Abstract
Cooperative brood care is assumed to be the common driving factor leading to sociality. While this seems to be true for social Hymenoptera and many cooperatively breeding vertebrates, the importance of brood care for the evolution of eusociality in termites is unclear. A first step in elucidating this problem is an assessment of the ancestral condition in termites. We investigated this by determining the overall level of brood care behaviour across four termite species that cover the phylogenetic diversity of the lower termites. Brood care was low in the three species (all from different families) that had an ancestral wood-dwelling lifestyle of living in a single piece of wood that serves as food and shelter. In the fourth species, a lower termite that evolved outside foraging, brood care was more common. Together with data for higher termites, this suggests that brood care in termites only becomes important when switching from a wood-dwelling to a foraging lifestyle. These results imply that early social evolution in termites was driven by benefits of increased defence, while eusociality in Hymenoptera and cooperative breeding in birds and mammals are primarily based on brood care.
Keywords: altruism, brood care, social evolution, cooperatively breeding, eusociality, social insects
1. Introduction
The study of social evolution and cooperation/altruism is a major focus in evolutionary biology that is intensively studied in cooperatively breeding birds and mammals, as well as social insects [1–4]. Among the latter, Hymenoptera, and in particular the ants and the honeybee Apis mellifera, became favourite model organisms to test (successfully) the factors influencing social evolution [5–7], while the other large group of social insects, the termites, received less attention. Social insects live in complex societies in which only a few individuals reproduce (queen/king) while a large majority (workers/soldiers) forgo reproduction, at least temporarily. This altruistic behaviour of the non-reproducing castes can be explained by kin selection theory (i.e. the propagation of the corresponding alleles through non-offspring relatives) [8]. In general, brood care of siblings has emerged as the common factor favouring social life in ants [3,4,9], bees [10,11] and many wasps [4,12]. The importance of brood care for social evolution was also supported by studies on cooperatively breeding vertebrates [1,2,13].
For the remaining large group of social insects, the termites, evidence for the importance of brood care in the evolution of social life is more limited and largely restricted to its most apical family, the Termitidae (reviewed by Korb [14]), which comprise about 75 per cent of all extant termite species [15]. Their apparent similarity to the ground-dwelling ants led to the assumption of convergent evolution driven by alloparental brood care between these taxonomically disparate groups of social insects [16]. Subsociality in the sister taxon of the termites, the woodroaches (Cryptocercidae; figure 1), in which parents care for their offspring for extended periods, further supported this hypothesis [26]. Compared with woodroaches, termites are characterized by two synapomorphies: (i) the evolution of sterile soldiers, which makes termites eusocial; and (ii) the evolution of neotenic reproduction (i.e. offspring can become wingless reproductives within the natal nest via a single moult). According to the former, termites have been classified as fortress-defenders [6,27], which raises the question of how important alloparental brood care by workers was for their transition to eusociality. Our present study makes this question explicit and tries to answer it.
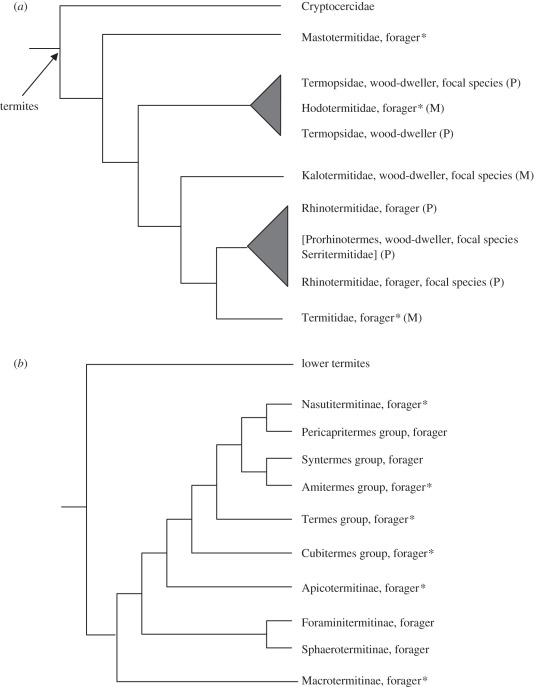
Figure 1.
(a) Phylogeny of termite families and (b) phylogeny of the Termitidae (both after Inward et al. [17]), with lifestyle (forager: foraging species; wood-dweller: wood-dwelling species) and studied focal species shown. The trees are cladograms so that branch lengths are arbitrary. Asterisks indicate taxa known to have brood care. M, monophyletic family; P, paraphyletic family. References for brood care in Termitidae: Nasutitermitinae [18–21]; Cubitermes [22]; Macrotermitinae [23–25].
Studying the history of social evolution is largely hampered by the difficulty that we can hardly reconstruct the relevant ecological conditions under which the first common ancestors of eusocial lineages evolved. A useful indirect approach is to trace the history of traits through robust phylogenetic trees [28–30], but this approach has only recently become feasible for termites because systematic behavioural data covering all major lineages were missing, and accurate molecular phylogenies were unavailable. The occurrence of different types of workers (true workers and pseudergates) has been plotted on termite phylogenies [31,32], but termite worker terminology has been inconsistently used in the termite literature. This implies that definitions vary, implicitly or explicitly, according to the authors, and are based on a mixture of ontogenetical, anatomical, morphological and behavioural criteria (for a full discussion, see [33]). The terminology does not reflect the degree of altruism and this has caused misunderstandings about the evolution of termite workers [33]. Hence, there is a need for direct behavioural data that measure the degree of brood care altruism, and also a need to interpret these data in a phylogenetic context.
The degree of social complexity differs greatly among different termite lineages (figure 1). In wood-dwelling termites, which nest in a single piece of wood that serves both as shelter and food source (also called one-piece-type nesters by Abe [34]) and which form small colonies [35], the workers are totipotent immatures (sometimes also called ‘pseudergates’) that can explore all caste options [36]. These older instars can function as workers and they can develop into soldiers or two types of reproductives: winged sexuals that found a new nest elsewhere or neotenic reproductives that inherit the natal breeding position when the current reproductives are unhealthy or die. This life type is considered to be phylogenetically basal [17,18], and characteristic for dampwood termites (Termopsidae), drywood termites (Kalotermitidae) and a few Rhinotermitidae (e.g. Prorhinotermes; figure 1a).
The wood-dwellers' lifestyle contrasts with that of termite species who sooner or later leave the nest to forage for food (foraging species [36]; also called separate and intermediate-type nesters according to Abe [34]; figure 1). Foraging termites are characterized by an increasingly restrictive development and an early separation into two developmental pathways: an apterous and a nymphal line, which give rise to wingless (mainly workers and soldiers) and winged (winged sexuals) individuals, respectively [37]. Although workers from the apterous line commonly reproduce in Mastotermitidae and most Rhinotermitidae, this potential is greatly reduced in the higher termites (Termitidae) [38] (figure 1). The workers of the latter clearly are an altruistic caste that is highly engaged in brood care [39] (figure 1b), and they have few opportunities to reproduce [37]. Higher termites can have colony sizes of up to a few million individuals with sophisticated division of labour (e.g. fungus-growers) [40,41] and they are the dominant extant species both in terms of species richness and biomass [15].
Recently, the importance of brood care for the evolution of eusociality in termites was questioned when it was found that ‘workers’ of several wood-dwelling termites seem to lack brood care of eggs and larvae [42]. Rather they seem to be hopeful reproductives that become either winged sexuals or neotenic replacement reproductives [43]. The importance to gain direct fitness benefits through nest inheritance was also stressed for the occurrence of workers in another wood-dwelling species that has been studied intensively: the dampwood termite, Zootermopsis nevadensis [44,45]. Yet the high microbial load of the nest environment of dampwood termites [46] might have selected for hygienic brood care behaviour, such as allogrooming [43]. Using a standardized behavioural protocol, we set up a series of observational experiments to investigate the occurrence and degree of brood care in representative species of lower termite lineages (figure 1a). We selected three wood-dwelling species, one from each family that contains wood-dwellers (Termopsidae: Zootermopsis nevadensis; Kalotermitidae: Cryptotermes secundus; Rhinotermitidae: Prorhinotermes simplex), and one foraging species (Reticulitermes flavipes) from the single family that has wood-dwellers as well as foraging species, the Rhinotermitidae. By mapping our behavioural results on currently available phylogenetic trees [17,31] (figure 1), this set-up enabled us to test the importance of brood care in lower termites and to gain novel insights into the incipient phases of termite social evolution.
2. Material and methods
(a). Study species
(i). Cryptotermes secundus (Kalotermitidae; drywood termite)
Cryptotermes secundus colonies were collected in 2002 near Channel Island, Darwin Harbour (Northern Territory, Australia; 12°30′ S 131°00′ E). Dead trees of Ceriops tagal were chopped from the mangroves and carefully split with hammer and chisel to obtain complete colonies (for more details, see [47]). The colonies were housed in standardized wood blocks of Pinus radiata providing abundant food conditions [47] and shipped back to Germany to be kept in climate chambers under appropriate conditions (12 h day–night cycle, 70% relative humidity, 28°C; for more details, see [48]).
(ii). Zootermopsis nevadensis (Termopsidae, dampwood termite)
Zootermopsis nevadensis colonies were originally collected from the Del Monte Forest in Pebble Beach, California, in 2006 and 2008. They were extracted from rotten logs and transferred into layers of Douglas fir wood bolted together. The wood was regular moistened. Temperature of the culturing room was maintained at 21°C. The collected termites belong to the subspecies Zootermopsis nevadensis nuttingi according to location and cuticular hydrocarbon spectrum [49,50]. In 2009, colonies were shipped from Arizona, USA, to Germany and then kept under the same conditions as in the USA until behavioural observations started.
(iii). Prorhinotermes simplex (Rhinotermitidae; wood-dwelling type)
In 2008, nine Prorhinotermes simplex colonies were obtained from Jan Sobotnik, University of Prague, Czech Republic. They were originally collected in Soroa (Piňar del Rio, Cuba) in 1964 and kept in the laboratory in Prague and Germany at 26 ± 1°C provided with spruce and pine wood blocks and high moisture [51].
(iv). Reticulitermes flavipes (Rhinotermitidae, foraging type)
Samples of Reticulitermes (santonensis) flavipes were collected in April 2008 from wood fragments or tree stumps on the island of Oléron (France) from the Saumonard and the Saint Trojan forests (80% Pinus maritima). The samples were kept inside their natural wood at room temperature with watering twice a month until extraction. Using three colonies transferred to Plexiglas boxes (90 × 60 × 50 mm; Boite Lab Ltd, France) with moistured Fontainebleau sand and pieces of wood, we prepared three subcolonies (one from each colony) of 200–350 individuals with several nymphs and neotenics present. The species was determined by morphological and chemical identification as described previously [52] and confirmed by molecular data as in [53]. These colonies were transferred to Germany; where they were kept under the same conditions until experiments started.
(b). Behavioural observations
Wood blocks were carefully split with hammer and chisel, and the colonies were extracted. Colony composition was determined and the individuals transferred to a new wood block that had a predrilled chamber where individuals could be observed (for details, see [48]). Wood block and chamber size were adjusted to termite body size and colony size, providing abundant food conditions and approximately equal social interaction probabilities (see also [48]: per 10 individuals: C. secundus: 100 cm³ wood and 2.8 cm² chamber area; Z. nevadensis: 200 cm³ wood and 5.6 cm² chamber area; P. simplex and R. flavipes: 50 cm³ wood and 1.4 cm² chamber area). In total, ten colonies of C. secundus (colony size: 20–160; observed in 2003), four of Z. nevadensis (colony size: 48–175; observed in 2009), nine of P. simplex (colony size: 41–233 individuals; observed in 2008) and three of R. flavipes (colony size: 202–339 individuals; observed in 2009) were used. The larger laboratory colony sizes in R. flavipes reflect their larger colony sizes in nature. All colonies had reproductives, soldiers and brood (eggs and larvae) available in proportion to what is normally found in field colonies. The relative proportions differed between species, but this reflects the natural composition (brood proportion relative to workers: C. secundus, 1–9%; Z. nevadensis, 2–10%; P. simplex, 6–44%; R. flavipes, 10–36%). There was no correlation between the proportion of brood and brood care behaviour (Pearson correlation: always p > 0.300).
Termite workers (individuals beyond the third instar) were individually marked with two colour dots (Revell, Germany). After a resting time of at least 24 h, standardized behavioural observations were done [48]. Each marked individual was observed for 30 min using focal sampling. The behavioural repertoire of all species included the solitary behaviours sitting, wood feeding and moving around, and the interactive behaviours antennation, butting (i.e. fast backward and forward movements of the termite body), allogrooming and proctodeal trophallaxis (i.e. anal feeding), and (rarely) stomodeal trophallaxis (i.e. mouth to mouth feeding). Sitting and wood feeding were combined as resting in the later analyses as it was impossible to unambiguously differentiate among these two behaviours during observation. In addition, interactions with eggs (mainly transport; egg care) were observed for Z. nevadensis, P. simplex and R. flavipes. Behaviours which lasted more than 3 s (resting, running, allogrooming, proctodeal and stomodeal trophallaxis, brood transport) were measured in seconds. For the remaining behaviours, the frequency was recorded. We also noted whether a focal individual performed the behaviour (donor; behaviour ‘given’) or was the recipient of the behaviour (recipient; behaviour ‘received’). This was not possible for stomodeal trophallaxis as it was often ambiguous to determine who was the donor and who the recipient. Differences in the statistical results between given and received behaviours are known from previous studies [54] and can be explained by lower statistical power in the given behaviour as opposed to the received; interactions received by a single focal worker from multiple other workers are typically less variable (reduced noise) than the interactions initiated by each of the single workers alone [54]. In total, 98 (about 10 per colony), 122 (at least 30 per colony), 90 (10 per colony) and 75 (about 25 per colony) workers were observed in C. secundus, Z. nevadensis, P. simplex and R. flavipes, respectively. The experiments were performed under the species-specific temperature and moisture conditions detailed above.
(c). Statistical analyses
Data were tested for assumptions of parametric testing and analyses were done accordingly. We first compared the frequency or duration of behaviours between species with linear mixed model analyses using ‘colony’ as random factor and ‘species’ as fixed factor. These models never revealed a significant contribution of ‘colony’. Thus, we reran the analyses with univariate analysis of variation (ANOVA) using ‘species’ as fixed factor. Comparison of the Akaike information criterion (AIC) between both tests always revealed the ANOVA as the more powerful model owing to the non-significant effect of ‘colony’. Therefore, ANOVA results are presented. Qualitatively, we obtained the same results with linear mixed models or when applying non-parametrical tests. Differences between species pairs were analysed with Tamhane's T2 post hoc comparisons, as variances between species differed.
We performed a principal component analysis to compare the overall behavioural profiles between species. The Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy gave a value of 0.705, classified as ‘middling’ after Kaiser [55]. The Bartlett test of sphericity rejected the null hypothesis that the correlation matrix was an identity matrix with p < 0.0001. Both analyses thus showed that principal component analysis (PCA) was appropriate. All analyses were done with the statistical package PASW SPSS 18 and all tests are two-tailed.
3. Results
(a). Quantitative differences between species in behavioural repertoire
The broad comparison of time budgets for all behaviours gave significant differences across species (table 1, figures 2 and 3; see also electronic supplementary material, figures S1 and S2). This included overall activity levels. The lowest level of movement was shown by Z. nevadensis, while R. flavipes was moving more than three times as much (see electronic supplementary material, figure S1a). Cryptotermes secundus and P. simplex had similar intermediate moving activity. Cryptotermes secundus did most resting, followed by Z. nevadensis, while P. simplex and R. flavipes had low resting durations, less than 50 per cent of those observed in C. secundus (see electronic supplementary material, figure S1b).
Table 1.
Comparison of time budgets of behaviours across species, showing the results of univariate ANOVAs using ‘species’ as fixed factor.
| behaviour | F3, 381(error) | p |
|---|---|---|
| moving | 30.19 | <0.001 |
| resting | 36.77 | <0.001 |
| egg care | 8.7 | <0.001 |
| stomodeal trophallaxis | 6.69 | <0.001 |
| proctodeal trophallaxis | ||
| donor | 3.16 | 0.025 |
| recipient | 7.56 | <0.001 |
| allogrooming | ||
| donor | 38.9 | <0.001 |
| recipient | 81.84 | <0.001 |
| antennation | ||
| donor | 136.99 | <0.001 |
| recipient | 213.98 | <0.001 |
| butting | ||
| donor | 34.86 | <0.001 |
| recipient | 162.11 | <0.001 |
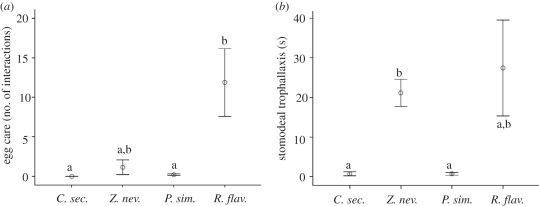
Figure 2.
Results of the behavioural comparison between species. Shown are mean values (±s.e.) of the frequency/duration of different behaviours during 30 min of focal observation of (a) egg care (number of interactions with eggs), (b) stomodeal trophallaxis (in seconds). Different letters indicate significant difference between species (p < 0.05).
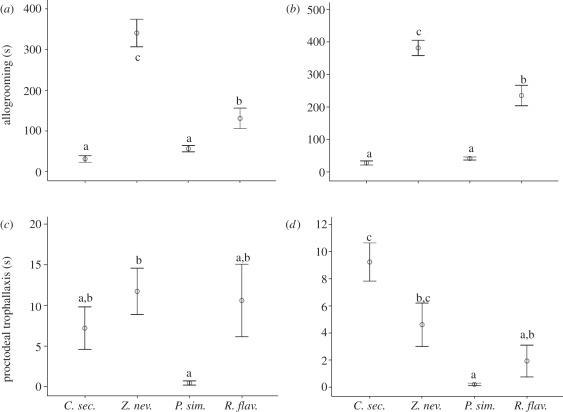
Figure 3.
Results of the comparison of interactive behaviours between species. Shown are mean values (±s.e.) of the duration (in seconds) during 30 min of focal observation for (a,c) donors and (b,d) recipients of (a,b) allogrooming and (c,d) proctodeal trophallaxis. Lower-case letters indicate significant differences between species (p < 0.05).
The most specific and central question of our study concerned the difference in the time budget and display of helping behaviour. According to their putative functions, the interactive behaviours can be grouped into altruistic helping (egg care, proctodeal and stomodeal trophallaxis, allogrooming) and communicative behaviours (antennation and butting), and both were compared across the species.
(b). Altruistic behaviours
Egg care, proctodeal and stomodeal trophallaxis, and allogrooming are all behaviours where it is supposed that the receiver primarily benefits from the interaction, making them forms of altruistic behaviour [35]. A qualitative difference was found in egg care behaviour. In contrast to all other species, C. secundus did not display any egg care. Even though duration of egg care in P. simplex and Z. nevadensis did not differ significantly from C. secundus, it did occur 6 and 32 times in P. simplex and Z. nevadensis, respectively (figure 2a). However, R. flavipes had 5–10 times higher duration of egg care than Z. nevadensis and 50–70 times as much as P. simplex (figure 2a). This cannot be explained by brood availability, since P. simplex together with R. flavipes had the largest amounts of brood per colony.
The dampwood termite Z. nevadensis had the highest allogrooming durations, which lasted about 3–10 times longer than in all other species (figure 3a,b). Cryptotermes secundus and P. simplex, which both nest in hardwood, had comparable, very low levels of allogrooming, while the subterranean nesting R. flavipes had intermediate levels. Differences between species were less pronounced for giving proctodeal trophallaxis (figure 3c): the values were similar, and no significant differences were found except between P. simplex and Z. nevadensis, which had the lowest and highest values, respectively. Strikingly, receiving proctodeal trophallaxis revealed a different pattern (figure 3d): while P. simplex still had the lowest values, C. secundus had the highest durations of receiving food, significantly different from P. simplex and R. flavipes. However, the duration of stomodeal trophallaxis was low both in C. secundus and P. simplex, while Z. nevadensis and R. flavipes had mutual mouth-to-mouth feeding for longer periods (figure 2b). For R. flavipes, this difference to other species was not significant owing to the large variability between individuals.
(c). Communicative behaviours
Antennation frequencies were lowest in C. secundus and highest in R. flavipes (see electronic supplementary material, figure S2a,b). Zootermopsis nevadensis and P. simplex had intermediate frequencies. Butting frequencies were lowest in C. secundus and Z. nevadensis, and highest in R. flavipes (see electronic supplementary material, figure S2c). Butting other individuals did not differ between P. simplex and R. flavipes, while being butted was significantly less common in the former than the latter species (see electronic supplementary material, figure S2d). Frequencies of communicative behaviours are often side-effects of colony or body size, but our results are unlikely to be primarily caused by body size and group size differences between species as we adjusted chamber sizes to account for these differences (see §2). Furthermore, Z. nevadensis was by far the largest species, and R. flavipes and P. simplex were the smallest, yet this body size difference did not correspond to the occurrence of the communicative behaviours. Only for butting behaviour did the frequency roughly correspond to the colony sizes, which were similarly small for C. secundus and Z. nevadensis, and largest in R. flavipes. Within species, there was never a correlation between colony size and the occurrence of butting or antennation (Spearman rank test: always p > 0.100; but note the small sample sizes here). This suggests that there are true species-specific differences in the occurrence of communicative behaviours and that R. flavipes is the most communicative species.
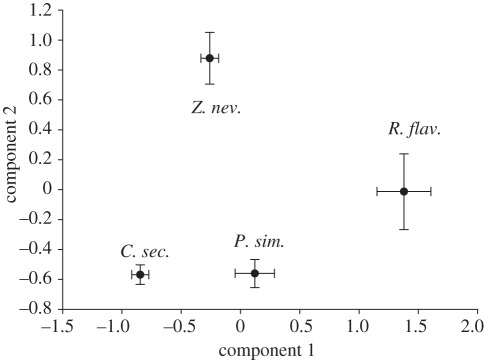
Including all behaviours, the PCA revealed three components with an eigenvalue larger than 1 (table 2). The first component explained 27.7 per cent of the total variance. Antennation (given and received), butting (given and received), moving and resting had the highest loadings on this component (table 2). The second component explained 16.9 per cent of the variance, with the more altruistic behaviours—allogrooming (given and received), proctodeal trophallaxis (given) and stomodeal trophallaxis—having the highest loadings (table 2). The remaining behaviours (receiving proctodeal trophallaxis and brood care) had their highest loadings on the third component, which explained 9.9 per cent of the variance. Along the first component, the species were separated in the order C. secundus, Z. nevadensis, P. simplex and R. flavipes, with the first two species clustering closely together (figure 4). The second component mainly separated Z. nevadensis from the other species, while R. flavipes had an intermediate position (figure 4). The third component did not reveal any obvious pattern.
Table 2.
Results of the principal component analysis using all behaviours show are the loadings of each behaviour on the three extracted components.
| variables | component 1 | component 2 | component 3 |
|---|---|---|---|
| eigenvalue (expl. variance) | 3.32 (27.70%) | 2.02 (16.85%) | 1.19 (9.88%) |
| moving | 0.637 | −0.449 | 0.315 |
| resting | −0.575 | −0.291 | −0.419 |
| egg care | 0.313 | 0.188 | 0.476 |
| stomodeal trophallaxis | 0.093 | 0.452 | −0.219 |
| proctodeal trophallaxis | |||
| donor | 0.033 | 0.361 | 0.245 |
| recipient | −0.169 | 0.076 | 0.412 |
| allogrooming | |||
| donor | 0.139 | 0.775 | 0.292 |
| recipient | 0.144 | 0.753 | −0.338 |
| antennation | |||
| donor | 0.882 | 0.081 | −0.129 |
| recipient | 0.785 | 0.155 | −0.398 |
| butting | |||
| donor | 0.698 | −0.349 | 0.069 |
| recipient | 0.729 | −0.2 | −0.168 |
Figure 4.
Results of a principal component analysis including all behaviours. The first component (x-axis) had an eigenvalue of 3.32. The second component (y-axis) had an eigenvalue of 2.02. Shown are the mean values ±95% CI. See text for details.
4. Discussion
Our results showed clear differences in the behaviours expressed by the four termite species studied. Brood care behaviour was, in general, rarely or not expressed in three species (P. simplex, Z. nevadensis and C. secundus), while one species (R. flavipes) displayed it frequently. The low level of brood care with egg carrying as its main component has strong implications for the importance of brood care for the evolution of eusociality in termites.
(a). Brood care in lower termites
Because the importance of brood care for the evolution of eusociality in termites is unclear [14,33,42,44,45,56], it is important to assess the overall level of brood care behaviour across species from different phylogenetic groups to get an impression of the ancestral condition. The four species chosen cover the phylogenetic diversity of the lower termites, except the Mastotermitidae (figure 1a), which are represented by a single extant species, Mastotermes darwiniensis. This species is derived in its lifestyle [31,57] and hence it is predicted to have brood care (see also below). Therefore, we assumed that features required for the first steps in the evolution of eusociality should be present in all four studied species. Brood care behaviour occurred in all species except C. secundus. It also was not found in previous studies on C. secundus [42] and two other Cryptotermes species [43]. This suggests that Cryptotermes species are unusual in their complete lack of brood care behaviour. The degree of egg care was very low in the other wood-dwellers, Z. nevadensis and P. simplex, so that no significant difference to C. secundus was found (figure 2a). This clearly differed from the subterranean termite R. flavipes, where brood care was more common. However, even here brood care was generally not very sophisticated, since it remained primarily restricted to carrying of egg and larvae, involving some licking.
The lack of brood care behaviour in C. secundus could indicate either that (i) no alloparental care is the ancestral condition, provided that Cryptotermes and the Kalotermitidae are the most basal group among the wood-dwellers [31]; or (ii) C. secundus secondarily lost brood care. The available phylogenies are ambiguous with regard to the position of the Termopsidae and Kalotermitidae (see also figure 1a): Legendre et al. [31] placed the Kalotermitidae in a more basal position, while it is the reverse in the study of Inward et al. [17]. These most recent phylogenies reflect a long-standing debate about the relative positions of these lower termite families [58], which might take a long time to resolve, owing to the fast pace of Dictyopteran and termite evolution [59]. Even if the second scenario is more likely, the lack of brood care by workers in C. secundus and the other Cryptotermes species provides evidence for the principle that alloparental brood care is no necessary prerequisite for termite eusociality.
(b). Termites as fortress defenders versus social Hymenoptera as brood care providers
The situation in termites strongly contrasts with the general pattern in eusocial Hymenoptera. Alloparental care is considered a major prerequisite for the evolution of eusociality in the latter group [10,60]. The major role of the altruistic helpers in Hymenopteran societies is the provisioning and raising of brood (classified as life insurers by Queller & Strassmann [6]). In termites, however, the reproductively altruistic caste, the soldiers, primarily engage in nest defence. This can be considered brood care as well, but it is still qualitatively different from the provisioning in eusocial Hymenoptera. Hence, wood-dwelling termites are always clearly fortress defenders [6,27].
(c). The worker role in lower termites is malleable by environmental conditions
The role of workers as life insurers and brood care providers differs among lower termite species, as does the developmental flexibility of workers [33], and the trait seems to be flexibly shaped by environmental conditions: in contrast to R. flavipes, P. simplex had few altruistic behaviours (allogrooming, proctodeal and stomodeal trophallaxis), although both species belong to the most derived family studied here. This fits the wood-dwelling life style of P. simplex, while R. flavipes is a foraging species (figure 1a). On the other hand, Z. nevadensis had the highest degree of allogrooming (figure 3a,b) despite its phylogenetic basal position (figure 1a), in line with an extremely high pathogen load in its nest [46]. This suggests that worker altruism relating to allogrooming and trophallaxis in lower termites is a malleable trait that changes easily, depending on prevailing environmental conditions. The high pathogen load of soils and the high allogrooming rates in the subterranean R. flavipes further strengthen the hypothesis that pathogen load is an important factor promoting altruistic worker behaviour in lower termites. Yet more studies are needed to understand the importance of phylogeny, ecology and species idiosyncrasies in order to understand the social evolution of termites.
(d). Foraging termites have altruistic workers and are comparable with social Hymenoptera
Altruistic behaviour and the importance of alloparental brood care in the termites and eusocial Hymenoptera converges in the higher termites. In all higher termites studied so far, there is intensive egg and brood care (figure 1b), associated with true worker castes that combine the specialized task of foraging with brood care, often starting with the latter early in life and changing to the former when they become older (age polyethism [40]). In R. flavipes, the amount of stomodeal trophallaxis varied widely among individuals, which might also be indicative of division of labour among workers. In wood-dwelling termites, foraging tasks are obsolete as the termites live inside their food. This changes with the transition to the foraging lifestyle in termites, as in R. flavipes, because they sooner or later have to leave the nest and forage for food. Increased foraging activity of workers seems to be paralleled by an increasing proportion of neotenic reproductives in some Rhinotermitidae, such as Reticulitermes [61].
The need for external foraging introduces two differences that dramatically change the trajectory of eusocial evolution [7,17,39,43]. First, the outside foraging is associated with much larger mortality risks, increasing the level of altruism. Second, and most importantly, the transition to foraging termites requires alloparental brood care because larvae no longer have food around them but rely on food collected by foragers. Hence, workers can increase the reproductive success of their parents through food provisioning of siblings and become more altruistic. As the most basal extant termite, M. darwiniensis, has a lifestyle similar to that of higher termites, with workers foraging outside their nests, we predicted that this species has brood care. Accordingly, its workers were also classified as true workers (figure 1a). Termite societies with external foraging therefore changed their evolutionary trajectory and are no longer shaped by the selective forces that wood-dwelling termite societies are primarily exposed to. Foraging termite species, which include R. flavipes, thus resemble social Hymenoptera concerning food provisioning and follow similar evolutionary trajectories, which lead to much more sophisticated societies than wood-dwellers, as fortress defenders, ever evolved.
Similarly, in cooperatively breeding vertebrates, helpers can reduce the workload of their parents through food provisioning of the young [2,62,63], and although other factors (e.g. group augmentation effects and improved defence) can contribute to explaining cooperative breeding, brood care was revealed as a major factor for social evolution in birds and mammals [1,2,27].
For cooperatively breeding vertebrates, and to a lesser extent for social Hymenoptera (especially for wasps; see [64]), it has been proposed that cooperative breeding is the result of a two-step process [13]. In the first step, constraints (often ecological) select against offspring dispersal and result in family living. In the second step, cooperative breeding arises if helpers receive sufficient direct and/or indirect fitness benefits. One could argue that wood-dwelling termites resemble the first step and foraging termites the second step. From this point of view, termites are not that different from these cooperative breeders except that the first truly altruistic caste to evolve in termites was a soldier caste. Hence, the first step still occurs in extant wood-dwelling termites with soldiers as an altruistic caste, while it is generally absent in cooperative breeders and social Hymenoptera. Wood-dwelling termites, as fortress defenders, are more similar to social aphids, thrips and mole rats (where brood care is also less important than colony defence [65]) than to the large majority of social Hymenoptera and cooperatively breeding vertebrates [27]. Only when termite colonies evolved the use of external resources did helpers supporting parents in brood care become more important [7,17,39,43]. This parallels the several independent origins in various groups of bees, wasps, ants and most cooperatively breeding vertebrates.
5. Conclusion
Our study suggests that, in contrast to ants, bees and most cooperative breeding vertebrates, brood care by workers is no necessary prerequisite for termite eusociality and that the degree of worker altruism largely differs between species. All lower termites are fortress defenders with an altruistic sterile soldier caste making them eusocial. This suggests that social evolution was driven by the need for increased defensive abilities rather than cooperative brood care in wood-dwelling termites. The degree of altruistic worker brood care seems to depend on environmental conditions, and especially pathogen load appears to be a major factor promoting altruistic allogrooming in workers. This implies that defence against pathogens might have been an additional important driver of termite social evolution.
Acknowledgements
We thank two anonymous referees for helpful comments on the manuscript, Pebble Beach Company for permission to collect Z. nevadensis, Simon Dupont for preparation of the microcolonies of R. (santonensis) flavipes and Jan Sobotnik for donation of the P. simplex colonies. This project was partly supported by Agriculture and Food Research Initiative Competitive Grant no. 2007-35302-18172 from the USDA National Institute of Food and Agriculture and the German Science Foundation (DFG KO1895/6).
References
- 1.Koenig W. D., Dickinson J. 2004. Ecology and evolution of cooperative breeding in birds. Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Russell A. F. 2004. Mammals: comparisons and contrasts. In Ecology and evolution of cooperative breeding birds (eds Koenig W. D., Dickinson J.), pp. 210–227 Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Bourke A. F. G., Franks N. R. 1995. Social evolution in ants. Princeton, NJ: Princeton University Press [Google Scholar]
- 4.Crozier R. H., Pamilo P. 1996. Evolution of social insect colonies: sex allocation and kin selection. Oxford, UK: Oxford University Press [Google Scholar]
- 5.Boomsma J. J., Franks N. R. 2006. Social insects: from selfish genes to self organisation and beyond. Trends Ecol. Evol. 21, 303–308 10.1016/j.tree.2006.04.001 (doi:10.1016/j.tree.2006.04.001). [DOI] [PubMed] [Google Scholar]
- 6.Queller D. C., Strassmann J. E. 1998. Kin selection and social insects. BioScience 48, 165–175 10.2307/1313262 (doi:10.2307/1313262) [DOI] [Google Scholar]
- 7.Boomsma J. J. 2009. Lifetime monogamy and the evolution of eusociality. Phil. Trans. R. Soc. B 364, 3191–3207 10.1098/rstb.2009.0101 (doi:10.1098/rstb.2009.0101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton W. D. 1964. The genetical evolution of social behaviour I & II. J. Theoret. Biol. 7, 1–52 10.1016/0022-5193(64)90038-4 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 9.Hölldobler B., Wilson E. O. 1990. The ants. Cambridge, MA: Harvard University Press [Google Scholar]
- 10.Strohm E., Liebig J. 2008. Why are so many bees but so few digger wasps social? The effect of provisioning mode and helper efficiency on the distribution of sociality among the Apoidea. In Ecology of social evolution (eds Korb J., Heinze J.), pp. 109–128 Heidelberg, Germany: Springer [Google Scholar]
- 11.Michener C. D. 2000. The bees of the world. Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 12.West-Eberhard M.-J., Turillazzi S. 1996. Natural history and evolution of paper wasps. Oxford, UK: Oxford University Press [Google Scholar]
- 13.Dickinson J., Hatchwell B. J. 2004. Fitness consequences of helping. In Ecology and evolution of cooperative breeding in birds (eds Koenig W., Dickinson J.), pp. 48–66 Cambridge, UK: Cambridge University Press [Google Scholar]
- 14.Korb J. 2008. The ecology of social evolution in termites. In Ecology of social evolution (eds Korb J., Heinze J.), pp. 151–174 Heidelberg, Germany: Springer [Google Scholar]
- 15.Eggleton P. 2000. Global patterns of termite diversity. In Termites: evolution, sociality, symbiosis and ecology (eds Abe T., Bignell D. E., Higashi M.), pp. 25–51 Dordrecht, The Netherlands: Kluwer Academic Publishers [Google Scholar]
- 16.Noirot C. 1985. Pathways of caste development in the lower termites. In Caste differentiation in social insects (eds Watson J. A. L., Okot-Kotber B. M., Noirot C.), pp. 41–58 Oxford, UK: Pergamon Press [Google Scholar]
- 17.Inward D. J. G., Vogler A. P., Eggleton P. 2007. A comprehensive phylogenetic analysis of termites (Isoptera) illuminates key aspects of their evolutionary biology. Mol. Phylogen. Evol. 44, 953–967 10.1016/j.ympev.2007.05.014 (doi:10.1016/j.ympev.2007.05.014) [DOI] [PubMed] [Google Scholar]
- 18.Thorne B. L. 1997. Evolution of eusociality in termites. Ann. Rev. Ecol. Syst. 28, 27–54 10.1146/annurev.ecolsys.28.1.27 (doi:10.1146/annurev.ecolsys.28.1.27) [DOI] [Google Scholar]
- 19.Adam R. A., Mitchell J. D. 2009. Energetics and development of incipient colonies of the harvester termite, Trinervitermes trinervoides (Sjostedt) (Termitidae, Nasutitermitinae). Insectes Soc. 56, 21–27 10.1007/s00040-008-1032-3 (doi:10.1007/s00040-008-1032-3) [DOI] [Google Scholar]
- 20.Grassé P. P., Noirot C. 1951. La sociotomie: migration et fragmentation de la termitière chez les Anoplotermes et les Trinervitermes. Behaviour 3, 146–166 10.1163/156853951X00241 (doi:10.1163/156853951X00241) [DOI] [Google Scholar]
- 21.Thorne B. L. 1985. Numerical and biomass caste proportions in colonies of the termites Nasutitermes corniger and N. ephratae (isoptera: Termitidae). Insectes Soc. 32, 411–426 10.1007/BF02224018 (doi:10.1007/BF02224018) [DOI] [Google Scholar]
- 22.Williams R. M. C. 1959. Colony development in Cubitermes ugandensis Fuller (Isoptera, Termitidae). Insectes Soc. 6, 291–304 10.1007/BF02224412 (doi:10.1007/BF02224412) [DOI] [Google Scholar]
- 23.Gerber C., Badertscher S., Leuthold R. H. 1988. Polyethism in Macrotermes bellicosus (Isoptera). Insectes Soc. 35, 226–240 10.1007/BF02224056 (doi:10.1007/BF02224056) [DOI] [Google Scholar]
- 24.Hinze B., Crailsheim K., Leuthold R. H. 2002. Polyethism in food processing and social organisation in the nest of Macrotermes bellicosus (Isoptera, Termitidae). Insectes Soc. 49, 31–37 10.1007/s00040-002-8275-1 (doi:10.1007/s00040-002-8275-1) [DOI] [Google Scholar]
- 25.Grassé P. P. 1982. Termitologia: anatomie-physiologie-biologie-systematique des termites. Anatomie-physiologie-reproduction. Tome I. Paris, France: Masson [Google Scholar]
- 26.Nalepa C. A. 1988. Cost of parental care in the woodroach Cryptocercus punctulatus Scudder (Dictyoptera: Cryptocercidae). Behav. Ecol. Sociobiol. 23, 135–140 10.1007/BF00300348 (doi:10.1007/BF00300348) [DOI] [Google Scholar]
- 27.Korb J., Heinze J. 2008. The ecology of social life: a synthesis. In Ecology of social evolution (eds Korb J., Heinze J.), pp. 245–260 Heidelberg, Germany: Springer [Google Scholar]
- 28.Brady S. G., Sipes S., Pearson A., Danforth B. N. 2006. Recent and simultaneous origins of eusociality in halictid bees. Proc. R. Soc. B 273, 1643–1649 10.1098/rspb.2006.3496 (doi:10.1098/rspb.2006.3496). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellens R., D'Haese C. A., Belles X., Piulachs M. D., Legendre F., Wheeler W. C., Grandcolas P. 2007. The evolutionary transition from subsocial to eusocial behaviour in Dictyoptera: phylogenetic evidence for modification of the ‘shift-in-dependent-care’ hypothesis with a new subsocial cockroach. Mol. Phylogen. Evol. 43, 616–626 10.1016/j.ympev.2006.12.017 (doi:10.1016/j.ympev.2006.12.017). [DOI] [PubMed] [Google Scholar]
- 30.Farrell B. D., Sequeira A. S., O'Meara B. C. O., Normark B. B., Chung J. H., Jordal B. H. 2001. The evolution of agriculture in beetles (Curculionidae: Scolytinae and Platypodinae). Evolution 55, 2011–2027 [DOI] [PubMed] [Google Scholar]
- 31.Legendre F., Whiting M. F., Bordereau C., Cancello E. M., Evans T. A., Grandcolas P. 2008. The phylogeny of termites (Dictyoptera: Isoptera) based on mitochondrial and nuclear markers: implications for the evolution of the worker and pseudergate castes, and foraging behaviors. Mol. Phylogen. Evol. 48, 615–627 10.1016/j.ympev.2008.04.017 (doi:10.1016/j.ympev.2008.04.017). [DOI] [PubMed] [Google Scholar]
- 32.Thompson G. J., Kitade O., Lo N., Crozier R. H. 2000. Phylogenetic evidence for a single, ancestral origin of a ‘true’ worker caste in termites. J. Evol. Biol. 13, 869–881 10.1046/j.1420-9101.2000.00237.x (doi:10.1046/j.1420-9101.2000.00237.x) [DOI] [Google Scholar]
- 33.Roisin Y., Korb J. 2011. Social organisation and the status of workers in termites. In Biology of termites: a modern synthesis (eds Bignell D. E., Roisin Y., Lo N.), pp. 133–164 Heidelberg, Germany: Springer [Google Scholar]
- 34.Abe T. 1987. Evolution of life types in termites. In Evolution and coadaptation in biotic communities (eds Kawano S., Connell J. H., Hidaka T.), pp. 125–148 Tokyo, Japan: University of Tokyo Press [Google Scholar]
- 35.Shellman-Reeve J. S. 1997. The spectrum of eusociality in termites. In The evolution of social behavior in insects and arachnids (eds Choe J. C., Crespi B. J.), pp. 52–93 Cambridge, UK: Cambridge University Press [Google Scholar]
- 36.Korb J., Hartfelder K. 2008. Life history and development: a framework for understanding the ample developmental plasticity in lower termites. Biol. Rev. 83, 295–313 10.1111/j.1469-185X.2008.00044.x (doi:10.1111/j.1469-185X.2008.00044.x) [DOI] [PubMed] [Google Scholar]
- 37.Roisin Y. 2000. Diversity and evolution of caste patterns. In Termites: evolution, sociality, symbiosis and ecology (eds Abe T., Bignell D. E., Higashi M.), pp. 95–119 Dordrecht, The Netherlands: Kluwer Academic Publishers [Google Scholar]
- 38.Noirot C. 1990. Sexual castes and reproductive strategies in termites. In An evolutionary approach to castes and reproduction (ed. Engels W.), pp. 5–35 Berlin, Germany: Springer [Google Scholar]
- 39.Higashi M., Yamamura N., Abe T., Burns T. P. 1991. Why don't all termite species have a sterile worker caste? Proc. R. Soc. Lond. B 246, 25–29 10.1098/rspb.1991.0120 (doi:10.1098/rspb.1991.0120) [DOI] [PubMed] [Google Scholar]
- 40.Traniello J. F., Leuthold R. H. 2000. Behavior and ecology of foraging in termites. In Termites: evolution, sociality, symbiosis and ecology (eds Abe T., Bignell D. E., Higashi M.), pp. 141–168 Dordrecht, The Netherlands: Kluwer Academic Publishers [Google Scholar]
- 41.Aanen D. K., Eggleton P., Rouland-Lefevre C., Guldberg-Froslev T., Rosendahl S., Boomsma J. J. 2002. The evolution of fungus-growing termites and their mutualistic fungal symbionts. Proc. Natl. Acad. Sci. USA 99, 14 887–14 892 10.1073/pnas.222313099 (doi:10.1073/pnas.222313099). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korb J. 2007. Workers of a drywood termite do not work. Front. Zool. 4, e7. 10.1186/1742-9994-4-7 (doi:10.1186/1742-9994-4-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korb J. 2008. Termites, hemimetabolous diploid white ants? Front. Zool. 5, e15. 10.1186/1742-9994-5-15 (doi:10.1186/1742-9994-5-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thorne B. L., Breisch N., Muscedere M. 2003. Evolution of eusociality and the soldier caste in termites: influence of intraspecific competition and accelerated inheritance. Proc. Natl Acad. Sci. USA 100, 12 808–12 813 10.1073/pnas.2133530100 (doi:10.1073/pnas.2133530100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johns P. M., Howard K. J., Breisch N. L., Rivera A., Thorne B. L. 2009. Nonrelatives inherit colony resources in a primitive termite. Proc. Natl Acad. Sci. USA 106, 17 452–17 456 10.1073/pnas.0907961106 (doi:10.1073/pnas.0907961106). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosengaus R. B., Moustakas J. E., Calleri D. V., Traniello J. F. A. 2003. Nesting ecology and cuticular microbial loads in dampwood (Zootermopsis angusticollis) and drywood termites (Incisitermes minor, I. schwarzi, Cryptotermes cavifrons). J. Insect Sci. 3, e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korb J., Lenz M. 2004. Reproductive decision-making in the termite, Cryptotermes secundus (Kalotermitidae), under variable food conditions. Behav. Ecol. 15, 390–395 10.1093/beheco/arh033 (doi:10.1093/beheco/arh033) [DOI] [Google Scholar]
- 48.Korb J., Schmidinger S. 2004. Help or disperse? Cooperation in termites influenced by food conditions. Behav. Ecol. Sociobiol. 56, 89–95 10.1007/s00265-004-0757-x (doi:10.1007/s00265-004-0757-x) [DOI] [Google Scholar]
- 49.Haverty M. I., Page M., Nelson L. J., Blomquist G. J. 1988. Cuticular hydrocarbons of dampwood termites, Zootermopsis: intracolony and intercolony variation and potential as taxonomic characters. J. Chem. Ecol. 14, 1035–1058 10.1007/BF01018791 (doi:10.1007/BF01018791) [DOI] [PubMed] [Google Scholar]
- 50.Liebig J., Eliyahu D., Brent C. 2009. Cuticular hydrocarbon profiles indicate reproductive status in the termite Zootermopsis nevadensis. Behav. Ecol. Sociobiol. 63, 1799–1807 10.1007/s00265-009-0807-5 (doi:10.1007/s00265-009-0807-5) [DOI] [Google Scholar]
- 51.Hanus R., Sobotnik J., Valterova I., Lukas J. 2006. The ontogeny of soldiers in Prorhinotermes simplex (Isoptera, Rhinotermitidae). Insectes Soc. 53, 249–257 10.1007/s00040-006-0865-x (doi:10.1007/s00040-006-0865-x) [DOI] [Google Scholar]
- 52.Clément J.-L., Bagnères A.-G., Uva P., Wilfert L., Quintana A., Reinhard J., Dronnet S. 2001. Biosystematics of Reticulitermes termites in Europe: morphological, chemical and molecular data. Insectes Soc. 48, 202–215 10.1007/PL00001768 (doi:10.1007/PL00001768) [DOI] [Google Scholar]
- 53.Perdereau E., Dedeine F., Christidès J.-P., Dupont S., Bagnères A.-G. 2011. Competition between invasive and indigenous species: an insular case study of subterranean termites. Biol. Invasions 13, 1457–1470 10.1007/s10530-010-9906-5 (doi:10.1007/s10530-010-9906-5) [DOI] [Google Scholar]
- 54.Korb J., Weil T., Hoffmann K., Foster K. R., Rehli M. 2009. A gene necessary for reproductive suppression in termites. Science 324, 758–758 10.1126/science.1170660 (doi:10.1126/science.1170660) [DOI] [PubMed] [Google Scholar]
- 55.Kaiser H. F. 1974. An index of factorial simplicity. Psychometrika 39, 31–36 10.1007/BF02291575 (doi:10.1007/BF02291575) [DOI] [Google Scholar]
- 56.Nalepa C. A. 2011. Altricial development in wood-feeding cockroaches: the key antecedent to termite eusociality. In Biology of termites: a modern synthesis (eds Bignell D. E., Roisin Y., Lo N.), pp. 69–96 Heidelberg, Germany: Springer [Google Scholar]
- 57.Watson J. A. L., Sewell J. J. 1985. Caste development in Mastotermes and Kalotermes: which is primitive? In Caste differentiation in social insects (eds Watson J. A. L., Okot-Kotber B. M., Noirot C.), pp. 27–40 Oxford, UK: Pergamon Press [Google Scholar]
- 58.Kambhampati S., Eggleton P. 2000. Taxonomy and phylogeny of termites. In Termites: evolution, sociality, symbiosis and ecology (eds Abe T., Bignell D. E., Higash M.), pp. 1–23 Dordrecht, The Netherlands: Kluwer Academic Publishers [Google Scholar]
- 59.Nalepa C., Bandi C. 2000. Characterizing the ancestors: paedomorphosis and termite evolution. In Termites: evolution, sociality, symbiosis and ecology (eds Abe T., Bignell D. E., Higashi M.), pp. 53–76 Dordrecht, The Netherlands: Kluwer Academic Press [Google Scholar]
- 60.Wilson E. O. 1971. Insect societies. Cambridge, MA: Harvard University Press [Google Scholar]
- 61.Leniaud L., Darrouzet E., Dedeine F., Ahn K., Huang Z., Bagnères A.-G. 2011. Ontogenic potentialities of the worker caste in two subterranean termites. Evol. Dev. 13, 138–148 10.1111/j.1525-142X.2011.00464.x (doi:10.1111/j.1525-142X.2011.00464.x) [DOI] [PubMed] [Google Scholar]
- 62.Heinsohn R. G. 2004. Parental care, load-lightening, and costs. In Ecology and evolution of cooperative breeding in birds (eds Koenig W., Dickinson J.), pp. 67–80 Cambridge, UK: Cambridge University Press [Google Scholar]
- 63.Russell A. F., Langmore N. E., Cockburn A., Astheimer L. B., Kilner R. M. 2007. Reduced egg investment can conceal helper effects in cooperatively breeding birds. Science 317, 941–944 10.1126/science.1146037 (doi:10.1126/science.1146037) [DOI] [PubMed] [Google Scholar]
- 64.Field J. 2008. The ecology and evolution of helping in hover wasps (Hymenoptera: Stenogastrinae). In Ecology of social evolution (eds Korb J., Heinze J.), pp. 85–108 Heidelberg, Germany: Springer [Google Scholar]
- 65.Costa J. T. 2006. The other social insect societies. Cambridge, MA: Harvard University Press [Google Scholar]