of Technology.
References
- 1.Glynn P. W. 1991. Coral reef bleaching in the 1980s and possible connections with global warming. Trends Ecol. Evol. 6, 175–179 10.1016/0169-5347(91)90208-F (doi:10.1016/0169-5347(91)90208-F) [DOI] [PubMed] [Google Scholar]
- 2.Brown P. G. 1997. Stewardship of climate. Clim. Change 37, 329–334 10.1023/A:1005315618569 (doi:10.1023/A:1005315618569) [DOI] [Google Scholar]
- 3.Hoegh-Guldberg O. 1999. Climate change, coral bleaching and the future of the world's coral reefs. Mar. Freshwater Res. 50, 839–866 10.1071/MF99078 (doi:10.1071/MF99078) [DOI] [Google Scholar]
- 4.Donner S. D., Skirving W. J., Little C. M., Oppenheimer M., Hoegh-Guldberg O. 2005. Global assessment of coral bleaching and required rates of adaptation under climate change. Global Change Biol. 11, 2251–2265 10.1111/j.1365-2486.2005.01073.x (doi:10.1111/j.1365-2486.2005.01073.x) [DOI] [PubMed] [Google Scholar]
- 5.Carpenter K. E., et al. 2008. One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321, 560–563 10.1126/science.1159196 (doi:10.1126/science.1159196) [DOI] [PubMed] [Google Scholar]
- 6.Purvis A., Jones K. E., Mace G. M. 2000. Extinction. Bioessays 22, 1123–1133 (doi:10.1002/1521-1878(200012)22:12<1123::AID-BIES10>3.0.CO;2-C) [DOI] [PubMed] [Google Scholar]
- 7.Davies T. J., et al. 2011. Extinction risk and diversification are linked in a plant biodiversity hotspot. PLoS Biol. 9, e1000620. 10.1371/journal.pbio.1000620 (doi:10.1371/journal.pbio.1000620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller K. G., et al. 2005. The Phanerozoic record of global sea-level change. Science 310, 1293–1298 10.1126/science.1116412 (doi:10.1126/science.1116412) [DOI] [PubMed] [Google Scholar]
- 9.Budd A. F., Johnson K. G. 1999. Origination preceding extinction during late Cenozoic turnover of Caribbean reefs. Paleobiology 25, 188–200 [Google Scholar]
- 10.Budd A. F., Klaus J. S., Johnson K. G. 2011. Cenozoic diversification and extinction patterns in Caribbean reef corals. A review. Paleontol. Soc. Pap. 17, 79–93 [Google Scholar]
- 11.Budd A. F., Petersen R. A., McNeill D. F. 1998. Stepwise faunal change during evolutionary turnover: a case study from the Neogene of Curacao, Netherlands antilles. Palaios 13, 170–188 10.2307/3515488 (doi:10.2307/3515488) [DOI] [Google Scholar]
- 12.Klaus J. S., Budd A. F. 2003. Comparison of Caribbean coral reef communities before and after Plio-Pleistocene faunal turnover: analyses of two Dominican Republic reef sequences. Palaios 18, 3–21 (doi:10.1669/0883-1351(2003)018<0003:COCCRC>2.0.CO;2) [DOI] [Google Scholar]
- 13.Hubbell S. P. A. 2001. A unified theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press [Google Scholar]
- 14.Johnson K. G., Jackson J. B. C., Budd A. F. 2008. Caribbean reef development was independent of coral diversity over 28 million years. Science 319, 1521–1523 10.1126/science.1152197 (doi:10.1126/science.1152197) [DOI] [PubMed] [Google Scholar]
- 15.Klaus J. S., Lutz B. P., McNeill D. F., Budd A. F., Johnson K. G., Ishman S. E. 2011. Rise and fall of Pliocene free-living corals in the Caribbean. Geology 39, 375–378 10.1130/g31704.1 (doi:10.1130/g31704.1) [DOI] [Google Scholar]
- 16.Sumpter D. J. T., Pratt S. C. 2009. Quorum responses and consensus decision making. Phil. Trans. R. Soc. B 364, 743–753 10.1098/rstb.2008.0204 (doi:10.1098/rstb.2008.0204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veron J. E. N., Stafford-Smith M. 2000. Corals of the world. Townsville, Australia: Australian Institute of Marine Sciences [Google Scholar]
- 18.Fukami H., Budd A. F., Paulay G., Solé-Cava A., Chen C. A., Iwao K., Knowlton N. 2004. Conventional taxonomy obscures deep divergence between Pacific and Atlantic corals. Nature 427, 832–835 10.1038/nature02339 (doi:10.1038/nature02339) [DOI] [PubMed] [Google Scholar]
- 19.Pinzón J. H., LaJeunesse T. C. 2011. Species delimitation of common reef corals in the genus Pocillopora using nucleotide sequence phylogenies, population genetics and symbiosis ecology. Mol. Ecol. 20, 311–325 10.1111/j.1365-294X.2010.04939.x (doi:10.1111/j.1365-294X.2010.04939.x) [DOI] [PubMed] [Google Scholar]
- 20.Flot J-F., Blanchot J., Charpy L., Cruaud C., Licuanan W. Y., Nakano Y., Payri C., Tillier S. 2011. Incongruence between morphotypes and genetically delimited species in the coral genus Stylophora: phenotypic plasticity, morphological convergence, morphological stasis or interspecific hybridization? BMC Ecol. 11, 22. 10.1186/1472-6785-11-22 (doi:10.1186/1472-6785-11-22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pillay K. R. M., Aasahida T., Chen C. A., Terashima H., Ida H. 2006. ITS Ribosomal DNA distinctions and the genetic structures of populations of two sympatric species of Pavona (Cnidaria: Scleractinia) from Mauritius. Zool. Stud. 45, 132–144 [Google Scholar]
- 22.Forsman Z. H., Barshis D. J., Hunter C. L., Toonen R. J. 2009. Shape-shifting corals: molecular markers show morphology is evolutionarily plastic in Porites . BMC Evol. Biol. 9, 45. 10.1186/1471-2148-9-45 (doi:10.1186/1471-2148-9-45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budd A. F., Johnson K. G., Stemann T. A., Tompkins B. H. 1999. Poioce to Pleistocene reef coral assemblages in the Limon Group of Costa Rica. In A paleobiotic survey of the Caribbean faunas from the Neogene of the Isthmus of Pana (eds Collins L. S., Coates A. G.), pp. 119–158 Ithaca, NY: Bulletins of American Paleontology [Google Scholar]
- 24.Klaus J. S., Budd A. F., McNeill D. F., Johnson K. G. 2008. Assessing community change in Miocene to Pliocene coral assemblages of the northern Dominican Republic. In Evolutionary stasis and change in the Dominican Republic (eds Nehm R. H., Budd A. F.), pp. 193–224 New York, NY: Springer [Google Scholar]
- 25.Budd A. F., Wallace C. C. 2008. First record of the Indo-Pacific reef coral genus Isopora in the Caribbean Region: two new species from the Neogene of Curacao, Netherlands Antilles. Palaeontology 51, 1387–1401 10.1111/j.1475-4983.2008.00820.x (doi:10.1111/j.1475-4983.2008.00820.x) [DOI] [Google Scholar]
- 26.McNeill D. F., Coates A. G., Budd A. F., Borne P. F. 2000. Integrated paleontologic and paleomagnetic stratigraphy of the upper Neogene deposits around Limon, Costa Rica: a coastal emergence record of the Central American Isthmus. Geol. Soc. Am. Bull. 112, 963–981 (doi:10.1130/0016-7606(2000)112<963:IPAPSO>2.0.CO;2) [DOI] [Google Scholar]
- 27.McNeill D. F., Klaus J. S., Budd A. F., Lutz B. P., Ishman S. E. 2012. Late Neogene chronology and sequence stratigraphy of mixed carbonate-siliciclastic deposits of the Cibao Basin, Dominican Republic. Geol. Soc. Am. Bull. 124, 35–58 10.1130/B30391.1 (doi:10.1130/B30391.1) [DOI] [Google Scholar]
- 28.Lunn D., Spiegelhalter D., Thomas A., Best N. 2009. The BUGS project: evolution, critique and future directions. Stat. Med. 28, 3049–3067 10.1002/sim.3680 (doi:10.1002/sim.3680) [DOI] [PubMed] [Google Scholar]
- 29.Firth D. 1993. Bias reduction of maximum-likelihood estimates. Biometrika 80, 27–3831. 10.1093/biomet/80.1.27 (doi:10.1093/biomet/80.1.27) [DOI] [Google Scholar]
- 30.Heinze G. 2006. A comparative investigation of methods for logistic regression with separated or nearly separated data. Stat. Med. 25, 4216–4226 10.1002/sim.2687 (doi:10.1002/sim.2687) [DOI] [PubMed] [Google Scholar]
- 31.Mattila N., Kotiaho J. S., Kaitala V., Komonen A. 2008. The use of ecological traits in extinction risk assessments: a case study on geometrid moths. Biol. Conserv. 141, 2322–2328 10.1016/j.biocon.2008.06.024 (doi:10.1016/j.biocon.2008.06.024) [DOI] [Google Scholar]
- 32.Olden J. D., Poff N. L., Bestgen K. R. 2008. Trait synergisms and the rarity, extirpation, and extinction risk of desert fishes. Ecology 89, 847–856 10.1890/06-1864.1 (doi:10.1890/06-1864.1) [DOI] [PubMed] [Google Scholar]
- 33.Murray K. A., Rosauer D., McCallum H., Skerratt L. F. 2011. Integrating species traits with extrinsic threats: closing the gap between predicting and preventing species declines. Proc. R. Soc. B 278, 1515–1523 10.1098/rspb.2010.1872 (doi:10.1098/rspb.2010.1872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loya Y., Sakai K., Yamazato K., Nakano Y., Sambali H., van Woesik R. 2001. Coral bleaching: the winners and the losers. Ecol. Lett. 4, 122–131 10.1046/j.1461-0248.2001.00203.x (doi:10.1046/j.1461-0248.2001.00203.x) [DOI] [Google Scholar]
- 35.McClanahan T. R. 2004. The relationship between bleaching and mortality of common corals. Mar. Biol. 144, 1239–1245 10.1007/s00227-003-1271-9 (doi:10.1007/s00227-003-1271-9) [DOI] [Google Scholar]
- 36.van Woesik R., De Vantier L. M., Glazebrook J. S. 1995. Effects of cyclone ‘Joy’ on nearshore coral communities of the Great Barrier Reef. Mar. Ecol. Prog. Ser. 128, 261–270 10.3354/meps128261 (doi:10.3354/meps128261) [DOI] [Google Scholar]
- 37.Takahashi S., Nakamura T., Sakamizu M., van Woesik R., Yamasaki H. 2004. Repair machinery of symbiotic photosynthesis as the primary target of heat stress for reef-building corals. Plant Cell Physiol. 45, 251–255 10.1093/pcp/pch028 (doi:10.1093/pcp/pch028) [DOI] [PubMed] [Google Scholar]
- 38.McClanahan T. R. 2008. Response of the coral reef benthos and herbivory to fishery closure management and the 1998 ENSO disturbance. Oecologia 155, 169–177 10.1007/s00442-007-0890-0 (doi:10.1007/s00442-007-0890-0) [DOI] [PubMed] [Google Scholar]
- 39.Sheppard C. R. C. 2003. Predicted recurrences of mass coral mortality in the Indian Ocean. Nature 425, 294–297 10.1038/nature01987 (doi:10.1038/nature01987) [DOI] [PubMed] [Google Scholar]
- 40.Riegl B. 2002. Effects of the 1996 and 1998 positive sea-surface temperature anomalies on corals, coral diseases and fish in the Arabian Gulf (Dubai, UAE). Mar. Biol. 140, 29–40 10.1007/s002270100676 (doi:10.1007/s002270100676) [DOI] [Google Scholar]
- 41.Kavousi J., Seyfabadi J., Rezai H., Fenner D. Coral reefs and communities of Qeshm Island, the Persian Gulf. Zool. Stud. 50, 276–283 [Google Scholar]
- 42.Pagani M., Liu Z. H., LaRiviere J., Ravelo A. C. 2010. High earth-system climate sensitivity determined from Pliocene carbon dioxide concentrations. Nat. Geosci. 3, 27–30 10.1038/ngeo724 (doi:10.1038/ngeo724) [DOI] [Google Scholar]
- 43.Dowsett H. J., Robinson M. M. 2009. Mid-Pliocene equatorial Pacific sea surface temperature reconstruction: a multi-proxy perspective. Phil. Trans. R. Soc. A 367, 109–125 10.1098/rsta.2008.0206 (doi:10.1098/rsta.2008.0206) [DOI] [PubMed] [Google Scholar]
- 44.Haug G. H., Tiedemann R., Zahn R., Ravelo A. C. 2001. Role of Panama uplift on oceanic freshwater balance. Geology 29, 207–210 (doi:10.1130/0091-7613(2001)029<0207:ROPUOO>2.0.CO;2) [DOI] [Google Scholar]
- 45.Jain S., Collins L. S. 2007. Trends in Caribbean Paleoproductivity related to the Neogene closure of the Central American Seaway. Mar. Micropaleontol. 63, 57–74 10.1016/j.marmicro.2006.11.003 (doi:10.1016/j.marmicro.2006.11.003) [DOI] [Google Scholar]
- 46.Sarnthein M., Bartoli G., Prange M., Schmittner A., Schneider B., Weinelt M., Anderson N., Garbe-Schonberg D. 2009. Mid-Pliocene shifts in ocean overturning circulation and the onset of Quaternary-style climates. Clim. Past 5, 269–283 10.5194/cp-5-269-2009 (doi:10.5194/cp-5-269-2009) [DOI] [Google Scholar]
- 47.O'Dea A., Jackson J. B. C., Fortunato H., Smith J. T., D'Croz L., Johnson K. G., Todd J. A. 2007. Environmental change preceded Caribbean extinction by 2 million years. Proc. Natl Acad. Sci. USA 104, 5501–5506 10.1073/pnas.0610947104 (doi:10.1073/pnas.0610947104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith J. T., Jackson J. B. C. 2009. Ecology of extreme faunal turnover of tropical American scallops. Paleobiology 35, 77–93 10.1666/07054.1 (doi:10.1666/07054.1) [DOI] [Google Scholar]
- 49.Berkelmans R., van Oppen M. 2006. The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc. R. Soc. B 273, 2305–2312 10.1098/rspb.2006.3567 (doi:10.1098/rspb.2006.3567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.LaJeunesse T. C. 2005. ‘Species’ radiations of symbiotic dinoflagellates in the Atlantic and Indo-Pacific since the Miocene-Pliocene transition. Mol. Biol. Evol. 22, 570–581 10.1093/molbev/msi042 (doi:10.1093/molbev/msi042) [DOI] [PubMed] [Google Scholar]
- 51.van Woesik R., Sakai K., Ganase A., Loya Y. 2011. Revisiting the winners and the losers a decade after coral bleaching. Mar. Ecol. Prog. Ser. 434, 67–76 10.3354/meps09203 (doi:10.3354/meps09203) [DOI] [Google Scholar]
- 52.Thompson D. M., van Woesik R. 2009. Corals escape bleaching in regions that recently and historically experienced frequent thermal stress. Proc. R. Soc. B 276, 2893–2901 10.1098/rspb.2009.0591 (doi:10.1098/rspb.2009.0591) [DOI] [PMC free article] [PubMed] [Google Scholar]