Abstract
Cuttlefish rapidly change their appearance in order to camouflage on a given background in response to visual parameters, giving us access to their visual perception. Recently, it was shown that isolated edge information is sufficient to elicit a body pattern very similar to that used when a whole object is present. Here, we examined contour completion in cuttlefish by assaying body pattern responses to artificial backgrounds of ‘objects’ formed from fragmented circles, these same fragments rotated on their axis, and with the fragments scattered over the background, as well as positive (full circles) and negative (homogenous background) controls. The animals displayed similar responses to the full and fragmented circles, but used a different body pattern in response to the rotated and scattered fragments. This suggests that they completed the broken circles and recognized them as whole objects, whereas rotated and scattered fragments were instead interpreted as small, individual objects in their own right. We discuss our findings in the context of achieving accurate camouflage in the benthic shallow-water environment.
Keywords: cephalopod, occlusion, contour completion, non-human visual perception, invertebrate vision
1. Introduction
As humans, we effortlessly make sense of ambiguous visual information as we go about our daily lives. For example, we can effectively detect visual contours even when local image information fails to provide complete cues about luminance and texture [1]. The ability to assemble spatially separated fragments of visual information into a coherent whole via filling-in seems fundamental to visual processing, and is demonstrated by a range of perceptual completion phenomena [1–3]. The best known of these are modal and amodal completion, wherein the former non-existent boundaries are filled in by ‘illusory’ contours in order to separate a foreground object from a background of similar contrast intensity, resulting in a perceptual contrast enhancement of the foreground object (e.g. as in the ‘Kanizsa triangle’ [4]), and in the latter an occluded background object is perceived in its entirety but without a perceptive sensation of the missing contours [5]. Perceptual completion has been demonstrated in other vertebrate taxa, including non-human primates [6,7], rodents [8], birds [9,10] and fishes [11,12]. Furthermore, it has been shown that bees are able to perform modal completion, meaning that such phenomena are not limited to the vertebrates [13,14]. (It is worth noting that as we cannot know the true perceptual experience of a non-human animal, distinguishing between completion phenomena in these studies is somewhat subjective and based on our own experiences associated with the stimuli tested, as outlined above.) In the above-mentioned studies, subjects were generally tested for their ability to discriminate, for example, between real geometrical figures and illusory figures following training. Here, we show evidence for contour completion in the European cuttlefish (Sepia officinalis L.) via experiments using their innate ability for adaptive camouflage.
Cuttlefish depend on their rapid, visually driven adaptive camouflage for survival as they move from one background to another [15,16]. They are able to match the visual characteristics of their environment with speed and complexity from hatching via chromatophores that are under direct neural control [17–21]. The animals make decisions about what body pattern should be used in a given visual environment by integrating cues about local and global visual characteristics such as contrast, overall illumination and texture, thereby offering a unique insight to non-human visual perception [22–27]. Notably, in the presence of defined objects (e.g. pebbles on a sandy seafloor), the relative scale and contrast of these objects determines whether the cuttlefish will use a high-contrast body pattern made up of large-scale components, known as the disruptive body pattern. In the presence of smaller scale or less-defined objects, a body pattern of smaller scale distinct components, known as the mottle body pattern, or one of a homogeneous colour (the uniform body pattern) is used [17,18,26,28–32] (see fig. 1 of Zylinski et al. [26] for example). Researchers have been able to use the general body pattern responses of cuttlefish to conduct detailed studies into the perception of artificial stimuli that can be well-defined and precisely modulated [18,20,26]. Most relevant to the results presented here, Zylinski et al. [33] showed that cuttlefish use isolated edge fragments as evidence for the existence of objects when choosing the appropriate body pattern to use, demonstrating that large fragments (approx. one-fourth of total diameter) of circle edge elicit a disruptive body pattern, whereas smaller fragments (approx. one-eighth of total diameter) do not elicit a disruptive pattern, but instead result in a body pattern associated with small-scale objects (see also Chiao et al. [30]). Here, we test whether S. officinalis is able to reconstruct fragmented information and perform contour completion when presented with incomplete boundary information.
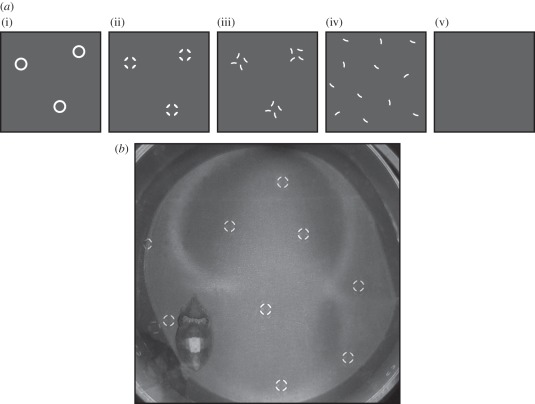
Figure 1.
(a) Details of stimuli used in the experiment. (i) Full circles (positive control); (ii) fragmented circles; (iii) rotated yet still clustered fragments of circles; (iv) scattered fragments of circles; and (v) uniform grey background (negative control). (b) A cuttlefish resting on the fragmented circles stimulus in the test arena to show relative size and density of presentation.
2. Material and methods
(a). Subjects
Sepia officinalis cuttlefish were hatched from eggs laid by wild-caught females trapped off Luc-sur-Mer, Calvados, France and kept in large tanks (1500 l) at the Centre de Recherches en Environnement Côtier, Luc-sur-Mer, France. They were kept in a group in sensory enriched tanks [34] and fed ad libitum with crabs and shrimps. At the start of the experiments, cuttlefish (n = 18) were 6–8 weeks old with a mean dorsal mantle length of 22.2 ± 0.8 mm (mean ± s.e.m.).
(b). Stimuli
Grey scale stimuli were made in Adobe Illustrator CS5, printed onto paper using an inkjet printer (Epson SX510W) and laminated prior to presentation. We tested a positive control based on object sizes and contrasts known from previous work to be associated with disruptive body pattern responses [17,26,28]. This therefore consisted of outlines of white circles (5 mm inner diameter, 6 mm outer diameter) scattered across a 50 per cent grey background (figure 1a(i)), creating high-contrast ‘objects’ of an area approximately 90 per cent of the mean area of the test animal's white square component. We determined that the cuttlefish responded to this stimulus with a strong disruptive body pattern. We then used this as the basis of our test stimuli (figure 1a(ii–v),b). Test stimuli were as follows: (ii) white fragmented circles made of four fragments; (iii) the same fragments of the circle randomly rotated on their axis; and (iv) with the fragments randomly scattered across the background. A uniform grey background served as a negative control (v). For (i) and (ii), circle diameter and the number of circles were identical. For (iii), the number clusters of fragments were the same as in (ii). For (iv), the overall number of fragments was the same as for (ii) and (iii).
(c). Experimental set-up and procedure
Eighteen cuttlefish were tested individually in a circular arena of 15.5 cm diameter, 10 cm deep and filmed from above with a Panasonic digital video camera via a 45° inclined mirror to prevent disturbance to the animal. Test stimuli were placed under and around the edges of the arena (figure 1b). Animals were introduced to the test arena and allowed to settle until a stable body pattern was produced and excessive movement had ceased. Stimuli were presented at random, one per day for each animal over five consecutive days. Images were collected every 5 min during the 30 min of recording, in total six images per cuttlefish per stimulus. Images of the cuttlefish were then cut from the background using GIMP2 (image manipulation software) and randomized to reduce potential order affects and grading bias (for details, see Zylinski et al. [33]). The expression of 11 body pattern components of the disruptive coloration was graded by eye for each image as described in Chiao et al. [29] on a four-point scale, where 0 = not expressed and 3 = strongly expressed. The sum of these grades gave an overall disruptive score for each image. Grading was performed independently by two observers (A-S.D. and S.Z.) and then averaged across the six images and both graders resulting in a single disruptive score for each animal on each stimulus.
(d). Statistics
The mean disruptive scores of the cuttlefish on the five patterns were compared using one-way ANOVA for correlated samples followed by Tukey's HSD tests for post hoc multiple comparisons.
3. Results
We scored the expression of disruptive components in the body pattern responses of 18 juvenile cuttlefish to artificial backgrounds of ‘objects’ formed from fragmented circles (figure 1a(ii)), these same fragments rotated at random on their axis (figure 1a(iii)), and with the fragments scattered over the background (figure 1a(iv)), as well as positive and negative controls (figure 1a(i,v)).
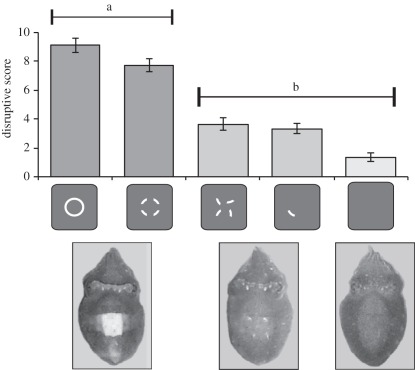
Animals showed two primary responses to the test stimuli: a disruptive body pattern (corresponding with a high disruptive score) on some, and a mottle/uniform body pattern (corresponding with a low disruptive score) on others (figure 2; one-way ANOVA for correlated samples: F4,17 = 8.77, p < 0.0001). There were no significant differences between the body pattern responses to the fragmented circles and to the white circle outlines (positive control; post hoc test: p = 0.764; figure 2). Conversely, animals on the backgrounds composed of rotated or scattered fragments showed a more similar pattern to that used on the uniform grey negative control (post hoc tests: uniform/rotated, p = 0.941; uniform/scattered, p = 0.978; rotated/scattered, p = 1; figure 2). Furthermore, significant differences were found between the responses to the fragmented circles and the rotated fragments (post hoc test: p = 0.035; figure 2), and between the responses to the fragmented circles and scattered fragments (post hoc test: p = 0.021; figure 2).
Figure 2.
Graph showing mean disruptive scores determined from body pattern responses to stimuli tested, with error bars giving the standard error of mean. Images below the graph illustrate the general body patterns typical for given stimuli. One-way ANOVA for correlated samples with Tukey's HSD post hoc testing found differences between groups a and b (p < 0.05) but not within them (p > 0.05). Note that the negative control tended to result in a very low disruptive score, corresponding to uniform body pattern, but this was not significantly different from group b responses.
4. Discussion
Cuttlefish assembled the spatially separated circle fragments and treated them as complete objects, as demonstrated by the high disruptive score associated with both the fragmented circle stimulus and the positive control (figure 1a(i,ii)). Crucial in this interpretation is that when fragments of the same number and size were viewed in an anomalous configuration (rotated; iii) or individually (scattered; iv), the animals no longer perceived a larger complete object but responded to the fragments as separate small objects, as demonstrated by the low disruptive score to these. From this, we conclude that the cuttlefish are able to interpolate missing visual information and perform contour completion.
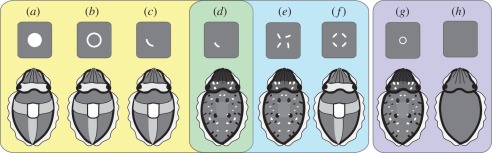
Zylinski et al. [33] showed that cuttlefish respond to partial edges of circles as if complete objects were present; the important and intriguing difference between these results and the findings presented here is that here the fragments (of circle edges the animals are required to complete in order to perceive the circle) are smaller than those used to determine the presence of edges alone. In other words, when these fragments are presented as individual entities, the animals respond with mottle/uniform body patterns, as predicted for an essentially homogeneous background [18,28], and as found by Zylinski et al. [33] when edge information was reduced to one-eighth of a circle. Figure 3 summarizes how our findings on contour completion complement and add to previous findings on edge detection in S. officinalis.
Figure 3.
Summary of body pattern responses from the findings reported here and from previous work. (a–c) Findings from Zylinski et al. [33] showing cuttlefish respond equally with disruptive components to whole printed two-dimensional ‘objects’, the outlines of such objects (i.e. object edges with no corresponding area), and one-fourth circle fragments of the object edges (note that high-passed edges were used in these experiments, but our positive control (b) confirms the response to this stimulus without high-pass filtering). (d) The bridge between previous work and the worked presented in this study. When edges are reduced further the disruptive components are no longer expressed and mottle-type patterns are used instead. We based our fragments for the experiments reported here on this relative size of edge, showing that when these are scattered on the background (figure 1a(iv)) the response is of the mottle type. (e) Crucially to our test here, when these fragments are clustered in an anomalous configuration (rotated randomly on their axes; figure 1a(iii)) they are still treated as separate pieces of visual information, with the response being a mottle. (f) When fragments are orientated to form a circle (figure 1a(ii)) the response changes to one containing disruptive components. (g,h) Illustrate how small objects and uniform backgrounds result in mottle and uniform patterns, respectively (e.g. negative control stimulus here (figure 1a(v)), see also earlier studies [26,28]).
The literature on contour completion in animal visual ecology has highlighted its potential role in camouflage breaking and in the detection of partially occluded predators and prey [4,10], and here we show it is of importance to the dynamically camouflaging animal. Cuttlefish depend on defeating their predators visual search abilities as their primary defence mechanism. In order to camouflage in visually complex environments effectively, they must make accurate decisions as to the true form of objects in their immediate surroundings; inaccuracies in the interpretation of this visual information may lead to errors in body pattern usage rendering them conspicuous to the potential predator. Here, we show that the cuttlefish not only interpolate information missing from the visual input, but also translate this reconstructed visual information into a body pattern output. This is an elegant solution to the challenge facing a benthic cephalopod camouflaging from a predator searching from above (e.g. by a swimming fish), where it is hindered by an alternative viewing angle and incomplete information pertaining to the wider visual environment [20,26,33].
This is, to our knowledge, the first demonstration of contour completion in an invertebrate outside of the insects, and the first empirical demonstration of non-human contour completion determined through an innate response rather than via training and discrimination tasks [10]. We are not able to determine what completion phenomenon cuttlefish experience when interpreting the stimulus tested here, but as our understanding of the subtleties of the visual hierarchy controlling their camouflage body displays increases [35–37], we believe it will be possible to investigate and distinguish between modal and amodal completion in future work.
Visual interpolation for contour completion has not only been widely demonstrated in vertebrates [4], but also shown in insects [13]. Here, we provide evidence for contour completion in cephalopods, a lineage deeply isolated from either of these groups. Cephalopods are renowned for their sophisticated vision, having a camera-type eye highly convergent with the vertebrate eye [15,38]; Zylinski & Osorio [27] propose that low-level visual mechanisms, such as edge detection, object recognition and texture perception, are also surprisingly similar to those of vertebrates (see also earlier studies [18,20]). The phenomena involved in contour completion and interpolation are of great interest in vision research because of the involvement of long-range neural and cortical interactions between different tiers of the visual pathway identified in primates, providing an opportunity to study unique aspects of the physiology of perception [3,5,39–41], a subject with little comparable knowledge in invertebrate counterparts. Given the remarkable convergence of visual processing in cephalopods and vertebrates shown thus far, our findings may be indicative of similar long-range interaction in the cephalopod optic lobe to assemble fragmented visual information in the absence of complete luminance borders.
Acknowledgements
We acknowledge the support from Office of Naval Research MURI grant no. N00014-09-1-1053 to S.Z. and Israeli Science Foundation grant no. 1081/10 to N.S. The authors thank the staff of the Marine Station of Luc-sur-Mer (France) for their technical assistance. We thank Tom Troscianko for his helpful comments and suggestions.
References
- 1.Halko M. A., Mingolla E., Somers D. C. 2008. Multiple mechanisms of illusory contour perception. J. Vis. 8, 11. 10.1167/8.11.17 (doi:10.1167/8.11.17) [DOI] [PubMed] [Google Scholar]
- 2.Pessoa L., Thompson E., Noë A. 1998. Finding out about filling-in: a guide to perceptual completion for visual science and the philosophy of perception. Behav. Brain. Sci. 21, 723–802 [DOI] [PubMed] [Google Scholar]
- 3.Peterhans E., von der Heydt R. 1991. Subjective contours: bridging the gap between psychophysics and physiology. Trends Neurosci. 14, 112–119 10.1016/0166-2236(91)90072-3 (doi:10.1016/0166-2236(91)90072-3) [DOI] [PubMed] [Google Scholar]
- 4.Nieder A. 2002. Seeing more than meets the eye: processing of illusory contours in animals. J. Comp. Physiol. A 188, 249–260 10.1007/s00359-002-0306-x (doi:10.1007/s00359-002-0306-x) [DOI] [PubMed] [Google Scholar]
- 5.Murray M. M. 2004. Setting boundaries: brain dynamics of modal and amodal illusory shape completion in humans. J. Neurosci. 24, 6898–6903 10.1523/jneurosci.1996-04.2004 (doi:10.1523/jneurosci.1996-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato A., Kanazawa S., Fujita K. 1997. Perception of object unity in a chimpanzee (Pan troglodytes). Jpn Psychol. Res. 39, 191–199 10.1111/1468-5884.00053 (doi:10.1111/1468-5884.00053) [DOI] [Google Scholar]
- 7.Deruelle C., Barbet I., Depy D., Fagot J. 2000. Perception of partly occluded figures by baboons (Papio papio). Perception 29, 1483–1497 10.1068/p3071 (doi:10.1068/p3071) [DOI] [PubMed] [Google Scholar]
- 8.Kanizsa G., Renzi P., Conte S., Compostela C., Guerani L. 1993. Amodal completion in mouse vision. Perception 22, 713–721 10.1068/p220713 (doi:10.1068/p220713) [DOI] [PubMed] [Google Scholar]
- 9.Nieder A., Wagner H. 1999. Perception and neuronal coding of subjective contours in the owl. Nat. Neurosci. 2, 660–663 10.1038/10217 (doi:10.1038/10217) [DOI] [PubMed] [Google Scholar]
- 10.Tvardíková K., Fuchs R. 2010. Tits use amodal completion in predator recognition: a field experiment. Anim. Cogn. 13, 609–615 10.1007/s10071-010-0311-3 (doi:10.1007/s10071-010-0311-3) [DOI] [PubMed] [Google Scholar]
- 11.Sovrano V. A., Bisazza A. 2007. Recognition of partly occluded objects by fish. Anim. Cogn. 11, 161–166 10.1007/s10071-007-0100-9 (doi:10.1007/s10071-007-0100-9) [DOI] [PubMed] [Google Scholar]
- 12.Sovrano V. A., Bisazza A. 2009. Perception of subjective contours in fish. Perception 38, 579–590 10.1068/p6121 (doi:10.1068/p6121) [DOI] [PubMed] [Google Scholar]
- 13.Horridge G., Zhang S., O'Carroll D. 1992. Insect perception of illusory contours. Phil. Trans. R. Soc. Lond. B 337, 59–64 10.1098/rstb.1992.0083 (doi:10.1098/rstb.1992.0083) [DOI] [Google Scholar]
- 14.Van Hateren J. H., Srinivasan M. V., Wait P. B. 1990. Pattern recognition in bees: orientation discrimination. J. Comp. Physiol. A 167, 649–654 [Google Scholar]
- 15.Hanlon R. T., Messenger J. B. 1996. Cephalopod behaviour. Cambridge, UK: Cambridge University Press [Google Scholar]
- 16.Holmes W. 1940. The color changes and color patterns of Sepia officinalis, L. Proc. Zool. Soc. Lond. A 110, 2–35 [Google Scholar]
- 17.Barbosa A., Mäthger L. M., Chubb C., Florio C., Chiao C.-C., Hanlon R. T. 2007. Disruptive coloration in cuttlefish: a visual perception mechanism that regulates ontogenetic adjustment of skin patterning. J. Exp. Biol. 210, 1139–1147 10.1242/jeb.02741 (doi:10.1242/jeb.02741) [DOI] [PubMed] [Google Scholar]
- 18.Chiao C.-C., Chubb C., Buresch K., Siemann L., Hanlon R. T. 2009. The scaling effects of substrate texture on camouflage patterning in cuttlefish. Vision Res. 49, 1647–1656 10.1016/j.visres.2009.04.002 (doi:10.1016/j.visres.2009.04.002) [DOI] [PubMed] [Google Scholar]
- 19.Hanlon R. T., Messenger J. 1988. Adaptive coloration in young cuttlefish (Sepia officinalis, L.): the morphology and development of body patterns and their relation to behaviour. Phil. Trans. R. Soc. Lond. B 320, 437–487 10.1098/rstb.1988.0087 (doi:10.1098/rstb.1988.0087) [DOI] [Google Scholar]
- 20.Kelman E. J., Osorio D., Baddeley R. J. 2008. A review of cuttlefish camouflage and object recognition and evidence for depth perception. J. Exp. Biol. 211, 1757–1763 10.1242/jeb.015149 (doi:10.1242/jeb.015149) [DOI] [PubMed] [Google Scholar]
- 21.Poirier R., Chichery R., Dickel L. 2005. Early experience and postembryonic maturation of body patterns in cuttlefish (Sepia officinalis). J. Comp. Psychol. 119, 230–237 10.1037/0735-7036.119.2.230 (doi:10.1037/0735-7036.119.2.230) [DOI] [PubMed] [Google Scholar]
- 22.Chiao C.-C., Hanlon R. T. 2001. Cuttlefish cue visually on area: not shape or aspect ratio of light objects in the substrate to produce disruptive body patterns for camouflage. Biol. Bull. 201, 269–270 10.2307/1543359 (doi:10.2307/1543359) [DOI] [PubMed] [Google Scholar]
- 23.Chiao C.-C., Hanlon R. T. 2001. Cuttlefish camouflage: visual perception of size, contrast and number of white squares on artificial checkerboard substrata initiates disruptive coloration. J. Exp. Biol. 204, 2119–2125 [DOI] [PubMed] [Google Scholar]
- 24.Hanlon R. T. 2007. Cephalopod dynamic camouflage. Curr. Biol. 17, R400–R404 10.1016/j.cub.2007.03.034 (doi:10.1016/j.cub.2007.03.034) [DOI] [PubMed] [Google Scholar]
- 25.Mäthger L. M., Chiao C.-C., Barbosa A., Buresch K. C., Kaye S., Hanlon R. T. 2007. Disruptive coloration elicited on controlled natural substrates in cuttlefish, Sepia officinalis. J. Exp. Biol. 210, 2657–2666 10.1242/jeb.004382 (doi:10.1242/jeb.004382) [DOI] [PubMed] [Google Scholar]
- 26.Zylinski S., Osorio D., Shohet A. J. 2009. Edge detection and texture classification by cuttlefish. J. Vis. 9, 13. (doi:10.1167/9.13.13) [DOI] [PubMed] [Google Scholar]
- 27.Zylinski S., Osorio D. 2011. What can camouflage tell us about non-human visual perception? A case study of multiple cue use in the cuttlefish Sepia officinalis. In Animal camouflage: mechanisms and function (eds Stevens M., Merilaita S.), pp. 164–185 Cambridge, UK: Cambridge University Press [Google Scholar]
- 28.Barbosa A., Mäthger L. M., Buresch K. C., Kelly J., Chubb C., Chiao C.-C., Hanlon R. T. 2008. Cuttlefish camouflage: the effects of substrate contrast and size in evoking uniform, mottle or disruptive body patterns. Vision Res. 48, 1242–1253 10.1016/j.visres.2008.02.011 (doi:10.1016/j.visres.2008.02.011) [DOI] [PubMed] [Google Scholar]
- 29.Chiao C.-C., Chubb C., Hanlon R. 2007. Interactive effects of size, contrast, intensity and configuration of background objects in evoking disruptive camouflage in cuttlefish. Vision Res. 47, 2223–2235 10.1016/j.visres.2007.05.001 (doi:10.1016/j.visres.2007.05.001) [DOI] [PubMed] [Google Scholar]
- 30.Chiao C.-C., Kelman E., Hanlon R. 2005. Disruptive body patterning of cuttlefish (Sepia officinalis) requires visual information regarding edges and contrast of objects in natural substrate backgrounds. Biol. Bull. 208, 7–11 [DOI] [PubMed] [Google Scholar]
- 31.Hanlon R. T., Chiao C.-C., Mäthger L. M., Barbosa A., Buresch K. C., Chubb C. 2008. Cephalopod dynamic camouflage: bridging the continuum between background matching and disruptive coloration. Phil. Trans. R. Soc. B 364, 429–437 10.1098/rstb.2008.0270 (doi:10.1098/rstb.2008.0270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelman E. J., Baddeley R. J., Shohet A. J., Osorio D. 2007. Perception of visual texture and the expression of disruptive camouflage by the cuttlefish, Sepia officinalis. Proc. R. Soc. B 274, 1369–1375 10.1098/rspb.2007.0240 (doi:10.1098/rspb.2007.0240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zylinski S., Osorio D., Shohet A. J. 2009. Perception of edges and visual texture in the camouflage of the common cuttlefish, Sepia officinalis. Phil. Trans. R. Soc. B 364, 439–448 10.1098/rstb.2008.0264 (doi:10.1098/rstb.2008.0264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dickel L., Boal J., Budelmann B. 2000. Effect of early experience on learning and memory in cuttlefish. Dev. Psychobiol. 36, 101–110 10.1002/(SICI)1098-230236:2 (doi:10.1002/(SICI)1098-230236:2) [DOI] [PubMed] [Google Scholar]
- 35.Barbosa A., Allen J. J., Mäthger L. M., Hanlon R. T. 2011. Cuttlefish use visual cues to determine arm postures for camouflage. Proc. R. Soc. B 279, 84–90 10.1098/rspb.2011.0196 (doi:10.1098/rspb.2011.0196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buresch K., Mäthger L., Allen J., Bennice C., Smith N., Schram J., Chiao C.-C., Chubb C., Hanlon R. 2011. The use of background matching versus masquerade for camouflage in cuttlefish Sepia officinalis. Vision Res. 51, 2362–2368 10.1016/j.visres.2011.09.009 (doi:10.1016/j.visres.2011.09.009) [DOI] [PubMed] [Google Scholar]
- 37.Zylinski S., How M. J., Osorio D., Hanlon R. T., Marshall N. J. 2011. To be seen or to hide: visual characteristics of body patterns for camouflage and communication in the Australian giant cuttlefish Sepia apama. Am. Nat. 177, 681–690 10.1086/659626 (doi:10.1086/659626) [DOI] [PubMed] [Google Scholar]
- 38.Land M., Nilsson D.-E. 2002. Animal eyes. Oxford, UK: Oxford University Press [Google Scholar]
- 39.von der Heydt R., Peterhans E., Baumgartner G. 1984. Illusory contours and cortical neuron responses. Science 224, 1260–1262 10.1126/science.6539501 (doi:10.1126/science.6539501) [DOI] [PubMed] [Google Scholar]
- 40.Peterhans E., von der Heydt R. 1989. Mechanisms of contour perception in monkey visual cortex. II. Contours bridging gaps. J. Neurosci. 9, 1749–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von der Heydt R., Peterhans E. 1989. Mechanisms of contour perception in monkey visual cortex. I. Lines of pattern discontinuity. J. Neurosci. 9, 1731–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]