Abstract
A key determinant of the relationship between diet and longevity is the balance of protein and carbohydrate in the diet. Eating excess protein relative to carbohydrate shortens lifespan in solitary insects. Here, we investigated the link between high-protein diet and longevity, both at the level of individual ants and colonies in black garden ants, Lasius niger. We explored how lifespan was affected by the dietary protein-to-carbohydrate ratio and the duration of exposure to a high-protein diet. We show that (i) restriction to high-protein, low-carbohydrate diets decreased worker lifespan by up to 10-fold; (ii) reduction in lifespan on such diets was mainly due to elevated intake of protein rather than lack of carbohydrate; and (iii) only one day of exposure to a high-protein diet had dire consequences for workers and the colony, reducing population size by more than 20 per cent.
Keywords: nutrition, lifespan, ant, protein
1. Introduction
Social insects offer three important opportunities for studying longevity compared with the usual model systems for research on lifespan and ageing. First, they show a striking difference in longevity between short-lived, sterile workers and long-lived reproductives (review in earlier studies [1–4]). Second, because many social insects have a pronounced division of labour between similarly sized sterile worker castes [5], they are ideal for studying the direct effects of extrinsic mortality on the evolution of ageing rates, independent of reproductive effort and genotype. Third, social insects as ‘superorganisms’ [5,6] offer the intriguing possibility to consider the relationship between longevity at the individual and collective (colony) level. The interface between individual and colony longevity also has applied significance, notably in relation to a problem that is currently facing bee-keepers in many regions of the world: colony collapse disorder (CCD).
Social insects not only offer these opportunities for research on lifespan and ageing, but they also share some fundamental features with other non-social organisms. For instance, social insects regulate their intake of both protein and carbohydrate when suitable foods are available [7–11] and have higher mortality rates when confined to diets that have an increased proportion of protein relative to requirements (ants [8,10,12] and honeybees [13]), as has also been shown for other insects (cockroaches [14], flies [15–19] and crickets [20]). Elevated protein or specific amino acids have also been linked to shortened lifespan in mammals [21]. However, none of the published studies performed on mortality in relation to macronutrient balance in social insects has lasted long enough to measure differences in colony longevity [8,10,13].
In this study, we investigated the link between protein and longevity, both at the level of individual workers and colonies. We used the geometric framework (GF) for nutrition [22] to show how lifespan was affected by the concentration and ratio of protein to carbohydrate in available foods, and by the duration of exposure to a high-protein diet. This approach has recently provided a new understanding of the nutritional correlates and causes of lifespan in solitary species [21–23]. These studies have involved mapping lifespan, reproductive effort and metabolic consequences as response surfaces onto arrays of macronutrient intakes generated by restricting study organisms to one of a large number of diets differing systematically in the ratios and concentrations of macronutrients. Here, we use GF experiments to show that worker ants are especially susceptible to protein over-feeding, and that a short exposure to a high-protein diet may have serious consequences for individuals and the colony.
2. Methods
(a). Species studied and rearing conditions
We chose to carry out experiments on the aphid-tending ant, Lasius niger. These ants obtain their protein and carbohydrate supplies from aphid colonies through honeydew and direct predation. Aphid-tending ants are consequently of particular interest to the study of how lifespan is affected by the concentration and ratio of protein to carbohydrate.
Twelve mother colonies of the black garden ant L. niger, each comprising around 10 000 workers, were collected in Marquefave (southwest France) in 2009. From these mother colonies, 128 queenless experimental colonies of 200 workers without brood were constructed. Workers for experimental colonies were collected from the foraging arena rather than within the nests of the mother colonies. All experimental colonies were created the same day for each experiment. All experiments started in June 2009.
Each experimental colony was housed in a plastic box (diameter: 100 mm), the bottom of which was covered by a layer of cotton moistened by a cotton plug soaking in a water reservoir underneath. The box was connected to an arena (diameter: 120 mm) with walls coated with Fluon to prevent ants from escaping. The nests were regularly moistened and the colonies were kept at room temperature (24–26°C) under a 12 L : 12 D photoperiod. Before starting the experiments, colonies were fed ad libitum with honey solution (15%) and prey (mealworms Tenebrio molitor) for a week, except for experiment 4.
(b). Synthetic foods
In the field, black garden ants scavenge for dead insects and collect honeydew from sap-feeding Homoptera. Accordingly, these ants experience foods that vary widely in their ratio of protein to carbohydrates, from nearly pure sources to mixtures. For the experiment described below, we used synthetic foods varying in the ratio and concentration of protein and digestible carbohydrate. The protein content of all the foods consisted of a mixture of casein (Nutrimuscle), whey protein (Nutrimuscle) and egg powder (Nutrimuscle), whereas glucose was used as a digestible carbohydrate source [24]. The quantity of whole egg was kept constant in each food in order to keep the quantity of fat and minerals identical. Each food contained 0.5 per cent of vitamins (Vanderzant vitamin mixture for insects; Sigma). The foods were presented to the ants in a 1 per cent agar solution at a 4.5 : 1 ratio of agar solution to dry mass of ingredients. Further preparation details are given by Dussutour & Simpson [24].
(c). Experiments
(i). Experiment 1: the effect on survival of the dietary protein-to-carbohydrate ratio
We confined 32 experimental colonies to one of four diets differing in their ratio of protein (P) and digestible carbohydrates (C), with a fixed total P + C of 200 g l−1. The four P : C ratios used were 5 : 1, 3 : 1, 1 : 3 and 1 : 5. For each treatment, we used eight experimental colonies originating from eight different mother colonies.
(ii). Experiment 2: the effect on survival of a protein-biased diet
Experiment 1 showed reduced survival on the high-protein diet (5 : 1). The objective of experiment 2 was to discover whether survival was affected by the high quantity of protein, the low quantity of carbohydrate or the interaction of these two (the ratio of protein to carbohydrate). We confined 32 experimental colonies to one of four diet treatments. The first group of eight experimental colonies received a diet (referred to as ‘C’) in which the protein mix was removed from the 5 : 1 diet. In order to keep the fat content constant, we were constrained to keep the egg yolk part of the diet, which contains a small amount of protein. Consequently, the C diet was characterized by a ratio of protein to carbohydrates of 1 : 5 with a fixed total P + C of 40 g l−1 (six times more diluted than the diet 1 : 5 used in the first experiment). The second group received a diet (referred to as ‘P’) in which glucose was omitted, yielding a diet with a total protein content of 167 g l−1 and no carbohydrate. The third group received diets C and P, each in separate dishes (this treatment is referred to as ‘P versus C’). This diet treatment allowed us to determine whether colonies were able to compose a diet that would maximize lifespan when provided with two highly unbalanced diets. A final control group received the original 5 : 1 diet (referred to as ‘P : C’), with a fixed total P + C of 200 g l−1. For each treatment, we used eight experimental colonies from eight different mother colonies.
(iii). Experiment 3: the effect on survival of the nutrient concentration in a diet with a high protein-to-carbohydrate ratio
If the reduction in longevity observed on a high-protein, low-carbohydrate diet is due to limited access to carbohydrates, increasing the total concentration of nutrients in the diet at a fixed high-protein, low-carbohydrate ratio would be expected to affect longevity. To test this, we confined 24 experimental colonies to one of three diets differing in their nutrient concentration (100, 200 and 400 g l−1; i.e. 1.7%, 3.3% and 6.7% of carbohydrates; respectively) with a fixed total P : C ratio of 5 : 1. For each treatment, we used eight experimental colonies arising from eight mother colonies.
(iv). Experiment 4: the effect on survival of the duration of exposure to a high-protein diet
Having demonstrated that a high-protein diet reduced longevity, a fourth experiment was conducted to investigate the effect of duration of exposure to high-protein diet on survival. We confined 40 experimental colonies to one of five treatments. The first group was confined to a protein-to-carbohydrate ratio of 1 : 5 for the duration of the experiment and served as a control. The other groups received the diet 5 : 1 for either 1, 2, 3 or 4 days and then were offered the diet 1 : 5 for the remaining duration of the experiment. All colonies were starved for 5 days before the beginning of the experiment to encourage food consumption from the first day of the experiment. The eight experimental colonies per treatment came from separate mother colonies.
(d). Measures
(i). Longevity
To assess mortality in all experiments, the number of dead ants within each colony was counted every day and removed from the colony until all ants had died.
(ii). Food consumption
All colonies had ad libitum access to food that was replenished daily. Colonies never collected all the food offered before it was renewed. For all experiments, we measured colony intake for the first three weeks of the experiment. The food was placed in the foraging arena in two small containers (diameter, 15 mm; height, 5 mm). The ants had access only to one container; the second was used as a control for measuring and correcting for evaporation. We also provisioned the nest with moistened cotton wool to minimize the water loss. In order to evaluate the colony's intake, the small containers with the food were weighed every day before they were placed in the foraging arena and again after they were removed. We adjusted colony intake to the number of ants in each colony, to take into account differences in mortality between colonies.
(iii). Activity
For all experiments, the total number of ants in the foraging arena and the number of ants feeding were counted once per day (2 h after replenishing the food) for the first three weeks of each experiment.
(e). Statistical analysis
All statistical tests were conducted with Statistica (v. 8.0) and R (v. 2.9.0). For all experiments, normality was assessed for each variable using a Kolmogorov–Smirnov test. The probabilities given in the text are always two-tailed.
Longevity data across treatments were compared using Cox regression analysis, with treatment (diet fed to the ants), mother colony and experimental colony as categorical variables. Treatment was included in the analysis as a factor, whereas colony was included as a clustered term (nested factor).
We used Lande–Arnold regression approaches to estimate parametric nonlinear response surfaces [15]. These comprise linear and quadratic components for protein and carbohydrate intake (or concentration) and the cross-product of P and C. Response surfaces for lifespan (median value per colony), proportion of foragers (over three weeks) and proportion of foragers feeding (over three weeks) were fitted over protein–carbohydrate intake (over three weeks) or concentration arrays. Response surfaces are best visualized by using non-parametric techniques that do not constrain the shape of the surface [13]. We fitted non-parametric thin-plate splines using the Fields package in R (v. 2.9.0).
We used multiple regression analysis to investigate the effect of number of ants feeding, protein concentration, carbohydrate concentration and colony on colony intake. Colony was treated as a random factor. For the purpose of the analysis, independent variables were centred on their means [25].
For all experiments, we used two-way ANOVAs with repeated measures to test for the effect of diet and time on (i) the amount of food collected per individual, (ii) the proportion of ants in the foraging arena and (iii) the proportion of foragers feeding.
3. Results
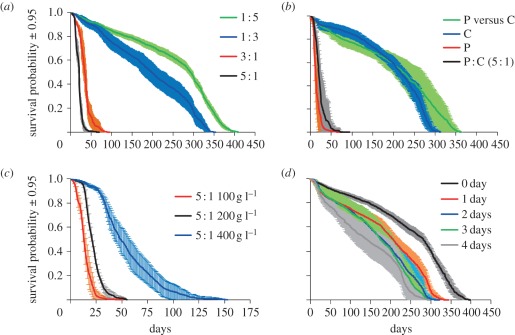
The first experiment was designed to establish whether there is a ratio and rate of protein and carbohydrate collected by foragers that maximizes worker and colony lifespan. Ants lived longest on a diet comprising a 1 : 5 ratio of protein to carbohydrate, and survived proportionately less long as this ratio rose (figure 1a; electronic supplementary material, table S1). Colonies fed with the high-protein diets collected substantial excesses of protein relative to the amount collected on a 1 : 5 diet, had more ants in the foraging arena and had more foragers engaged in feeding, presumably in an effort to maintain a constant carbohydrate intake (electronic supplementary material, figures S1–S4A).
Figure 1.
Ant lifespan on different diets. (a) The response to the ratio of protein to carbohydrate in the diet at a fixed total concentration of nutrients. (b) Exploration of the effect of the P : C 5 : 1 diet: complete 5 : 1 mix of protein and carbohydrate (P : C); the main source of protein omitted, leaving only residual protein in other ingredients and a highly dilute food medium (C); carbohydrate omitted (P); and choice of protein and carbohydrate diets offered in separate dishes (P versus C). (c) The effect of concentration of nutrients in the high-protein diet (5 : 1). (d) The response to duration of exposure to the high-protein diet (5 : 1). Experimental colonies of 200 individuals per treatment (n = 8). Mortality dynamics was consistent between colonies of the same treatment.
Having established that survival was lower on P : C 5 : 1 than on the other diets, we next explored if reduced survival on the 5 : 1 diet was caused by the elevated proportion of protein, the low level of carbohydrate or a combination of these two (i.e. the ratio of protein to carbohydrate). Ants lived longest on P versus C, and survived for a much shorter time on P (figure 1b; electronic supplementary material, table S2). Colonies fed P versus C were then able to compose a diet that would maximize their lifespan when provided with two unbalanced but complementary foods. On treatments C and P versus C, colonies increased food collection from the C food in an effort to maintain carbohydrate intake (electronic supplementary material, figures S1–S4B).
The reduction in longevity observed on a high-protein diet could be because of a shortage of carbohydrates due to their dilution by protein in the diet. If so, then increasing the total concentration of nutrient in the diet might improve longevity. Indeed, we observed an increase in colony longevity as nutrient concentration was increased (figure 1c; electronic supplementary material, table S3). Ants did not increase food collection on the most diluted foods (electronic supplementary material, figure S2C). The proportion of foragers was higher when nutrients was highly diluted (electronic supplementary material, figure S3C), but the foraging efficiency (i.e. the number of foragers actually feeding) was very low (electronic supplementary material, figure S4C).
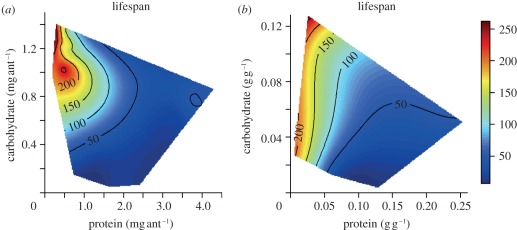
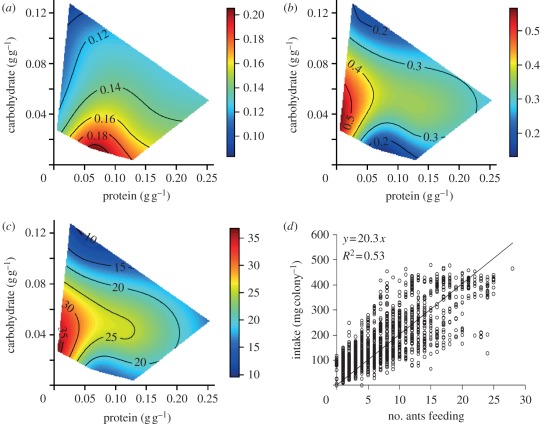
Taken together, these experiments allowed us to generate a map of protein–carbohydrate intake space, on which response surfaces for variables related to colony performance (lifespan, storage, foraging activity and foraging efficiency) could be regressed. Survival depended on protein and carbohydrate intakes, and on the ratio of protein to carbohydrate eaten (figure 2a; electronic supplementary material, table S4). Ants lived longest on diets comprising four times more carbohydrate than protein (figure 2a–c; electronic supplementary material, table S4). A colony's lifespan was strongly influenced by nutrient concentrations and the nutrient ratio of the diet, falling sharply at protein concentrations above 5 per cent (figure 2c; electronic supplementary material, table S5). When nutrients were highly diluted, colonies had more ants in the foraging arena (i.e. higher foraging activity; figure 3a; electronic supplementary material, table S5) and a higher proportion of foragers were engaged in feeding (i.e. higher foraging efficiency; figure 3b; electronic supplementary material, table S5), thereby increasing food collection to the colony (figure 3c; electronic supplementary material, table S5) and compensating for nutrient dilution. The number of ants feeding is a good predictor of colony intake (figure 3d; electronic supplementary material, table S6), whereas foraging activity alone is not a good predictor of foraging efficiency (electronic supplementary material, table S5).
Figure 2.
Performance response surfaces. Data were recorded for ants confined throughout their life to treatments varying in both the ratio and the total amount of protein and carbohydrate (diets used for experiments 1–4). Response surfaces were visualized using non-parametric thin-plate splines, which were fitted using the Fields package in the statistical software R. Red indicates the highest values for the experimental variable on a given response surface, with values descending to lowest values in dark blue regions. (a) Effects of nutrient intake on ant survival (median value for each experimental colony). (b) Effects of nutrient concentration on ant survival. The response surface regression analyses yielded significant relationships as follows: r² = 0.88, F12,115 = 77.92, p < 0.001, and r² = 0.92, F12,107 = 116.69, p < 0.001 for survival as a function of diet intake and diet concentration, respectively (electronic supplementary material, tables S4 and S5).
Figure 3.
Activity responses. Effects of dietary composition on (a) proportion of ants in the foraging arena (exploring activity) and (b) proportion of ants in the foraging arena that are feeding (feeding activity; mean value for each experimental colony, n = 21 days of sampling). The response surface regression analyses yielded significant relationships as follows: r² = 0.85, F12,107 = 57.92, p < 0.001, and r² = 0.87, F12,107 = 67.70, p < 0.001 for proportion of ants in the foraging arena and proportion of ants in the foraging arena that are feeding, respectively (electronic supplementary material, table S5). (c) Effects of nutrient concentration on the total diet intake measured across 21 days (r² = 0.95, F12,107 = 177.87, p < 0.001; electronic supplementary material, table S5). (d) Effect of number of ant feeding on colony diet intake. The model investigating the effect of number of ants feeding, protein concentration, carbohydrates concentration and colony on the colony intake was significant (F13,2506 = 106.37, p < 0.001), and accounted for 36% of the variance. Examination of the standardized regression coefficients shows that the main effect on colony intake was due to number of ants feeding (see electronic supplementary material, table S6).
We then explored the role of duration of exposure to high-protein diet on survival. The aim was to discover if colonies fed a high-protein diet for a certain period of time were able to recover when returned to a balanced diet. Surprisingly, only one day of exposure to a high-protein diet was sufficient to affect lifespan, and reduced it by at least 20 per cent (figure 1d; electronic supplementary material, table S7). The longer the exposure to a high-protein diet, the more colony lifespan was reduced. When colonies were exposed to a high-protein diet, a substantial decrease in survival probability occurred early in the experiment (after 10 days) and lasted for 20 days. The survival probability thereafter decreased more slowly, following the time course observed for colonies not exposed to high-protein diet. As in experiment 1, when the colonies were exposed to high-protein diet, food collection increased, more ants were observed in the foraging arena and more foragers were engaged in feeding (electronic supplementary material, figures S2–S4D).
4. Discussion
We have demonstrated that the key determinant of the relationship between diet and longevity in ants is the balance of protein and non-protein energy ingested. Lifespan was reduced when ants were fed with high-protein, low-carbohydrate diets. We were able to demonstrate that the reduction in lifespan was mainly due to elevated intake of protein and to a far lesser extent to the lack of carbohydrates. Remarkably, colonies paid the longevity cost of a high-protein diet after only one day of exposure, even if they were subsequently shifted to an optimal, high-carbohydrate diet.
Regulation of nutrient intake requires assessment of the nutritional quality of available foods and sensors indicating the nutritional state of the regulating entity. In the case of ants, the assessment and collection of food is undertaken on behalf of the colony by the foraging ants, which can be specialized for the collection of different food types. Ants collect both protein and carbohydrate in the environment. The major sink for collected protein is growing larvae, whereas worker ants require mainly carbohydrate for energy [26–32]. When larvae are present in the colony, protein comprised a higher proportion of the macronutrients collected [10]. In our study, larvae were absent, partly explaining why ants survived better on a highly carbohydrate-biased diet.
Ants had a pronounced ability to compensate for dietary sugar dilution, as demonstrated by their collecting six times the total quantity of food when confined to diet C (2% sugar) compared with the 1 : 5 diet (12% sugar). Colonies compensated for the dilution at a collective level, recruiting more ants to collect more food. Previous studies have shown that after a long period of food deprivation (e.g. 5–8 days), colonies exhibit a higher rate of ant mobilization and an increased tendency to leave the nest and explore [33–36]. For high-protein diets, however, dilution of nutrients strongly affected lifespan, with a higher mortality and a lack of compensatory feeding behaviour on highly diluted diets. We did, however, observe a higher proportion of foragers on high-protein diets. Interestingly, these foragers were not observed feeding, but instead appeared to be engaged in ‘exploratory’ behaviour.
Data from the choice experiment (P versus C) indicated that ants have the capacity to regulate intake of both protein and carbohydrate separately even if food sources are highly unbalanced, as had been found in other ants observed over shorter periods of time with more balanced foods [7,8,10,11]. Ants were able to select a diet composition that maximized lifespan, confirming that in social insect colonies, interactions among individuals somehow coordinate the activities of the entire colony so that it acts as a nutrient-regulating ‘superorganism’ [10].
Our results, indicating a lasting deleterious effect of short exposure to a high-protein diet, contrast strikingly with results from Drosophila, in which there appear to be no persevering effects of diet on longevity—flies that have their diet switched immediately adopt the mortality rates and longevity prospects of their new regime, with no ‘memory’ of the previous diet [37,38]. However, in one of those studies [37], flies were shifted from one concentration of food to another without modifying the P : C ratio, whereas in the second study [38] flies were moved from a high-carbohydrate diet to a high-protein diet (i.e. from low-mortality conditions to high-mortality conditions). In our study, ants were moved from high-mortality conditions (high-P diet) to low-mortality conditions (high-C diet). Colonies exposed to high-protein diet showed a delayed but substantial decrease in survival probability even after they were shifted to an optimal high-carbohydrate diet. Only after several weeks did the survival probability follow the time course observed for colonies that were not previously exposed to a high-protein diet.
An effect of dietary history on mortality was also reported in Caenorhabditis elegans [39], with worm mortality being a cumulative function of all past mortality risks. Adult age-specific mortality may be induced by two types of factors: those that are intrinsic to the system and induce accumulation of irreversible damage; and those that are either external to the system or reflect an inherent instability in the system and cause short-term vulnerability to the risk of dying [37–39]. In this formulation, placing ants onto a high-protein diet increases the short-term risk of dying and causes lasting damage.
It remains unclear how high-protein diets increase mortality rates in both the short and long term. In yeast, roundworms, flies and mice, the target of rapamycin (TOR) pathway, which signals systemic nutrient levels and is especially sensitive to branch-chain amino acids, has been shown to regulate lifespan (review by Simpson & Raubenheimer [21]). For example, amino acid restriction mediates lifespan extension via the TOR pathway [40]. Conversely, elevated levels of nutrients such as proteins that increase TOR signalling are expected to be life-shortening. Moreover, in ants, workers, unlike larvae, have only a limited ability to digest proteinaceous foods in the midgut because of a combination of their narrow waist (petiole) separating the thorax from the abdomen and their producing only very small amounts of proteases in their midguts [41–44]. Life-shortening effects of protein could also be linked to the elimination of nitrogenous waste.
Recently, CCD has emerged as a major threat to honeybees in the USA and has caused 30 to 40 per cent loss of bee colonies each year since the autumn of 2006. CCD-affected colonies have greatly reduced adult bee populations, with only a few hundred workers and the queen left, but with many frames of brood, which suggests rapid depopulation of adults. The cause of CCD remains unknown. Our data on ants may offer a clue that nutrition, particularly protein over-nutrition, may be involved. It is known that, as in ants, a high-protein diet reduces longevity in worker honeybees [13,45]. Bee colonies are now increasingly commonly provisioned with a commercially available protein supplement, aiming to increase the production of brood. Such ‘pollen substitutes’ are high-protein mixtures (typically around 40% protein, when compared with 25% in pollen) that are used to replace or augment pollen in the diet, and typically include such ingredients as soybean flour, powdered skimmed milk, egg powder and brewer's yeast [46–49]. Consequently, protein levels in the haemolymph of bees fed pollen substitute were significantly protein-elevated compared with controls [50]. It would therefore be interesting to investigate how lifespan in honeybees is affected using pollen substitute and whether patterns of CCD incidence globally correlate with the practice of protein supplementation.
Acknowledgements
A.D. was supported by a Fyssen Foundation research grant, by the Centre National de la Recherche Scientifique and by an ANR grant 11 JSV7 009 01 (NUTRIANT). S.J.S. was supported by an Australian Research Council Laureate Fellowship.
References
- 1.Heinze J., Schrempf A. 2008. Aging and reproduction in social insects: a mini-review. Gerontology 54, 160–167 10.1159/000122472 (doi:10.1159/000122472) [DOI] [PubMed] [Google Scholar]
- 2.Parker J. D. 2010. What are social insects telling us about aging? Myrmecol. News 13, 103–110 [Google Scholar]
- 3.Page R. E., Peng C. Y. S. 2001. Aging and development in social insects with emphasis on the honey bee, Apis mellifera L. Exp. Gerontol. 36, 695–711 10.1016/S0531-5565(00)00236-9 (doi:10.1016/S0531-5565(00)00236-9) [DOI] [PubMed] [Google Scholar]
- 4.Holldobler B., Wilson E. O. 2009. The superorganism: the beauty, elegance, and strangeness of insect societies. New York, NY: W. W. Norton & Company Ltd [Google Scholar]
- 5.Chapuisat M., Keller L. 2002. Division of labour influences the rate of ageing in weaver ant workers. Proc. R. Soc. Lond. B 269, 909–913 10.1098/rspb.2002.1962 (doi:10.1098/rspb.2002.1962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Southwick E. E., Moritz R. F. 1992. Bees as superorganisms: an evolutionary reality. New York, NY: Springer [Google Scholar]
- 7.Cook S. C., Behmer S. T. 2010. Macronutrient regulation in the tropical terrestrial ant, Ectatomma ruidum: a field study. Biotropica 42, 135–139 10.1111/j.1744-7429.2009.00616.x (doi:10.1111/j.1744-7429.2009.00616.x) [DOI] [Google Scholar]
- 8.Cook S. C., Eubanks M. D., Gold R. E., Behmer S. T. 2010. Colony-level macronutrient regulation in ants: mechanisms, hoarding and associated costs. Anim. Behav. 79, 429–437 10.1016/j.anbehav.2009.11.022 (doi:10.1016/j.anbehav.2009.11.022) [DOI] [Google Scholar]
- 9.Dussutour A., Simpson S. J. 2008. Carbohydrate regulation in relation to colony growth in ants. J. Exp. Biol. 211, 2224–2232 10.1242/jeb.017509 (doi:10.1242/jeb.017509) [DOI] [PubMed] [Google Scholar]
- 10.Dussutour A., Simpson S. J. 2009. Communal nutrition in ants. Curr. Biol. 19, 740–744 10.1016/j.cub.2009.03.015 (doi:10.1016/j.cub.2009.03.015) [DOI] [PubMed] [Google Scholar]
- 11.Christensen K. L., Gallacher A. P., Martin L., Tong D., Elgar M. A. 2010. Nutrient compensatory foraging in a free-living social insect. Naturwissenschaften 10, 941–944 10.1007/s00114-010-0705-8 (doi:10.1007/s00114-010-0705-8) [DOI] [PubMed] [Google Scholar]
- 12.Kay A. D., Zumbusch T. B., Heinen J. L., Marsh T. C., Holway D. A. 2010. Nutrition and interference competition have interactive effects on the behavior and performance of Argentine ants. Ecology 91, 57–64 10.1890/09-0908.1 (doi:10.1890/09-0908.1) [DOI] [PubMed] [Google Scholar]
- 13.Pirk C. W. W., Boodhoo C., Human H., Nicolson S. W. 2010. The importance of protein type and protein to carbohydrate ratio for survival and ovarian activation of caged honeybees (Apis mellifera scutellata). Apidologie 41, 62–72 10.1051/apido/2009055 (doi:10.1051/apido/2009055) [DOI] [Google Scholar]
- 14.Hamilton R. L., Cooper R. A., Schal C. 1990. The influence of nymphal and adult dietary protein on food intake and reproduction in female brown-banded cockroaches. Entomol. Exp. Appl. 55, 23–31 10.1111/j.1570-7458.1990.tb01344.x (doi:10.1111/j.1570-7458.1990.tb01344.x) [DOI] [Google Scholar]
- 15.Lee K. P., Simpson S. J., Clissold F. J., Brooks R., Ballard J. W. O., Taylor P. W., Soran N., Raubenheimer D. 2008. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc. Natl Acad. Sci. USA 105, 2498–2503 10.1073/pnas.0710787105 (doi:10.1073/pnas.0710787105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prabhu V., Perez-Staples D., Taylor P. W. 2008. Protein: carbohydrate ratios promoting sexual activity and longevity of male Queensland fruit flies. J. Appl. Entomol. 132, 575–582 10.1111/j.1439-0418.2007.01265.x (doi:10.1111/j.1439-0418.2007.01265.x) [DOI] [Google Scholar]
- 17.Fanson B. G., Weldon C. W., Perez-Staples D., Simpson S. J., Taylor P. W. 2009. Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni). Aging Cell 8, 514–523 10.1111/j.1474-9726.2009.00497.x (doi:10.1111/j.1474-9726.2009.00497.x) [DOI] [PubMed] [Google Scholar]
- 18.Grandison R. C., Piper M. D. W., Partridge L. 2009. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 462, 1061–1064 10.1038/nature08619 (doi:10.1038/nature08619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kristensen T. N., Overgaard J., Loeschcke V., Mayntz D. 2011. Dietary protein content affects evolution for body size, body fat and viability in Drosophila melanogaster. Biol. Lett. 7, 269–272 10.1098/rsbl.2010.0872 (doi:10.1098/rsbl.2010.0872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maklakov A. A., Simpson S. J., Zajitschek F., Hall M. D., Dessmann J., Clissold F., Raubenheimer D., Bonduriansky R., Brooks R. C. 2008. Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Curr. Biol. 18, 1062–1066 10.1016/j.cub.2008.06.059 (doi:10.1016/j.cub.2008.06.059) [DOI] [PubMed] [Google Scholar]
- 21.Simpson S. J., Raubenheimer D. 2009. Macronutrient balance and lifespan. Aging 1, 875–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpson S. J., Raubenheimer D. 2012. The nature of nutrition: a unifying framework from animal adaptation to human obesity. Princeton, NJ: Princeton University Press [Google Scholar]
- 23.Piper M. D. W., Partridge L., Raubenheimer D., Simpson S. J. 2011. Dietary restriction and aging: a unifying perspective. Cell Metab. 14, 154–160 10.1016/j.cmet.2011.06.013 (doi:10.1016/j.cmet.2011.06.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dussutour A., Simpson S. J. 2008. Description of a simple synthetic diet for studying nutritional responses in ants. Insect Soc. 55, 329–333 10.1007/s00040-008-1008-3 (doi:10.1007/s00040-008-1008-3) [DOI] [Google Scholar]
- 25.Aiken L. S., West S. G. 1991. Multiple regression: testing and interpreting interactions. Newbury Park, CA: Sage Publications [Google Scholar]
- 26.Brian M. V., Abbott A. 1977. The control of food flow in a society of the ant Myrmica rubra L. Anim. Behav. 25, 1047–1055 10.1016/0003-3472(77)90055-0 (doi:10.1016/0003-3472(77)90055-0) [DOI] [Google Scholar]
- 27.Sorensen A. A., Busch T. M., Vinson S. B. 1985. Control of food influx by temporal subcastes in the fire ant, Solenopsis invicta. Behav. Ecol. Sociobiol. 17, 191–198 10.1007/BF00300136 (doi:10.1007/BF00300136) [DOI] [Google Scholar]
- 28.Sorensen A. A., Vinson S. B. 1981. Quantitative food distribution studies within laboratory colonies of the imported fire ant, Solenopsis invicta Buren. Insect Soc. 28, 129–160 10.1007/BF02223701 (doi:10.1007/BF02223701) [DOI] [Google Scholar]
- 29.Markin G. P. 1970. Food distribution within laboratory colonies of the Argentine ant, Iridomyrmex humilis (Mayr). Insect Soc. 17, 127–158 10.1007/BF02223074 (doi:10.1007/BF02223074) [DOI] [Google Scholar]
- 30.Howard D. F., Tschinkel W. R. 1981. The flow of food in colonies of the fire ant, Solenopsis invicta: a multifactorial study. Physiol. Entomol. 6, 297–306 10.1111/j.1365-3032.1981.tb00274.x (doi:10.1111/j.1365-3032.1981.tb00274.x) [DOI] [Google Scholar]
- 31.Abbott A. 1978. Nutrient dynamics of ants. In Production ecology of ants and termites (ed. Brian M. V.), pp. 233–244 Cambridge, UK: Cambridge University Press [Google Scholar]
- 32.Cassill D. L., Tschinkel W. R. 1999. Regulation of diet in the fire ant, Solenopsis invicta . J. Insect Behav. 12, 307–328 10.1023/A:1020835304713 (doi:10.1023/A:1020835304713) [DOI] [Google Scholar]
- 33.Wallis D. I. 1962. The relation between hunger, activity and worker function in an ant colony. Proc. Zool. Soc. Lond. B 139, 589–605 10.1111/j.1469-7998.1962.tb01595.x (doi:10.1111/j.1469-7998.1962.tb01595.x) [DOI] [Google Scholar]
- 34.Howard D. F., Tschinkel W. R. 1980. The effect of colony size and starvation on food flow in the fire ant, Solenopsis invicta (Hymenoptera: Formicidae). Behav. Ecol. Sociobiol. 7, 293–300 10.1007/BF00300670 (doi:10.1007/BF00300670) [DOI] [Google Scholar]
- 35.Torres-Contreras H., Niemeyer H. M. 2009. Fasting and chemical signals affect recruitment and foraging efficiency in the harvester ant, Pogonomyrmex vermiculatus. Behaviour 146, 923–938 10.1163/156853908X396773 (doi:10.1163/156853908X396773) [DOI] [Google Scholar]
- 36.Mailleux A. C., Devigne C., Deneubourg J. L., Detrain C. 2010. Impact of starvation on Lasius niger’ exploration. Ethology 116, 248–256 10.1111/j.1439-0310.2009.01736.x (doi:10.1111/j.1439-0310.2009.01736.x) [DOI] [Google Scholar]
- 37.Mair W., Goymer P., Pletcher S. D., Partridge L. 2003. Demography of dietary restriction and death in Drosophila. Science 301, 1731–1733 10.1126/science.1086016 (doi:10.1126/science.1086016) [DOI] [PubMed] [Google Scholar]
- 38.Good T. P., Tatar M. 2001. Age-specific mortality and reproduction respond to adult dietary. J. Insect Physiol. 47, 1467–1473 10.1016/S0022-1910(01)00138-X (doi:10.1016/S0022-1910(01)00138-X) [DOI] [PubMed] [Google Scholar]
- 39.Wu D., Rea S. L., Cypser J. R., Johnson T. E. 2009. Mortality shifts in Caenorhabditis elegans: remembrance of conditions past. Aging Cell 8, 666–675 10.1111/j.1474-9726.2009.00523.x (doi:10.1111/j.1474-9726.2009.00523.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kapahi P., Chen D., Rogers A. N., Katewa S., Wai-Lun Li P., Kockel L. 2010. Less is more with TOR. The emerging role of the conserved nutrient sensing TOR pathway in aging. Cell Metab. 11, 453–465 10.1016/j.cmet.2010.05.001 (doi:10.1016/j.cmet.2010.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hölldobler B., Wilson E. O. 1990. The ants. Cambridge, MA: Harvard University Press [Google Scholar]
- 42.Ricks B. L., Vinson S. B. 1972. Digestive enzymes of the imported fire ant, Solenopsis richteri (Hymenoptera: Formicidae). Entomol. Exp. Appl. 15, 329–334 [Google Scholar]
- 43.Glancey B. M., Vander Meer R. K., Glover A., Lofgren C. S., Vinson S. B. 1981. Filtration of microparticles from liquids ingested by the red imported fire ant, Solenopsis invicta Buren. Insect Soc. 28, 395–401 [Google Scholar]
- 44.Petralia R. S., Sorensen A. A., Vinson S. B. 1980. The labial gland system of larvae of the imported fire ant, Solenopsis invicta Buren: ultrastructure and enzyme analysis. Cell Tissue Res. 206, 145–156 [DOI] [PubMed] [Google Scholar]
- 45.Altaye S. Z., Pirk C. W., Crewe R. M., Nicolson S. W. 2010. Convergence of carbohydrate-biased intake targets in caged worker honeybees fed different protein sources. J. Exp. Biol. 213, 3311–3318 10.1242/jeb.046953 (doi:10.1242/jeb.046953) [DOI] [PubMed] [Google Scholar]
- 46.Prakash S., Bhat N. S., Naik M. I., Hanumantha S. B. C. 2010. Evaluation of pollen supplement and substitute on honey and pollen stores of honeybee, Apis cerana Fabricius. Karnataka J. Agric. Sci. 20, 155–156 [Google Scholar]
- 47.Rogala R., Symas B. 2004. Nutritional value for bees of pollen substitute enriched with synthetic amino acids. J. Apic. Sci. 48, 19–27 [Google Scholar]
- 48.Saffari A., Kevan P. G., Atkinson J. 2010. Consumption of three dry pollen substitutes in commercial apiaries. J. Apic. Sci. 54, 5–12 [Google Scholar]
- 49.Brodschneider R., Crailsheim K. 2010. Nutrition and health in honey bees. Apidologie 41, 278–294 10.1051/apido/2010012 (doi:10.1051/apido/2010012) [DOI] [Google Scholar]
- 50.De Jong D., Da Silva E. J., Kevan P. G., Atkinson J. L. 2009. Pollen substitutes increase bee haemolymph protein levels as much or more than does pollen. J. Apic. Res. 48, 34–37 10.3896/IBRA.1.48.1.08 (doi:10.3896/IBRA.1.48.1.08) [DOI] [Google Scholar]