Abstract
The most dramatic gradient in global biodiversity is between marine and terrestrial environments. Terrestrial environments contain approximately 75–85% of all estimated species, but occupy only 30 per cent of the Earth's surface (and only approx. 1–10% by volume), whereas marine environments occupy a larger area and volume, but have a smaller fraction of Earth's estimated diversity. Many hypotheses have been proposed to explain this disparity, but there have been few large-scale quantitative tests. Here, we analyse patterns of diversity in actinopterygian (ray-finned) fishes, the most species-rich clade of marine vertebrates, containing 96 per cent of fish species. Despite the much greater area and productivity of marine environments, actinopterygian richness is similar in freshwater and marine habitats (15 150 versus 14 740 species). Net diversification rates (speciation–extinction) are similar in predominantly freshwater and saltwater clades. Both habitats are dominated by two hyperdiverse but relatively recent clades (Ostariophysi and Percomorpha). Remarkably, trait reconstructions (for both living and fossil taxa) suggest that all extant marine actinopterygians were derived from a freshwater ancestor, indicating a role for ancient extinction in explaining low marine richness. Finally, by analysing an entirely aquatic group, we are able to better sort among potential hypotheses for explaining the paradoxically low diversity of marine environments.
Keywords: biodiversity, evolution, fish, freshwater, marine, richness
1. Introduction
The most dramatic gradient in global biodiversity is between marine and terrestrial environments. Marine environments cover approximately 70 per cent of Earth's surface, but have only a fraction of the diversity of terrestrial environments (approx. 15–25% of all estimated species; [1–3]). Given the depth at which marine environments can be inhabited, oceans also include approximately 90–99% of the volume of the habitable biosphere [2,4]. Many hypotheses have been proposed to explain this dramatic difference in diversity relative to area. These hypotheses variously suggest that terrestrial environments are more diverse because they have higher net primary productivity, larger primary producers (macroscopic versus mostly microscopic), less extensive herbivory on individual primary producers, more complex habitats, and more effective barriers to dispersal, as well as species with narrower ecological specialization and smaller geographical range sizes [1,2,5]. However, there have been few (if any) large-scale quantitative analyses that address the evolutionary and ecological causes of the disparity in richness between marine and terrestrial environments.
Actinopterygian (ray-finned) fish are an important group for addressing the question of why marine environments have so few species. Actinopterygians contain roughly half of all vertebrate species, and approximately 96 per cent of all ‘fish’ [4,6]. They are unquestionably the most diverse group of marine vertebrates, but also have many species in freshwater [4,6]. Clearly, terrestrial and freshwater environments are not necessarily synonymous. However, the fact that non-marine actinopterygians occur in freshwater rather than on land may actually give them some advantages for addressing the causes of this richness gradient, because the similarity between freshwater and saltwater habitats may help identify properties that are unique to marine environments, and not simply characteristics of aquatic environments in general. Thus, actinopterygians may offer a complementary perspective on the diversity of marine environments relative to only comparing marine and truly terrestrial (non-aquatic) organisms. Surprisingly, no previous studies have addressed global patterns of fish diversity in freshwater versus saltwater habitats, nor the evolutionary and ecological causes of these patterns.
In this study, we take a phylogenetic approach to address the relative diversity of actinopterygian fishes in saltwater versus freshwater habitats, and their implications for explaining the overall disparity in biodiversity between marine and terrestrial environments. Ultimately, differences in species richness between environments must be related to differences in rates and patterns of speciation and extinction in each environment (i.e. net diversification), and/or to different amounts of time spent in each environment [7]. Here, we first quantify the species diversity of actinopterygian fish in freshwater versus saltwater. We then test whether the occurrence of a clade in freshwater versus saltwater influences its rate of net diversification (speciation–extinction). We also address the relative time that actinopterygians have been present in each environment, and the potential role of time in explaining these diversity patterns.
2. Material and methods
(a). Patterns of diversity
We quantified the number of species in each habitat using FishBase [8]. We did not treat subspecies as separate species, even if some might eventually prove to be. We initially scored each species for occurrence in freshwater, brackish or saltwater environments. Many species occurred in some combination of these three habitat types. To estimate the richness in each environment, species whose range of habitats included freshwater were considered to be freshwater (e.g. diadromous species; following Berra [9]), and all others were considered saltwater (to avoid counting species that occur in both freshwater and saltwater twice). Relatively few species occur exclusively in brackish water habitats. Alternate ways of categorizing species as freshwater versus saltwater (e.g. any occurrence in the sea as marine) give similar estimates for overall richness in each habitat [10]. Our summary suggests that only 720 (4%) of actinopterygian species are found in both freshwater and saltwater, so different ways of categorizing these species have little impact on diversity patterns across the group.
We recognize that many new species of fish continue to be described, and that the species numbers we use are therefore underestimates. However, new fish species are being described at similar rates in marine and freshwater environments (with a slightly higher rate in freshwater; [10]). Thus, extrapolating from these rates, the true richness patterns should parallel our current estimates, with similar numbers in each environment (but slightly more in freshwater; see §3).
(b). Phylogeny
For phylogenetic analyses, we used the time-calibrated phylogeny from Alfaro et al. [11]. This phylogeny is based on extensive taxon sampling across vertebrates using the slow-evolving nuclear gene RAG-1, a locus that is widely used in studies of higher level vertebrate phylogeny and dating times (e.g. [12]). Clade ages were estimated by those authors [11] using multiple fossil calibration points (both inside and outside actinopterygians) and a relaxed clock model with BEAST [13,14]. The phylogeny [11] includes 124 actinopterygian species, representing 20 per cent of actinopterygian families (97 of 476; [8]). The phylogeny is generally strongly supported, and the topology and divergence dates are largely concordant with traditional estimates of phylogeny and clade ages [11]. Furthermore, the number of species sampled from each of the 22 major clades (see below) relative to the total number of species sampled is proportional to the relative richness of these clades among actinopterygians (r2 = 0.984; p < 0.0001).
(c). Diversification rates
We tested whether habitat influences rates of net diversification of actinopterygian clades. The net diversification rate reflects the combined influence of speciation and extinction on diversity (without distinguishing between them), and provides an index that can be used to compare clades of different ages (even if these rates are not constant over time within a clade). We estimated and compared the net diversification rate for 22 non-overlapping higher level clades (corresponding to orders and superorders) that together include nearly all extant actinopterygian species (table 1). The clades used are effectively the same as those used by Alfaro et al. ([11]; their fig. 1), except that we do not treat Scombridae as separate from Percomorpha. Although more extensive phylogenies will doubtless become available in the future, the diversity, ages and relationships of these 22 clades should be relatively stable.
Table 1.
Species richness, habitat distribution, age and estimated diversification rates for 22 clades of actinopterygian fishes.
| clade | freshwater richness | saltwater richness | % marine | total richness |
|---|---|---|---|---|
| Amiiformes | 1 | 0 | 0 | 1 |
| Argentiniformes | 0 | 19 | 100 | 19 |
| Aulopiformes | 1 | 254 | 99.6 | 255 |
| Beryciformes | 0 | 254 | 100 | 254 |
| Chondrostei | 29 | 0 | 0 | 29 |
| Clupeomorpha | 159 | 224 | 58.4 | 383 |
| Elopomorpha | 47 | 931 | 95.2 | 978 |
| Esociformes | 12 | 0 | 0 | 12 |
| Galaxiiformes | 51 | 0 | 0 | 51 |
| Lampriformes | 0 | 24 | 100 | 24 |
| Myctophiformes | 0 | 254 | 100 | 254 |
| Ophidiiformes | 7 | 498 | 98.6 | 505 |
| Osmeriiformes | 41 | 206 | 83.4 | 247 |
| Ostariophysi | 9267 | 83 | 0.8 | 9350 |
| Osteoglossomorpha | 216 | 0 | 0 | 216 |
| Percomorpha | 5090 | 11535 | 69.3 | 16625 |
| Percopsiformes | 9 | 0 | 0 | 9 |
| Polymixiiformes | 0 | 10 | 100 | 10 |
| Polypteriformes | 12 | 0 | 0 | 12 |
| Salmoniiformes | 207 | 0 | 0 | 207 |
| Stomiiformes | 0 | 411 | 100 | 411 |
| Zeiformes | 0 | 33 | 100 | 33 |
| totals | 15 149 | 14 736 | 29 885 | |
| clade | stem age | div. rate (e = 0) | div. rate (e = 0.50) | div. rate (e = 0.90) |
| Amiiformes | 231.12 | 0 | 0 | 0 |
| Argentiniformes | 166.84 | 0.0176 | 0.0138 | 0.0062 |
| Aulopiformes | 152.72 | 0.0363 | 0.0318 | 0.0214 |
| Beryciformes | 127.94 | 0.0433 | 0.0379 | 0.0256 |
| Chondrostei | 269.98 | 0.0125 | 0.0100 | 0.0049 |
| Clupeomorpha | 150.22 | 0.0396 | 0.0350 | 0.0244 |
| Elopomorpha | 203.98 | 0.0338 | 0.0304 | 0.0225 |
| Esociformes | 112.52 | 0.0221 | 0.0166 | 0.0066 |
| Galaxiiformes | 133.90 | 0.0294 | 0.0243 | 0.0134 |
| Lampriformes | 124.45 | 0.0255 | 0.0203 | 0.0096 |
| Myctophiformes | 147.56 | 0.0375 | 0.0329 | 0.0222 |
| Ophidiiformes | 117.67 | 0.0529 | 0.0470 | 0.0335 |
| Osmeriiformes | 114.27 | 0.0482 | 0.0422 | 0.0284 |
| Ostariophysi | 150.22 | 0.0609 | 0.0563 | 0.0455 |
| Osteoglossomorpha | 195.17 | 0.0275 | 0.0240 | 0.0160 |
| Percomorpha | 110.57 | 0.0879 | 0.0816 | 0.0671 |
| Percopsiformes | 108.70 | 0.0202 | 0.0148 | 0.0054 |
| Polymixiiformes | 131.73 | 0.0175 | 0.0129 | 0.0049 |
| Polypteriformes | 298.32 | 0.0083 | 0.0063 | 0.0025 |
| Salmoniiformes | 112.52 | 0.0474 | 0.0413 | 0.0273 |
| Stomiiformes | 114.27 | 0.0527 | 0.0466 | 0.0327 |
| Zeiformes | 108.70 | 0.0322 | 0.0261 | 0.0132 |
We estimated the net diversification rate for each clade using the method-of-moments estimator for stem-group ages [15]. We focused on stem-group ages given that for most clades too few species were included in the phylogeny to allow confident estimation of crown-group ages. We used three different measures for the relative rate of speciation and extinction (epsilon, or e), including arbitrarily low (0) and high (0.90) values and an intermediate value (0.50). All three values gave similar estimates for the relationship between diversification rate and habitat (see §3). In theory, we could estimate values of e separately for each clade, but such estimates are often problematic for many clades [11] and different values of e seem unlikely to influence the comparisons of net diversification rates among clades. We also confirmed that estimated diversification rates are significantly correlated with the richness of clades (e = 0: r2 = 0.498, p = 0.0002; e = 0.50: r2 = 0.532, p = 0.0001; e = 0.90: r2 = 0.625, p < 0.0001), such that these net rates are potentially relevant to explaining richness patterns [7].
If marine environments generally decrease speciation or increase extinction (for example), then clades with more marine species should have a lower net rate of diversification. We quantified the proportion of marine species that each clade contained, again considering marine species as those not occurring in freshwater at all.
Given that clades are not necessarily independent data points (owing to phylogeny), the final estimate of the relationship between diversification rate and habitat was based on phylogenetic generalized least-squares analysis (PGLS; [16]). PGLS analyses were implemented in the R-package CAIC [17], using R version 2.12.2 [18]. The tree for these analyses was obtained by pruning the 124-species tree so that each higher clade was represented by a single species (the species chosen are unimportant, since the branch length will be the same for any species in the clade).
To test if these estimated relationships between habitat and diversification were contingent on the particular clades used, we repeated this analysis using the 97 families sampled by Alfaro et al. [11] as data points (see the electronic supplementary material, table S1). This analysis gave similar results to those using higher clades as terminal units (see §3). However, because only 20 per cent of actinopterygian families were included in the phylogeny, we did not use this as our primary analysis.
In theory, we could have performed additional analyses of the dynamics of diversification over time in actinopterygians. However, our limited sampling of species (124 of approx. 29 000 species) makes these approaches potentially problematic, and our primary focus here was on patterns of species richness in different environments, not richness over time.
(d). Time and ancestral environment
One habitat may have more species than another simply because it has been inhabited by the group for a longer period of time, allowing more time for speciation to occur there and build up richness (e.g. [7,19]). We first tested whether there was any tendency for predominantly marine clades to be older, and whether older clades tend to have more species. Given that clade age does not evolve like other biological attributes, we used linear regression for these analyses, rather than PGLS.
We also used the phylogeny of 124 sampled species to estimate the ancestral habitat for the common ancestor of living actinopterygians, and to estimate the relative amount of time that actinopterygians have been present in each environment. We coded each of the sampled species with a single character state, either primarily freshwater (state 0: freshwater or freshwater + brackish), primarily marine (state 1: marine or marine + brackish) or widespread (state 2: freshwater + brackish + marine). We then reconstructed the ancestral states using maximum likelihood. We estimated the likelihood and parameters for two models using BayesTraits ([20]; http://www.evolution.reading.ac.uk/BayesTraits.html). First, using a model with a single rate for all transitions. Second, using a model with a separate rate for all six possible transitions (q01, q10, q02, q20, q12 and q21). We then estimated the Akaike information criterion (AIC) [21] for each model (with AIC = 2k − (2(logL)), where k is the number of parameters in each model and L is the likelihood. The model with the lowest AIC was considered to have the best fit, and was used in reconstructions. We found that the one-rate model has a better fit than the six-rate model (one rate: logL = −94.43, AIC = 190.86; six-rate: logL = −90.79, AIC = 193.58).
We then visualized the ancestral state for each node using Mesquite version 2.74 [22]. A state was considered to be unambiguously supported at a given node using the standard-likelihood decision threshold of 2.0, when the difference between the log likelihoods with and without the state fixed at that node is 2 units or more. We then determined the oldest unambiguous occurrence of each habitat on the tree. The full tree is shown in electronic supplementary material, figure S1. We also performed a limited set of analyses incorporating fossil taxa (see electronic supplementary material, appendix S1).
A potential criticism of these analyses is that the effects of habitat on diversification of clades might bias our ancestral reconstructions [23]. However, our results (see below) show that there is no evidence that habitat influences diversification (and these latter analyses are not contingent on ancestral-state reconstructions). Furthermore, current methods that integrate ancestral-state reconstruction and trait-dependent rates of diversification would be problematic to apply given the very large number of actinopterygian species (e.g. [24]).
3. Results
The number of actinopterygian species in freshwater habitats is similar to that in saltwater environments (15 149 versus 14 736, respectively; table 1), despite the striking disparity in the surface area and volume of each environment. There is no relationship between the net diversification rates of the 22 higher clades and the proportion of saltwater species that they contain (e = 0: r2 = 0.040, p = 0.354; e = 0.50; r2 = 0.037, p = 0.391; e = 0.90, r2 = 0.022; p = 0.507). Using 97 families instead of 22 higher clades also shows no relationship between habitat and net diversification rate (e = 0: r2 = 0.005, p = 0.458; e = 0.50: r2 = 0.001, p = 0.708; e = 0.90: r2 = 0.001, p = 0.709). There is a non-significant trend for clades with more freshwater species to be older (r2 = 0.149; p = 0.076), but no tendency for older clades to have more species (r2 = 0.028, p = 0.453; see also [25]).
Likelihood reconstructions on the time-calibrated phylogeny suggest that the common ancestor of living actinopterygians occurred in freshwater (either partly or exclusively), roughly 300 Ma (figure 1; but note that this clade may be considerably older; [26]). Indeed, the three successive extant clades closest to the root occur predominantly in freshwater, as do many other clades near the actinopterygian root (figure 1). Among the species sampled, the oldest clades that are reconstructed as unambiguously marine are approximately 180 Myr old. Reconstructions incorporating fossil taxa also tentatively support the hypothesis that the most recent common ancestor of actinopterygians occurred in freshwater (see electronic supplementary material, appendix S1 and figure S2).
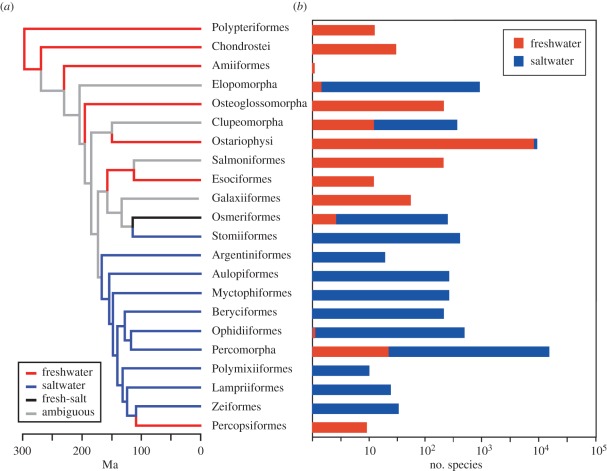
Figure 1.
Summary of actinopterygian phylogeny, habitats and diversity patterns. (a) Phylogeny of 22 major clades of actinopterygians [11], showing maximum-likelihood reconstruction of habitats (using the best-fitting one-rate Mk1 model), summarized from the full 124-species tree (see electronic supplementary material, figure S1). (b) Patterns of species richness in the 22 major clades, indicating the proportion of freshwater and saltwater species. Note that in (a) species are coded as freshwater (freshwater only or fresh + brackish), saltwater (saltwater only or salt + brackish), or both, but in (b) all species are assigned to one habitat or another (i.e. freshwater based on any occurrence in freshwater, saltwater if there is no occurrence in freshwater).
Species richness in both environments is dominated by two relatively recent clades (figure 1). Specifically, ostariophysans have 9350 species (99% freshwater; including 61% of all freshwater species) and have a stem age of 150 Myr, whereas percomorphs have 16 016 species (69% saltwater, including 77% of all marine species), with a stem age of 111 Myr (table 1). The presence of ostariophysans in freshwater habitats may have been inherited from the ancestor of living actinopterygians (even though many intervening branches are ambiguously reconstructed; figure 1). In contrast, the phylogeny shows clearly that there have been repeated invasions of freshwater environments from marine ancestors within Percomorpha in the past approximately 100 Myr (accounting for roughly 33% of all freshwater species; see electronic supplementary material, figure S1). Overall, our results show that most of extant marine actinopterygian biotas are derived from a relatively recent radiation (percomorphs), whereas freshwater biotas are a mixture of ancient lineages (e.g. gars), a major radiation (ostariophysans), and multiple invasions from marine environments that have spawned many major and minor radiations (e.g. cichlids, percids and poeciliids).
4. Discussion
In this paper, we analyse the origins of patterns of diversity in the largest clade of vertebrates (actinopterygian fish) in order to help address the causes of diversity differences between marine and other environments. Overall, species richness of actinopterygians is similar in freshwater and marine habitats, despite the fact that freshwater environments occupy approximately 2 per cent of the Earth's surface and marine environments occupy approximately 70 per cent [1]. Thus, the question to answer is not why there are so many fish species in the sea, but why there are so few. We find no evidence that either environment alone has a significant influence on net diversification rates (speciation–extinction) in actinopterygians. We also find little evidence that either marine or freshwater clades are generally older, or that the age of these clades is related to their richness. The oldest clade reconstructed unambiguously as freshwater is much older than the oldest clade unambiguously reconstructed as marine (300 versus 180 Myr), which suggests that greater age in freshwater environments has not led to dramatically higher species richness (contrary to the time-for-speciation hypothesis; [7,19]). Instead, species richness in both environments is dominated by two relatively recent clades (ostariophysans and percomorphs) that diversified over roughly similar time frames (relative to the age of actinopterygians) to produce similar richness in each environment.
Remarkably, our ancestral reconstructions suggest that all extant marine actinopterygian fish are derived from a freshwater ancestor (i.e. the most recent ancestor of all living actinoptyergians). Although this result clearly depends on taxon sampling (and other factors), our sampling of extant higher level actinopterygian clades is relatively complete [11], and analyses with fossil taxa also support this hypothesis (electronic supplementary material, appendix S1 and figure S2). Surveys of the fossil record [27] show that actinopterygians have been present in both marine and freshwater environments for long periods of time, but with many extinctions, and the high modern diversity in both environments is not reflected in the older fossil record (i.e. less than 100 genera in each environment through most of the Mesozoic; [27]). Even if the earliest actinopterygians were primitively marine, our results nevertheless suggest that extant marine fishes were derived from the living descendants of the early freshwater lineages (figure 1). In addition, although some groups that are predominately freshwater today may have included marine species in the past (e.g. Amiiformes [28]) or had marine ancestors (electronic supplementary material, figure S2), this observation further supports the role of ancient marine extinctions in driving modern patterns of actinopterygian richness. We hypothesize that ancient extinction in marine environments may help explain low marine fish diversity, even though the impact of extinction is not apparent from the net diversification rates of the surviving clades. Interestingly, many other major marine vertebrate groups are derived from terrestrial ancestors and have subsequently gone extinct (e.g. plesiosaurs, ichthyosaurs and mosasaurs) only to be replaced by other marine clades of terrestrial origin (e.g. cetaceans; [6]). We speculate that the relatively low diversity of other ancient marine clades (e.g. sponges, cnidarians and chondrichthyans; [5–6]) might also reflect the long-term impacts of extinction (and/or slow recovery from extinctions). Testing this hypothesis quantitatively will be an important area for future research.
Our results may offer insights on the general question of why there are so few species in marine environments (e.g. [1–2,5]). By focusing on a group that occurs in both marine and freshwater environments (but with low richness per unit area in marine environments), we can evaluate which of the many proposed hypotheses for lower marine richness can explain the relatively limited diversity of marine fish. Our comparisons do not support hypotheses for low marine diversity based primarily on the physical medium of water (recent review in Vermeij & Grosberg [2]), given that marine fish have low diversity per unit area relative to freshwater overall. We also do not support hypotheses based on differences in net primary productivity (NPP in Petagrams of carbon/year): NPP for the oceans is 48.5 Pg/yr and is 56.4 Pg/yr for terrestrial environments [29], but only 0.4 Pg/yr for lakes and streams ([30]). Although freshwater habitats can also have significant inputs from adjacent terrestrial environments [31], these inputs would have to nearly match the NPP of all terrestrial environments combined to allow freshwater productivity to match that of marine environments, which is clearly not possible (and terrestrial inputs into streams and rivers can also contribute to marine productivity). Thus, actinopterygian fish illustrate that global diversity patterns need not be determined primarily by area or productivity. However, we note that there is considerable heterogeneity in the richness, area and productivity of different marine habitats, such as coral reefs versus open ocean, that remains to be untangled. Similarly, higher rates of herbivory in marine environments are unlikely to explain low marine diversity of fish, since these rates are high in freshwater habitats as well [32]. Instead, we suggest that those hypotheses based on more effective barriers to dispersal (promoting speciation, endemism and provincialism [1,2,5]) might apply to both terrestrial and freshwater environments, and might help explain low marine richness in fish and other groups (despite some debate regarding the magnitude and the extent of differences between marine versus terrestrial dispersal and speciation; [33–36]). Furthermore, within marine environments, actinopterygian fish (and many other marine groups) show high species richness in the geographically complex Indo-Pacific ocean region and relatively low richness in most other regions [37]. This pattern potentially supports the importance of spatial habitat heterogeneity and associated limits on dispersal (and lack thereof in many other marine regions), although the causes of high diversity in this region are not fully resolved [37–39]. We speculate that these geographical effects may be particularly important in how they affect diversification after major extinction events, given that for some groups the oceans may have been recolonized from freshwater or terrestrial habitats.
Finally, our results suggest many intriguing questions for future studies of both fish and other terrestrial, freshwater and marine organisms. Are freshwater taxa a reasonable proxy for those in terrestrial organisms, or might they be even more sensitive to dispersal barriers than most terrestrial organisms? What specific properties of marine and terrestrial environments and organisms make them more or less prone to dispersal? Do other marine clades show signatures of re-colonization from terrestrial or freshwater environments (or lower diversity relative to age, for those clades that have remained continuously in marine environments)? What properties of the ostariophysans and percomorphs explain their remarkable radiations relative to other actinopterygian clades? Are other major clades in freshwater as ancient as the basal actinopterygians? Are they primarily derived from marine or terrestrial ancestors? These and other questions should become increasingly tractable as more large-scale time-calibrated phylogenies [11] and databases of ecological traits [8] become available.
Acknowledgements
We thank A. Dornburg for providing the tree from Alfaro et al. [11]. We thank G. Carvalho, M. Dawson, M. Friedman, J. Levinton and two anonymous reviewers for helpful comments that improved the manuscript, and M. Alfaro and F. Santini for advice and encouragement.
References
- 1.May R. M. 1994. Biological diversity: differences between and land and sea. Phil. Trans. R. Soc. Lond. B 343, 105–111 10.1098/rstb.1994.0014 (doi:10.1098/rstb.1994.0014) [DOI] [Google Scholar]
- 2.Vermeij G. J., Grosberg R. K. 2010. The great divergence: when did diversity on land exceed that in the sea? Int. Comp. Biol. 50, 675–682 10.1093/icb/icq078 (doi:10.1093/icb/icq078) [DOI] [PubMed] [Google Scholar]
- 3.Mora C., Tittensor D. P., Adl S., Simpson A. G. B., Worm B. 2011. How many species are there on Earth and in the Ocean? PLoS Biol. 9, e1001127. 10.1371/journal.pbio.1001127 (doi:10.1371/journal.pbio.1001127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helfman G. S., Collette B. B., Facey D. E., Bowen B. W. 2009. The diversity of fishes, 2nd edn West Sussex, UK: Wiley-Blackwell [Google Scholar]
- 5.Benton M. J. 2001. Biodiversity on land and in the sea. Geol. J. 36, 211–230 10.1002/gj.877 (doi:10.1002/gj.877) [DOI] [Google Scholar]
- 6.Pough F. H., Janis C. M., Heiser J. B. 2009. Vertebrate life, 8th edn. San Francisco, CA: Pearson Benjamin Cummings [Google Scholar]
- 7.Wiens J. J. 2011. The causes of species richness patterns across space, time, and clades and the role of ‘ecological limits’. Q. Rev. Biol. 86, 75–96 10.1086/659883 (doi:10.1086/659883) [DOI] [PubMed] [Google Scholar]
- 8.Froese R., Pauly D. Editors. 2011. FishBase. World Wide Web electronic publication www.fishbase.org (accessed January 2011) [Google Scholar]
- 9.Berra T. M. 2001. Freshwater fish distribution. San Diego, CA: Academic Press [Google Scholar]
- 10.Eschmeyer W. N., Fricke R., Fong J. D., Polack D. A. 2010. Marine fish diversity: history of knowledge and discovery (Pisces). Zootaxa 2525, 19–50 [Google Scholar]
- 11.Alfaro M. E., Santini F., Brock C. D., Alamillo H., Dornburg A., Carnevale G., Rabosky D. L., Harmon L. J. 2009. Nine exceptional radiations plus high turnover explain species diversity in jawed vertebrates. Proc. Natl Acad. Sci. USA 106, 13 410–13 414 10.1073/pnas.0811087106 (doi:10.1073/pnas.0811087106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hugall A. F., Foster R., Lee M. S. Y. 2007. Calibration choice, rate smoothing, and the pattern of tetrapod diversification according to the long nuclear gene RAG-1. Syst. Biol. 56, 543–563 10.1080/10635150701477825 (doi:10.1080/10635150701477825) [DOI] [PubMed] [Google Scholar]
- 13.Drummond A. J., Ho S. Y. W., Phillips M. J., Rambaut A. 2006. Relaxed phylogenetics and dating with confidence. PLoS Biol. 4, e88. 10.1371/journal.pbio.0040088 (doi:10.1371/journal.pbio.0040088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drummond A. J., Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214. 10.1186/1471-2148-7-214 (doi:10.1186/1471-2148-7-214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magallon S., Sanderson M. J. 2001. Absolute diversification rates in angiosperm clades. Evolution 55, 1762–1780 10.1111/j.0014-3820.2001.tb00826.x (doi:10.1111/j.0014-3820.2001.tb00826.x) [DOI] [PubMed] [Google Scholar]
- 16.Martins E. P., Hansen T. F. 1997. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 149, 646–667 10.1086/286013 (doi:10.1086/286013) [DOI] [Google Scholar]
- 17.Orme C. D. 2007. CAIC (v1.0.4–94) package for R. See http://r-forge.rproject.org/projects/caic/
- 18.R Development Core Team 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 19.Stephens P. R., Wiens J. J. 2003. Explaining species richness from continents to communities: the time-for-speciation effect in emydid turtles. Am. Nat. 161, 112–128 10.1086/345091 (doi:10.1086/345091) [DOI] [PubMed] [Google Scholar]
- 20.Pagel M., Meade A., Barker D. 2004. Bayesian estimation of ancestral character states on phylogenies. Syst. Biol. 53, 673–684 10.1080/10635150490522232 (doi:10.1080/10635150490522232) [DOI] [PubMed] [Google Scholar]
- 21.Akaike H. 1983. Information measures and model selection. Int. Stat. Inst. 22, 277–291 [Google Scholar]
- 22.Maddison W. P., Maddison D. R. 2010. Mesquite: a modular system for evolutionary analysis. Version 2.72. See http://mesquiteproject.org
- 23.Maddison W. P. 2006. Confounding asymmetries in evolutionary diversification and character change. Evolution 60, 1743–1746 10.1111/j.0014-3820.2006.tb00517.x (doi:10.1111/j.0014-3820.2006.tb00517.x) [DOI] [PubMed] [Google Scholar]
- 24.FitzJohn R. G., Maddison W. P., Otto S. P. 2009. Estimating trait dependent speciation and extinction rates from incompletely resolved phylogenies. Syst. Biol. 58, 595–611 10.1093/sysbio/syp067 (doi:10.1093/sysbio/syp067) [DOI] [PubMed] [Google Scholar]
- 25.Rabosky D. L. 2009. Ecological limits on clade diversification in higher taxa. Am. Nat. 173, 662–674 10.1086/597378 (doi:10.1086/597378) [DOI] [PubMed] [Google Scholar]
- 26.Friedman M., Blom H. 2006. A new actinopterygian from the Famennian of East Greenland and the interrelationships of Devonian ray-finned fishes. J. Paleontol. 80, 1186–1204 10.1666/0022-3360(2006)80[1186:ANAFTF]2.0.CO;2 (doi:10.1666/0022-3360(2006)80[1186:ANAFTF]2.0.CO;2) [DOI] [Google Scholar]
- 27.Cavin L., Forey P. L., Lécuyer C. 2007. Correlation between environment and Late Mesozoic ray-finned fish evolution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 245, 353–367 10.1016/j.palaeo.2006.08.010 (doi:10.1016/j.palaeo.2006.08.010) [DOI] [Google Scholar]
- 28.Grande L., Bemis W. E. 1998. A comprehensive phylogenetic study of amiid fishes (Amiidae) based on comparative skeletal anatomy. An empirical search for interconnected patterns of natural history. Soc. Vert. Paleontol. Mem. 4, 1–690 [Google Scholar]
- 29.Field C. B., Behrenfeld M. J., Randerson J. T., Falkowski P. 1998. Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281, 237–240 10.1126/science.281.5374.237 (doi:10.1126/science.281.5374.237) [DOI] [PubMed] [Google Scholar]
- 30.Amthor J. S. and members of the Ecosystems Working Group 1998. Terrestrial ecosystem responses to global change: a research strategy. ORNL Technical Memorandum 1998/27, 37 pp Oak Ridge National Laboratory, Oak Ridge, TN [Google Scholar]
- 31.Begon M., Townsend C. R., Harper J. L. 2006. Ecology. From individuals to ecosystems, 4th edn. Malden, MA: Blackwell Publishing [Google Scholar]
- 32.Cyr H., Pace M. L. 1993. Magnitude and patterns of herbivory in terrestrial and aquatic ecosystems. Nature 361, 148–150 10.1038/361148a0 (doi:10.1038/361148a0) [DOI] [Google Scholar]
- 33.Palumbi S. R. 1994. Genetic divergence, reproductive isolation, and marine speciation. Ann. Rev. Ecol. Syst. 25, 547–557 10.1146/annurev.es.25.110194.002555 (doi:10.1146/annurev.es.25.110194.002555) [DOI] [Google Scholar]
- 34.Paulay G., Meyer C. 2002. Diversification in the Tropical Pacific: comparisons between marine and terrestrial systems and the importance of founder speciation. Integr. Comp. Biol. 42, 922–934 10.1093/icb/42.5.922 (doi:10.1093/icb/42.5.922) [DOI] [PubMed] [Google Scholar]
- 35.Kinlan B. P., Gaines S. D. 2003. Propagule dispersal in marine and terrestrial environments: a community perspective. Ecology 84, 2007–2020 10.1890/01-0622 (doi:10.1890/01-0622) [DOI] [Google Scholar]
- 36.Dawson M. N., Hamner W. M. 2008. A biophysical perspective on dispersal and the geography of evolution in marine and terrestrial systems. J. R. Soc. Interface 5, 135–150 10.1098/rsif.2007.1089 (doi:10.1098/rsif.2007.1089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tittensor D., Mora C., Jetz W., Lotze H. K., Ricard D., vanden Berghe E., Worm B. 2010. Global patterns and predictors of marine biodiversity across taxa. Nature 466, 1098–1101 10.1038/nature09329 (doi:10.1038/nature09329) [DOI] [PubMed] [Google Scholar]
- 38.Benzie J. A. H. 1997. Genetic structure of marine organisms and SE Asian biogeography. In Biogeography and geological evolution of SE Asia (eds Hall R., Holloway J. D.), pp. 197–209 Leiden, UK: Backhuys Publishers [Google Scholar]
- 39.Barber P. H. 2009. The challenge of understanding the Coral Triangle biodiversity hotspot. J. Biogeography 36, 260–265 10.1111/j.1365-2699.2009.02198.x (doi:10.1111/j.1365-2699.2009.02198.x) [DOI] [Google Scholar]