Abstract
In Drosophila melanogaster, biological rhythms, aggression and mating are modulated by group size and composition. However, the fitness significance of this group effect is unknown. By varying the composition of groups of males and females, we show that social context affects reproductive behaviour and offspring genetic diversity. Firstly, females mating with males from the same strain in the presence of males from a different strain are infecund, analogous to the Bruce effect in rodents, suggesting a social context-dependent inbreeding avoidance mechanism. Secondly, females mate more frequently in groups composed of males from more than one strain; this mitigates last male sperm precedence and increases offspring genetic diversity. However, smell-impaired Orco mutant females do not increase mating frequency according to group composition; this indicates that social context-dependent changes in reproductive behaviour depend on female olfaction, rather than direct male–male interactions. Further, variation in mating frequency in wild-type strains depends on females and not males. The data show that group composition can affect variance in the reproductive success of its members, and that females play a central role in this process. Social environment can thus influence the evolutionary process.
Keywords: Drosophila melanogaster, social behaviour, reproduction, mate choice, sperm competition, Bruce effect
1. Introduction
The adaptive value of group life has been illustrated in many species by field and laboratory studies [1,2]. Group membership can facilitate biological functions including reproduction, foraging and protection against predators [1]. Such traits are controlled by the genotype of an individual as well as by social partners, a phenomenon termed indirect genetic effects (IGEs) [3,4]. IGEs are predicted to affect the evolutionary process when interactions with social partners affect variance in the individual's fitness, known as social selection [3–6].
Drosophila melanogaster swarm on food substrates, where they feed and breed [7,8]. Members of these groups derive benefits from this social environment. For instance, females lay eggs communally [9], which increases larval survival [10]. Group composition is an important parameter influencing Drosophila social life. Studies on wild populations and laboratory lines suggest that the genotype of a minority can influence behaviour in the whole group [11–13]. For example, mixing arrhythmic period mutants with wild-type flies resets the locomotor activity rhythms, changes the blend of pheromones, and increases mating frequency of the wild-types [14–16] as well as modulates gene expression in their brain, and in their pheromone-producing cells [15]. Social interactions within D. melanogaster groups are thus clearly subjected to IGEs [16], allowing for the phenotype of others to affect the behaviour of an individual. However, little is known about the fitness consequences of these group interactions.
Genotypic frequency in a population is affected by mate preference. In the laboratory, mate choice is often tested by observing interactions between a single male and female. However, genetic monogamy is rare; both males and females of many species mate with multiple individuals in their lifetime [17–19]. In species where females have large broods, such as in D. melanogaster, multiple matings in one reproductive episode may lead to multiple paternities, but each male may not sire equal amounts of offspring [20,21]. For instance, when a female re-mates, the second male sires a majority of her offspring by out-competing his predecessor, a phenomenon called last male sperm precedence [20,21]. Because of male sperm competition, remating could have little benefit on females [22–24]. Yet, females mating with many males in a single reproductive episode and polyandry have been regularly reported in D. melanogaster [15,25–28], so the adaptive significance of this behaviour remains unclear. In other species, multiple matings and/or polyandry are reported to have a positive effect on female fitness, including an increase in the number of offspring produced [17] and offspring genetic diversity [18].
We have previously reported high incidence of remating when flies are housed continuously for 24 h in groups of six males and six females [5]. This high mating frequency is dramatically higher than what is observed in most laboratory assays of remating in D. melanogaster, which include a single male and female [22,29,30]. This discrepancy between assays suggests that social context affects mating frequency in D. melanogaster [31] and provides a possible example of social selection—if group composition affects variance in reproductive success.
Here, we show that the social environment affects the reproductive success of individual flies. Group assays with six males and six females demonstrate high frequencies of remating. We show that housing males from different strains to increase social heterogeneity further increases remating frequency and affects female mate choice. Females are central to this effect of the social environment, assessing group composition by olfactory mechanisms. We also report that females can block fecundity with certain males or cause a breakdown in last male sperm precedence in a social context-dependent manner. This study shows that the social environment can affect variance in reproductive success and thus cause an impact on the evolutionary process.
2. Material and methods
(a). Fly rearing
Flies were reared on a medium containing agar, glucose, sucrose, yeast, cornmeal, wheat germ, soya flour, molasses, propionic acid and Tegosept in a 12 L : 2 D cycle at 25°C. Virgin adults were collected using CO2 anaesthesia and aged in same-sex groups of 20 in food vials for 5–6 days.
(b). Group-mating assays
Group-mating assays were performed in disposable 55 × 8 mm Petri dishes containing a fly food slice (22 × 5 mm). Assays were set up by sequentially introducing six virgin females followed by different types and numbers of virgin males using a mouth pipette. We report four different variations on this assay.
Experiment 1 tests the effect of group composition on individual mating and reproductive success over 8 h. Two common laboratory wild-type strains, Canton-S and Oregon-R, were assayed in groups of six females housed with six males. We tested these strains for male cuticular hydrocarbons [15]: our Canton-S are characterized by a ratio of 7-tricosene to 7-pentacosene of 5 : 1 and Oregon-R of 2 : 1. Five group compositions were tested: groups of six males comprising only Canton-S or Oregon-R males (homogeneous groups) or heterogeneous groups comprising both Canton-S and Oregon-R males at ratios of 2 : 4, 3 : 3 and 4 : 2, respectively. Flies were marked with acrylic paint (pink, blue, yellow, gold, white or black) on the thorax upon collection for identification. Assays ran from Zeitgeber Time (ZT) ZT1 (10.00 h) to ZT8 (18.00 h) and were continuously recorded using a Sony AVCHD camcorder. An observer, blind to the flies’ colour code, scored the time to mating, and identity of mating partners using the Picture Motion Browser software (Sony, Inc.). Females were extracted after the assays and placed individually in food vials for egg-laying at 25°C. Vials were changed daily for 5 days; females were left in the last vial for another 5 days. Freshly eclosed adult offspring were counted daily to determine female fecundity, then aged in fresh food vials for 3–7 days before paternity analysis (see §2c).
The unit of replication is the group. Individual measurements in a given group were averaged by sex, strain or mating history to generate one value per replicate. Differences in reproductive behaviour in different social contexts were tested using the following mixed effect linear model run with JMP v. 9.0 (SAS):
where X is the number of matings, reproductive success or offspring genotype in figure 1a–c, respectively. Sti is the effect of male strain, Socj is the effect of social context (male strain ratios), StSocij is the effect of the interaction of strain with social context and Gr is the effect of replicate groups. Group was modelled as a random effect whereas all other factors were fixed.
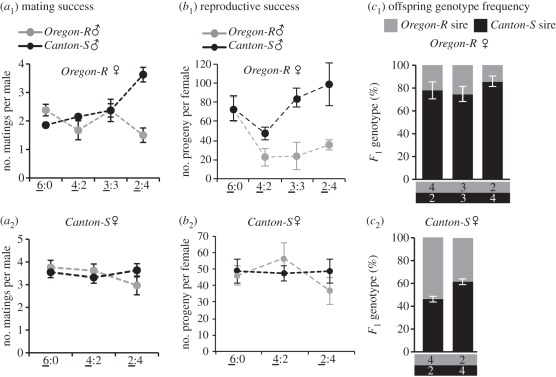
Figure 1.
Social context affects (a) male-mating success, (b) reproductive success and (c) the frequency of genotypes in the F1 offspring. A group of six Canton-S males (black circles) and/or Oregon-R males (grey circles) were housed at a range of ratios (indicated below the x-axis) with either six Oregon-R females (a1–c1) or six Canton-S females (a2–c2) to vary social contexts. The first digit (underlined) of each ratio indicates the males for which the data are presented; the second digit represents the males with which they were housed. (a) Mean numbers of matings per male of the indicated strain were determined in different social contexts. Replicate groups: 10–18. (b) Number of offspring of females who mated exclusively with Canton-S (black) or Oregon-R males (grey) in different social contexts. Number of replicate groups: 4–14. The female offspring were genotyped to determine the fraction (c) of offspring sired by each male type (Oregon-R, grey; Canton-S, black) in different socials contexts. Number of replicate groups: 9–14. Error bars: ±s.e.m.
Experiment 2 tests the effect of group composition on mating frequency over 24 h [15]. Frequency and time of mating of Canton-S and Oregon-R, as well as Orco mutants (née Or83b) [32] in a Canton-S genetic background were assayed. Orco encodes an odorant co-receptor required for the functioning of most olfactory receptor neurons [32]. The original w1118 + Orco2 was outcrossed to a Canton-S-isogenized white stock for five generations and the X and second chromosomes were replaced by those of Canton-S. Six females were housed with groups of males composed either of Canton-S or Oregon-R males, or of four males of these strains with two males mutant for the yellow and white genes (y,w males; unknown genetic background). Assays were started at ZT8 (17.00 h) in an incubator set at 25°C and at 12 L : 12 D. The ZoomBrowser EX software (Canon, Inc.) controlled a Canon S10 digital camera to take images of the assays at 2 min intervals for 24 h. Red light (wavelength above 620 nm) was used to monitor mating during the dark phase. The lighter body and eye colours of y,w mutants allowed discrimination from wild-type flies by the observer scoring mating events. Images were surveyed for copulating pairs and scored if a pair was observed for at least four consecutive frames.
Experiment 3 assesses the effect of multiple female matings on last male sperm precedence in groups over 8 h. Six Canton-S females were housed with three GFP-marked and three non-GFP-marked males. The GFP-marked males express the green fluorescent protein (GFP) ubiquitously via a homozygous Ubiquitin::GFP transgene. The non-GFP males do not have this transgene, but are otherwise in the same foragings wild-type background. These males were chosen because they had been shown to be equivalent despite the GFP transgene [33]. This experiment was otherwise performed and scored as in experiment 1. Colour tags permitted the identification of mate choice and mating order. Females were extracted at the end of the assay as in experiment 1 for fecundity and offspring paternity analysis, but paternity was determined through presence or absence of GFP using a Leica MZ10F fluorescence stereomicroscope. Maximum one female per group was used for statistical analysis.
Experiment 4 (figure 4) assays mating frequency of seven wild-type strains: Canton-S, Oregon-R, C0–3, TW-1, Amherst-3, Florida-9 and Urbana-S (the last five are from the Bloomington Drosophila stock centre) in groups composed of six females and six males, either all from the same strain, or in conditions where the sexes were from different strains. Experiments were set-up and scored as in Experiment 2.
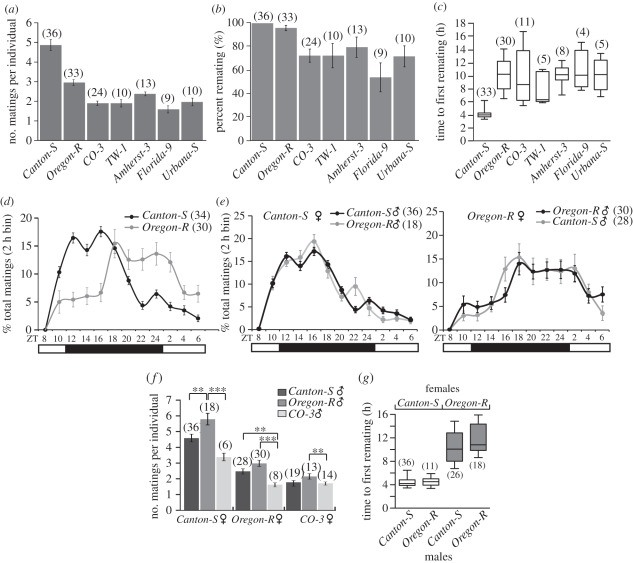
Figure 4.
Remating differs in (a,b) frequency and (c–e) temporal distribution between wild-type strains because of strain differences in female behaviour (f,g). (a) Mean number of matings over 24 h per individual in groups of six males and six females. Number of matings was estimated by dividing the sum of matings in each experiment by six (the number of potential couples). Each replicate was taken as a single data point. (b) Fraction of individuals that mated more than once. (c) Time to first remating. Values derived from the mean time to first six remating. The horizontal bars in box plots represent the median, boxes denote 25th–75th percentile range, and whiskers the minimum and maximum data points. (d) Temporal distribution of mating. Fraction of the total mating observed over 24 h binned per 2 h. (e) Mean number of mating per individual in groups of six males and six females from different strains. Asterisks indicate significant differences determined by ANOVA followed by Tukey–Kramer post hoc test (**p < 0.001; ***p < 0.0001). (f) Temporal distribution of mating as in (d), but with males and females from different strains. (g) Time to first remating measured as in (c), in combinations of females and males of the indicated genotypes. Box plots are as in (c). White or grey boxes indicate significant differences (ANOVA, p < 0.0001). Sample size is between brackets. Error bars: ±s.e.m. except for (c,g).
(c). Paternity analysis
Abdominal segments A6 and A7 are more darkly pigmented in Canton-S than in Oregon-R females. Daughters from an Oregon-R dam and a Canton-S sire inherit the dark pigmentation of Canton-S (electronic supplementary material, figure S2). Paternity was determined based on this pigmentation dimorphism. Daughters from Canton-S dams and Oregon-R sires, however, have the same pigmentation as Canton-S females when raised at 25°C, but displayed intermediate pigmentation when raised at 30°C. The eggs of Canton-S dams were therefore transferred to a 30°C incubator after having been laid by the female at 25°C, allowing unambiguous paternity identification.
(d). Statistical analysis
Statistical analyses were performed with SPSS software (v. 19.0), unless stated otherwise. ANOVAs were followed by the post hoc Tukey–Kramer test for multiple comparisons except when data did not fit a normal distribution or had unequal variances, in which case Kruskal–Wallis ANOVA was used followed by Dunn's post hoc test. Differences in temporal distribution of mating were tested using repeated-measurement ANOVA after degrees of freedom were corrected using Greenhouse–Geisser estimates of sphericity.
3. Results
(a). Experiment 1: social context modulates male-mating success, reproductive success and offspring genotype
We assayed mating behaviour of six females from Oregon-R or Canton-S wild-type strains housed with groups of six males comprising different mixes of Oregon-R or Canton-S to determine effects of the social environment on reproductive behaviour (see experiment 1 in §2). In 8 h, Oregon-R females mated on average twice and Canton-S females thrice (electronic supplementary material, figure S1a1,2). Housing females in different social contexts neither affected the mating frequency of Oregon-R (F3,90.7 = 1.47, p = 0.22), nor of Canton-S (F2,56.82 = 0.68, p = 0.51) females (electronic supplementary material, figure S1a1–2), but did affect male-mating success (figure 1a). The interaction of male strain and social context had a significant effect (F3,88.07 = 8.4, p < 0.0001), indicating that a male's mating success is not only determined by his strain identity, but also by the identity of other males in his group (figure 1a1). The social environment mainly affected Canton-S males, which gained more mating when in a 2 : 4 minority than in any other social contexts (estimate = 0.81, s.e. = 0.18, p < 0.001; figure 1a1). The influence of the social context on a male's mating success depends on the females’ strain because the effect was not observed when males were housed with Canton-S females (figure 1a2). Male-mating success was similar for both strains (F1,60.48 = 0.05, p = 0.82), was unaffected by social context (F2,54.73 = 0.67, p = 0.52) and there was no significant interaction between strain and social context (F2,60.51 = 1.29, p = 0.28; figure 1a2).
Changes in social context had dramatic effects on reproductive success. In homogeneous groups, Oregon-R females had similar numbers of offspring with Canton-S and Oregon-R males, indicating equal male fecundity and no reproductive incompatibility (electronic supplementary material, figure S1b1). However, Oregon-R females’ fecundity changed in different social contexts (F3,52.02 = 3.47, p = 0.022; electronic supplementary material, figure S1b1). Comparison of females who mated solely with one male type (Canton-S or Oregon-R) in different social contexts revealed that the strain of the male they mated with has a significant effect on their fecundity (F1,39.02 = 28.84, p < 0.0001). This effect is dependent on social context (F3,45.85 = 4.42, p = 0.0081), and social context and the strain of the males interact to determine reproductive success (F3,44.88 = 4.42, p = 0.0146). The fecundity of Oregon-R females was affected by the social context both when mating only with Canton-S males (F3,33.72 = 10.60, p < 0.001), or only with Oregon-R (F3,26 = 3.67, p = 0.0250; figure 1b1). Oregon-R females who mated with Oregon-R males in the presence of Canton-S males, were on average 70 per cent less fecund than when they mated with Oregon-R males in the absence of Canton-S males (figure 1b1). The social context in which individuals mate can be as important as mating itself because mating does not ensure reproductive success in all social environments (figure 1b1). Interaction between social context and male strain affecting reproductive success was not observed when similar groups of males were housed with Canton-S females (F2,39.97 = 0.50, p = 0.60; figure 1b2), indicating that influence of the social context on male reproductive success depends on the females. Decreased fecundity of Oregon-R and not Canton-S females in heterogeneous groups is not linked to a general fecundity problem because Oregon-R females are significantly more fecund than Canton-S females when mating with either Canton-S or Oregon-R in homogeneous groups (two-way ANOVA F1,41 = 8.0, p = 0.007; electronic supplementary material, figure S1a1,2).
We determined paternity ratios in the offspring of females that mated in different social contexts to determine the fitness of different male strains (see §2). In heterogeneous groups, Canton-S males consistently sired approximately 80 per cent of offspring and Oregon-R males 20 per cent (figure 1c1). There was a significant deviation in paternity success from a predicted ratio of 50 : 50 in all social contexts (for 2 : 4, χ2 (1,n = 281.93) = 136.26, p < 0.0001; for 3 : 3 ratio, χ2(1,n = 293.4) = 110.29, p < 0.0001; for 4 : 2 ratio, χ2 (1,n = 398.03) = 193.14, p < 0001). Despite changes in mating and reproductive success (figure 1a1 and b1), we observed no significant difference in the pattern of offspring produced by Oregon-R females across mixed social contexts (F2,30 = 1.95, p = 0.16). We highlight that this stable percentage is a group property and is not found in any one female. This effect was not observed when males were housed with Canton-S females (figure 1c2). Offspring genotypic frequency of Canton-S females was similar in different social contexts (F1,15.92 = 2.94, p = 0.10) and offspring were sired equally by Canton-S and Oregon-R males in the 4 : 2 context (χ2 (1, n = 342.26) = 171.13, p = 0.24)), but not in the 2 : 4 context (χ2(1, n = 293.06) = 40.09, p < 0.0001; figure 1c2). We conclude that social environment can influence mate choice and fecundity, and notably genotypic diversity in the next generation.
(b). Experiment 2: Social heterogeneity affects mating frequency
Male strain diversity strongly influences mating behaviour of Oregon-R but not Canton-S females, leaving the generality of this phenomenon unclear. We extended the assay from 8 to 24 h to determine a potential long-term effect of the social environment on Canton-S female-mating behaviour, and created socially heterogeneous groups by housing mutant males with wild-type males and female Canton-S or Oregon-R (see experiment 2, §2). Mutant males (y,w) are sluggish courters with low mating success [34], making them unlikely to directly influence mating frequency. This allows testing their indirect effect on the mating success of the wild-type males by comparing mating patterns in homogeneous groups composed of six females and males from either the Canton-S or the Oregon-R strain with that of socially heterogeneous groups comprising four males (either all Canton-S or Oregon-R) and two y,w mutant males (figure 2).
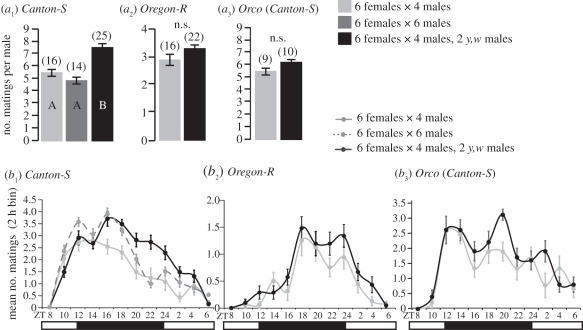
Figure 2.
Social heterogeneity affects mating frequency and is perceived by female olfaction. (a) Mean number of matings per male in 24 h, excluding y,w males, in groups of six females whose genotype is indicated above each graph in either homogenous groups of males (light grey bars) or in mixed groups comprising four wild-type males and two y,w mutant males (black). The four males were from the same strain as the females in (a1,2). In the Orco females’ experiment (a3), the males were Canton-S. Number of matings per male was estimated by dividing the total number of matings by the number of wild-type males (four or six). (a1) Bars labelled by the same letter (A or B) are not statistically different (ANOVA followed by Tukey–Kramer test, p > 0.05). (a2,3) n.s. indicates no significant difference between groups determined by a two-tailed Student's t-test (p > 0.05). (b) Temporal distribution of mating in groups of the indicated genotypes and composition as in (a). Data points indicate the mean number of matings observed per 2 h time bins over 24 h. Numbers of group replicates are between brackets. Error bars: ±s.e.m.
The presence of y,w males indirectly increased Canton-S males’ mating success (figure 2a1). Canton-S males mated 38 per cent more in socially heterogeneous groups than in groups comprising no y,w males (figure 2a1), indicating that social heterogeneity can also affect Canton-S females’ reproductive behaviour. Because the mating success of the two y,w males is negligible (less then 3% of all matings in Canton-S groups), we reasoned that the mating frequency of four Canton-S males with whom they are housed is better compared with that of four Canton-S males without y,w males, but that changes social density. We ruled out a confound of social density because homogeneous groups of Canton-S flies with either four or six Canton- S males were not different in terms of individual male-mating frequency (figure 2a1).
No significant increase was observed in mating frequency when Oregon-R flies were mixed with mutant males (p = 0.11; figure 2a2). Differences in response to social heterogeneity between Canton-S and Oregon-R between experiments 1 and 2 may be partially caused by a temporal difference in behaviour. Graphing the temporal distribution of mating over the 24 h of experiment 2 reveals that the effect of mixing y,w males in Canton-S groups becomes apparent 10–12 h after the start of the experiment (after ZT18; figure 2b1). Further, Canton-S and Oregon-R have different temporal distributions of mating (figure 2b1,2).
Increased mating frequency in socially heterogeneous groups of Canton-S flies (figure 2a1) may be caused by Canton-S females perceiving male diversity and increasing receptivity. Because flies use chemical communication during social interactions [35], we hypothesized that smell-impaired Canton-S females would not increase mating frequency between homogeneous and heterogeneous groups. We re-tested the mating behaviour of Canton-S flies in the presence of y,w males, using Canton-S females carrying a null Orco allele, impairing their olfaction. Unlike groups including Canton-S females (figure 2a1), groups including Orco females did not show increased mating frequency (figure 2a3) indicating that social context perception is mediated by an olfactory cue perceived by females. Orco females behaved like Canton-S females in homogeneous groups in terms of mating frequency (figure 2a1,3) and temporal distribution of mating (figure 2b1,3) indicating no basic reproductive defects caused by the mutation. We conclude that effects of social heterogeneity on mating frequency are mediated by females.
(c). Experiment 3: female-mating frequency affects last male sperm precedence
The function of Canton-S increased mating frequency in heterogeneous groups is unclear (figure 2a1). Females produce large broods (approx. 100), so increasing number of mates could increase offspring genetic diversity. However, males possess mechanisms ensuring sperm precedence [21] predicting that female mating will not achieve greater offspring diversity because the last male will sire most offspring [24]. We tested this prediction by examining the percentage of offspring sired by the last male when mating with Canton-S females who had already mated with one, two, three or four males (see experiment 3, §2). Groups were composed of three males genetically marked with a GFP transgene and three unmarked housed with six Canton-S females, allowing determination of paternity in offspring by observing GFP. We observed classical instances of last male sperm precedence in twice-mated females, with the last male siring a majority of offspring (figure 3a1,2). Last male precedence was, however, not observed in females that mated more than twice, and the portion of offspring sired by the last male was reduced to one-third or less (figure 3a1,2). This rejects the prediction that increased remating does not change offspring diversity. A one-way ANOVA with the proportion of offspring sired by the last male as the dependent variable showed that last male sperm precedence is significantly affected by the number of times a female mated (for GFP male: F2,52 = 17.52, p < 0.0001; for non-GFP males: F2,52 = 13.35, p < 0.0001). The higher percentage of offspring sired by non-GFP (figure 3a1) compared with GFP (figure 3a2) last males is probably linked to an effect of the ubiquitous expression of GFP, which may reduce the ability of the sperm from GFP males to compete with sperm from non-GFP males [21]. Female fecundity was not significantly affected by numbers of mating or male types, indicating that multiple matings do no affect female fecundity (electronic supplementary material, figure S3).
Figure 3.
Female mating affects pattern of last male sperm precedence. (a) Mean percentage of offspring sired by the last male in a mating series ranging from one to five matings. Mating series are indicated below the graphs. Numbers between brackets indicate females recorded with this particular mating series. Histograms labelled by different letters (A, B or C) are statistically different (ANOVA followed by Tukey–Kramer test, p > 0.05). (b) Effect of male mating history on last male precedence in thrice mated females. Offspring percentages are those presented in (a) for thrice-mated females. Data were separated between offspring sired by males having mated with other females twice or less before and those that had mated more, as indicated below the x-axis. Asterisks indicate significant differences determined by a two-tailed Student's t-test. Error bars: ±s.e.m. Number of broods from individual female tested is between brackets. Black bars, non-GFP male; grey bars, GFP male.
Males reportedly transfer fewer sperm in successive copulations, until exhaustion after four to five matings [26]. We observed on average 3.89 mating per female (s.e.m. = 0.08; n = 464), each spaced by an average 135 min (s.e.m. = 3 min). GFP males mated 3.4 times (s.e.m. = 0.12; n = 217) and non-GFP males mated 4.9 times (s.e.m. = 0.13; n = 230). This remating frequency may allow females to affect male–male sperm competition through sperm depletion. We indirectly checked sperm depletion in males who had mated last with three times mated females by comparing those males who had previously mated with no more than two females (‘fresh’ males) and those who had mated with three or more (‘exhausted’ males). The ability of ‘exhausted’ non-GFP males to sire offspring was reduced when compared with ‘fresh’ ones, suggesting that sperm depletion is a factor in last male precedence reduction (figure 3b). However, it cannot be the only factor as the percentage of offspring sired by ‘fresh’ non-GFP males in twice-mated females (figure 3a) and three-time mated females (figure 3b) was significantly reduced in three-times mated females (t-test p = 0.03, d.f. = 31). The sperm depletion effect was not observed in GFP males (figure 3b). The effect of female mating history must therefore involve an interaction between sperm depletion and additional female cryptic effects.
(d). Experiment 4: variation in remating behaviour between wild-type strains
As discussed in §1, the high female remating frequencies we observed are at odds with several reports of low female remating frequency in D. melanogaster. We tested seven laboratory wild-type strains to better characterize between-strain variability in remating behaviour in our assay.
Mating frequency is strain-specific and ranges on average from two to five matings per individual (figure 4a). The percentage of individuals that mated more than once is also strain-specific and ranged from 50 to 100 per cent (figure 4b). We also observed strain differences in timing between the first and second mating (figure 4c). Canton-S mate for the second time (first remating) 4 h after the virginal mating, while other strains, such as Oregon-R, mate for the second time 10 h later (figure 4c). Temporal distributions of remating over 24 h of the two wild-type strains with the highest remating frequencies (Oregon-R and Canton-S) are clearly different (repeated-measure ANOVA: F1,7.5 = 19.08, p < 0.001; figure 4d). Canton-S re-mated at the highest frequency in the first half of the night, while Oregon-R peaked in the second half of the night and in the early morning (figure 4d).
We investigated the contribution of each sex to mating frequency. We selected three strains with high, medium and low mating frequency (Canton-S, Oregon-R and CO-3, respectively; figure 4a) and quantified mating in groups composed of the nine possible permutations of males and females (figure 4f). In this factorial design, Canton-S females showed the highest mating frequency, while Oregon-R and CO-3 showed medium and low frequency, respectively (figure 4f). Oregon-R males mated more than Canton-S or CO-3 males with females from a given strain (figure 4f). A two-way ANOVA revealed that the strain of females significantly affects mating frequency and accounted for 47 per cent of the variance between groups (F2,124 = 133.87, p < 0.0001). Strain of the male also significantly affects mating frequency but accounts for only 11 per cent of the variance (F2,124 = 30.33, p < 0.0001). The male-by-female genotypic interaction was small and accounted for only 3 per cent of the variance (F4,124 = 4.77, p = 0.001). Temporal distribution of mating also tracks the distribution observed in the strain of the female and not that of the male (figure 4e) and so does the time to first remating (figure 4g). We conclude that remating is a common behaviour in groups, but both the frequency and temporal distribution of mating vary between strains. Although males exert some influence, females are the main determinant of when and how often remating occurs.
4. Discussion
We investigated the effect of group composition on mating behaviour and offspring production to determine the connection between an individual's social environment and its reproductive success. In groups, male reproductive success was predicted not only by strain identity, but also by the strain of the other males (figures 1 and 2). Thus, the contribution of the strain of an individual male to his own reproductive success can be small relative to the contribution of group composition. Because effects of group composition on male reproductive success were dependent on the type of females they were housed with (figure 1), we suggest that females may assess all potential mates in their social environment and adjust mate preference and remating frequency as well as fecundity accordingly (figures 1 and 2). The social environment can thus influence sexual selection.
A general result across our assays is that increase in social heterogeneity is accompanied by changes in female reproductive behaviour resulting in an increase in offspring genetic diversity. This happens either via disassortative mate choice and selective fecundity (figure 1), or by increase in remating frequency diluting last male sperm precedence (figures 2 and 3). Several studies have indicated that female remating may increase the production of genetically diverse progeny, which may increase offspring fitness, for instance, through greater resistance to parasites [18,19]. One interpretation of our data may be that females adapt reproductive behaviour to male diversity to maximize offspring fitness via a greater progeny genetic diversity.
Females from different strains behave differently in similar conditions, perhaps reflecting different adaptations in the natural populations from which they were originally derived, or could have been picked up in the laboratory owing to different levels of genetic heterogeneity among stocks (figures 1, 2 and 4) [36,37]. However, females from the same strain also change reproductive behaviour when housed with a mixture of males, indicating that females can assess male diversity. Female ability to assess the genetic diversity of potential mates would offer an interpretation for our observations. For instance, Canton-S females increase mating frequency with Canton-S males in the presence of y,w mutant males, but Oregon-R females do not (figure 2); y,w males may not be perceived as different enough from Oregon-R males to warrant extra mating. Oregon-R females blocking offspring production when mating with males from their own strain in the presence of unrelated males may stem from the ability to determine genetic relatedness (figure 1b1). Alternatively, both direct and indirect contributions of the males to these effects are difficult to reconcile with our observations. In the context of the blocking of offspring production by Oregon-R females, indirect male–male interactions like sperm competition are not possible because we report on females who only mated with Oregon-R males (figure 1b1). Direct male–male interactions, such as Canton-S males interrupting Oregon-R mating, preventing ejaculate transfer and causing a reduction in female fecundity, can be excluded because mating length does not differ between male strains or between social contexts (electronic supplementary material, figure S4). A focus on female mechanisms, such as the active discarding of sperm [21,38], appears more relevant to explain the offspring production block. This post-mating phenomenon recalls the ‘Bruce effect’ in which female rodents terminate pregnancy if exposed to a male from a different strain than the original stud male [39]. This similarity is intriguing because the Bruce effect is mediated by the smell of the ‘other’ male [39]. Smell-impaired Drosophila females do not adapt to a mix of males in their group (figure 2), indicating that male discrimination occurs via smell in Drosophila as well. It remains to be determined what other similarities are shared between the phenomenon we report in Drosophila and the Bruce effect.
We studied the effect of the social context on reproductive success by measuring the frequency of remating. The observation of high remating frequency contrasts with several studies reporting low levels of remating in D. melanogaster. These studies employ assays where a female is mated with a single male, isolated for 24 h and subsequently housed with a different male, typically resulting in very low remating frequencies [22,29–31]. Our assay differs in the number of flies, length and presence of food, explaining discrepancies between reports. Our observation of high female remating frequencies does not appear to be an experimental artefact because it matches data on polyandry in the wild. Wild-caught D. melanogaster females commonly carry sperm from several males and will have offspring from four to five different sires [25,27], indicating that our assay may better model the natural situation than classical assays. Differences in mating frequency may also be linked to genetic variation between populations [36], as implied by the strain specificity of this phenotype (figure 4). Strain differences also extend to temporal distribution of mating (figure 4). Temporal preference in mating may create reproductive barriers between species [40]. We may speculate that strain-specific temporal differences in remating may also limit reproduction between populations, to the extent that patterns of variance observed in laboratory strains reflect differences between wild populations [37].
Drosophila melanogaster is a model for last male sperm precedence, but this phenomenon has mainly been explored in the context of females mating twice and with long intervals between copulations (1–2 days) [21,24]. In this context, females mating three times failed to show an effect of increased mating on last male sperm precedence [24]. That high remating frequency in our assay dilutes last male precedence (figure 3) is therefore at odds with previous publications. This discrepancy may be partially owing to ejaculate depletion resulting from fast mating frequency [19], which would not be an issue in classical assays. However, this factor is not sufficient to fully explain the breakdown in sperm precedence we observe. Studies in other arthropods have shown that last male sperm precedence breaks down when females mate more than twice, or at high frequencies [41,42], showing precedents for this effect.
Observations in this study support the evolutionary theory of IGEs, which states that the reproductive success of an individual can be influenced by the genotype of another, thereby affecting allelic distribution in the next generation and in populations [3,4]. Evolutionary changes may occur whenever the breeding value of one individual covaries with the phenotype of its social partners [5]. Here, we show that the reproductive success of a given male can depend more on the genotypes and abundance of other males in the group than his own genotype. Females appear to mediate this social effect creating a behavioural equivalent of other variation-generating mechanisms such as meiotic recombination and sex itself.
Acknowledgements
We thank J. Schneider and J. Boonekamp for help with Statistics and J. C. Hall, M. de la Paz Fernández, M. E. Maan, F. H. Rodd, L., Rowe, M. F. Wolfner, J. Atallah and A. J. Moore for comments on the manuscript. The Orco mutant strain was a gift from L. Vosshall (Rockefeller University). J.-C.B. was supported by a fellowship for advanced researcher from the Swiss National Science Foundation. This work was supported by grants from the Canadian Institutes of Health Research, Canada Research Chair grants and NSERC awarded to J.D.L.
References
- 1.Allee W. C. 1938. The social life of animals. New York, NY: W.W. Norton and Co [Google Scholar]
- 2.Clutton-Brock T. 2002. Breeding together: kin selection and mutualism in cooperative vertebrates. Science 296, 69–72 10.1126/science.296.5565.69 (doi:10.1126/science.296.5565.69) [DOI] [PubMed] [Google Scholar]
- 3.Moore A., Brodie E., III, Wolf J. 1997. Interacting phenotypes and the evolutionary process. I. Direct and indirect genetic effects of social interactions. Evolution 51, 1352–1362 [DOI] [PubMed] [Google Scholar]
- 4.Wolf J., Brodie E., III, Cheverud J., Moore A., Wade M. 1998. Evolutionary consequences of indirect genetic effects. Trends Ecol. Evol. 13, 64–69 10.1016/S0169-5347(97)01233-0 (doi:10.1016/S0169-5347(97)01233-0) [DOI] [PubMed] [Google Scholar]
- 5.Mcglothlin J. W., Moore A. J., Wolf J. B., Brodie, E., III 2010. Interacting phenotypes and the evolutionary process. III. social evolution. Evolution 64, 2558–2574 [DOI] [PubMed] [Google Scholar]
- 6.Formica V. A., Mcglothlin J. W., Wood C. W., Augat M. E., Butterfield R. E., Barnard M. E., Brodie E. D., III 2011. Phenotypic assortment mediates the effect of social selection in a wild beetle population. Evolution 65, 2771–2781 10.1111/j.1558-5646.2011.01340.x (doi:10.1111/j.1558-5646.2011.01340.x) [DOI] [PubMed] [Google Scholar]
- 7.Spieth H. T. 1974. Courtship behavior in Drosophila. Annu. Rev. Entomol. 19, 385–405 10.1146/annurev.en.19.010174.002125 (doi:10.1146/annurev.en.19.010174.002125) [DOI] [PubMed] [Google Scholar]
- 8.Wertheim B., Allemand R., Vet L., Dicke M. 2006. Effects of aggregation pheromone on individual behaviour and food web interactions: a field study on Drosophila. Ecol. Entomol. 31, 216–226 10.1111/j.1365-2311.2006.00757.x (doi:10.1111/j.1365-2311.2006.00757.x) [DOI] [Google Scholar]
- 9.Sarin S., Dukas R. 2009. Social learning about egg-laying substrates in fruitflies. Proc. R. Soc. B. 276, 4323–4328 10.1098/rspb.2009.1294 (doi:10.1098/rspb.2009.1294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tinette S., Zhang L., Robichon A. 2004. Cooperation between Drosophila flies in searching behavior. Genes Brain Behav. 3, 39–50 10.1046/j.1601-183x.2003.0046.x (doi:10.1046/j.1601-183x.2003.0046.x) [DOI] [PubMed] [Google Scholar]
- 11.Petfield D., Chenoweth S. F., Rundle H. D., Blows M. W. 2005. Genetic variance in female condition predicts indirect genetic variance in male sexual display traits. Proc. Natl Acad. Sci. USA. 102, 6045–6050 10.1073/pnas.0409378102 (doi:10.1073/pnas.0409378102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hine E., Chenoweth S. F., Blows M. W. 2004. Multivariate quantitative genetics and the lek paradox: genetic variance in male sexually selected traits of Drosophila serrata under field conditions. Evolution 58, 2754–2762 [DOI] [PubMed] [Google Scholar]
- 13.Saltz J. B., Foley B. R. 2011. Natural genetic variation in social niche construction: social effects of aggression drive disruptive sexual selection in Drosophila melanogaster. Am. Nat. 177, 645–654 10.1086/659631 (doi:10.1086/659631) [DOI] [PubMed] [Google Scholar]
- 14.Levine J. D., Funes P., Dowse H. B., Hall J. C. 2002. Resetting the circadian clock by social experience in Drosophila melanogaster. Science 298, 2010–2012 10.1126/science.1076008 (doi:10.1126/science.1076008) [DOI] [PubMed] [Google Scholar]
- 15.Krupp J. J., Kent C., Billeter J.-C., Azanchi R., So A. K. C., Schonfeld J. A., Smith B. P., Levine J. D. 2008. Social experience modifies pheromone expression and mating behavior in male Drosophila melanogaster. Curr. Biol. 18, 1373–1383 10.1016/j.cub.2008.07.089 (doi:10.1016/j.cub.2008.07.089) [DOI] [PubMed] [Google Scholar]
- 16.Kent C., Azanchi R., Smith B., Formosa A., Levine J. D. 2008. Social context influences chemical communication in D. melanogaster males. Curr. Biol. 18, 1384–1389 10.1016/j.cub.2008.07.088 (doi:10.1016/j.cub.2008.07.088) [DOI] [PubMed] [Google Scholar]
- 17.Arnqvist G., Nilsson T. 2000. The evolution of polyandry: multiple mating and female fitness in insects. Anim. Behav. 60, 145–164 10.1006/anbe.2000.1446 (doi:10.1006/anbe.2000.1446) [DOI] [PubMed] [Google Scholar]
- 18.Jennions M., Petrie M. 2000. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. 75, 21–64 10.1017/S0006323199005423 (doi:10.1017/S0006323199005423) [DOI] [PubMed] [Google Scholar]
- 19.Birkhead T. R., Pizzari T. 2002. Postcopulatory sexual selection. Nat. Rev. Genet. 3, 262–273 10.1038/nrg774 (doi:10.1038/nrg774) [DOI] [PubMed] [Google Scholar]
- 20.Clark A. G., Begun D. J., Prout T. 1999. Female x male interactions in Drosophila sperm competition. Science 283, 217–220 10.1126/science.283.5399.217 (doi:10.1126/science.283.5399.217) [DOI] [PubMed] [Google Scholar]
- 21.Manier M. K., Belote J. M., Berben K. S., Novikov D., Stuart W. T., Pitnick S. 2010. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster . Science 328, 354–357 10.1126/science.1187096 (doi:10.1126/science.1187096) [DOI] [PubMed] [Google Scholar]
- 22.Brown W. D., Bjork A., Schneider K., Pitnick S. 2004. No evidence that polyandry benefits females in Drosophila melanogaster. Evolution 58, 1242–1250 [DOI] [PubMed] [Google Scholar]
- 23.Byrne P. G., Rice W. R. 2005. Remating in Drosophila melanogaster: an examination of the trading-up and intrinsic male-quality hypotheses. J. Evol. Biol. 18, 1324–1331 10.1111/j.1420-9101.2005.00918.x (doi:10.1111/j.1420-9101.2005.00918.x) [DOI] [PubMed] [Google Scholar]
- 24.Morrow E. H., Stewart A. D., Rice W. R. 2005. Patterns of sperm precedence are not affected by female mating history in Drosophila melanogaster. Evolution 59, 2608–2615 [PubMed] [Google Scholar]
- 25.Imhof M., Harr B., Brem G., Schlötterer C. 1998. Multiple mating in wild Drosophila melanogaster revisited by microsatellite analysis. Mol. Ecol. 7, 915–917 10.1046/j.1365-294x.1998.00382.x (doi:10.1046/j.1365-294x.1998.00382.x) [DOI] [PubMed] [Google Scholar]
- 26.Lefevre G., Jonsson U. 1962. sperm transfer, storage, displacement, and utilization in Drosophila melanogaster. Genetics 42, 1719–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochando M. D., Reyes A., Ayala F. J. 1996. Multiple paternity in two natural populations (orchard and vineyard) of Drosophila. Proc. Natl Acad. Sci. USA 93, 11 769–11 773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuijper B., Morrow E. 2009. Direct observation of female mating frequency using time-lapse photography. Fly 3, 1–3 10.4161/fly.3.1.7904 (doi:10.4161/fly.3.1.7904) [DOI] [PubMed] [Google Scholar]
- 29.Liu H., Kubli E. 2003. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 100, 9929–9933 10.1073/pnas.1631700100 (doi:10.1073/pnas.1631700100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chapman T., Bangham J., Vinti G., Seifried B., Lung O., Wolfner M. F., Smith H. K., Partridge L. 2003. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc. Natl Acad. Sci. USA 100, 9923–9928 10.1073/pnas.1631635100 (doi:10.1073/pnas.1631635100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yapici N. Y., Kim Y. J., Ribeiro C., Dickson B. J. 2007. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 451, 33–37 10.1038/nature06483 (doi:10.1038/nature06483) [DOI] [PubMed] [Google Scholar]
- 32.Larsson M. C., Domingos A. I., Jones W. D., Chiappe M. E., Amrein H., Vosshall L. B. 2004. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703–714 10.1016/j.neuron.2004.08.019 (doi:10.1016/j.neuron.2004.08.019) [DOI] [PubMed] [Google Scholar]
- 33.Fitzpatrick M. J., Feder E., Rowe L., Sokolowski M. B. 2007. Maintaining a behaviour polymorphism by frequency-dependent selection on a single gene. Nature 447, 210–212 10.1038/nature05764 (doi:10.1038/nature05764) [DOI] [PubMed] [Google Scholar]
- 34.Bastock M. 1956A. gene mutation which changes a behavior pattern. Evolution 10, 421–439 10.2307/2407002 (doi:10.2307/2407002) [DOI] [Google Scholar]
- 35.Ferveur J.-F. 2010. Drosophila female courtship and mating behaviors: sensory signals, genes, neural structures and evolution. Curr. Opin. Neurobiol. 20, 1–6 10.1016/S0959-4388(10)00021-8 (doi:10.1016/S0959-4388(10)00021-8) [DOI] [PubMed] [Google Scholar]
- 36.Giardina T. J., Beavis A., Clark A. G., Fiumera A. C. 2011. Female influence on pre- and post-copulatory sexual selection and its genetic basis in Drosophila melanogaster. Mol. Ecol. 20, 4098–4108 10.1111/j.1365-294X.2011.05253.x (doi:10.1111/j.1365-294X.2011.05253.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rice W. R., Stewart A. D., Morrow E. H., Linder J. E., Orteiza N., Byrne P. G. 2006. Assessing sexual conflict in the Drosophila melanogaster laboratory model system. Phil. Trans. R. Soc. B 361, 287–299 10.1098/rstb.2005.1787 (doi:10.1098/rstb.2005.1787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snook R. R., Hosken D. J. 2004. Sperm death and dumping in Drosophila. Nature 428, 939–941 10.1038/nature02455 (doi:10.1038/nature02455) [DOI] [PubMed] [Google Scholar]
- 39.Bruce H. M., Parrott D. M. 1960. Role of olfactory sense in pregnancy block by strange males. Science 131, 1526. 10.1126/science.131.3412.1526 (doi:10.1126/science.131.3412.1526) [DOI] [PubMed] [Google Scholar]
- 40.Tauber E., Roe H., Costa R., Hennessy J. M., Kyriacou C. P. 2003. Temporal mating isolation driven by a behavioral gene in Drosophila. Curr. Biol. 13, 140–145 10.1016/S0960-9822(03)00004-6 (doi:10.1016/S0960-9822(03)00004-6) [DOI] [PubMed] [Google Scholar]
- 41.Drnevich J. 2003. Number of mating males and mating interval affect last-male sperm precedence in Tenebrio molitor. Anim. Behav. 66, 349–357 10.1006/anbe.2003.2219 (doi:10.1006/anbe.2003.2219) [DOI] [Google Scholar]
- 42.Zeh J., Zeh D. 1994. Last-male sperm precedence breaks down when females mate with 3 males. Proc. R. Soc. Lond. B 257, 287–292 10.1098/rspb.1994.0127 (doi:10.1098/rspb.1994.0127) [DOI] [Google Scholar]