Abstract
The bottlenose dolphin, Tursiops truncatus, is one of very few animals that, through vocal learning, can invent novel acoustic signals and copy whistles of conspecifics. Furthermore, receivers can extract identity information from the invented part of whistles. In captivity, dolphins use such signature whistles while separated from the rest of their group. However, little is known about how they use them at sea. If signature whistles are the main vehicle to transmit identity information, then dolphins should exchange these whistles in contexts where groups or individuals join. We used passive acoustic localization during focal boat follows to observe signature whistle use in the wild. We found that stereotypic whistle exchanges occurred primarily when groups of dolphins met and joined at sea. A sequence analysis verified that most of the whistles used during joins were signature whistles. Whistle matching or copying was not observed in any of the joins. The data show that signature whistle exchanges are a significant part of a greeting sequence that allows dolphins to identify conspecifics when encountering them in the wild.
Keywords: Tursiops truncatus, acoustic communication, signature whistle, individual recognition
1. Introduction
Vocal learning is a skill that allows animals to expand their call repertoires and therefore the complexity of their communication systems. Most examples of vocal learning come from animals in which males produce learned songs to attract females such as song birds [1], baleen whales [2], phocid seals [3] and bats [4]. In animal song, most information is carried in the overall complexity of the vocal display. Only few animals use learned signals outside of song with each individual call having a specific meaning. The best-known examples for this are parrots and dolphins. While song birds’ signal identity by the repertoire of learned signals that they use [1] and by voice features that affect all calls [5,6], parrots [7,8] and dolphins [9] also have single, individually specific calls that broadcast identity. Furthermore, experimental studies have demonstrated that bottlenose dolphins [10] and grey parrots [11] can use learned artificial signals in a referential way to give information about their environment. To date, it is unclear whether and how these abilities are used in their own communication systems and information on contextual use of signals in the wild is sparse [12,13].
In bottlenose dolphins, each individual develops its own distinctive signature whistle in the first few months of life [14] and uses it when in isolation [9,15,16]. Individuals appear to use learning to create a novel whistle that, in most cases, does not overlap with existing whistles of close associates [17]. In females, signature whistles remain stable for at least a decade [18], but males can change their signature whistles after alliance formation, to resemble the whistle of an alliance partner [19]. The identity information is encoded in the frequency modulation pattern of the signature whistle [20], which is the part that is invented by the dolphin. Bottlenose dolphins also copy signature whistles of their conspecifics [16,21,22], suggesting they can use them to address specific individuals. Around 50 per cent of all whistles recorded from wild dolphins are signature whistles [23]. Given that signature whistles transmit identity information, one would expect animals to exchange signature whistles when meeting at sea. In this study, we investigated whether such exchanges occurred when groups of free-ranging dolphins encountered each other.
2. Material and methods
Our study was conducted in St Andrews Bay off the northeast coast of Scotland between Arbroath and Fife Ness, from July to September in 2003 and 2004. Our subject animals were individuals of a bottlenose dolphin population known to travel large distances around the east Scotland coast from the Moray Firth to St Andrews Bay [24,25]. We conducted focal follows from a small boat in calm seas with no rain or fog and individually identified animals through photo-identification of the dorsal fin. We used a towed distributed hydrophone array to localize whistle producers [26]. The array consisted of three HTI-94-SSQ hydrophones and one HTI-96-MIN hydrophone all with a frequency response of 2 Hz to 30 kHz ± 1 dB, and was towed at 2 m depth around our observation boat in a rectangular formation (approx. 2 × 3 m) [26]. Recordings were made onto a Fostex D824 multi-track digital recorder during 2003 and an Alesis Adat HD24 multi-track digital recorder during 2004 (sampling frequency 48 kHz, 24 bit for the Fostex, 32 bit for the Alesis). This recording method enabled us to gain information on caller location for each whistle event, through passive acoustic localization [27–29] using time of arrival differences between pairs of hydrophone receivers, coupled with a function to determine the depth of the caller [26]. We chose a focal animal and followed it with its group for as long as we could each day. Spoken tracks of two observers (continuous sampling of location, group size and composition with point summaries every 2 min), one detailing the surface behaviour of the focal animal and its group members, and one the positions and behaviour of other, non-focal groups in the area if present, were also recorded on the multi-track recorder. We used a 10 m chain rule to define groups, so all individuals in a group were within 10 m of at least one other member of the group. Locations were given as distance and direction from the boat based on a standard clock face with the bow being 12 o'clock. This yielded a direction resolution of ±15°. Dorsal fin photo-identification of the focal and its associates was completed using a Canon Digital D30 SLR camera with a Sigma 100–300 mm apochromatic lens. Photos were taken throughout each follow whenever the focal group composition changed.
Traditionally, signature whistles have been identified by isolating animals from their group members [15]. This was not possible here, so we used the sequential signature identification (SIGID) method that is based on the temporal patterning of whistles to identify signature whistles [30]. In this method, the observer counts for each whistle type how often whistles of the same type occur within 1–10 s of each other. The percentage of whistles that fulfil this criterion for each whistle type changes with each additional emitted whistle of that type. If the value reaches 75 per cent at least once when or after the first four whistles of a type have been emitted, then previous analyses indicate that the given whistle type is a signature whistle [30]. To identify whistle exchanges, we searched for sections with stereotyped and repeated whistle vocalizations by visually scanning spectrograms of the hydrophone tracks without observer knowledge of group composition, behaviour sampling or recording history. For animals to exchange identity information, each animal needs to produce its signature whistle at least once. Because we needed sequences, to be certain that we were looking at signature whistles [30], we defined signature whistle exchanges (figure 1) as any occurrence of at least two different whistle types each repeated at least twice, and separated in time by 3 s or less. This time window was previously used successfully to demonstrate whistle matching in bottlenose dolphins [21]. When multiple sections were within the same recording, sections had to be at least 2 min apart to be used. This resulted in a set of exchanges that did not have any whistle types in common (see below). Any sections of recordings containing engine noise that was masking the frequency band above 2 kHz were discarded from the analysis.
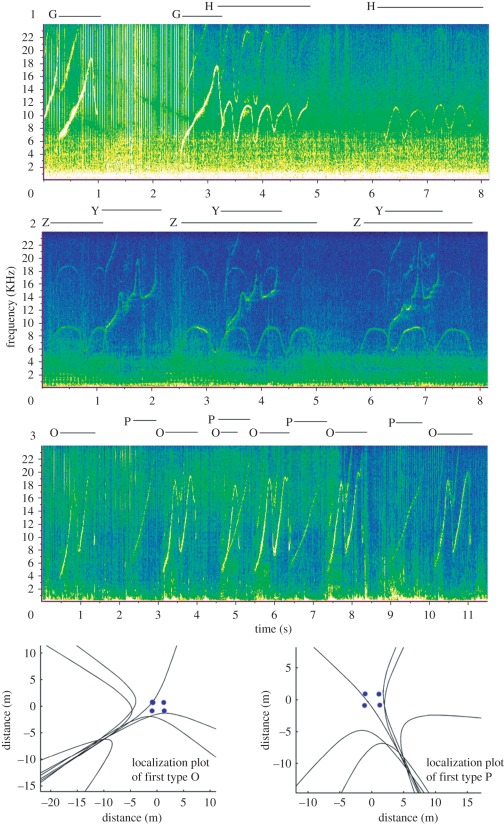
Figure 1.
Spectrograms of three separate exchange events including localization plots for the third event. Spectrograms showing examples of three stereotyped and repeated whistle sequences. Letters and bars above spectrograms indicate the two whistle types within the exchange event. Localization plots show position to where whistle types O and P in spectrogram 3 are localized. Blue circles in plots indicate positions of hydrophone receivers around boat. All examples of whistle type O within the event localized to a position of 7 o'clock from the boat. All examples of whistle type P localized to a position at 5 o'clock from the boat.
Onset time of each whistle event was logged, and candidate sections were formatted for localization analysis in the Matlab-based Toady program [26]. For each whistle, six hyperbolas were plotted to determine signal source (figure 1). Acoustic localization errors for directions were ±15° [26]. Whistles were discarded if (i) the signal was too weak to appear on all four hydrophone recordings; (ii) large portions of the whistles overlapped so that it was difficult to distinguish between them or filter out the overlapping whistle; (iii) three or more hyperbolas did not converge on the same caller position; and (iv) source location was at the engine position. In all instances, localization of all whistles was completed without reference to the position of hyperbolas of previously localized whistles or the visual observations of dolphin positions.
All whistle categorization was conducted by eye and then confirmed by using adaptive resonance theory (ART; ARTwarp) [31]. Frequency contours of each whistle were extracted from each whistle spectrogram (FFT 2048, frame length 512, overlap between frames 87.5%; Hanning window, time resolution 1.333 ms), using a peak algorithm that was supervised by the user. Contours were saved at 10 ms time resolution and fed into the ARTwarp software. ARTwarp uses an ART neural network and dynamic time warping to categorize contours based on a set degree of similarity or vigilance factor. This was set to 91 per cent, a value that was found to allow signature whistle detection reliably in larger samples [32]. ARTwarp analysis was conducted separately for each exchange sequence.
Times of each exchange event were compared with the synchronized time in each spoken track that detailed surface behaviour of all animals. We analysed details of group locations and behaviour at the start of the exchange and during the 2 min behavioural sample after the exchange event had ceased (table 1). If the animals were in two separate groups at the start of the exchange sequence and in one group in the 2 min behavioural sample after the exchange had ceased, then we counted a preceding whistle exchange as leading to a join.
Table 1.
Summary of all 11 whistle exchange events. Bold letters indicate whistle types that could be localized. Capital letters indicate confirmed signature whistles. For lower case letters, we do not know whether they are signature whistles or not. Outcomes were judged either by whistle localization (all whistle in 2 min after event coming from the same spot), visually (groups were observed to join at the surface) or both. Each letter represents a different whistle type.
| date and time | whistle type sequence (each letter stands for an individual whistle type) | group sizes group 1/2/3/4 | outcome (identification method) |
|---|---|---|---|
| 8 July 2003, 15.40 | AAbbAbb | 4/6 | join (localization) |
| 9 July 2003, 10.33 | CDCCDCD | 4/4 | join (visual) |
| 9 July 2003, 10.36 | EFEFFFFF | 8–10/3–4 | no join (localization and visual) |
| 23 July 2003, 10.15 | gghh | 10–15/2 | join (localization) |
| 23 July 2003, 10.28 | iijjii | 10–15/2 | no join (localization and visual) |
| 23 July 2003, 10.32 | klkl | 15–20/2 | join (visual) |
| 12 August 2003, 15.13 | MnMMnMnnMn | 5–7/3 | join (localization) |
| 16 August 2003, 11.44 | OPOPOOPOPOOPOP | 4/5 | join (visual) |
| 16 August 2003, 14.32 | qRRqsRRRsRtRsRtRssqustu | 8/3/5–6/7 | join (localization and visual) |
| 15 July 2004, 14.55 | vvvxvxvxv | 10–12/5 | join (visual) |
| 26 July 2004, 12.52 | YzYzYzYzzzYYzYzYzzYYzzzzzzzzYzY | 8–10/8–10 | join (visual) |
To test whether whistles from different groups could have co-occurred by chance we used an exact randomization test. First, we counted how often the whistle types observed in the exchanges occurred in the entire follow. For this, we used only sections of equal or lower background noise than that found in each exchange. For the test, the temporal position of each whistle of these types was then randomized 10 000 times over the duration of the follow during and after the exchange to determine the number of times the pairs were within 5 s of each other by chance. We used five rather than 3 s as an exchange criterion in the test to allow for whistle duration because the positioning of the whistles was conducted in 1 s bins. Values from the randomization were then compared with the observed values in the exchanges.
3. Results
In total, 17 separate whistle sections from 12 separate follows from 10 separate days (total recording time 414 min) were identified as possible stereotyped exchanges. From these 17 sections, two were discarded, one because its whistles were more than 3 s apart and the other because it was within 2 min of a previous exchange. This previous exchange had different whistle types but was too close in time to be considered a separate event. From the remaining 15 sections, 156 individual whistles were identified and run through the passive acoustic localization analysis. In a total of 170 min of unmasked recordings, 11 separate sections totalling 108 whistles—from 8 separate follows on 7 separate days—were of sufficient quality for localization. Four additional cases had to be discarded here because the presence of too many animals did not allow us to unequivocally link acoustic recordings to separate groups. The average whistle rate (±s.d.) during these exchanges was 4.6 ± 1.5 whistles per individual per minute.
For all whistle sequences consisting of at least two repeated whistle types, localizations of the different types corresponded to locations of separate groups, indicating that vocal exchanges tend to occur between separate groups. Different whistle types in exchanges were produced by different individuals because the maximum swimming speed of dolphins (7.5 m s−1) [33] would not have allowed them to cover the necessary distance between calling sites. Furthermore, at no time were individual animals observed to be continually switching between subgroups at each surfacing. It is possible that dolphins happen to whistle at the same time without being influenced by the other caller, so that exchanges would be a product of chance. We found that this was not the case (exact randomization test, p < 0.05 for exchanges with two whistle types) and that exchanges reflect a significant interaction between individuals. We also recorded one event that contained five whistle types. Unfortunately for this follow, no 1 min intervals without any masking were available after the exchange sequence. Therefore, we could not test its statistical significance.
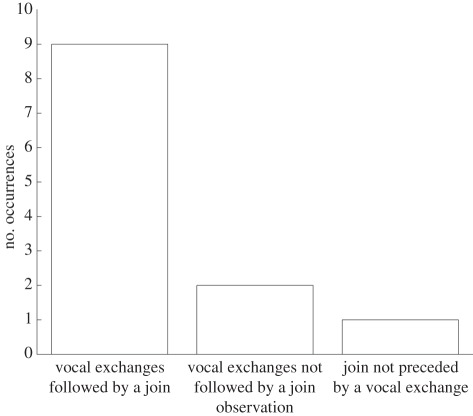
From convergence of caller position through localization or behavioural observations of surface group positions, results showed that exchanges occurred if different groups encounter each other at sea. Furthermore, nine of the 11 instances of vocal exchanges were followed by groups joining (figure 2 and table 1). For the remaining two events, surface observations did not conclusively point to groups joining, and caller positions did not converge during the localization. Only one other instance of a join, in the 170 min of recording where the background noise levels would have allowed whistles to be visible, was seen during the follows containing the exchange events. This join was not preceded by an exchange.
Figure 2.
Occurrence of joins and exchange events. Plot of the total number of observations of whistle exchanges and joins and how their occurrences related to each other.
Sequential SIGID analysis confirmed that approximately 50 per cent of whistles in exchanges were signature whistles. Four whistle types had to be excluded from this analysis, because they occurred less than four times in the follow. Of the remaining 21 whistles found in exchanges, 10 were identified as signature whistles by SIGID. It is important to note here that SIGID has a success rate of only 50 per cent in identifying signature whistles and that it does not create false positives. Thus, while we could confirm only the number given earlier, it is likely that all whistles used in these exchanges were signature whistles, because all whistles in exchanges occurred in the typical bout delivery reported for signature whistles [30].
Photo-identification data showed that there was some overlap in group composition between the events as is to be expected in a fission–fusion society. We identified 47 animals of the 143 animals involved in joins. Fifteen animals were seen in more than one event (seven in two events, three in three events, three in four events and two in six events). We were unable to localize whistles to individuals within groups, but whistle types were sufficiently different between events to suggest that all were produced by different individuals given that most of them were signature whistles. Additionally for the two follows where more than one sequence occurred, observations showed that the number of other, non-focal groups in the area increased during the course of the follow and different group compositions were involved in the joins.
All except two exchanges consisted of more than four whistles (table 1), and all except one exchange consisted of only two whistle types. However, in one exchange sequence, five whistle types from three subgroups were recorded. Bottlenose dolphins in St Andrews Bay often travel in large aggregations of several subgroups that swim a few hundred metres from each other. It is difficult to simultaneously monitor several groups, so it is possible that the observed joins are of animals that have not been apart for long periods of time. The times from the start of our observations to the joins give an indication of the minimum time they had been apart. The average of this time interval was 19 min (min–max range: 8–29 min).
4. Discussion
Our results clearly show that dolphins provide identity information through signature whistles when they encounter other groups at sea. Furthermore, exchanges are a crucial component of the initial social interaction between groups when they join. Only 10 per cent of joins occurred without vocal exchanges, suggesting that signature whistles are the main vehicle to provide identity information. Whistle rates were nine times higher during these exchanges than in peak whistle rates during general socializing [34], which is otherwise the context with the highest whistle rates [34].
Generally, whistles were repeated several times in each interaction. This repetition is not surprising because it improves the probability of correct identification. However, it could also mean that parameters other than the frequency modulation pattern carry additional information that is transmitted through the signature whistle, as bottlenose dolphins have been found to vary signature whistle parameters with context [35].
With one exception, only one whistle type was heard from each joining group during the exchange interactions. Because whistle copying is rare [16,21], this suggests that only one dolphin from each group signals before a join. There are four possible explanations for this. Firstly, it is possible that each group has a leading animal that makes decisions about movements. There have been three examples addressing this possibility. In mother–calf pairs, the mother decides on swimming directions when they are together [36]. An observation of scouting behaviour [37] suggests that even in temporary groups of adults, animals could have different roles and group-hunting dolphins clearly show role specialization [38]. Secondly, it could be that dolphins can recognize each other individually through echolocation and that signature whistles are only part of a greeting ritual that does not require the individual identification of every group member. Echolocation is a powerful tool for the animals, but we do not know how they use it in social situations. Thirdly, if separations have been relatively short, the animals may have an accurate expectation of who is in a group and do not need everyone to give identity information. This may seem unlikely in a fluid fission–fusion society, but we did not know separation durations. Finally, dolphins may not be very choosy about who they approach closely, in which case, individual identification may not be necessary for group joins. The exchanges would then be part of the interaction between those group members that engage in social behaviour that goes beyond swimming in close proximity and which leads to other animals following vocalizers and ending up in one joint group. However, dolphins live in fission–fusion societies [39] and individuals prefer some animals over others as social companions, suggesting that they are selective in their choice of who to approach. This makes this last possibility rather unlikely.
While dolphins sometimes match each others' whistles [21], none of the exchanges here contained any matching. One of the problems in whistle matching is that it can lead to confusion for a listener, whereas it is a powerful signal between the two individuals engaging in it. In a context when identities of group members are still unclear, we would not expect matching to occur. It is more likely to be either a signal between individuals that know who is present, or one used when animals are actively seeking a particular individual.
Our study showed that bottlenose dolphins exchange signature whistles before they join with others at sea. It also showed that these exchanges occur if animals encounter a new group at sea. These exchanges are different to any territorial interactions, which are common in birds and among some primate species, because bottlenose dolphins do not defend territories. They also do not resemble contact calls used by mothers and their offspring because the groups we observed did not consist of mothers and calves. The most similar vocal interactions to the those described here are those found in fission–fusion societies of primates, where individuals counter-call to stay in spatial proximity [40,41]. Because dolphins in St Andrews Bay were not in visual contact and no other exchanges have been recorded for on average 19 min before our observed joins, the observed dolphin encounters were in fact of individuals that had not been in contact. This sets them apart from interactions between members of a large primate group travelling together.
Bottlenose dolphins are also capable of vocal learning that gives their communication system flexibility beyond that of non-human primates [3]. Given their cognitive skills [42] and the similarities between humans and dolphins in the acquisition and use of sounds, it is of great interest to investigate how signals are used in wild dolphins. In this study, we demonstrated that whistle copying does not occur when animals encounter each other initially, which is what we would predict if copied signature whistles are used to address specific individuals. The first step should be to exchange information on who is present. However, from our study, it is now clear that signature whistles are a vehicle to negotiate approaches between groups of animals, as the exchanges occurred only during group encounters. We now need to investigate in more detail why only single individuals interact vocally in groups that consist of several animals. It is unclear whether the observed interactions are ritualized greeting ceremonies that involve only certain individuals in each group or whether the interactions are less organized. Following the same individual and observing its behaviour during encounters in different group compositions would be one way to investigate this further.
Acknowledgements
We thank Teresa Gridley, Danielle Harris and Peter Miles for assistance in the field. We acknowledge sponsorship received from E. P. Barrus and Mariner through the provision of boat equipment. We are also thankful for comments and feedback received during various parts of this study, particularly by Volker Deecke, Luke Rendell, Peter Slater and Ben Wilson. Funding for this study was provided by a Natural Environment Research Council studentship to N.J.Q. and a Royal Society University Research Fellowship to V.M.J.
References
- 1.Catchpole C. K., Slater P. J. B. 2008. Bird song: biological themes and variations, 2nd edn. Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Garland E. C., Goldizen A. W., Rekdahl M. L., Constantine R., Garrigue C., Hauser N. D., Poole M. M., Robbins J., Noad M. J. 2011. Dynamic horizontal cultural transmission of humpback whale song at the ocean basin scale. Curr. Biol. 21, 687–691 10.1016/j.cub.2011.03.019 (doi:10.1016/j.cub.2011.03.019) [DOI] [PubMed] [Google Scholar]
- 3.Janik V. M., Slater P. J. B. 1997. Vocal learning in mammals. Adv. Stud. Behav. 26, 59–99 10.1016/S0065-3454(08)60377-0 (doi:10.1016/S0065-3454(08)60377-0) [DOI] [Google Scholar]
- 4.Knörnschild M., Nagy M., Metz M., Mayer F., von Helversen O. 2010. Complex vocal imitation during ontogeny in a bat. Biol. Lett. 6, 156–159 10.1098/rsbl.2009.0685 (doi:10.1098/rsbl.2009.0685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lind H., Dabelsteen T., McGregor P. K. 1996. Female great tits can identify mates by song. Anim. Behav. 52, 667–671 10.1006/anbe.1996.0211 (doi:10.1006/anbe.1996.0211) [DOI] [Google Scholar]
- 6.Weary D. M., Krebs J. R. 1992. Great tits classify songs by individual voice characteristics. Anim. Behav. 43, 283–287 10.1016/S0003-3472(05)80223-4 (doi:10.1016/S0003-3472(05)80223-4) [DOI] [Google Scholar]
- 7.Berg K., Delgado S., Cortopassi K. A., Beissinger S. R., Bradbury J. W. 2011. Vertical transmission of vocal signatures in a wild parrot. Proc. R. Soc. B 279, 722–731 10.1098/rspb.2011.0932 (doi:10.1098/rspb.2011.0932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortopassi K. A., Bradbury J. W. 2006. Contact call diversity in wild orange-fronted parakeet pairs, Aratinga canicularis. Anim. Behav. 71, 1141–1154 10.1016/j.anbehav.2005.09.011 (doi:10.1016/j.anbehav.2005.09.011) [DOI] [Google Scholar]
- 9.Sayigh L. S., Esch H. C., Wells R. S., Janik V. M. 2007. Facts about signature whistles of bottlenose dolphins (Tursiops truncatus). Anim. Behav. 74, 1631–1642 10.1016/j.anbehav.2007.02.018 (doi:10.1016/j.anbehav.2007.02.018) [DOI] [Google Scholar]
- 10.Richards D. G., Wolz J. P., Herman L. M. 1984. Vocal mimicry of computer-generated sounds and vocal labeling of objects by a bottlenosed dolphin, Tursiops truncatus. J. Comp. Psychol. 98, 10–28 10.1037/0735-7036.98.1.10 (doi:10.1037/0735-7036.98.1.10) [DOI] [PubMed] [Google Scholar]
- 11.Pepperberg I. M. 1999. The Alex studies: cognitive and communicative abilities of grey parrots. Cambridge, MA: Harvard University Press [Google Scholar]
- 12.Bradbury J. W. 2003. Vocal communication in wild parrots. In Animal social complexity: intelligence, culture, and individualized societies (eds De Waal F. B. M., Tyack P. L.), pp. 293–316 Cambridge, MA: Harvard University Press [Google Scholar]
- 13.Janik V. M. 2009. Acoustic communication in delphinids. Adv. Stud. Behav. 40, 123–157 10.1016/S0065-3454(09)40004-4 (doi:10.1016/S0065-3454(09)40004-4) [DOI] [Google Scholar]
- 14.Caldwell M. C., Caldwell D. K. 1979. The whistle of the Atlantic bottlenosed dolphin (Tursiops truncatus): Ontogeny. In Behavior of marine animals: current perspectives in research, vol. 3. Cetaceans (eds Winn H. E., Olla B. L.), pp. 369–401 New York, NY: Plenum Press [Google Scholar]
- 15.Caldwell M. C., Caldwell D. K., Tyack P. L. 1990. Review of the signature-whistle-hypothesis for the Atlantic bottlenose dolphin. In The bottlenose dolphin (eds Leatherwood S., Reeves R. R.), pp. 199–234 San Diego, CA: Academic Press [Google Scholar]
- 16.Janik V. M., Slater P. J. B. 1998. Context-specific use suggests that bottlenose dolphin signature whistles are cohesion calls. Anim. Behav. 56, 829–838 10.1006/anbe.1998.0881 (doi:10.1006/anbe.1998.0881) [DOI] [PubMed] [Google Scholar]
- 17.Fripp D., Owen C., Quintana-Rizzo E., Shapiro A., Buckstaff K., Jankowski K., Wells R., Tyack P. 2005. Bottlenose dolphin (Tursiops truncatus) calves appear to model their signature whistles on the signature whistles of community members. Anim. Cogn. 8, 17–26 10.1007/s10071-004-0225-z (doi:10.1007/s10071-004-0225-z) [DOI] [PubMed] [Google Scholar]
- 18.Sayigh L. S., Tyack P. L., Wells R. S., Scott M. D. 1990. Signature whistles of free-ranging bottlenose dolphins, Tursiops truncatus: mother-offspring comparisons. Behav. Ecol. Sociobiol. 26, 247–260 [Google Scholar]
- 19.Watwood S. L., Tyack P. L., Wells R. S. 2004. Whistle sharing in paired male bottlenose dolphins, Tursiops truncatus. Behav. Ecol. Sociobiol. 55, 531–543 10.1007/s00265-003-0724-y (doi:10.1007/s00265-003-0724-y) [DOI] [Google Scholar]
- 20.Janik V. M., Sayigh L. S., Wells R. S. 2006. Signature whistle contour shape conveys identity information to bottlenose dolphins. Proc. Natl Acad. Sci. USA 103, 8293–8297 10.1073/pnas.0509918103 (doi:10.1073/pnas.0509918103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janik V. M. 2000. Whistle matching in wild bottlenose dolphins (Tursiops truncatus). Science 289, 1355–1357 10.1126/science.289.5483.1355 (doi:10.1126/science.289.5483.1355) [DOI] [PubMed] [Google Scholar]
- 22.Tyack P. 1986. Whistle repertoires of two bottlenosed dolphins, Tursiops truncatus: mimicry of signature whistles? Behav. Ecol. Sociobiol. 18, 251–257 10.1007/BF00300001 (doi:10.1007/BF00300001) [DOI] [Google Scholar]
- 23.Cook M. L. H., Sayigh L. S., Blum J. E., Wells R. S. 2004. Signature-whistle production in undisturbed free-ranging bottlenose dolphins (Tursiops truncatus). Proc. R. Soc. Lond. B 271, 1043–1049 10.1098/rspb.2003.2610 (doi:10.1098/rspb.2003.2610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson B., Hammond P. S., Thompson P. M. 1999. Estimating size and assessing trends in a coastal bottlenose dolphin population. Ecol. Appl. 9, 288–300 10.1890/1051-0761(1999)009[0288:ESAATI]2.0.CO;2 (doi:10.1890/1051-0761(1999)009[0288:ESAATI]2.0.CO;2) [DOI] [Google Scholar]
- 25.Wilson B., Reid R. J., Grellier K., Thompson P. M., Hammond P. S. 2004. Considering the temporal when managing the spatial: a population based range expansion impacts protected areas-based management for bottlenose dolphins. Anim. Cons. 7, 331–338 10.1017/S1367943004001581 (doi:10.1017/S1367943004001581) [DOI] [Google Scholar]
- 26.Quick N. J., Rendell L. E., Janik V. M. 2008. A mobile acoustic localization system for the study of free-ranging dolphins during focal follows. Mar. Mammal Sci. 24, 979–989 10.1111/j.1748-7692.2008.00231.x (doi:10.1111/j.1748-7692.2008.00231.x) [DOI] [Google Scholar]
- 27.Freitag L. E., Tyack P. L. 1993. Passive localization of the Atlantic bottlenose dolphin using whistles and echolocation clicks. J. Acoust. Soc. Am. 93, 2197–2205 10.1121/1.406681 (doi:10.1121/1.406681) [DOI] [PubMed] [Google Scholar]
- 28.Janik V. M., Van Parijs S. M., Thompson P. M. 2000. A two-dimensional acoustic localization system for marine mammals. Mar. Mammal Sci. 16, 437–447 10.1111/j.1748-7692.2000.tb00935.x (doi:10.1111/j.1748-7692.2000.tb00935.x) [DOI] [Google Scholar]
- 29.Watkins W. A., Schevill W. E. 1974. Listening to Hawaiian spinner porpoises, Stenella cf. longirostris, with a three-dimensional hydrophone array. J. Mammal 55, 319–328 10.2307/1379001 (doi:10.2307/1379001) [DOI] [Google Scholar]
- 30.Janik V. M., King S. L., Sayigh L. S., Wells R. S. 2012. Identifying signature whistles from recordings of groups of unrestrained bottlenose dolphins (Tursiops truncatus). Mar. Mammal Sci. (doi:10.1111/j.1748-7692.2011.00549.x) [Google Scholar]
- 31.Deecke V. B., Janik V. M. 2006. Automated categorization of bioacoustic signals: avoiding perceptual pitfalls. J. Acoust. Soc. Am. 119, 645–653 10.1121/1.2139067 (doi:10.1121/1.2139067) [DOI] [PubMed] [Google Scholar]
- 32.Quick N. J. 2006. Vocal behaviour and abundance of bottlenose dolphins (Tursiops truncatus) in St Andrews Bay. PhD thesis St Andrews: University of St Andrews; pp. 160. [Google Scholar]
- 33.Lang T. G., Norris K. S. 1966. Swimming speed of a Pacific bottlenose dolphin. Science 151, 588–590 10.1126/science.151.3710.588 (doi:10.1126/science.151.3710.588) [DOI] [PubMed] [Google Scholar]
- 34.Quick N. J., Janik V. M. 2008. Whistle rates of wild bottlenose dolphins: influences of group size and behavior. J. Comp. Psychol. 122, 305–311 10.1037/0735-7036.122.3.305 (doi:10.1037/0735-7036.122.3.305) [DOI] [PubMed] [Google Scholar]
- 35.Janik V. M., Dehnhardt G., Todt D. 1994. Signature whistle variations in a bottlenosed dolphin, Tursiops truncatus. Behav. Ecol. Sociobiol. 35, 243–248 10.1007/BF00170704 (doi:10.1007/BF00170704) [DOI] [Google Scholar]
- 36.Mann J., Smuts B. B. 1999. Behavioral development of wild bottlenose dolphin newborns. Behaviour 136, 529–566 10.1163/156853999501469 (doi:10.1163/156853999501469) [DOI] [Google Scholar]
- 37.Evans W. E., Dreher J. J. 1962. Observations on scouting behavior and associated sound production by the Pacific bottlenosed porpoise (Tursiops gilli Dall). Bull. Southern California Acad. Sci. 61, 217–226 [Google Scholar]
- 38.Gazda S. K., Connor R. C., Edgar R. K., Cox F. 2005. A division of labour with role specialization in group-hunting bottlenose dolphins (Tursiops truncatus) off Cedar Key, Florida. Proc. R. Soc. B 272, 135–140 10.1098/rspb.2004.2937 (doi:10.1098/rspb.2004.2937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wells R. S. 2003. Dolphin social complexity: lessons from long-term study and life history. In Animal social complexity: intelligence, culture, and individualized societies (eds de Waal F. B. M., Tyack P. L.), pp. 32–56 Cambridge, MA: Harvard University Press [Google Scholar]
- 40.Biben M., Symmes D., Masataka N. 1986. Temporal and structural analysis of affiliative vocal exchanges in squirrel monkeys (Saimiri sciureus). Behaviour 98, 259–273 10.1163/156853986X00991 (doi:10.1163/156853986X00991) [DOI] [Google Scholar]
- 41.Cheney D. L., Seyfarth R. M., Palombit R. 1996. The function and mechanisms underlying baboon ‘contact’ barks. Anim. Behav. 52, 507–518 10.1006/anbe.1996.0193 (doi:10.1006/anbe.1996.0193) [DOI] [Google Scholar]
- 42.Herman L. M., Pack A. A., Morrel-Samuels P. 1993. Representational and conceptual skills of dolphins. In Language and communication: comparative perspectives (eds Roitblat H. L., Herman L. M., Nachtigall P. E.), pp. 403–442 Hillsdale, NJ: Lawrence Erlbaum Associates [Google Scholar]