Abstract
Seabird populations of the Southern Ocean have been responding to climate change for the last three decades and demographic models suggest that projected warming will cause dramatic population changes over the next century. Shift in species distribution is likely to be one of the major possible adaptations to changing environmental conditions. Habitat models based on a unique long-term tracking dataset of king penguin (Aptenodytes patagonicus) breeding on the Crozet Islands (southern Indian Ocean) revealed that despite a significant influence of primary productivity and mesoscale activity, sea surface temperature consistently drove penguins' foraging distribution. According to climate models of the Intergovernmental Panel on Climate Change (IPCC), the projected warming of surface waters would lead to a gradual southward shift of the more profitable foraging zones, ranging from 25 km per decade for the B1 IPCC scenario to 40 km per decade for the A1B and A2 scenarios. As a consequence, distances travelled by incubating and brooding birds to reach optimal foraging zones associated with the polar front would double by 2100. Such a shift is far beyond the usual foraging range of king penguins breeding and would negatively affect the Crozet population on the long term, unless penguins develop alternative foraging strategies.
Keywords: habitat model, penguins, polar front, distribution shift, IPCC climate models
1. Introduction
Recent climate changes have already impacted the phenology, physiology and distribution of a wide range of organisms [1]. Projected global climate change is expected to force species distributions towards higher altitudes or latitudes [2,3], leading to range contraction [4] and sometimes to the extinction of species whose optimal breeding or foraging habitats disappear [5]. Predicting the impact of future environmental changes on biodiversity is today a major challenge for ecologists and there is an increasing number of studies that predict future population trends or distributions. These predictions have been made under multiple scenarios of climate change and using different types of modelling approaches such as demographic models [6–8], habitat and bioclimatic models [9–12], or spatial and population dynamics models [13].
Over the last few decades, polar regions have been warming at a faster rate than other parts of the world [14]. Arctic ecosystems have been extensively affected [15] as well as some regions of Antarctica and the surrounding Southern Ocean [16]. Climate simulations from coupled ocean–atmosphere general circulation models (GCMs) predict a sustained and accelerated warming of the Southern Ocean under enhanced greenhouse conditions over the twenty-first century [16,17]. However, there is little information on the possible ecological impact of future climate changes on the Southern Ocean ecosystems.
Numerous studies have pointed out the role of seabirds as convenient indicators of change in marine ecosystems because they are sensitive to physical and biological changes at several spatial and temporal scales [18,19]. Demographic studies have shown that seabird populations of the Southern Ocean have responded to climate change over the past three decades [20,21]. According to demographic models, the projected warming is expected to cause dramatic population changes in marine predators [6,22]. The observed population declines are thought to be mediated by modification of biotic and abiotic oceanographic conditions that drive changes in marine resources available to predators on their foraging grounds. Consequently, it is crucial to predict how future warming will affect species' foraging distributions. To date, few studies have modelled marine predator distributions in relation to environmental conditions to make predictions on their future geographical ranges (polar bear [10]; 14 large pelagic fish species [11]). Both studies suggested a poleward shift and a range contraction of predators over the twenty-first century.
Among Southern Ocean seabirds, king penguins (Aptenodytes patagonicus) are major consumers, with millions of individuals breeding on sub-Antarctic islands [23]. They have been the focus of comprehensive studies with respect to the oceanography of their foraging habitat [24–27], feeding habits [28] and diving behaviour [29,30]. During summer, their foraging strategy is strongly influenced by hydrological features as their diet relies essentially on myctophid fish that are concentrated at the polar front [27,31]. These trophic and habitat specializations, together with life-history traits such as long life expectancy, long generation time and low reproductive output [32], make king penguins potentially more vulnerable to climate change in the long term than a generalist species with high reproductive output capable of rapid adaptive changes [33].
With several million individuals, the Crozet Islands hold the world's largest population of king penguins [34]. Long-term monitoring has indicated that the population increased between the 1960s and the early 1990s, but has been decreasing since 1994 [34]. Variations in breeding population sizes and demographic parameters have been related to both density-dependent processes and environmental variability forced by El Niño Southern Oscillations (ENSO) [34,35]. Changes in resource acquisition are suspected to be responsible for the decline in breeding success and survival of king penguins during or following years of warm climatic conditions [35].
In this study, we used a unique long-term tracking dataset of king penguin breeding on Possession Island (Crozet Islands) to (i) characterize and model the foraging habitat of king penguins during incubation and brooding, and (ii) predict how projected warming of the Southern Ocean would affect their foraging range over the twenty-first century. To do so, we first identified key oceanographic variables driving the foraging distribution of king penguins to model spatial patterns of habitat use and investigate interannual variability in penguins’ accessibility to marine resources. Subsequently, we used sea surface temperature (SST) projections provided by a set of GCMs contributing to the Intergovernmental Panel on Climate Change Fourth Assessment Report (IPCC AR4) to predict king penguins' foraging range over the twenty-first century, under three IPCC scenarios (B1, A1B and A2).
2. Material and methods
(a). Study site and species biology
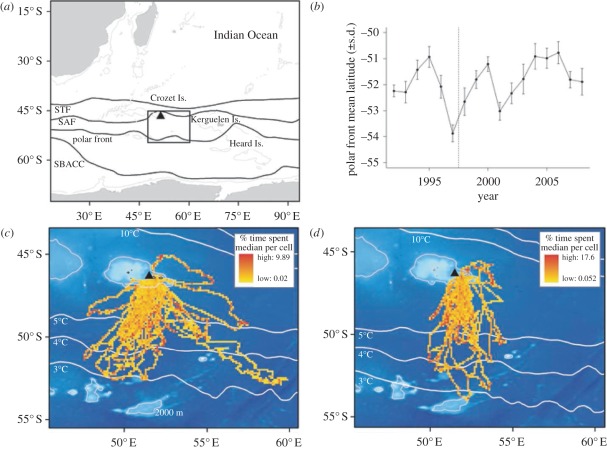
The study was carried out on Possession Island, in the Crozet Islands, southern Indian Ocean (figure 1a). The breeding cycle of the king penguin takes nearly 1 year, with females laying a single egg between November and February, and a chick fledging in November–December of the following year [32]. Energetic costs associated with breeding and moulting force penguins to alternate between foraging trips at sea and fasting periods on shore. During the incubation period, each parent alternately incubates the egg and restores its body reserves by foraging on marine resources. After hatching, the parental energy demand increases greatly since the chick must be provisioned regularly; this results in shorter trip durations [23,38]. The bounding box in figure 1a corresponds to the foraging range of adult king penguins during the summer breeding period. The position of the main frontal structures (figure 1a) varies in latitude depending on season and year [36]. Figure 1b shows the latitudinal variations of the 4°C surface isotherm, indicative of the polar front in summer [37].
Figure 1.
(a) Map of the study area and king penguins' range (47–60° E, 45–54° S). The mean annual position of the fronts (STF: subtropical front, SAF: sub-Antarctic front and SBACC: southern boundary of the Antarctic circumpolar current) are illustrated following Moore et al. [36] and Park et al. [37]. (b) Time series of the mean position of the 4°C isotherm corresponding to the polar front. The mean latitudinal position (±s.d.) was calculated for February within king penguins' longitudinal range (47–60° E). (c,d) Observed habitat use of king penguins tracked from 1998 to 2008 during the incubation and brooding period, respectively. Bathymetry and mean annual position of the isotherms (1998–2008) are displayed in the background. Triangle corresponds to Crozet Is.
(b). Tracking data
Breeding adults were fitted with Argos platform transmitter terminals (PTTs; models Toyocom and Sirtrack Kiwisats) during each austral summer (December to March) from 1992 to 2008 (see electronic supplementary material, table S1). The PTTs were fixed to the lower back feathers with cyanoacrylate glue and secured with cable ties. They weighed 208 g (i.e. approx. 1.8% of bird body weight) and were hydrodynamically streamlined. Birds were equipped just after they had been relieved by their partner. Previous studies showed that PTT equipment may have a deleterious effect on penguin swimming ability and slightly increase trip duration [39,40]. However, meal size was comparable among control and instrumented birds, and no significant differences in breeding failure were observed during the study period. We selected tracks from January–February for the incubation and February–March for the brooding period. The Argos fixes were filtered following Cotté et al. [40].
(c). Habitat modelling
Habitat models were built to explain and then predict spatial distribution of king penguins' foraging effort within their summer range. The proportion of time spent in each cell of a regular grid (0.1° × 0.1°) was used as a proxy of foraging effort. We postulate that a bird actively exploiting a prey patch spends more time in a certain area than when commuting between foraging patches [41]. The suitability of this proxy was supported by studies indicating that high relative residence time was associated with periods when the penguins slow down and dive repeatedly to increase their food intake [30,40]. In the case of king penguins, residency time has been clearly correlated with the mass of food ingested, indicative of foraging success [24]. Time spent was then converted into percentage of time spent in relation to the total trip duration and log-transformed to meet normality. This proxy was matched temporally and spatially to eight environmental variables that were likely to influence penguin foraging behaviour. We included bathymetry (BAT), SST, chlorophyll a concentrations (CHLA), sea surface height deviation (SSHd) and eddy kinetic energy (EKE) [42]. Additionally, spatial gradients of bathymetry, sea surface temperature and chlorophyll a concentration (BATG, SSTG and CHLAG, respectively) were calculated. The source, calculation, resolution and interpretation of oceanographic variables are given in electronic supplementary material, table S2. All these environmental variables were normalized and reinterpolated on a grid of 0.1 × 0.1° to match the habitat-use proxy. Since some oceanographic variables were not available prior to 1998, the tracking dataset analysis was limited to the period 1998–2008.
We used linear mixed-effects models with the log-transformed percentage of time spent in a cell as response variable and non-correlated oceanographic variables (|r2| < 0.7) as explanatory variables. By including spatial autocorrelation structure and random effects (‘Year’ as a random intercept term and the ‘Individual’ identity nested within ‘Year’), we accounted for the hierarchical structure of the tracking data [43,44]. Equations and details on modelling procedure are given in the electronic supplementary material, §S1. Each breeding stage was modelled separately.
Model selection was performed using a maximum-likelihood approach to select the best model by minimizing the Akaike information criterion (AIC). Models with all combinations of variables were ranked according to their respective Akaike weight (wi), which represents the relative likelihood of each candidate model. As there was no obvious evidence of a single best model (wi > 0.90), we applied a model averaging procedure to account for uncertainties in model selection [45,46]. For each breeding stage, a set of models totalling 95 per cent of the Akaike weights was kept and used to calculate model parameters (see [46] for details).
Model validation was performed by checking model residuals and calculating model goodness-of-fit by cross-validation (n = 100). Models were fitted using a 70 per cent subset of the tracking data and then evaluated with the remaining 30 per cent using Spearman rank correlation coefficients. Finally, validated models were generalized to predict habitat-use probability within the spatial extent accessible to king penguins from 1998 to 2008, in January and February for incubation and brooding, respectively.
(d). Future trends in water mass temperature
Since there are many sources of uncertainty about the trajectories of future greenhouse gas emissions and predictions of the different GCMs, we used three different scenarios and adopted an ‘ensemble approach’ to describe the future warming trend within king penguin range. This approach is commonly used to derive a consensus from multiple model outputs [6,11,22]. The average warming trend within the penguins' range was calculated according to the projections of 12 GCMs (hereafter ‘climate models’) contributing to the IPCC AR4 [14]. These models were part of the World Climate Research Programme's (WCRP's) Coupled Model Intercomparison Project phase 3 (CMIP3) multi-model dataset, available on the PCMDI website at http://www-pcmdi.llnl.gov/. For each climate model, we downloaded SST projections forced by three different scenarios (B1, A1B and A2; see electronic supplementary material, §S2 for details). These three scenarios correspond to increasing levels in greenhouse gas emissions over the twenty-first century.
When comparing time series of SST projections to satellite-derived SST from 2000 to 2009 within the king penguins’ range, we noticed that most models over- or under-estimated SST within our study area. However, since we were more interested in the rate of warming than in its baseline level, we applied a correction factor to scale each time series to the same SST baseline (see electronic supplementary material, §S3 for correction factor calculation and table S3). The rate of warming from 2000 to 2100 was then calculated for each model and scenario to derive average trends and CI. The 4°C surface isotherm is considered a good proxy of polar front position in summer [37]. Hence, we applied the same correction method and extracted the mean latitude of this isotherm monthly from 2000 to 2100 to detect potential shifts in polar front position (see electronic supplementary material, §S3 and table S4).
(e). Future predictions of king penguins' foraging range
Since SST was the most important variable predicting the foraging effort of king penguins and the only one available through IPCC climate models at a suitable resolution, we fitted a univariate linear mixed-effects model with SST as single explanatory variable to predict the future distribution of penguins' foraging effort (see electronic supplementary material, §S1). Since SST was available prior to 1998, we used the entire tracking dataset (starting in 1992) to fit this model. An important assumption when predicting foraging range in the future is that there will be no adaptation that will change habitat preferences.
The spatial resolution and reliability of the SST projections varied widely between climate models within the penguins’ range (see electronic supplementary material, figure S2 and table S4). Hence, we selected the most reliable climate model using three objective criteria: finest spatial resolution, smallest latitudinal correction factor and highest correlation with the average shift of the polar front (see electronic supplementary material, table S4). The climate model that best matched these criteria was the one provided by the US National Center for Atmospheric Research (NCAR CCSM3.0; see electronic supplementary material, figure S2 and table S4). SST projections of the NCAR model were interpolated onto a regular grid of 0.5 × 0.5° using the inverse weighting distance method before being included as an input of our habitat model.
Habitat models were run for the two breeding stages (January for incubation and February for brooding) from 2000 to 2100. A threshold of 0.7 was selected to define a contour of preferred foraging areas and calculate a minimal travelled distance between the colony and optimal foraging zones annually over the twenty-first century.
3. Results
(a). Distribution of foraging effort
A total of 42 and 21 king penguins were tracked during incubation and brooding periods, respectively (see electronic supplementary material, table S1). Distribution of foraging effort indicated that king penguins mainly exploit pelagic waters located 300–500 km south of the colony, an area corresponding to the polar front (figure 1c,d). Their longitudinal range was more extended during incubation (48–60° E) than brooding (50–55° E), whereas their maximal latitudinal range was similar during the two breeding stages (see electronic supplementary material, table S1).
(b). Habitat modelling of observed foraging effort
Seven out of the eight non-correlated oceanographic variables were retained in the linear mixed-effect models (BATG, SST, SSTG, CHLA, CHLAG, SSHd and EKE). Habitat model selection resulted in a set of 95% confidence including 7 and 10 models with different combinations of variables for the incubation and brooding periods, respectively (see electronic supplementary material, table S5). Model residuals were normally distributed and the cross-validation procedure indicated that the averaged models provided a good fit to the observed data (r2test = 0.44 and 0.40 for incubation and brooding, respectively). SST was the most important variable predicting the king penguins' foraging zones during both breeding stages (table 1). The negative coefficients of SST and BATG indicated that king penguins foraged in cold oceanic waters typical of the polar front, where hydrography is not influenced by high topographic features.
Table 1.
Coefficient estimates and s.e. for environmental variables included in the averaged models of king penguins habitat use during incubation and brooding.
| incubation January–February | brooding February–March | |
|---|---|---|
| no. foraging tracks | 42 | 21 |
| no. models within 95% CI | 7 | 10 |
| standardized coefficients estimates of the averaged model (± s.e.): | ||
| intercept | −0.650 ± 0.084 | −0.532 ± 0.142 |
| + BATG | −0.008 ± 0.019 | −0.031 ± 0.024 |
| + SST | −0.119 ± 0.028 | −0.080 ± 0.043 |
| + SSTG | 0.035 ± 0.016 | −0.018 ± 0.020 |
| + CHLA | −0.024 ± 0.025 | 0.037 ± 0.031 |
| + CHLAG | −0.031 ± 0.019 | −0.008 ± 0.024 |
| + SSHd | −0.049 ± 0.027 | −0.003 ± 0.033 |
| + EKE | −0.016 ± 0.022 | −0.017 ± 0.025 |
| cross-validation: mean r2 | ||
| 70% train dataset | 0.42 | 0.41 |
| 30% test dataset | 0.45 | 0.41 |
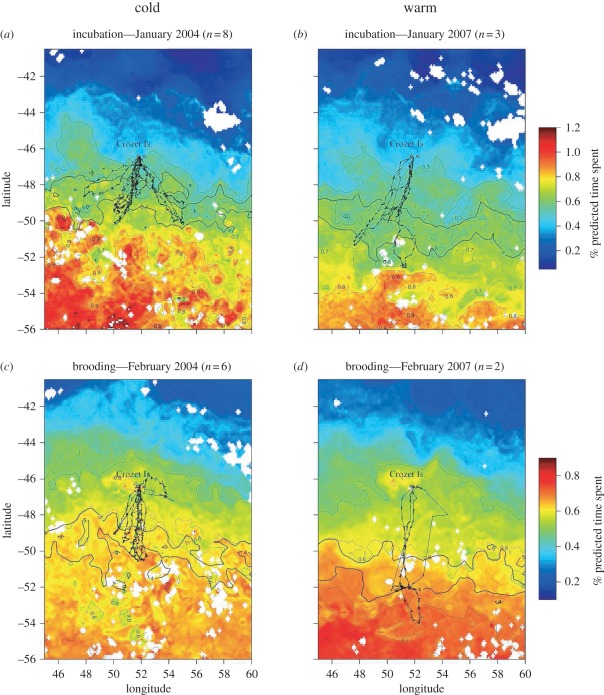
Validated habitat models were then generalized to predict spatial patterns of foraging effort annually from 1998 to 2008. During both stages, the predicted foraging areas were located further south during warmer years, indicating that penguins had to cover larger distances. Figure 2 illustrates the spatial predictions of habitat use for 2 years of contrasting SST conditions, indicating a southward shift of the penguins’ foraging zones during the relatively warmer summer of 2007 compared with the colder summer of 2004 (figure 2). Importantly, predicted foraging areas coincided with the polar front (figure 2).
Figure 2.
Spatial distribution of king penguin habitat use during (a,b) incubation and (c,d) brooding, as predicted by the linear mixed-effects models for 2 years of contrasting oceanographic conditions ((a,c) 2004 and (b,d) 2007). Thick black lines correspond to 4°C and 5°C isotherms, medium black lines with points represent king penguin tracks and thin lines correspond to contours of predicted habitat suitability. Blanks correspond to areas where predictions could not be made because of cloud cover.
(c). Future warming of the surface waters
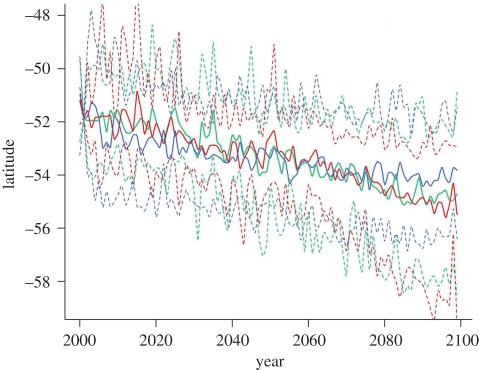
Projected warming trends within the geographical range of king penguins varied depending on climate models and scenarios (see electronic supplementary material, figure S1 and table S3). The ensemble approach revealed an average warming rate of 0.117, 0.173 and 0.184°C per decade for the B1, A1B and A2 scenarios, respectively. This warming would lead to a southward shift of the surface water masses over the twenty-first century. Consequently, projections suggested an average southward shift of the surface signature of the polar front of 24.9 km per decade, 39.9 km per decade and 40 km per decade for the B1, A1B and A2 scenarios, respectively (figure 3 and electronic supplementary material, table S4) in summer.
Figure 3.
Projected changes in the mean latitudinal position of the 4°C isotherm (i.e. polar front) under the scenarios B1, A1B and A2. These future trends and 90% CI were calculated in January within king penguins' range from a multi-model dataset. Blue line, B1; green line, A1B; red line, A2.
(d). Future predictions of king penguins' foraging range
Future predictions of foraging ground distribution were based on the SST-only models. Slope coefficients of the SST parameter were −0.099 and −0.077 for incubation and brooding, respectively. The goodness of fit was slightly lower for the SST-only model than for the complete averaged model, but remained acceptable (r2test = 0.41 and 0.38 for incubation and brooding, respectively).
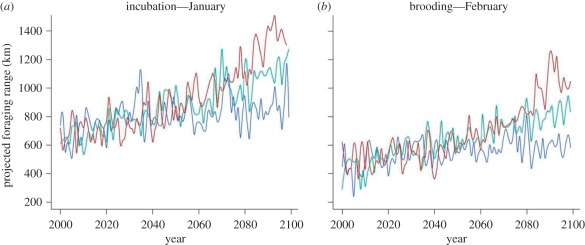
Spatial predictions of the future foraging range of king penguins were made under the three scenarios using the NCAR CCSM3.0 climate model. This climate model tended to over-estimate the rate of warming for the A2 scenario and under-estimate this rate for the B1 scenario compared with the ensemble model (see electronic supplementary material, table S4). As expected, the SST-only model predicted a southward shift of the penguins' foraging range. Under the A1B (intermediate) scenario, the foraging range of king penguins was predicted to double by 2100 for both breeding stages (figure 4a,b). This trend was less pronounced under the B1 scenario (17.8 and 14.9 km per decade) and more pronounced under the A2 scenario (73 and 61 km per decade), compared with the A1B scenario (52.8 and 43.4 km per decade, for incubation and chick rearing, respectively).
Figure 4.
Projected changes in the distance between the colony and the optimal foraging zones of (a) incubating and (b) brooding penguins under the three different scenarios. Spatial patterns of habitat suitability were predicted by the SST-only habitat model and the NCAR CCSM3.0 model. Red lines, A2; green lines, A1B; blue lines, B1.
4. Discussion
Our study suggests that the warming of the Southern Ocean could represent a major challenge for oceanic top-predators such as king penguins. Based on a unique long-term tracking dataset, the habitat models confirmed the consistent role played by SST over years in structuring the foraging habitat of king penguins during incubation and brooding. During the last 17 years, king penguins from Possession Island spent most of their time foraging in cold waters typical of the polar front. Their foraging range fluctuated as a result of the variations in the position of this dynamic frontal structure. Habitat models predicted a southward shift of the favourable foraging grounds during warmer years, such as 2007. By 2100, the predicted warming of the water masses should thus lead to a gradual southward shift of approximately 400 km of the more profitable foraging zones. Such a shift is far beyond the usual summer foraging range of king penguins and would undoubtedly have consequences on the Crozet Islands population.
King penguins targeted cold waters typical of the polar front, where they feed on aggregated stenothermal myctophid fish [27,31,47]. The habitat models captured the southward shift of the polar front as the summer season progressed. This progressive warming results in higher pressure on brooding birds that need to swim further to gather more food over a shorter time period [48].
Despite the variability in SST predictions provided by the IPCC climate models, all the climate models predicted a warming leading to a southward shift of the water masses. Under the A2 scenario, the ensemble model suggested an average warming of 0.18°C per decade within the penguins’ range, a trend comparable to the predicted warming reported in Antarctica by Ainley et al. [22]. A direct consequence of this warming would be a latitudinal shift of approximately 400 km in the position of the polar front by 2100 south of the Crozet Islands. In fact, the polar front is not constrained by bathymetric features south of the Crozet Islands, which allows large variations in its latitudinal range [49]. This predicted southward shift (40 km per decade under the A1B scenario) is consistent with the southward shift of approximately 60 km previously reported in the average positions of the Antarctic circumpolar currents between 1992 and 2007 [49].
Assuming that the statistical relationships of the habitat models fitted in the present study remain unchanged over the next century, king penguins from the Crozet Islands would have to travel, on average, 43.4 km further south per decade to reach profitable foraging zones during the chick rearing period (under the A1B scenario, NCAR climate model). Similarly, predictions by Hobday [11] suggested a future southward shift of about 40 km per decade on average for large pelagic fishes off Australia. Although our predictive habitat model did not include the other environmental variables for future projections of the king penguins’ foraging range, SST was the most important parameter of our habitat models. Since SST is likely to be the best predictor of environmental suitability for the pelagic ectothermic fish preyed upon by penguins, our analysis may be relatively robust.
A demographic study conducted at the Crozet Islands revealed that breeding success decreased during warmer years [35]. This decrease was supposed to be the result of reduced foraging efficiency during warmer years. During historic warm events such as the 1997–1998 El Niño event, king penguins had to extend their travel by 250 km compared with previous years (C. A. Bost 1992–1998, unpublished data). As central place foragers when breeding, the foraging success of king penguins is strongly constrained by the location of food resources. The minimal distance to foraging zones is thus a crucial factor affecting king penguin population dynamics. Previous studies indicated that chick survival was greatly reduced after an absence of the provisioning parent exceeding 20 days during the brooding period [50]. This fast duration corresponds to a maximal foraging range of 722 km before breeding failure occurred (C. A. Bost 1992–1998, unpublished data). Our predictive model suggested that such a distance could frequently occur from the year 2050 onwards, which may cause chronic decreases in the average breeding success and result in the long-term decline of the Crozet Islands population.
This projected decline could only be averted through major changes in foraging strategy or breeding phenology. The foraging strategy of king penguins may have evolved as a result of the thinning of the surface mixed layer and a more accessible thermocline by heading south [51], rather than staying at lower latitudes where the prey are probably deeper. However, they can dive down to 440 m to find myctophids in winter [30], so they may be able to track prey in deeper waters closer to the colonies, instead of following horizontally the southward shift of the polar front. Strong and maintained selection pressure on foraging strategies could indeed act on penguin morphology and/or physiology, and drive evolutionary changes, if these traits are to some extent heritable. However, microevolution is difficult to predict, especially at this rate of change and for species with long generation times [52]. Phenotypic plasticity is the most common adaptive response to climate change [53]. The most likely adaptation without assuming genetic evolution would then be a change in foraging strategy by prospecting in different water masses, diving deeper or shifting diet. Climate-induced shift in diet has already been reported in penguins [33], but seems unlikely for king penguins given (i) the rate of change, (ii) the importance of myctophid fish in their diet (98% by mass) and (iii) the lack of alternative resources in their pelagic food web [31]. Another plastic response of the Crozet Islands population would be a change in breeding phenology. However, the breeding cycle of king penguins is complex [32]; there are many critical periods when penguins need to rely on predictable and abundant resources (incubation, brooding, chick fledging and pre-moulting). Predicting phenological adjustments to resource availability would thus require a better understanding of prey distribution and accessibility throughout the year. Furthermore, we cannot exclude some dispersal by permanent emigration or natal dispersal [32] (see [33] for a review on the response of penguins to climate change).
Many studies suggest that most responses to climate changes occur at the border of current geographical distribution, where penguins operate at the limit of their environmental tolerance [33]. Although the northernmost breeding localities such as the Crozet Islands (46° S) could see their populations shrinking over the next century, this might not be the case in other locations. For example, the southward shift of the polar front may reduce the foraging range of king penguins breeding on colonies located south of the polar front, such as south Georgia, located at 54° S [26]. Kerguelen and Heard islands, located in Kerguelen plateau (49° S and 53° S, respectively), hold about 350 000 breeding pairs [23]. In contrast to Crozet Islands, bathymetric features of Kerguelen plateau constrain polar front position and reduce interannual variability in Antarctic circumpolar currents [49,54]. Such features may help these populations overcome future environmental changes.
In addition to latitudinal shifts of surface water masses, future warming and positive Southern Annular Mode anomalies may lead to a deepening of the mixed layer depth, with negative consequences on the overall level of biological productivity [55]. Changes in the vertical structure of the water masses following positive ENSO events have already been reported in the east equatorial Pacific [13]. Such changes could lead to a deeper vertical distribution of prey and force penguins to dive deeper. Another critical point is whether ectothermic prey with a strict thermal optimum, such as myctophid fish [56], will be able to track the shift of the water masses to maintain their thermal optimum. In other parts of the world, several studies indicated that fish populations are responding to climate change by shifting their geographical distribution [4,57,58]. Unfortunately, myctophid fish are poorly studied in the sub-Antarctic waters and their response to future warming is unknown.
Future studies should favour community and ecosystem approaches, including both physical and biotic interactions across trophic levels [59,60], as very little is known about large-scale oceanographic mechanisms driving long-term changes in primary [61] and secondary productivity in sub-Antarctic regions.
Acknowledgements
This study was carried out at Crozet Is. and funded by the French Polar Institute (IPEV) with programmes 394 (C.A. Bost) and 109 (H. Weimerskirch), and two ANR programmes: ‘REMIGE’ and 07 BIODIV ‘GLIDES’. It was approved by the Ethics Committee of the French Polar Institute. We are very grateful to all the fieldworkers involved in the long-term satellite tracking programme since 1992, and to Y. Cherel, C. Barbraud and M. Authier for their constructive comments. We address special thanks to Y. le Maho (progr IPEV 137) for his support at the beginning of the tracking study. We acknowledge the modelling groups, the Programme for Climate Model Diagnosis and Intercomparison (PCMDI) and the World Climate Research Programme's (WCRP's) Working Group on Coupled Modeling (WGCM) for their roles in making available the WCRP CMIP-3 multi-model dataset. We thank Kylie Pitt for reviewing the English and two anonymous referees for helpful comments on the manuscript.
References
- 1.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Ann. Rev. Ecol. Evol. Syst. 37, 637–669 10.1146/annurev.ecolsys.37.091305.110100 (doi:10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 2.Li J., Hilbert D. W., Parker T., Williams S. 2009. How do species respond to climate change along an elevation gradient? A case study of the grey-headed robin (Heteromyias albispecularis). Global Change Biol. 15, 255–267 10.1111/j.1365-2486.2008.01737.x (doi:10.1111/j.1365-2486.2008.01737.x) [DOI] [Google Scholar]
- 3.Chen I.-C., Hill J. K., Ohlemüller R., Roy D. B., Thomas C. D. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026 10.1126/science.1206432 (doi:10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
- 4.Worm B., Tittensor D. P. 2011. Range contraction in large pelagic predators. Proc. Natl Acad. Sci. USA 108, 11 942–11 947 10.1073/pnas.1102353108 (doi:10.1073/pnas.1102353108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas C. D., et al. 2004. Extinction risk from climate change. Nature 427, 145–148 10.1038/nature02121 (doi:10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- 6.Jenouvrier S., Caswell H., Barbraud C., Holland M., Strove J., Weimerskirch H. 2009. Demographic models and IPCC climate projections predict the decline of an emperor penguin population. Proc. Natl Acad. Sci. USA 106, 11 425. 10.1073/pnas.0806638106 (doi:10.1073/pnas.0806638106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf S. G., Snyder M. A., Sydeman W. J., Doak D. F., Croll D. A. 2010. Predicting population consequences of ocean climate change for an ecosystem sentinel, the seabird Cassin's auklet. Global Change Biol. 16, 1923–1935 10.1111/j.1365-2486.2010.02194.x (doi:10.1111/j.1365-2486.2010.02194.x) [DOI] [Google Scholar]
- 8.Barbraud C., Rivalan P., Inchausti P., Nevoux M., Rolland V., Weimerskirch H. 2010. Contrasted demographic responses facing future climate change in Southern Ocean seabirds. J. Anim. Ecol. 80, 89–100 10.1111/j.1365-2656.2010.01752.x (doi:10.1111/j.1365-2656.2010.01752.x) [DOI] [PubMed] [Google Scholar]
- 9.Thuiller W. 2003. BIOMOD—optimizing predictions of species distributions and projecting potential future shifts under global change. Global Change Biol. 9, 1353–1362 10.1046/j.1365-2486.2003.00666.x (doi:10.1046/j.1365-2486.2003.00666.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durner G. M., et al. 2009. Predicting 21st-century polar bear habitat distribution from global climate models. Ecol. Monogr. 79, 25–58 10.1890/07-2089.1 (doi:10.1890/07-2089.1) [DOI] [Google Scholar]
- 11.Hobday A. J. 2010. Ensemble analysis of the future distribution of large pelagic fishes off Australia. Progr. Oceanogr. 86, 291–301 10.1016/j.pocean.2010.04.023 (doi:10.1016/j.pocean.2010.04.023) [DOI] [Google Scholar]
- 12.Witt M. J., Hawkes L. A., Godfrey M. H., Godley B. J., Broderick A. C. 2010. Predicting the impacts of climate change on a globally distributed species: the case of the loggerhead turtle. J. Exp. Biol. 213, 901–911 10.1242/jeb.038133 (doi:10.1242/jeb.038133) [DOI] [PubMed] [Google Scholar]
- 13.Lehodey P., Senina I., Sibert J., Bopp L., Calmettes B., Hampton J., Murtugudde R. 2010. Preliminary forecasts of Pacific bigeye tuna population trends under the A2 IPCC scenario. Progr. Oceanogr. 86, 302–315 10.1016/j.pocean.2010.04.021 (doi:10.1016/j.pocean.2010.04.021) [DOI] [Google Scholar]
- 14.Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., Miller H. L. 2007. Climate Change 2007: the Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press [Google Scholar]
- 15.Greene C. H., Pershing A. J., Cronin T. M., Ceci N. 2008. Arctic climate change and its impacts on the ecology of the north Atlantic. Ecology 89, S24–S38 10.1890/07-0550.1 (doi:10.1890/07-0550.1) [DOI] [PubMed] [Google Scholar]
- 16.Mayewski P. A., et al. 2009. State of the Antarctic and Southern Ocean system. Rev. Geophys. 47, RG1003. 10.1029/2007RG000231 (doi:10.1029/2007RG000231) [DOI] [Google Scholar]
- 17.Liu J. P., Curry J. A. 2010. Accelerated warming of the Southern Ocean and its impacts on the hydrological cycle and sea ice. Proc. Natl Acad. Sci. USA 107, 14 987–14 992 10.1073/pnas.1003336107 (doi:10.1073/pnas.1003336107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piatt J. F., Sydeman W. J., Wiese F. 2007. Introduction: a modern role for seabirds as indicators. Mar. Ecol. Progr. Ser. 352, 199–204 10.3354/meps07070 (doi:10.3354/meps07070) [DOI] [Google Scholar]
- 19.Durant J. M., Hjermann D. O., Frederiksen M., Charrassin J. B., Le Maho Y., Sabarros P. S., Crawford R. J. M., Stenseth N. C. 2009. Pros and cons of using seabirds as ecological indicators. Clim. Res. 39, 115–129 10.3354/cr00798 (doi:10.3354/cr00798) [DOI] [Google Scholar]
- 20.Croxall J. P., Trathan P. N., Murphy E. J. 2002. Environmental change and Antarctic seabird populations. Science 297, 1510–1514 10.1126/science.1071987 (doi:10.1126/science.1071987) [DOI] [PubMed] [Google Scholar]
- 21.Jenouvrier S., Barbraud C., Weimerskirch H. 2005. Long-term contrasted responses to climate of two Antarctic seabird species. Ecology 86, 2889–2903 10.1890/05-0514 (doi:10.1890/05-0514) [DOI] [Google Scholar]
- 22.Ainley D., Russell J., Jenouvrier S., Woehler E., Lyver P. O., Fraser W. R., Kooyman G. L. 2010. Antarctic penguin response to habitat change as Earth's troposphere reaches 2 degrees C above preindustrial levels. Ecol. Monogr. 80, 49–66 10.1890/08-2289.1 (doi:10.1890/08-2289.1) [DOI] [Google Scholar]
- 23.Bost C. A., Delord K., Barbraud C., Cotté C., Péron C., Weimerskirch H. In press The King Penguin: life history, current status and priority conservation action. [Google Scholar]
- 24.Bost C. A., et al. 1997. Foraging habitat and food intake of satellite-tracked king penguins during the austral summer at Crozet Archipelago. Mar. Ecol. Prog. Ser. 150, 21–33 10.3354/meps150021 (doi:10.3354/meps150021) [DOI] [Google Scholar]
- 25.Sokolov S., Rintoul S. R., Wienecke B. 2006. Tracking the Polar Front south of New Zealand using penguin dive data. Deep Sea Res. I 53, 591–607 10.1016/j.dsr.2005.12.012 (doi:10.1016/j.dsr.2005.12.012) [DOI] [Google Scholar]
- 26.Trathan P. N., Bishop C., Maclean G., Brown P., Fleming A., Collins M. A. 2008. Linear tracks and restricted temperature ranges characterise penguin foraging pathways. Mar. Ecol. Progr. Ser. 370, 285–294 10.3354/meps07638 (doi:10.3354/meps07638) [DOI] [Google Scholar]
- 27.Bost C. A., Cotté C., Bailleul F., Cherel Y., Charrassin J. B., Guinet C., Ainley D. G., Weimerskirch H. 2009. The importance of oceanographic fronts to marine birds and mammals of the southern oceans. J. Mar. Syst. 78, 363–376 10.1016/j.jmarsys.2008.11.022 (doi:10.1016/j.jmarsys.2008.11.022) [DOI] [Google Scholar]
- 28.Cherel Y., Verdon C., Ridoux V. 1993. Seasonal importance of oceanic Myctophids in King Penguin diet at Crozet islands. Polar Biol. 13, 355–357 [Google Scholar]
- 29.Kooyman G. L., Cherel Y., Le Maho Y., Croxall J. P., Thorson P. H., Ridoux V. 1992. Diving behavior and energetics during foraging cycles in King Penguins. Ecol. Monogr. 62, 143–163 10.2307/2937173 (doi:10.2307/2937173) [DOI] [Google Scholar]
- 30.Charrassin J. B., Le Maho Y., Bost C. A. 2002. Seasonal changes in the diving parameters of king penguins (Aptenodytes patagonicus). Mar. Biol. 141, 581–589 10.1007/s00227-002-0843-4 (doi:10.1007/s00227-002-0843-4) [DOI] [Google Scholar]
- 31.Pakhomov E. A., Perissinotto R., McQuaid C. D. 1994. Comparative structure of the macrozooplankton micronekton communities of the subtropical and Antarctic polar fronts. Mar. Ecol. Progr. Ser. 111, 155–169 10.3354/meps111155 (doi:10.3354/meps111155) [DOI] [Google Scholar]
- 32.Weimerskirch H., Stahl J. C., Jouventin P. 1992. The breeding biology and population dynamics of King Penguins Aptenodytes patagonica on the Crozet Islands. Ibis 134, 107–117 10.1111/j.1474-919X.1992.tb08387.x (doi:10.1111/j.1474-919X.1992.tb08387.x) [DOI] [Google Scholar]
- 33.Forcada J., Trathan P. N. 2009. Penguin responses to climate change in the Southern Ocean. Global Change Biol. 15, 1618–1630 10.1111/j.1365-2486.2009.01909.x (doi:10.1111/j.1365-2486.2009.01909.x) [DOI] [Google Scholar]
- 34.Delord K., Barbraud C., Weimerskirch H. 2004. Long-term trends in the population size of king penguins at Crozet archipelago: environmental variability and density dependence? Polar Biol. 27, 793–800 10.1007/s00300-004-0651-z (doi:10.1007/s00300-004-0651-z) [DOI] [Google Scholar]
- 35.Le Bohec C., Durant J. M., Gauthier-Clerc M., Stenseth N. C., Park Y. H., Pradel R., Grémillet D., Gendner J. P., Le Maho Y. 2008. King penguin population threatened by Southern Ocean warming. Proc. Natl Acad. Sci. USA 105, 2493–2497 10.1073/pnas.0712031105 (doi:10.1073/pnas.0712031105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore J. K., Abbott M. R., Richman J. G. 1999. Location and dynamics of the Antarctic Polar Front from satellite sea surface temperature data. J. Geophys. Res. 104, 3059–3073 [Google Scholar]
- 37.Park Y. H., Gambéroni L., Charriaud E. 1993. Frontal structures, water masses, and circulation in the Crozet Basin. J. Geophys. Res. Oceans 98, 12 361–12 385 [Google Scholar]
- 38.Guinet C., Koudil M., Bost C. A., Durbec J. P., Georges J. Y., Mouchot M. C., Jouventin P. 1997. Foraging behaviour of satellite-tracked king penguins in relation to sea-surface temperatures obtained by satellite telemetry at Crozet Archipelago: a study during three austral summers. Mar. Ecol. Progr. Ser. 150, 11–20 10.3354/meps150011 (doi:10.3354/meps150011) [DOI] [Google Scholar]
- 39.Wilson R. P., Kreye J. A., Lucke K., Urquhart H. 2004. Antennae on transmitters on penguins: balancing energy budgets on the high wire. J. Exp. Biol. 207, 2649–2662 10.1242/jeb.01067 (doi:10.1242/jeb.01067) [DOI] [PubMed] [Google Scholar]
- 40.Cotté C., Park Y. H., Guinet C., Bost C. A. 2007. Movements of foraging king penguins through marine mesoscale eddies. Proc. R. Soc. B 274, 2385–2391 10.1098/rspb.2007.0775 (doi:10.1098/rspb.2007.0775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kareiva P., Odell G. 1987. Swarms of predators exhibit ‘preytaxis’ if individual predators use area-restricted search. Am. Nat. 130, 233–270 10.1086/284707 (doi:10.1086/284707) [DOI] [Google Scholar]
- 42.Zainuddin M., Kiyofuji H., Saitoh K., Saitoh S. I. 2006. Using multi-sensor satellite remote sensing and catch data to detect ocean hot spots for albacore (Thunnus alalunga) in the northwestern North Pacific. Deep Sea Res. II 53, 419–431 10.1016/j.dsr2.2006.01.007 (doi:10.1016/j.dsr2.2006.01.007) [DOI] [Google Scholar]
- 43.Dormann F., et al. 2007. Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30, 609–628 10.1111/j.2007.0906-7590.05171.x (doi:10.1111/j.2007.0906-7590.05171.x) [DOI] [Google Scholar]
- 44.Bolker B. M., Brooks M. E., Clark C. J., Geange S. W., Poulsen J. R., Stevens M. H., White J. S. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135 10.1016/j.tree.2008.10.008 (doi:10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 45.Burnham K. P., Anderson D. R. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer Verlag [Google Scholar]
- 46.Louzao M., Bécares J., Rodríguez B., Hyrenbach K. D., Ruiz A., Arcos J. M. 2009. Combining vessel-based surveys and tracking data to identify key marine areas for seabirds. Mar. Ecol. Prog. Ser. 391, 183–197 10.3354/meps08124 (doi:10.3354/meps08124) [DOI] [Google Scholar]
- 47.Duhamel G., Koubbi P., Ravier C. 2000. Day and night mesopelagic fish assemblages off the Kerguelen Islands (Southern Ocean). Polar Biol. 23, 106–112 10.1007/s003000050015 (doi:10.1007/s003000050015) [DOI] [Google Scholar]
- 48.Charrassin J. B., Bost C. A., Putz K., Lage L., Dahier T., Zorn T., Le Maho Y. 1998. Foraging strategies of incubating and brooding king penguins Aptenodytes patagonicus. Oecologia 114, 194–201 10.1007/s004420050436 (doi:10.1007/s004420050436) [DOI] [PubMed] [Google Scholar]
- 49.Sokolov S., Rintoul S. R. 2009. Circumpolar structure and distribution of the Antarctic Circumpolar Current fronts: 2. Variability and relationship to sea surface height. J. Geophys. Res. 114, C11019. 10.1029/2008JC005248 (doi:10.1029/2008JC005248) [DOI] [Google Scholar]
- 50.Gauthier-Clerc M., Le Maho Y., Gendner J. P., Handrich Y. 2002. Moulting fast and time constraint for reproduction in the king penguin. Polar Biol. 25, 288–295 [Google Scholar]
- 51.Charrassin J. B., Bost C. A. 2001. Utilisation of the oceanic habitat by king penguins over the annual cycle. Mar. Ecol. Prog. Ser. 221, 285–297 10.3354/meps221285 (doi:10.3354/meps221285) [DOI] [Google Scholar]
- 52.Gienapp P., Teplitsky C., Alho J. S., Mills J. A., Merila J. 2008. Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167–178 10.1111/j.1365-294X.2007.03413.x (doi:10.1111/j.1365-294X.2007.03413.x) [DOI] [PubMed] [Google Scholar]
- 53.Davis M. B., Shaw R. G., Etterson J. R. 2005. Evolutionary responses to changing climate. Ecology 86, 1704–1714 10.1890/03-0788 (doi:10.1890/03-0788) [DOI] [Google Scholar]
- 54.Park Y. H., Roquet F., Durand I., Fuda J. L. 2008. Large-scale circulation over and around the Northern Kerguelen Plateau. Deep Sea Res. II 55, 566–581 10.1016/j.dsr2.2007.12.030 (doi:10.1016/j.dsr2.2007.12.030) [DOI] [Google Scholar]
- 55.Sallée J. B., Speer K. G., Rintoul S. R. 2010. Zonally asymmetric response of the Southern Ocean mixed-layer depth to the Southern annular mode. Nat. Geosci. 3, 273–279 10.1038/NGEO812 (doi:10.1038/NGEO812) [DOI] [Google Scholar]
- 56.Hulley P. A. 1990. Fish of the southern ocean, pp. 146–178 Grahamstown, South Africa: Institute of Ichtyologie [Google Scholar]
- 57.Cheung W. W. L., Lam V. W. Y., Sarmiento J. L., Kearney K., Watson R., Zeller D., Pauly D. 2010. Large-scale redistribution of maximum fisheries catch potential in the global ocean under climate change. Global Change Biol. 16, 24–35 10.1111/j.1365-2486.2009.01995.x (doi:10.1111/j.1365-2486.2009.01995.x) [DOI] [Google Scholar]
- 58.Sumaila U. R., Cheung W. W. L., Lam V. W. Y., Pauly D., Herrick S. 2011. Climate change impacts on the biophysics and economics of world fisheries. Nat. Clim. Change 1, 449–456 10.1038/NCLIMATE1301 (doi:10.1038/NCLIMATE1301) [DOI] [Google Scholar]
- 59.Walther G. R. 2010. Community and ecosystem responses to recent climate change. Phil. Trans. R. Soc. B 365, 2019–2024 10.1098/rstb.2010.0021 (doi:10.1098/rstb.2010.0021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van der Putten W. H., Macel M., Visser M. E. 2010. Predicting species distribution and abundance responses to climate change: why it is essential to include biotic interactions across trophic levels. Phil. Trans. R. Soc. B 365, 2025–2034 10.1098/rstb.2010.0037 (doi:10.1098/rstb.2010.0037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boyce D. G., Lewis M. R., Worm B. 2010. Global phytoplankton decline over the past century. Nature 466, 591–596 10.1038/nature09268 (doi:10.1038/nature09268) [DOI] [PubMed] [Google Scholar]