Abstract
There are two highly interconnected clusters of visually responsive areas in the primate cortex. These two clusters have relatively few interconnections with each other, though those interconnections are undoubtedly important. One of the two main clusters (the dorsal stream) links the primary visual cortex (V1) to superior regions of the occipito-parietal cortex, while the other (the ventral stream) links V1 to inferior regions of the occipito-temporal cortex. According to our current understanding of the functional anatomy of these two systems, the dorsal stream's principal role is to provide real-time ‘bottom-up’ visual guidance of our movements online. In contrast, the ventral stream, in conjunction with top-down information from visual and semantic memory, provides perceptual representations that can serve recognition, visual thought, planning and memory offline. In recent years, this interpretation, initially based chiefly on studies of non-human primates and human neurological patients, has been well supported by functional MRI studies in humans. This perspective presents empirical evidence for the contention that the dorsal stream governs the visual control of movement without the intervention of visual awareness.
Keywords: vision, behaviour, neuroscience, consciousness
1. Introduction
There are two highly interconnected clusters of visually responsive areas in the primate cortical mantle. These two clusters have relatively few interconnections with each other, though those interconnections are undoubtedly important. One of the two main clusters (commonly known as the dorsal stream) links the primary visual cortex (V1) to superior regions of the occipito-parietal cortex, while the other (the ventral stream) links V1 to inferior regions of the occipito-temporal cortex [1–6]. According to our current understanding of the functional anatomy of these two systems, the dorsal stream's principal role is to provide real-time ‘bottom-up’ visual guidance of our movements online. In contrast, the ventral stream, in conjunction with top-down information from visual and semantic memory, provides perceptual representations that can serve recognition, visual thought, planning and memory offline [7–11]. In recent years, this interpretation, initially based chiefly on studies of non-human primates and human neurological patients, has been well supported by functional MRI studies in humans [12,13]. The well-documented inter-stream connections [4,10] can be assumed to play an important, though as yet poorly understood, role in the constant interplay between perception and visuomotor control that underpins our integrated visual life [10,14].
We have argued [10,15] that the course of primate evolution has resulted in these two visual streams operating on different computational principles, and that it has resulted concomitantly in them yielding qualitatively different visual products. Specifically, we have proposed that the visual products of dorsal stream1 processing are not available to conscious awareness—that they exist only as evanescent raw materials to provide the unconscious moment-to-moment sensory calibration of our movements. This article is intended to provide a brief review of the evidence from this claim, concentrating on three bodies of data, based on studies of (i) visual form agnosia, (ii) visual extinction and (iii) functional MRI.
2. Evidence from visual form agnosia
Patient D.F. is an intensively studied patient who is quite unable to distinguish different shapes or to report the size or orientation of items she is presented with. This so-called visual form agnosia is a rare condition, and D.F. is particularly unusual in having well-preserved cognitive and motor functions. Of particular interest is that she is able to perform an amazingly full repertoire of visually guided acts tailored to the size, shape or orientation of different objects, despite being quite unable to tell us anything about those same geometrical properties [16–19]. Clearly, these observations imply that such figural information must be processed somewhere in D.F.'s brain, despite its inaccessibility for conscious report. We subsequently gained clear evidence from functional MRI as to where this processing takes place. D.F. was scanned while performing reaching and grasping actions to objects of different sizes and shapes [20]. A region lying in the anterior part of the dorsal stream, the anterior intraparietal area (AIP or aIPS), was found to be activated more strongly during reaching-to-grasp than during reaching movements alone (actually reaching with a closed fist), just as in healthy control subjects. This preserved net activation in aIPS strongly suggests that visual information about shape and contour undergoes transformations into the motor parameters required to execute well-formed grasping actions in D.F., using the normal neural pathways. Thus, D.F.'s dorsal stream evidently provides visual processing that, despite being inaccessible to conscious report, enables preserved visually guided prehension to take place.
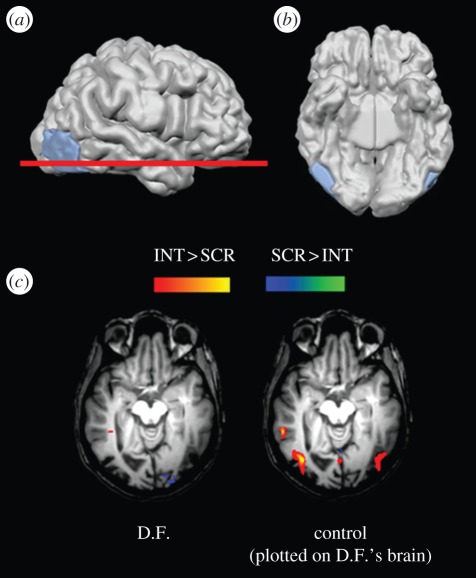
That same study [20] provided clear evidence as to why D.F. has no conscious perception of visual shapes. The capacity to perceive and recognize shapes is known to be mediated by a brain region, the lateral occipital area (LO), that is located within the ventral stream. This area is mapped by comparing the pattern of activation that is observed when pictures or drawings of whole objects are viewed, with that observed when scrambled versions of the same images are viewed [21,22]. A high-resolution structural MRI scan of D.F.'s brain indicated a close correspondence between the location of her most dense brain damage (on both sides), and the expected location of area LO based on data from healthy control subjects [20]. In striking confirmation of this discovery, when D.F. herself viewed a series of line drawings of objects, versus fragmented versions of them, in the MRI scanner, no net activations could be detected in her brain (figure 1). In other words, D.F.'s brain damage seems to have completely destroyed area LO bilaterally—certainly functionally, and probably also anatomically [20]. These observations thus provide a straightforward explanation of D.F.'s visual form agnosia: she has lost the neural machinery for constructing percepts of shapes and patterns from their component features. As an aside, D.F. does better at identifying pictures when colour and fine surface detail are provided, and this is nicely paralleled by fMRI observations on the activation patterns in her ventral stream [23,24]. Ventral-stream regions outside the lesion location, in more medial parts of the occipito-temporal cortex, show robust net activations when, and only when, these surface features are present in images of objects presented to her [20].
Figure 1.
Functional and structural MRI data on patient D.F. (a) A right lateral view of D.F.'s brain, showing a lesion marked in blue that encompasses area LO. (b) A ventral view of D.F.'s cerebral hemispheres, showing that the lesions of area LO are bilateral. (c) fMRI activation for line drawings (versus scrambled drawings) plotted on a horizontal section through D.F.'s brain at the level of the red line on (a). D.F. shows no selective activation for line drawings either in area LO or in neighbouring areas. In contrast, a control subject shows robust activation to the same drawings. The activation in the control subject's brain, which has been mathematically morphed to fit onto D.F.'s brain, coincides well with her LO lesions. Reprinted from Milner & Goodale [10].
The converse behavioural picture is seen in patients with unilateral or bilateral damage to their dorsal stream, who have difficulties in reaching for and grasping visual objects, while in most cases remaining able to report their perceptions and to make discriminations based on them [25–28]. Deficits in tailoring the grasp to the object, as opposed to deficits in reaching accuracy alone, appear to depend on whether the damage extends anteriorly to include area aIPS [29], in agreement with fMRI studies. These complementary dissociations following damage to the human ventral or dorsal stream demonstrate rather clearly that visual information about shape and contour can be used by the brain for guiding actions, despite being unavailable for conscious visual perception. They also tie this unconscious visuomotor processing of object geometry specifically to areas within the dorsal stream.
3. Evidence from visual extinction
The earlier-mentioned conclusions, reached from behavioural and neuroimaging observations of patient D.F., apply only to the visual processing of objects that formed the targets for simple actions—solid geometric shapes, for example, that she was asked to reach out for and pick up. But our acts of prehension in everyday life typically need to take into account much more visual information than just the properties of the target object. Clearly, if Milner & Goodale's proposal [10] that we have no conscious access to the visual products of dorsal stream processing is to have any credibility, it must apply to all aspects of the visual control of our voluntary movements.
For example, we generally also use online visual feedback from the moving hand during the act of reaching out. This information serves to improve the accuracy of our reaches by providing moment-to-moment visual feedback about the relative spatial coordinates of the hand and its target during the movement. A second complication arises from the fact that the target objects that we need to interact with in real life are seldom present in isolation. In contrast to the impoverished stimulus arrays that characterize most research on visuomotor control, we often need to skirt around potential obstacles that lie in the vicinity of the target object or adjacent to the route of our intended action. It has been established that even when there is little or no chance of collision or damage taking place, our reaching movements still show small but consistent displacements away from non-target objects [30,31].
The question as to whether the brain, in processing this non-target information to guide our reaching movements through space, uses visual representations that can reach our conscious visual perception, cannot be addressed by testing patients like D.F. This is because her brain damage is concentrated in area LO, a brain module dedicated to object–shape perception rather than to static or dynamic spatial processing. Instead, we have carried out extensive testing of a patient with visual extinction. Extinction, which is a symptom often associated with spatial neglect, refers to a failure to detect a sensory stimulus presented on the side of space opposite to a brain lesion, when and only when there is simultaneously a stimulus present on the ‘good’ (ipsi-lesional) side as well. The definition requires that a unilateral stimulus on either side can be detected alone, thus ruling out a simple sensory failure. Extinction is believed to be caused by a pathological imbalance of attentional resources, which reveals itself as a loss of awareness on the contra-lesional side of space under competitive conditions. As a rule, extinction occurs most reliably when the sensory stimulation is presented very briefly, presumably because longer presentations allow time for attention to be switched from one side to the other. This time-dependence of extinction makes it an ideal ‘experiment of nature’ for studying the causal role of sensory awareness in behaviour, because at intermediate stimulus durations, the patient will sometimes detect, and sometimes not detect, the very same contra-lesional stimulus. By fixing the exposure duration judiciously, it thus becomes possible to collect and compare behavioural data on trials with identical physical stimulation but qualitatively different visual phenomenology.
Our patient, V.E., was aged in his early 70s at the time of testing, and a right parieto-temporal stroke (figure 2) 1 year earlier had left him with a persistent left-side visual (and tactile) extinction, but with no other detectable neuropsychological impairment. In particular, we undertook careful screening for visual field loss, and found no indication of any areas of blindness.
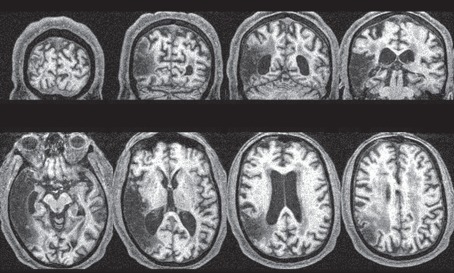
Figure 2.
MRI scan of patient V.E.'s brain. Cross sections are shown in axial and coronal planes, with the right hemisphere shown on the left. Extensive infarction is present within the right middle cerebral artery distribution region, with substantial involvement of the temporal lobe, though sparing medial temporal and hippocampal structures. Reprinted from McIntosh et al. [32].
(a). Obstacle avoidance
Our studies of obstacle avoidance have concentrated on the small automatic adjustments of our reach trajectories that occur when we reach towards an object in the presence of one or more non-target objects [30,31]. We investigated patient V.E.'s reaching behaviour using the arrangement illustrated in figure 3 [32].
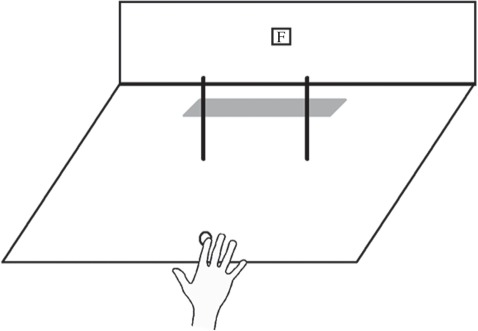
Figure 3.
Stimulus array for studying obstacle avoidance in extinction. The patient (V.E.) was asked to reach out from a fixed starting point on each trial and quickly touch the grey strip at the back of the board with his right forefinger, while holding his gaze steady on the fixation point (F). Reprinted from McIntosh et al. [32].
On any given trial, one or two thin rods would be present on the testing board, and the task was simply to reach out and touch the grey strip at the back of the board, passing the hand between the two rods. First, however, we needed to contrive the conditions such that V.E. would show extinction of the left rod. We therefore asked him to wear ‘shutter’ goggles with liquid-crystal lenses that could be switched between translucency and opacity instantaneously by means of an electrical pulse, so that his view of the board could be restricted reliably to a brief exposure. We first established what exposure time would be brief enough to result in visual extinction on a substantial proportion of trials, but without being so brief that accurate reaching between the two rods would be difficult. We found that at 500 ms, he failed to detect the left-hand rod on a majority of occasions when both rods were present, despite his reporting its presence on 90 per cent of occasions when it appeared alone. This exposure time was also sufficient to allow V.E., after a little practice, to reach between the two rods without colliding with either of them.
Unpredictably from trial to trial in the experiments proper, V.E. was presented with either the left rod alone, the right rod alone, both rods together or neither rod present. On each trial, he made his reaches as quickly as possible, and also reported immediately after doing so which rods (if any) he had seen on the board. As shown in figure 4, his reaches veered strongly away from the left rod (average trajectory shown in black) or right rod (shown in green), whenever either was presented alone. The points at which these reaches crossed the imaginary line joining the rods differed from each another at a highly reliable statistical level, and also differed reliably from the reaches made when both rods were present (shown in blue and red). Crucially, when both rods were present, a subset of reaches made when V.E. reported seeing only the right one (red) were statistically indistinguishable from those where he reported seeing both rods (blue). They were entirely different from the reaches he made when only the right rod actually was present (shown in green), despite the fact that his reported visual experience on these trials was the same. Thus, the reaches that V.E. made when he experienced visual extinction still took full account of the ‘unseen’ left rod, exactly like those he made when both rods were seen and reported.
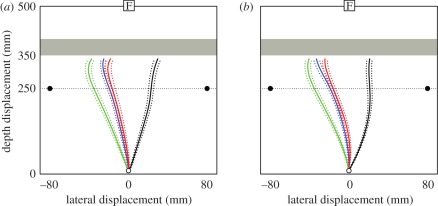
Figure 4.
Reaching routes followed by patient V.E. V.E.'s spatially averaged trajectories in each of two test conditions: (a) manual reach followed by verbal report; (b) verbal report preceding reach (dotted lines indicate standard errors). Green: reaches with only the right rod present; black: reaches with only the left rod present. Blue: reaches with both rods present, V.E. reports seeing both rods; red: reaches with both rods present, V.E. reports seeing only right rod (dotted lines indicate standard errors). The zero lateral coordinate is aligned with V.E.'s mid-sagittal axis. Reprinted from McIntosh et al. [32].
As figure 4 shows, it made no difference whether V.E. was asked to say after reaching what he had seen on that trial, or whether instead he was asked to say what he saw immediately before initiating his reach. This latter control condition rules out the possibility that in ‘after-report’ he might have ‘forgotten’ seeing a rod on the left, even though he actually had been aware of it at the moment of making his reach. In other words, it could have been that his visual short-term memory might itself have suffered from a kind of extinction, whereby his memory of what he saw on the left was blotted out by his recall of what was on the right. In practice, in both test conditions, his behaviour when he experienced extinction closely resembled his behaviour when he reported seeing both rods, and it differed completely from his behaviour when he correctly reported the presence of only the right rod.
These results strongly support the idea that the motor adjustments made when we take account of potential obstacles during reaching are guided by visual information that is not, or does not have to be, conscious. Furthermore, in separate studies, we have strong independent evidence that these adjustments to one's reaching behaviour depend crucially on the integrity of the dorsal stream. We found that two patients with bilateral damage to the dorsal stream (A.T. and I.G.) made no detectable adjustments at all to their reaching movements when reaching between two potential obstacles whose locations varied from trial to trial [33]. This indicates that the integrity of the dorsal stream is necessary for this behaviour to survive after brain damage. In addition, we have found in other experiments that neurological patients with severe visual difficulties, but whose dorsal stream is structurally spared, still perform normally on this task. As well as our patient with extinction (V.E.), the list includes patients with spatial neglect [34], and two patients (D.F. and S.B.) with visual form agnosia [35]. Finally, there is now complementary data from functional MRI showing that the dorsal stream is selectively activated during obstacle avoidance in healthy subjects [36].
Taken together, these experiments provide convincing evidence that obstacle avoidance, of this automatic variety at least, depends on the integrity of the dorsal stream, and that it proceeds without the need for conscious perception of the stimuli that guide it. Of course, there is little doubt that our healthy controls in these experiments were visually aware of both potential obstacles as they reached out between them. What the data from our extinction patient V.E. [32] show is that the controls did not need to be aware of the obstacles. Their awareness was presumably generated in a quite separate brain system (either in the ventral stream or in higher circuitry that receives inputs from the ventral stream) from the one that was guiding their movements (the dorsal stream and its associated visuomotor areas).
(b). Visual feedback during reaching
The second study we undertook with patient V.E. was designed to investigate the benefits of visual feedback received from the hand during reaching out towards an object. The accuracy of reaching generally improves when one can see one's hand [37,38], particularly if the hand is visible during the deceleration phase of the movement, while it is ‘zeroing in’ on the target. In fact, under normal viewing conditions, the brain continuously registers the visual locations of both the reaching hand and the target, incorporating these two visual elements within a single ‘loop’ that operates like a servomechanism to progressively reduce their mutual separation in space (the ‘error signal’) as the movement unfolds. When the need to use such visual feedback is increased by the occasional introduction of unnoticed perturbations in the location of the target during the course of a reach, a healthy subject will make the necessary adjustments to the parameters of his or her movement quite seamlessly [39]. In contrast, a patient with damage to the dorsal stream (patient I.G.) was quite unable to take such target changes on board: she first had to complete the reach towards the original location, before then making a post hoc switch to the new target location [40,41]. I.G.'s deficit was present even when she could perceive the changed location of the target very well. It thus seems very likely that the ability to exploit the error signal between hand and target during reaching is dependent on the integrity of the dorsal stream. And indeed different neural circuitry in the dorsal stream is known to be activated during reaching with versus without visual feedback from the hand, in both human and non-human primates [42–44].
Are we aware of the visual information that the dorsal stream uses about the location of the hand as it moves towards the target to achieve these instantaneous online adjustments? We addressed this question by contriving a task in which patient V.E. would often, by virtue of his visual extinction, be unaware of the visual information coming from his hand while executing reaching movements [45]. Because V.E. showed extinction for left-sided stimuli only, we had to ask him to reach with his left hand, which despite his earlier stroke, he could by this stage do without difficulty. In order to manipulate feedback from V.E.'s moving hand, we attached a small LED to his index finger while he reached out in the dark towards a second LED that served as the target. The target light always came on briefly at the beginning of each trial, and then during the reach itself, one or other of the visual stimuli (target or finger), or both together, was switched on for a short time. Of course, having both stimuli (target and finger) illuminated would ‘close the loop’, but at the same time would tend to induce extinction of the hand LED. As it turned out, 500 ms presentations were again perfect for our purpose: this duration was long enough to provide significant feedback benefits for reaching accuracy, while at the same time being short enough to result in extinction of the hand LED on more than half of the test trials.
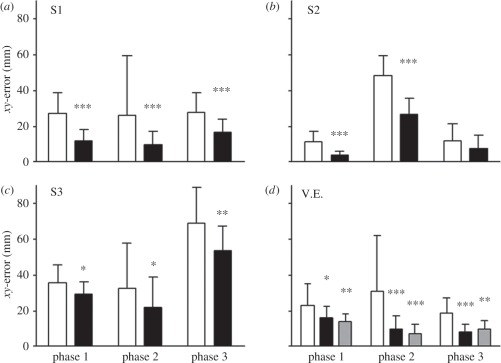
The results are shown in figure 5. It is clear that throughout the three phases of the experiment,2 the benefits of visual feedback were strong and reliable in all of our subjects. This was just as true in patient V.E. as in all of the healthy control subjects we tested. Crucially, however, this benefit was equally strong and reliable whether V.E. was aware of the visual feedback on a given trial or not. There were no statistically reliable differences in reaching accuracy between the ‘aware’ trials and the ‘unaware’ trials. In other words, we can conclude that whether or not V.E. was conscious of the light stimulus on his finger was a matter quite irrelevant to the accuracy of his movements. The feedback still benefited his visuomotor system to the same extent, regardless of whether or not he was visually aware of its presence.
Figure 5.
The improvement in reaching accuracy resulting from visual feedback. Data are shown for three healthy subjects (a–c: white bars, feedback; black bars, no feedback) and for patient V.E. ((d) white bars, no feedback; black bars, feedback-extinction; grey bars, feedback-no extinction). The histograms depict the mean reaching error and its standard deviation. The open bars refer to the trials without visual feedback, the black bars the trials with visual feedback. In the case of V.E. (d), the feedback trials have been divided into those where he showed extinction (i.e. the feedback was unperceived: black bars) and those where he did not (i.e. the feedback was consciously perceived (shaded bars). The asterisks give the statistical significance of the difference in accuracy between feedback and no-feedback trials (*p < 0.05; **p < 0.01; ***p < 0.001). In patient V.E., those trials in which the visual feedback was perceived, and the trials in which it was consciously visible, both differed significantly from trials where there was no visual feedback. Trials where the visual feedback was conscious or unconscious did not differ significantly from each other. Modified from Schenk et al. [45].
By extension, we can infer that even in our healthy controls, this same visuomotor control loop was operating on the basis of unconsciously processed information from their reaching hand, even though, unlike V.E., they were always able to report seeing the light on the hand whenever it was switched on during a reach. The logic behind this apparently paradoxical state of affairs is similar to that in our earlier-mentioned argument based on V.E.'s behaviour in the obstacle avoidance experiment. There is no question of the controls being unaware of the light stimulus emanating from their reaching hand—clearly, they were fully aware of it. Our claim is that the processing of that stimulus for visuomotor guidance took place in a different brain system from that which generated the conscious visual experiences reported by the controls. The data support our contention that the normal brain routinely uses unconscious visual information about hand location during reaching.
4. Evidence from functional neuroimaging
Most of the evidence I have presented so far comes from studying brain-damaged patients. Evidence of this kind is very powerful, particularly in its ability to decide between different causal accounts—in this instance, regarding the role of unconscious visual processing in the guidance of behaviour. Explanations in cognitive neuroscience, however, are at their most convincing when they are supported by converging evidence from more than just one methodology. The directly converse approach to investigating how brain damage affects cognition and behaviour is to monitor the areas that are active during those same forms of cognition and behaviour in the intact brain. Currently, the most effective way to do this in humans is to use functional MRI [46]. Of course, all techniques that record the brain activity that accompanies behaviour and cognition inevitably suffer from the critical limitation of being correlational—they can never alone provide conclusive evidence for particular causal accounts of the phenomena in question. But although functional MRI cannot stand alone, it can be very powerful in convergent combination with data derived from other methodologies.
There is now strong evidence that neural activation levels in several ventral-stream areas correlate closely with visual awareness. One of the best-known examples comes from exploiting the well-known phenomenon of ‘binocular rivalry’, in which observers are presented dichoptically with two conflicting images (for example, a face to one eye and a building to the other). Under these circumstances, the observer experiences constantly alternating percepts of either the face alone or the house alone, depending from moment to moment on which eye's image is dominating perception. Yet of course despite these changing experiences, the two retinae (and the early parts of the visual system) are receiving the same visual stimulation throughout. Tong et al. [47] exploited this phenomenon by measuring fMRI activations in two eponymous areas of the human ventral stream known to be specialized for the processing of the stimuli used in their experiment: the fusiform face area (FFA) and parahippocampal place area (PPA), respectively. They found that activation in the FFA and PPA fluctuated up and down in a reciprocal fashion, in close correspondence with a subject's current awareness of the face or the building (which the subject signalled by pressing a key whenever a change occurred). In other words, the level of activity in each area was directly correlated with the presence or absence of visual awareness for stimuli within its visual specialization. Presumably whatever brain process determined the switching between the two alternative percepts did so by modulating the relative activity levels in these two parts of the ventral stream.
Importantly, a more recent fMRI study by Large et al. [48] has shown that not all ventral-stream areas show such a close correlation with visual awareness. Their subjects viewed two successive arrays of four faces, and had to judge whether one of the faces changed between the first and second views. Area FFA showed exactly the same adaptation effect when a real change went unnoticed as when there really was no change; that is, the FFA behaved in a way corresponding to conscious perception. However, an earlier area (the occipital face area) behaved quite differently, showing a lack of adaptation whenever a change occurred, irrespective of whether the subject noticed it, suggesting that this area reflects the retinal stimulation, not the conscious perception.
These and other studies provide strong evidence for a close association between different patterns of brain activity within the ventral stream and the contents of conscious perception. Notably, however, none of these studies tell us whether the differential fMRI activation associated with conscious versus unconscious processing is restricted to the ventral stream. Might not such differential effects also be present in the dorsal stream?
As mentioned earlier in this chapter, functional MRI has uncovered specific parts of the human dorsal stream that are concerned with visuomotor control, including ones that are specialized for the processing of object shape, size and orientation to guide our hand shaping when we reach to pick up objects. The best-known part of this ‘grasp’ circuitry (area AIP) lies anteriorly within the intraparietal sulcus, close to the border between the dorsal stream and primary sensorimotor cortex. In addition, a more posterior dorsal-stream area, cIPS, lying in the caudal intraparietal sulcus [49,50] is selectively activated by action-relevant information about the shape and orientation of objects, even when no overt action occurs [51]. The crucial question for present purposes, therefore, is whether the level of activation in any of these visuomotor areas is selectively associated with conscious visual experiences of shape, size or orientation. Because they are concerned with processing object information for calibrating grasping, areas including AIP and cIPS may be regarded as the dorsal-stream counterpart of the LO area in the ventral stream.
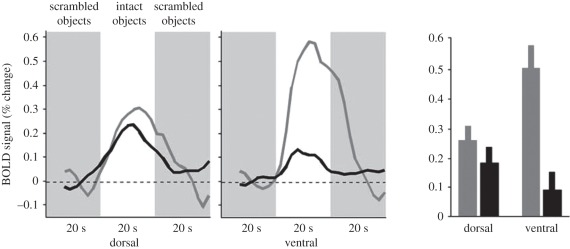
Fang & He [52] have directly compared the activation of area LO with the object-related areas in the dorsal stream, while presenting images of objects to one eye that could not be consciously perceived, owing to the presence of a simultaneous high-contrast dynamic noise pattern on the other eye. As shown in figure 6, they discovered that although observers were quite unaware of the object pictures as a result of this interocular suppression, the pictures still elicited substantial fMRI activation in the dorsal stream, and indeed this activation did not differ reliably from that recorded when the image was consciously perceived. In sharp contrast, as would be expected from previous work using binocular rivalry, Fang & He did find large differences in activation in the ventral stream (in and around area LO) between these ‘unaware’ and ‘aware’ conditions.
Figure 6.
The results of Fang & He's [52] experiment. The time courses and the average fMRI response (percentage change BOLD signals) from dorsal and ventral object sensitive areas in ‘visible’ (grey curves and bars) and ‘invisible’ (black curves and bars) conditions. Data (mean ± s.e.m.) were averaged across eight subjects. Reprinted from Fang & He [52].
Fang & He's results provide the first clear fMRI evidence that neural activity in the dorsal stream is not correlated with visual awareness. The fMRI data mesh nicely with the behavioural data of Roseboom & Arnold [53], who have shown that under this kind of high-contrast suppression, healthy observers can still orient their grasp appropriately for objects presented at different orientations, despite being quite unaware of the stimulus orientation. It can reasonably be concluded, therefore, that visual shape information gets through and is processed in the dorsal stream, even when it is suppressed in the ventral stream, and irrespective of whether or not it is consciously perceived.
Fang & He's data nicely support the conclusions that emerge from our studies of brain-damaged patients, namely that visual processing in the dorsal stream proceeds quite independently of the concurrent conscious perception of the observer. In fact, we can reasonably assume that the very same object-processing systems in the dorsal stream that were activated in Fang & He's experiment are functional in our form-agnosic patient D.F. too, thereby allowing her to perform perfectly well-formed grasping actions for objects of different shapes and sizes. In neither D.F., nor the healthy subjects of Fang & He's experiment, do these systems provide any conscious visual percepts of object form. Such conscious perception, when present, is evidently generated elsewhere—namely within the ventral stream or beyond.
Importantly, Fang & He's particular experimental conditions allowed them to demonstrate a significant statistical interaction between ‘visible/invisible’ and ‘dorsal/ventral’ in terms of fMRI activations. Such an interaction provides the most convincing argument possible for a differential role of the two streams in furnishing visual awareness. A more recent study by Hesselmann & Malach [54] did not find such an interaction, using a different kind of binocular suppression to induce unawareness. However, a negative result in a context like this is less powerful than a positive result. For example, in an extreme limiting case, if the suppression was sufficiently intrusive that no effective computation of form could be performed in visual cortex at all, then necessarily there would be reduced activation levels in both streams relative to a non-masked ‘visible’ condition, which is what Hesselmann & Malach found. Behavioural evidence strongly suggests that this happens when so-called ‘backward masking’, for example, is used [55]. In other words, the type and extent of suppression has to be tailored such as to permit effective form processing in both streams.
A second caveat should be noted. The present argument is not intended to imply that the dorsal stream plays no role whatever in determining conscious visual perception. In particular, the stimulus information that informs each of our percepts is obtained through a dynamic selection process that is governed by parietal and frontal areas concerned with the control of eye movements and shifts of visual attention. These areas (which notably include the lateral intraparietal area, LIP, within the dorsal stream) are also active in association with perceptual switching during binocular rivalry [56]. The important point for present purposes is that there is no evidence that these attention-related areas code anything about the specific contents of the alternating perceptual experiences. For example, the areas show an equally strong fMRI response whether one's experience switches from percept A to percept B or from percept B to percept A (e.g. Milner [57], pp. 195–196).
5. Conclusion
It is worth re-emphasizing that when we reach out to pick up an object, we may have full visual awareness of our arm moving and our hand configuring in a certain way, of the target object as having a certain shape and lying in a certain location, of the presence and nature of neighbouring objects and so on. In other words, we may be fully visually aware of our actions and of the detailed environmental context in which they are made. But the essence of Milner & Goodale's [10] interpretation is that this visual awareness accrues not from visuomotor processing in the dorsal stream, but from concurrent processing in the ventral stream. Conversely, according to the model, such ventral-stream processing plays no causal role in the real-time visual guidance of the action, despite our strong intuitive inclination to believe otherwise (what Clark [58] calls ‘the assumption of experienced-based control’). According to the Milner & Goodale model, that real-time guidance is provided through continuous visual monitoring by the dorsal stream of those very same visual inputs that we experience by courtesy of our ventral stream.
Acknowledgements
The author is grateful to Dr Cristiana Cavina-Pratesi and Dr Jason D Connolly for their comments on a draft of this paper.
Endnotes
The areas that constitute the primate dorsal stream include V6, V6A, 7a, medial intraparietal area (MIP), ventral intraparietal area (VIP), LIP, cIPS and AIP. Likely human homologues for almost all of these areas have now been found using functional neuroimaging.
In phase 2, prismatic glasses were worn, causing a rightward shift of 10° in the perceived position of the target. This was done in order to maximize the benefits of visual feedback. It is not relevant for the current argument.
Author profile
A. David Milner

David Milner was born in Leeds, and grew up in the neighbouring town of Pudsey. He was educated at Bradford Grammar School and Lincoln College, Oxford, where he read Psychology, Philosophy and Physiology, graduating in 1965. He then undertook postgraduate training in clinical psychology at the Institute of Psychiatry in London, where he subsequently completed a PhD on cross-modal transfer and matching in primates, under the supervision of Prof. George Ettlinger. In 1970, he moved to a lectureship at the University of St Andrews, where he worked for almost 30 years, before moving to a Chair in Cognitive Neuroscience at Durham in 2000. David Milner's research interests centre on the brain mechanisms underlying visual perception, visual attention and the visual guidance of action. He was elected a Fellow of the Royal Society of Edinburgh in 1992 and of the Royal Society of London in 2011.
References
- 1.Morel A., Bullier J. 1990. Anatomical segregation of two cortical visual pathways in the macaque monkey. Vis. Neurosci. 4, 555–578 10.1017/S0952523800005769 (doi:10.1017/S0952523800005769) [DOI] [PubMed] [Google Scholar]
- 2.Baizer J. S., Ungerleider L. G., Desimone R. 1991. Organization of visual inputs to the inferior temporal and posterior parietal cortex in macaques. J. Neurosci. 11, 168–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young M. P. 1992. Objective analysis of the topological organization of the primate cortical visual system. Nature 358, 152–155 10.1038/358152a0 (doi:10.1038/358152a0) [DOI] [PubMed] [Google Scholar]
- 4.Van Essen D. C. 2005. Corticocortical and thalamocortical information flow in the primate visual system. Prog. Brain Res. 149, 173–185 10.1016/S0079-6123(05)49013-5 (doi:10.1016/S0079-6123(05)49013-5) [DOI] [PubMed] [Google Scholar]
- 5.Borra E., Belmalih A., Calzavara R., Gerbella M., Murata A., Rozzi S., Luppino G. 2008. Cortical connections of the macaque anterior intraparietal (AIP) area. Cereb. Cortex 18, 1094–1111 10.1093/cercor/bhm146 (doi:10.1093/cercor/bhm146) [DOI] [PubMed] [Google Scholar]
- 6.Borra E., Ichinohe N., Sato T., Tanifuji M., Rockland K. S. 2010. Cortical connections to area TE in monkey: hybrid modular and distributed organization. Cereb. Cortex 20, 257–270 10.1093/cercor/bhp096 (doi:10.1093/cercor/bhp096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodale M. A., Milner A. D. 1992. Separate visual pathways for perception and action. Trends Neurosci. 15, 20–25 10.1016/0166-2236(92)90344-8 (doi:10.1016/0166-2236(92)90344-8) [DOI] [PubMed] [Google Scholar]
- 8.Jeannerod M., Rossetti Y. 1993. Visuomotor coordination as a dissociable visual function: experimental and clinical evidence. In Visual perceptual defects (Baillière's Clinical Neurology, vol.2, no.2) (ed. Kennard C.), pp. 439–460 London, UK: Baillière Tindall; [PubMed] [Google Scholar]
- 9.Jacob P., Jeannerod M. 2003. Ways of seeing: the scope and limits of visual cognition. Oxford, UK: Oxford University Press [Google Scholar]
- 10.Milner A. D., Goodale M. A. 2006. The visual brain in action, 2nd edn Oxford, UK: Oxford University Press [Google Scholar]
- 11.Milner A. D., Goodale M. A. 2008. Two visual systems re-viewed. Neuropsychologia 46, 774–785 10.1016/j.neuropsychologia.2007.10.005 (doi:10.1016/j.neuropsychologia.2007.10.005) [DOI] [PubMed] [Google Scholar]
- 12.Culham J. C., Danckert S. L., DeSouza J. F. X., Gati J. S., Menon R. S., Goodale M. A. 2003. Visually-guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Exp. Brain Res. 153, 180–189 10.1007/s00221-003-1591-5 (doi:10.1007/s00221-003-1591-5) [DOI] [PubMed] [Google Scholar]
- 13.Cavina-Pratesi C., Goodale M. A., Culham J. C. 2007. FMRI reveals a dissociation between grasping and perceiving the size of real 3D objects. PLoS ONE 2, e424. 10.1371/journal.pone.0000424 (doi:10.1371/journal.pone.0000424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schenk T., McIntosh R. D. 2010. Do we have independent visual streams for perception and action? Cognitive Neuroscience 1, 52–78 10.1080/17588920903388950 (doi:10.1080/17588920903388950) [DOI] [PubMed] [Google Scholar]
- 15.Milner A. D. 1995. Cerebral correlates of visual awareness. Neuropsychologia 33, 1117–1130 10.1016/0028-3932(95)00052-5 (doi:10.1016/0028-3932(95)00052-5) [DOI] [PubMed] [Google Scholar]
- 16.Milner A. D., et al. 1991. Perception and action in ‘visual form agnosia’. Brain 114, 405–428 10.1093/brain/114.1.405 (doi:10.1093/brain/114.1.405) [DOI] [PubMed] [Google Scholar]
- 17.Goodale M. A., Milner A. D., Jakobson L. S., Carey D. P. 1991. A neurological dissociation between perceiving objects and grasping them. Nature 349, 154–156 10.1038/349154a0 (doi:10.1038/349154a0) [DOI] [PubMed] [Google Scholar]
- 18.Goodale M. A., Meenan J. P., Bülthoff H. H., Nicolle D. A., Murphy K. J., Racicot C. I. 1994. Separate neural pathways for the visual analysis of object shape in perception and prehension. Curr. Biol. 4, 604–610 10.1016/S0960-9822(00)00132-9 (doi:10.1016/S0960-9822(00)00132-9) [DOI] [PubMed] [Google Scholar]
- 19.Carey D. P., Harvey M., Milner A. D. 1996. Visuomotor sensitivity for shape and orientation in a patient with visual form agnosia. Neuropsychologia 34, 329–338 10.1016/0028-3932(95)00169-7 (doi:10.1016/0028-3932(95)00169-7) [DOI] [PubMed] [Google Scholar]
- 20.James T. W., Culham J., Humphrey G. K., Milner A. D., Goodale M. A. 2003. Ventral occipital lesions impair object recognition but not object-directed grasping: a fMRI study. Brain 126, 2463–2475 10.1093/brain/awg248 (doi:10.1093/brain/awg248) [DOI] [PubMed] [Google Scholar]
- 21.Malach R., et al. 1995. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc. Natl Acad. Sci. USA 92, 8135–8139 10.1073/pnas.92.18.8135 (doi:10.1073/pnas.92.18.8135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanwisher N., Woods R. P., Iacoboni M., Mazziotta J. C. 1997. A locus in human extrastriate cortex for visual shape analysis. J. Cogn. Neurosci. 9, 133–142 10.1162/jocn.1997.9.1.133 (doi:10.1162/jocn.1997.9.1.133) [DOI] [PubMed] [Google Scholar]
- 23.Cavina-Pratesi C., Kentridge R. W., Heywood C. A., Milner A. D. 2010. Separate processing of texture and form in the ventral stream: evidence from fMRI and visual agnosia. Cereb. Cortex 20, 433–446 10.1093/cercor/bhp111 (doi:10.1093/cercor/bhp111) [DOI] [PubMed] [Google Scholar]
- 24.Cavina-Pratesi C., Kentridge R. W., Heywood C. A., Milner A. D. 2010. Separate form, texture and colour processing in the ventral stream: evidence from fMRI adaptation and visual agnosia. Cereb. Cortex 20, 2319–2332 10.1093/cercor/bhp298 (doi:10.1093/cercor/bhp298) [DOI] [PubMed] [Google Scholar]
- 25.Perenin M.-T., Vighetto A. 1983. Optic ataxia: a specific disorder in visuomotor coordination. In Spatially oriented behavior (eds Hein A., Jeannerod M.), pp. 305–326 New York, NY: Springer [Google Scholar]
- 26.Perenin M.-T., Vighetto A. 1988. Optic ataxia: a specific disruption in visuomotor mechanisms. I. Different aspects of the deficit in reaching for objects. Brain 111, 643–674 10.1093/brain/111.3.643 (doi:10.1093/brain/111.3.643) [DOI] [PubMed] [Google Scholar]
- 27.Jeannerod M., Decety J., Michel F. 1994. Impairment of grasping movements following bilateral posterior parietal lesion. Neuropsychologia 32, 369–380 10.1016/0028-3932(94)90084-1 (doi:10.1016/0028-3932(94)90084-1) [DOI] [PubMed] [Google Scholar]
- 28.Milner A. D., Dijkerman H. C., McIntosh R. D., Rossetti Y., Pisella L. 2003. Delayed reaching and grasping in patients with optic ataxia. Prog. Brain Res. 142, 225–242 10.1016/S0079-6123(03)42016-5 (doi:10.1016/S0079-6123(03)42016-5) [DOI] [PubMed] [Google Scholar]
- 29.Cavina-Pratesi C., Ietswaart M., Humphreys G. W., Lestou V., Milner A. D. 2010. Impaired grasping in a patient with optic ataxia: primary visuomotor deficit or secondary consequence of misreaching? Neuropsychologia 48, 226–234 10.1016/j.neuropsychologia.2009.09.008 (doi:10.1016/j.neuropsychologia.2009.09.008) [DOI] [PubMed] [Google Scholar]
- 30.Tipper S. P., Howard L. A., Jackson S. R. 1997. Selective reaching to grasp: evidence for distractor interference effects. Visual Cogn. 4, 1–38 10.1080/713756749 (doi:10.1080/713756749) [DOI] [Google Scholar]
- 31.Tresilian J. R. 1998. Attention in action or obstruction of movement? A kinematic analysis of avoidance behaviour in prehension. Exp. Brain Res. 120, 352–368 10.1007/s002210050409 (doi:10.1007/s002210050409) [DOI] [PubMed] [Google Scholar]
- 32.McIntosh R. D., McClements K. I., Schindler I., Cassidy T. P., Birchall D., Milner A. D. 2004. Avoidance of obstacles in the absence of visual awareness. Proc. R. Soc. Lond. B 271, 15–20 10.1098/rspb.2003.2545 (doi:10.1098/rspb.2003.2545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schindler I., Rice N. J., McIntosh R. D., Rossetti Y., Vighetto A., Milner A. D. 2004. Automatic avoidance of obstacles is a dorsal stream function: evidence from optic ataxia. Nat. Neurosci. 7, 779–784 10.1038/nn1273 (doi:10.1038/nn1273) [DOI] [PubMed] [Google Scholar]
- 34.McIntosh R. D., McClements K. I., Dijkerman H. C., Birchall D., Milner A. D. 2004. Preserved obstacle avoidance during reaching in patients with left visual neglect. Neuropsychologia 42, 1107–1117 10.1016/j.neuropsychologia.2003.11.023 (doi:10.1016/j.neuropsychologia.2003.11.023) [DOI] [PubMed] [Google Scholar]
- 35.Rice N. J., McIntosh R. D., Schindler I., Mon-Williams M., Démonet J.-F., Milner A. D. 2006. Intact automatic avoidance of obstacles in patients with visual form agnosia. Exp. Brain Res. 174, 176–188 10.1007/s00221-006-0435-5 (doi:10.1007/s00221-006-0435-5) [DOI] [PubMed] [Google Scholar]
- 36.Chapman C. S., Gallivan J. P., Culham J. C., Goodale M. A. 2011. Mental blocks: fMRI reveals top-down modulation of early visual cortex when obstacles interfere with grasp planning. Neuropsychologia 49, 1703–1717 10.1016/j.neuropsychologia.2011.02.048 (doi:10.1016/j.neuropsychologia.2011.02.048) [DOI] [PubMed] [Google Scholar]
- 37.Prablanc C., Échallier J. F., Komilis E., Jeannerod M. 1979. Optimal response of eye and hand motor systems in pointing to a visual target. I. Spatio-temporal characteristics of eye and hand movements and their relationships when varying the amount of visual information. Biol. Cybern. 35, 113–124 10.1007/BF00337436 (doi:10.1007/BF00337436) [DOI] [PubMed] [Google Scholar]
- 38.Prablanc C., Échallier J. F., Jeannerod M., Komilis E. 1979. Optimal response of eye and hand motor systems in pointing at a visual target. II. Static and dynamic visual cues in the control of hand movement. Biol. Cybern. 35, 183–187 10.1007/BF00337063 (doi:10.1007/BF00337063) [DOI] [PubMed] [Google Scholar]
- 39.Goodale M. A., Pélisson D., Prablanc C. 1986. Large adjustments in visually guided reaching do not depend on vision of the hand or perception of target displacement. Nature 320, 748–750 10.1038/320748a0 (doi:10.1038/320748a0) [DOI] [PubMed] [Google Scholar]
- 40.Pisella L., Gréa H., Tilikete C., Vighetto A., Desmurget M., Rode G., Boisson D., Rossetti Y. 2000. An ‘automatic pilot’ for the hand in human posterior parietal cortex: toward reinterpreting optic ataxia. Nat. Neurosci. 3, 729–736 10.1038/76694 (doi:10.1038/76694) [DOI] [PubMed] [Google Scholar]
- 41.Gréa H., Pisella L., Rossetti Y., Desmurget M., Tilikete C., Grafton S., Prablanc C., Vighetto A. 2002. A lesion of the posterior parietal cortex disrupts on-line adjustments during aiming movements. Neuropsychologia 40, 2471–2480 10.1016/S0028-3932(02)00009-X (doi:10.1016/S0028-3932(02)00009-X) [DOI] [PubMed] [Google Scholar]
- 42.Battaglia-Mayer A., Ferraina S., Genovesio A., Marconi B., Squatrito S., Molinari M., Lacquaniti F., Caminiti R. 2001. Eye-hand coordination during reaching. II. An analysis of the relationships between visuomanual signals in parietal cortex and parieto-frontal association projections. Cereb. Cortex 11, 528–544 10.1093/cercor/11.6.528 (doi:10.1093/cercor/11.6.528) [DOI] [PubMed] [Google Scholar]
- 43.Cavina-Pratesi C., Monaco S., Fattori P., Galletti C., McAdam T. D., Quinlan D. J., Goodale M. A., Culham J. C. 2010. Functional magnetic resonance imaging reveals the neural substrates of arm transport and grip formation in reach-to-grasp actions in humans. J. Neurosci. 30, 10 306–10 323 10.1523/JNEUROSCI.2023-10.2010 (doi:10.1523/JNEUROSCI.2023-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Filimon F., Nelson J. D., Huang R.-S., Sereno M. I. 2009. Multiple parietal reach regions in humans: cortical representations for visual and proprioceptive feedback during on-line reaching. J. Neurosci. 29, 2961–2971 10.1523/JNEUROSCI.3211-08.2009 (doi:10.1523/JNEUROSCI.3211-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schenk T., Schindler I., McIntosh R. D., Milner A. D. 2005. The use of visual feedback is independent of visual awareness: evidence from visual extinction. Exp. Brain Res. 167, 95–102 10.1007/s00221-005-0027-9 (doi:10.1007/s00221-005-0027-9) [DOI] [PubMed] [Google Scholar]
- 46.Kanwisher N., Duncan J. 2004. Attention and performance XX: functional neuroimaging of visual cognition. Oxford, UK: Oxford University Press [Google Scholar]
- 47.Tong F., Nakayama K., Vaughan J. T., Kanwisher N. 1998. Binocular rivalry and visual awareness in human extrastriate cortex. Neuron 21, 753–759 10.1016/S0896-6273(00)80592-9 (doi:10.1016/S0896-6273(00)80592-9) [DOI] [PubMed] [Google Scholar]
- 48.Large M. E., Cavina-Pratesi C., Vilis T., Culham J. 2008. The neural correlates of change detection in the face perception network. Neuropsychologia 46, 2169–2176 10.1016/j.neuropsychologia.2008.02.027 (doi:10.1016/j.neuropsychologia.2008.02.027) [DOI] [PubMed] [Google Scholar]
- 49.Sakata H., Taira M., Kusunoki M., Murata A., Tanaka Y. 1997. The parietal association cortex in depth perception and visual control of hand action. Trends Neurosci. 20, 350–357 10.1016/S0166-2236(97)01067-9 (doi:10.1016/S0166-2236(97)01067-9) [DOI] [PubMed] [Google Scholar]
- 50.James T. W., Humphrey G. K., Gati J. S., Menon R. S., Goodale M. A. 2002. Differential effects of viewpoint on object-driven activation in dorsal and ventral streams. Neuron 35, 793–801 10.1016/S0896-6273(02)00803-6 (doi:10.1016/S0896-6273(02)00803-6) [DOI] [PubMed] [Google Scholar]
- 51.Culham J. C., Valyear K. F. 2006. Human parietal cortex in action. Curr. Opin. Neurobiol. 16, 205–212 10.1016/j.conb.2006.03.005 (doi:10.1016/j.conb.2006.03.005) [DOI] [PubMed] [Google Scholar]
- 52.Fang F., He S. 2005. Cortical responses to invisible objects in the human dorsal and ventral pathways. Nat. Neurosci. 8, 1380–1385 10.1038/nn1537 (doi:10.1038/nn1537) [DOI] [PubMed] [Google Scholar]
- 53.Roseboom W., Arnold D. H. 2011. Learning to reach for ‘invisible’ visual input. Curr. Biol. 21, R493–R494 10.1016/j.cub.2011.05.036 (doi:10.1016/j.cub.2011.05.036) [DOI] [PubMed] [Google Scholar]
- 54.Hesselmann G., Malach R. 2011. The link between fMRI-BOLD activation and perceptual awareness is ‘stream-invariant’ in the human visual system. Cereb. Cortex 21, 2829–2837 10.1093/cercor/bhr085 (doi:10.1093/cercor/bhr085) [DOI] [PubMed] [Google Scholar]
- 55.Almeida J., Mahon B. Z., Nakayama K., Caramazza A. 2008. Unconscious processing dissociates along categorical lines. Proc. Natl Acad. Sci. USA 105, 15 214–15 218 10.1073/pnas.0805867105 (doi:10.1073/pnas.0805867105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knapen T., Brascamp J., Pearson J., van Ee R., Blake R. 2011. The role of frontal and parietal brain areas in bistable perception. J. Neurosci. 31, 10 293–10 301 10.1523/JNEUROSCI.1727-11.2011 (doi:10.1523/JNEUROSCI.1727-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Milner A. D. 2008. Visual awareness and human action. In Frontiers in consciousness research (eds Weiskrantz L., Davies M.). Oxford, UK: Oxford University Press [Google Scholar]
- 58.Clark A. 2011. Visual experience and motor action: are the bonds too tight? Phil. Rev. 110, 495–519 [Google Scholar]