Abstract
The limbless, primarily soil-dwelling and tropical caecilian amphibians (Gymnophiona) comprise the least known order of tetrapods. On the basis of unprecedented extensive fieldwork, we report the discovery of a previously overlooked, ancient lineage and radiation of caecilians from threatened habitats in the underexplored states of northeast India. Molecular phylogenetic analyses of mitogenomic and nuclear DNA sequences, and comparative cranial anatomy indicate an unexpected sister-group relationship with the exclusively African family Herpelidae. Relaxed molecular clock analyses indicate that these lineages diverged in the Early Cretaceous, about 140 Ma. The discovery adds a major branch to the amphibian tree of life and sheds light on both the evolution and biogeography of caecilians and the biotic history of northeast India—an area generally interpreted as a gateway between biodiversity hotspots rather than a distinct biogeographic unit with its own ancient endemics. Because of its distinctive morphology, inferred age and phylogenetic relationships, we recognize the newly discovered caecilian radiation as a new family of modern amphibians.
Keywords: caecilian amphibians, Chikilidae, new family, systematics, northeast India
1. Introduction
The ca 185 described extant species of caecilian amphibians (Gymnophiona) are concentrated in tropical regions of mostly Gondwanan origin. Like other amphibians, the limbless, mostly soil-dwelling caecilians have limited capacity for dispersal across salt-water barriers and are therefore expected to be useful in investigating vicariance in continental-scale biogeography. Additionally, because the distribution of modern caecilian lineages is highly regionalized [1] and almost entirely restricted to the wet tropics, the contemporary presence of caecilian lineages in a region can provide evidence of a long history of wet tropical environments in that region. Thus, our discovery of an ancient radiation of caecilians from northeast India prompts a re-evaluation of the biogeographic history and significance of this area.
The latest family-level classification of caecilians [1] recognized nine monophyletic, morphologically distinct and ancient (more than 60 Ma old) families. Only two of these families have representatives in Asia and both are thought to have reached Asia through continental drift by rafting on the Indian plate. The entirely Asian Ichthyophiidae dispersed ‘Out of India’ into southeast Asia [2]. By contrast, the teresomatan (i.e. non-rhinatrematid and non-ichthyophiid, or tail-less caecilians) Indotyphlidae has 12 species in the Western Ghats biodiversity hotspot in peninsular India [1,3,4] and only one poorly preserved and badly damaged specimen from Assam, northeast India, described in 1904 [5]. Our recent investigations of the caecilians of northeast India reveal the latter to be member of a previously unknown, yet widespread, radiation of teresomatan caecilians that is morphologically distinct from indotyphlids and more closely related to the African family Herpelidae than to any other caecilians. We propose that this radiation be recognized as a distinct new family.
2. Material and methods
During 2006–2010, we conducted, to our knowledge, the most extensive dedicated caecilian surveys ever attempted and the first in northeast India, comprising 1177 person-hours of digging soil at 238 localities. Vouchers were fixed in 4 per cent formalin and stored in 70 per cent ethanol, with tissue samples (liver and/or muscle) of representative specimens stored in absolute ethanol. Three main molecular phylogenetic analyses (using maximum likelihood and Bayesian inference) were carried out with various taxon and character sampling regimes; (i) representatives of all major caecilian lineages, including a single northeast Indian teresomatan, sequenced for the complete mitochondrial (mt) genome and the nuclear genes rag1 and slc8a1; (ii) representatives of all major caecilians, including 10 northeast Indian teresomatans from all major mt haplotype clades sequenced for mt cox1 and 16S; and (iii) 43 northeast Indian teresomatans sequenced for mt cox1 and 16S. Divergence times were estimated using a Bayesian relaxed clock method and all possible combinations of up to three calibrated nodes. Cranial osteology of four northeast Indian teresomatans was investigated using high-resolution X-ray computed tomography (voxel size 10 μm). Diagnostic cranial features were confirmed by gross dissection in single members of the other major mt haplotype clades. See the electronic supplementary material for detailed methods.
3. Results
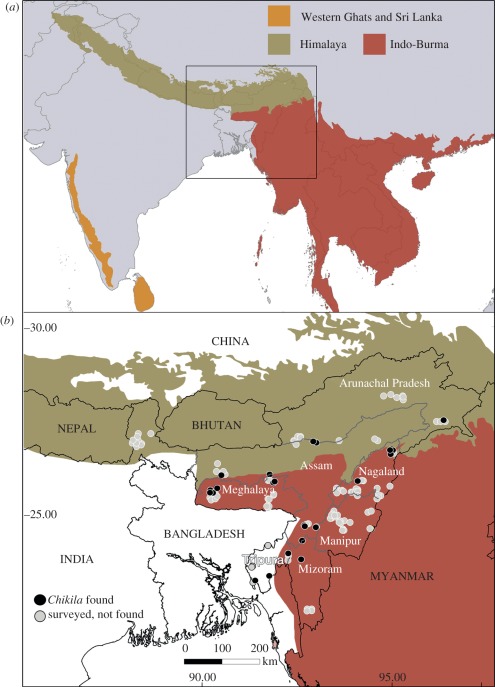
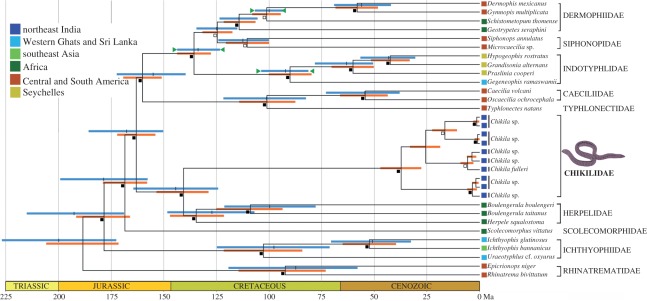
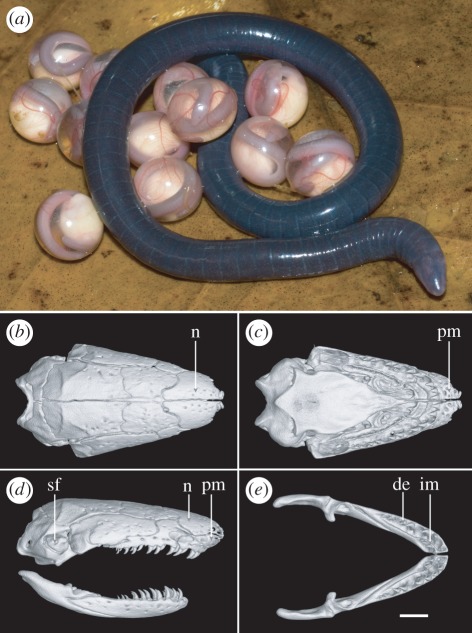
More than 500 teresomatan caecilians were encountered at 58 of the 238 localities surveyed (see the electronic supplementary material, table S1), including, to our knowledge, the first teresomatans discovered from the states of Arunachal Pradesh, Meghalaya, Nagaland and Tripura (figure 1). Phylogenetic analyses of a large dataset of DNA sequence data comprising complete mt genomes and two nuclear markers reveal that these caecilians are more closely related to the African Herpelidae than to the Indotyphlidae and that they and the Herpelidae diverged about 140 ± ca 20 Ma (figure 2). Morphologically, the northeast Indian teresomatans differ from indotyphlids in having perforate stapes and separate premaxillae and nasals (figure 3), and lack the multiple antotic foramina that diagnoses the Herpelidae [1]. Thus, we recognize the northeast Indian teresomatans as a distinct, tenth family of caecilians.
Figure 1.
Distribution of Chikilidae fam. nov. in northeast India. (a) Map of South Asia and Indochina showing three global biodiversity hotspots. (b) Map of northeast India showing geographical sampling effort and locations where Chikila gen. nov. were found.
Figure 2.
Timetree of caecilian amphibians. Age estimates and confidence intervals (horizontal bars) of the divergences among Chikila gen. nov. and other extant caecilians. The timetree depicted is the unique supertree that displays both of the entirely compatible trees found in two analyses: (i) analysis of 12 413 bp of complete mt genome and two nuclear genes (rag1 and slc8a1) for one Chikila fulleri plus 22 other caecilians, and (ii) analysis of 1982 bp of mt cox1 + 16S for 10 Chikila plus the 22 other caecilians. Blue and orange bars are confidence intervals from analyses (i) and (ii), respectively. Green triangles indicate calibrations (upper and lower bounds) for analysis (i). Filled squares next to nodes indicate maximal support for Bayesian inference (BI) and maximum likelihood (ML); open squares indicate maximal support for BI and high (>90%) for ML. Colour codes of leaves correspond to geographical region. See Estimation of Divergence Times in the electronic supplementary material for details.
Figure 3.
Morphology of Chikilidae fam. nov. (a) Chikila fulleri in life, brooding egg clutch (in captivity). Scale bar, 10 mm. (b–e) Volume reconstruction of high-resolution X-ray computed tomography data showing cranium and mandibles of C. fulleri. (b) Cranium in dorsal view. (c) Cranium in palatal view. (d) Cranium and mandible in right lateral view. (e) Mandibles in dorsal view. Scale bar (b,c), 1 mm. n, nasal; pm, premaxilla; sf, stapedial foramen; im, inner mandibular (i.e. ‘splenial’) tooth row; de, dentary tooth row. See the electronic supplementary material, figure S2 for more detailed labelling.
Amphibia L., 1758
Gymnophiona Rafinesque, 1814
Stegokrotaphia Cannatella & Hillis, 1993
Teresomata Wilkinson & Nussbaum, 2006
Chikilidae fam. nov.
Chikila gen. nov.
Etymology. Chikila is a northeast (Meghalaya state) Indian tribal name for the included caecilians.
Type species. Herpele fulleri Alcock, 1904.
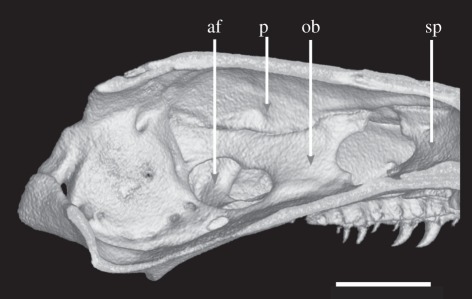
Diagnosis of family and genus. Caecilians that, like herpelids (Boulengerula and Herpele), have perforate stapes and no septomaxilla, but which differ from herpelids in having a single (undivided) antotic foramen on each side, frontals that do not contribute to the roof of the braincase posterior to the sphenethmoid and carotid foramina posterior to the crista marking the anterior limit of the insertion of the ventral trunk musculature onto the os basale (figures 3 and 4). They further differ from Herpele in lacking separate pterygoids, and from Boulengerula in retaining separate premaxillae and nasals, secondary annuli and annular scales. Additionally, Chikila are oviparous with direct development, lack prefrontals and have two rows of teeth in the lower jaws.
Figure 4.
Volume reconstruction of high-resolution X-ray computed tomography data showing sagittal section through braincase of Chikila fulleri (SDB 1304). The left internal wall of the braincase is seen from the midline, showing the single antotic foramen, and exclusion of the frontal from the roof of the braincase posterior to the sphenethmoid (formed by the parietal). af, antotic foramen; ob, os basale; p, parietal; sp, sphenethmoid. Scale bar, 1 mm.
Phylogenetic definition. All caecilians more closely related to Chikila fulleri than to Herpele squalostoma.
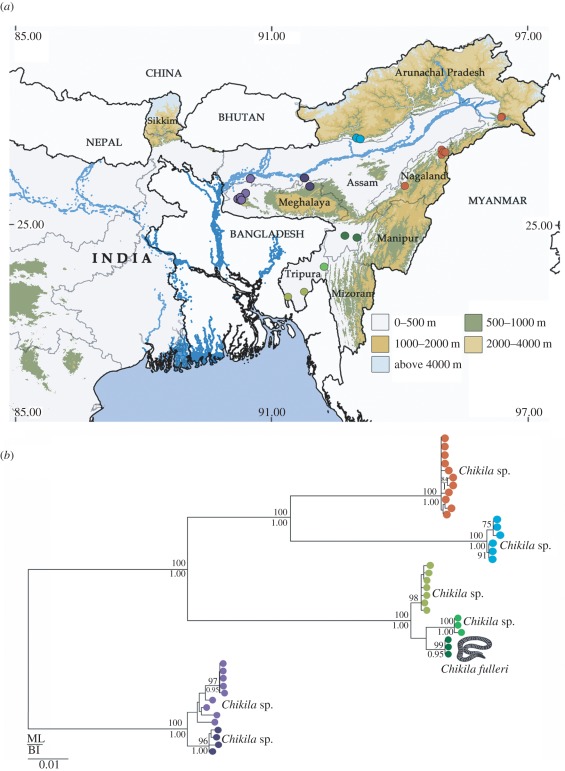
Distribution. Chikila is a small but widespread radiation of relatively common caecilians in the northeast Indian states of Arunachal Pradesh, Assam, Meghalaya, Nagaland and Tripura (figures 1b and 5a). DNA barcoding of 43 individuals from 38 localities spanning the known range of the genus reveals it to be a monophyletic, multi-species radiation of as many as seven strongly supported, allopatric mt haplotype clades (figure 5b).
Figure 5.
Genetic diversity of Chikila gen. nov. and collection localities of Chikila in northeast India. (a) Northeast India showing collection localities, relief and major drainage. (b) DNA phylogeny of Chikila based on the analysis of 696 bp of mt cox1 plus two (non-overlapping) fragments of mt 16S (463 and 823 bp). The tree is rooted according to the relationships depicted in figure 2, even though that part of the tree was not well supported. Coloured dots relate to collection localities indicated in the map. Numbers next to nodes represent support for internal branches from ML (above branch) and BI (below branch).
4. Discussion
Herpelidae comprises exclusively African caecilians whose distributions include the Eastern Arc (Boulengerula) and the Guinean Forests of West Africa (Herpele) biodiversity hotspots. Our timetree (figure 2) indicates that the divergence between Chikilidae and Herpelidae could have been caused by the separation of India from Africa 165–121 Ma [6]. By contrast, for a ‘Gondwanan group’ [7–9], it is striking how little signature of Gondwanan fragmentation is carried in the pattern and timing of other divergences between major caecilian lineages. In particular, the five oldest divergences appear to be independent of tectonic vicariance, and lead to several regionally restricted ancient lineages: rhinatrematids (South America), ichthyophiids (Asia), scolecomorphids (Africa) and caeciliids + typhlonectids (South America). Thus, if our timescale is correct, the caecilian fauna of Gondwana was markedly regionalized, and continental fragmentation largely reinforced spatial partitions rather than caused them.
Our analyses indicate that caecilian history is mostly characterized by the long persistence of geographically restricted lineages with limited dispersal and low net rates of speciation. Modern caecilians are mostly restricted to wet tropical regions, a constraint we interpret as suggesting that the presence of ancient caecilian lineages is an indicator of a continuous history of spatio-temporally connected wet tropical environments in that region.
Our discoveries indicate that Chikilidae was present in Greater India (Madagascar–India–Seychelles or Indigascar [10]) during isolation and northward drift of this landmass. However, the oldest divergence among extant chikilids probably occurred after India accreted with the rest of Asia ca 65–42 Ma [11] (figure 2). Many plant and animal groups that experienced isolation in Greater India are nowadays endemic to the Western Ghats–Sri Lanka biodiversity hotspot, suggesting that part of this mountain range acted as a refugium during the massive Deccan Traps volcanism around the Cretaceous–Tertiary (K–T) boundary [12]. The absence of chikilids in peninsular India and of any peninsular Indian indotyphlids (Gegeneophis, Indotyphlus) in northeast India suggests that both lineages radiated within their respective regions, potentially isolated by the Deccan Traps that separated these regions in the Early Cenozoic [13].
This in situ radiation of Chikilidae, combined with the apparently low rates of caecilian dispersal as evidenced by observed present and inferred past regionalism, is strongly suggestive of long-term relative environmental stability within northeast India. This identifies northeast India as an overlooked candidate unit in biogeography, containing a richer fauna than would be expected from a ‘gateway’ [14] lying between the Himalaya and Indo-Burma hotspots, and serving as a second refugium for fauna and flora during Cretaceous isolation of the Indian subcontinent. The Western Ghats is part of a relatively well-studied biodiversity hotspot [15]. By contrast, and as our research highlights, northeast India is very poorly documented and not well understood. Thus, although India is a recognized centre of global amphibian diversity and endemism [12,16] having more endemic families than any other country [17], this endemism has hitherto been documented only from the Western Ghats.
As well as having important implications for understanding amphibian evolution, the discovery of an endemic family of vertebrates, to our knowledge the first for northeast India, identifies this as a region with a potentially rich but still hidden biodiversity in need of improved inventories and robust phylogenies (currently lacking) of other taxa. If northeast India indeed harboured a special fauna and flora around the K-T transition, this region is likely to reveal more hidden treasures in the near future. Further explorations and conservation actions are urgent because the region's biodiversity is generally under high threat from the growing resident human population1 and rapid deforestation [18].
Acknowledgements
The research was supported by funding from the University of Delhi (support to faculty for strengthening R&D programme); DBT, Government of India; IUCN/ASG/SSC (lost amphibian species of India, 2007); The Percy Sladen Memorial Fund, The Linnaean Society, London to S.D.B; Critical Ecosystem Partnership Fund, Conservation International, USA; The Royal Society and Indian DST (to S.D.B. and D.J.G); Direct Senior Research Fellowship, Council of Scientific & Industrial Research (CSIR), India (9/45 (1082)/2011-EMR-I to R.G.K); European Union Marie Curie Mobility and Training Programme (PIEF-GA-2009-237658 to D.S.M.); Natural Environmental Research Council studentship (NE/F009011/1 to E.S.); European Research Council (Grant 204509, project TAPAS, to F.B.); the Fonds voor Wetenschappelijk Onderzoek Vlaanderen postdoctoral fellowship (FWOTM573 to I.V.B.). Forest departments (Arunachal Pradesh, Assam, Manipur, Meghalaya, Nagaland, Tripura and West Bengal) provided study permits to S.D.B.
Endnote
Between 1961 and 2001, the human population in northeast India grew by a whopping 24 million (Census of India, retrieved from http://censusindia.gov.in/), an increase of ca 0.6 million people per year. This excludes the population of three districts of West Bengal viz. Cooch Behar, Jalpaiguri and Darjeeling, which are considered as part of the biogeographic part of northeast India.
References
- 1.Wilkinson M., San Mauro D., Sherratt E., Gower D. J. 2011. A nine-family classification of caecilians (Amphibia: Gymnophiona). Zootaxa 2874, 41–64 [Google Scholar]
- 2.Gower D. J., et al. 2002. A molecular phylogeny of ichthyophiid caecilians (Amphibia: Gymnophiona: Ichthyophiidae): out of India or out of South East Asia? Proc. R. Soc. Lond. B 269, 1563–1569 10.1098/rspb.2002.2050 (doi:10.1098/rspb.2002.2050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giri V., Gower D. J., Gaikwad K., Wilkinson M. 2011. A second species of Gegeneophis Peters (Amphibia: Gymnophiona: Caeciliidae) lacking secondary annular grooves. Zootaxa 2815, 49–58 [Google Scholar]
- 4.Gower D. J., et al. 2011. Molecular systematics of caeciliid caecilians (Amphibia: Gymnophiona) of the Western Ghats, India. Mol. Phylogenet. Evol. 59, 698–707 10.1016/j.ympev.2011.03.002 (doi:10.1016/j.ympev.2011.03.002) [DOI] [PubMed] [Google Scholar]
- 5.Alcock A. 1904. Description and reflections upon a new species of apodous amphibian from India. Ann. Mag. Nat. Hist. 14, 267–273 [Google Scholar]
- 6.Sanmartin I., Ronquist F. 2004. Southern Hemisphere biogeography inferred by event-based models: plant versus animal patterns. Syst. Biol. 53, 216–243 10.1080/10635150490423430 (doi:10.1080/10635150490423430) [DOI] [PubMed] [Google Scholar]
- 7.Duellman W. E., Trueb L. 1986. Biology of amphibians. New York, NY: McGraw-Hill [Google Scholar]
- 8.Hedges S. B., Nussbaum R. A., Maxson L. R. 1993. Caecilian phylogeny and biogeography inferred from mitochondrial DNA sequences of the 12S rRNA and 16S rRNA genes (Amphibia: Gymnophiona). Herpetol. Monogr. 7, 64–76 10.2307/1466952 (doi:10.2307/1466952) [DOI] [Google Scholar]
- 9.Gower D. J., Wilkinson M. 2009. Caecilians (Gymnophiona). In The timetree of life (eds Hedges S. B., Kumar K.), pp. 369–372 Oxford, UK: Oxford University Press [Google Scholar]
- 10.Vidal N., et al. 2010. Blindsnake evolutionary tree reveals long history on Gondwana. Biol. Lett. 6, 558–561 10.1098/rsbl.2010.0220 (doi:10.1098/rsbl.2010.0220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briggs J. C. 2003. The biogeographic and tectonic history of India. J. Biogeogr. 30, 381–388 10.1046/j.1365-2699.2003.00809.x (doi:10.1046/j.1365-2699.2003.00809.x) [DOI] [Google Scholar]
- 12.Bossuyt F., Milinkovitch M. C. 2001. Amphibians as indicators of Early Tertiary ‘Out of India’ dispersal of vertebrates. Science 292, 93–95 10.1126/science.1058875 (doi:10.1126/science.1058875) [DOI] [PubMed] [Google Scholar]
- 13.Pande K. 2002. Age and duration of the Deccan Traps, India: a review of radiometric and palaeomagnetic constraints. Proc. Ind. Acad. Sci. (Earth Planet Sci.) 111, 115–123 [Google Scholar]
- 14.Mani M. S. 1995. Biogeography in India. Dehra Dun, Uttaranchal, India: Surya Publications [Google Scholar]
- 15.Bossuyt F., et al. 2004. Local endemism within the Western Ghats: Sri Lanka biodiversity hotspot. Science 306, 479–481 10.1126/science.1100167 (doi:10.1126/science.1100167) [DOI] [PubMed] [Google Scholar]
- 16.Biju S. D., Bossuyt F. 2003. New frog family from India reveals an ancient biogeographical link with the Seychelles. Nature 425, 711–714 10.1038/nature02019 (doi:10.1038/nature02019) [DOI] [PubMed] [Google Scholar]
- 17.Frost D. R. 2011. Amphibian species of the world: an online reference. Version 5.5 (31 January, 2011). New York, USA: American Museum of Natural History; See http://research.amnh.org/vz/herpetology/amphibia/ (accessed 30 October 2011) [Google Scholar]
- 18.Puyravaud J. P., Davidar P., Laurance W. F. 2010. Cryptic loss of India's native forests. Science 329, 32. 10.1126/science.329.5987.32-b (doi:10.1126/science.329.5987.32-b) [DOI] [PubMed] [Google Scholar]