Abstract
Maternal effects are widespread in ecology and can alter the dynamics of a population. We investigated the impact of maternal foraging strategies on offspring weaning mass—a proxy of maternal foraging success and of offspring survival—in southern elephant seals on îles Kerguelen. Using 4 years of data, we modelled pup weaning mass as a two-component mixture and used blood stable isotope values to discriminate between maternal foraging strategies previously identified from bio-logging studies. Carbon isotope ratio was a strong predictor of weaning mass, but the relationship was non-monotonic in contrast to a priori expectations. Females foraging in the interfrontal zone weaned pups with a smaller mass compared with females foraging in Antarctic waters. Pup mass was positively correlated with a proxy of global primary production in the interfrontal zone for small weanlings. Maternal effects, via a poor foraging efficiency in the 1970s, may help explain the large population decrease observed at that time on îles Kerguelen because of an overall decrease in pup weaning mass, survival and subsequent recruitment.
Keywords: maternal effects, Mirounga leonina, stable isotopes, mixture modelling
1. Introduction
Maternal effects can be broadly defined as the influence a mother's phenotype exerts on her offsprings' phenotype [1]. Acknowledging the occurrence of maternal effects sheds a new light on some classic ecological phenomena such as population cycles [2] or life-history trade-offs [3]. In mammalian species, females alone usually support their offspring. Yet, this biological burden may prove to be an evolutionary blessing in disguise as females, via maternal effects, have the potential to shape population dynamics or evolutionary responses [4]. Investigations of the influence of mother characteristics such as mass, body length or age on offspring phenotype are numerous, but the impacts of complex maternal phenotypes such as foraging behaviour are less elucidated [5].
Pinnipeds are marine carnivores that are well suited for studying maternal effects: females usually give birth to a single pup and provide all care until weaning. Lactation is especially important in phocid species in which weaning is usually abrupt and occurs after a short nursing period. Among pinnipeds, most phocids exhibit a capital-breeding strategy, whereby females accumulate energy stores prior to hauling-out and parturition; then fast while nursing their pup [6]. Hence, maternal foraging behaviour may have great leverage on both female and pup fitness. We investigated the role of maternal foraging strategies in the southern elephant seal (Mirounga leonina).
The southern elephant seal is the largest extant phocid species. Females only haul-out on land to breed in the early austral spring and to moult in the late summer. The bio-logging revolution has provided invaluable insights into the foraging ecology of these exceptional divers [7,8]. The main drawback of bio-logging studies is its costs: small sample size may weaken inferences [9]. Besides, animal movement analyses usually rely on the area-restricted search assumption that decreased speed and increased track sinuosity betray foraging [10,11]. This condition may be sufficient to infer foraging, while not being strictly necessary [12].
Stable isotopes are popular tools to trace the flow of molecules across food webs [13]. Commonly used elements are carbon (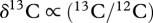 ) and nitrogen (
) and nitrogen (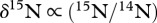 ), which can provide information about the diet's geographical origin and trophic position of a consumer, respectively [14].
), which can provide information about the diet's geographical origin and trophic position of a consumer, respectively [14].
Marine organisms can travel great distances between their marine foraging and terrestrial breeding grounds [15]. In the case of southern elephant seal breeding on îles Kerguelen, Bailleul et al. [8] identified two main foraging zones for females prior to hauling-out: the interfrontal zone (pelagic sub-Antarctic waters bounded by the sub-tropical front and the southern boundary of the Antarctic circumpolar current; electronic supplementary material, figure S1) and the Antarctic zone (area south of the southern boundary of the Antarctic circumpolar current; electronic supplementary material, figure S1). Based on tracking studies (n = 44 post-moult trips of females), the estimated proportion of females committed to each strategy is 73 and 22 per cent for the interfrontal zone and Antarctica, respectively (the remaining 5% accounts for individuals who remained on the Kerguelen plateau, C. Guinet 2010, unpublished data). Given the latitudinal gradient in δ13C values of particulate organic matter within the Southern Ocean [16], stable isotopes analysis can inform where southern elephant seals have been foraging (electronic supplementary material, figure S2). Ducatez et al. [17] showed how blood δ13C value of pups, which are easy to handle and to weigh, accurately reflected that of their mother. Inferring the foraging history of breeding females prior to haul-out is thus possible using stable isotope analysis of their pup's blood. δ13C value hints at the latitude, where an animal fed, but gives no information on the longitude [18]. However, stable isotopes can circumvent small sample size issues that plague bio-logging studies.
Our aims were twofold: we investigated (i) the representativeness of results from bio-logging studies and (ii) assessed the influence of maternal foraging strategies on their pup's fitness. We augment the work of Ducatez et al. [17] using more data and an explicit mixture modelling approach. Specifically, we modelled weaned pups as a mixture of two groups depending on their isotope ratio, then compared their respective proportions with those of maternal foraging behaviour estimated from tracking data.
McMahon et al. [19] found a positive relationship between a pup post-weaning survival and its weaning mass: females that have stored and transferred more energy to their pup prior to the spring haul-out had a larger reproductive fitness. Because any changes in patterns of maternal foraging strategies may affect pup survival, we assessed the effect of maternal foraging strategies on pup fitness by studying the relationships between pup weaning mass and blood isotope ratio. Using carbon stable isotope values in particular, we inferred maternal foraging grounds before hauling-out, and evaluated how it affected pup weaning mass.
2. Material and methods
(a). Materials
Field work was carried out during the austral spring (September–November) of 2006–2009 on the Courbet Peninsula, îles Kerguelen (49°30′ S, 69°30′ E). Except in 2007 due to logistics, a cohort of approximately 200 pups was monitored daily from birth to weaning (table 1). Each pup was individually marked upon birth on the trailing edge of one hind-flipper with a numbered plastic tag (Dalton Rototag, Nettlebed, UK), which was removed upon weaning. When spotted outside a harem, any marked pup was considered weaned, immediately captured, weighed and blood-sampled. Blood was collected from the dorsal venous sinus using 90 × 1.2 mm needles. Ethanol (70%) was added for sample preservation before laboratory analysis. Body weight was measured with a weighing scale with a precision of 0.1 kg in 2006–2008, but of 2 kg in 2009. This measurement error was taken into account in subsequent analyses.
Table 1.
Summary statistics of weaning mass (kg) of pups from îles Kerguelen. Bowley's skewness coefficient, which varies between −1 and 1 is reported [20]. The kurtosis estimator is computed according to An & Ahmed [21] (their 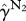 ), with the value 0 corresponding to the kurtosis of normal distribution. Both the skewness and kurtosis coefficients are dimensionless.
), with the value 0 corresponding to the kurtosis of normal distribution. Both the skewness and kurtosis coefficients are dimensionless.
| year | n | mean | median | s.d. | skewness | kurtosis |
|---|---|---|---|---|---|---|
| 2006 | 193 | 105 | 104 | 19 | 0.03 | −0.29 |
| 2007 | 57 | 106 | 106 | 19 | 0.08 | 0.27 |
| 2008 | 202 | 110 | 114 | 23 | −0.25 | 0.02 |
| 2009 | 234 | 111 | 112 | 23 | −0.14 | −0.18 |
| 2006–2009 | 686 | 109 | 110 | 22 | −0.11 | −0.13 |
Before stable isotope analysis, whole blood was oven-dried for 48 h at 50–60°C. Samples were weighted (range: 3–5 mg) into tin cups prior to combustion in an elemental analyser (Euro Vector EA 3024) coupled to a continuous flow mass spectrometer (Micromass Isoprime). Carbon to nitrogen (C/N) ratios were checked, and when above 3.7, lipids were extracted using cyclohexane. Lipids are depleted in 13C relative to proteins and carbohydrates [22], but lipid extraction is usually unnecessary because of the typically small lipid content of blood. Out of 686 samples, 67 were lipid-extracted and their nitrogen value was checked for consistency. Excluding those samples did not change any results. Stable isotope ratios are presented in the usual δ notation (in) relative to Vienna Pee Dee Belemnite and atmospheric N2 for δ13C and δ15N, respectively. Replicate measurements of laboratory standards (acetanilide, δ13C =−27.5‰ and δ15N = 10.3‰, two of every 23 samples) indicated precisions of 0.15‰ (carbon) and 0.20‰ (nitrogen). Measurement errors were accounted for in statistical analyses.
(b). Model building
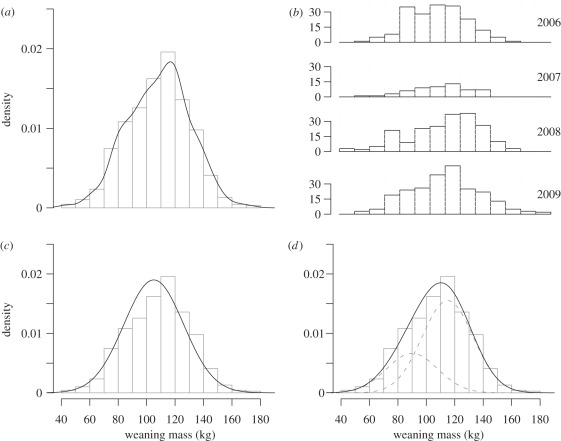
Using nitrogen stable isotope values, Bailleul et al. [8] used a mixture modelling approach to distinguish between large and small adults as assessed from their standard body length (snout-to-tail length, STL), and found a bimodal distribution of carbon stable isotope values in female adults and juvenile males. Kurtosis of the distribution of pup weaning mass tended to be negative, which was suggestive of bimodality (figure 1a,b and table 1; [23,24]). A bimodal distribution could reflect the two identified foraging strategies of females. We built a generalized linear mixed model (GLMM) to assess the relationship between pup weaning mass and stable isotope values:
| 2.1 |
where ɛ, the residuals, are assumed to follow a normal distribution of mean 0 and variance σresidual2.
Figure 1.
Distribution of the (a,b) raw data and (c,d) fitted models. The upper panels show the whole dataset (on a density scale, a) and the data broken down by year (on a class-size scale, b). On the lower panel, fitted values from two different models, (c) a GLMM and a (d) HMM (dotted curves represent each component of the mixture) are represented. The mode and the overall shape of the data are better described by a two-component mixture model.
Squared values of blood isotope values allowed for a nonlinear functional response, whose appropriateness was preliminarily checked with splines. For example, a convex function of δ13C value may be interpreted as higher or lower latitude waters being a highly profitable foraging zone for females as they would transfer more energy to and wean a larger pup than females foraging at the interfrontal zone. Both δ13C and δ15N values were included since their correlation was modest (ρ = 0.32, 95% CI [0.25, 0.39]). Weaning date was included in all models as (i) it was not largely correlated to any other covariates and (ii) it is a loose proxy for maternal age: older (and larger) females haul-out later than young (and smaller) ones [25]. Pup STL was not included as weaned pups are still growing: STL is an intermediate outcome and should not be controlled [26]. Weaning STL and mass were largely correlated (ρ = 0.63, 95% CI [0.58, 0.67]) with the distribution of STL also suggestive of bimodality (not shown).
We further hypothesized weaned pups to form a heterogeneous aggregate of small and large individuals. We modelled weaning mass (figure 1a,b) as a mixture of two normal distributions:
| 2.2 |
where 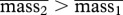 and p(1−p) denotes the proportion of large (small) weanlings.
and p(1−p) denotes the proportion of large (small) weanlings.
Equation (2.2) defines a hierarchical mixture model (HMM). Group membership (that is being a small or a large weanling) can be conceptualized as missing data to be estimated from stable isotope values:
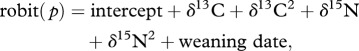 |
2.3 |
where the robit is a robust link function [27]. Other link functions (probit and logit) were initially considered but led to convergence problems.
(c). Environmental covariates
Considering how primary production is positively linked to the biological richness available to foraging predators [28,29], we investigated the effect of this environmental covariate. We suspected a priori the years with high primary production during active pregnancy (after blastocyst implantation) to be more profitable for females.
Chlorophyll a concentration maps were computed from SeaWiFs sea colour images with a ground resolution of 4×4 km (http://oceancolor.gsfc.nasa.gov/). Owing to cloud cover causing a large percentage of missing pixels, we used monthly data in the interfrontal zone (40° : 60° S to 0° : 125° E) and calculated chlorophyll a anomalies about the monthly mean for each pixel. A proxy of the total surface chlorophyll a production was then calculated every year from cumulated anomalies between October and May, the bulk period of main chlorophyll a production in sub-Antarctic waters [30].
We modelled a year effect j specific to each group iε1,2:
| 2.4 |
where η
j,i , the residuals for group i, are assumed to follow a normal distribution of mean 0 and variance 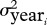 .
.
Dragon et al. [31] evidenced a positive correlation between sea ice extent and chlorophyll a concentration in Antarctic waters. Sea ice extent is assessed from microwave energy (whose measurement is unaffected by the majority of clouds) radiated from the Earth's surface. Sea ice extent is more readily available than chlorophyll a concentration in Antarctic waters. We investigated the influence of the former (extracted over the zone >60° S to 0° and 125° E; [31]) on weaning mass for pups whose blood δ13 C value was suggestive of an Antarctic signature (small δ13C values). We assessed the relevance of the chlorophyll a concentration and sea ice extent with a year-level R2-statistic [32].
(d). Model checking
We assessed model fit with posterior predictive checks [33], wherein each fitted model is used to predict (hypothetical) repetitions of the dataset. We then compared an observed summary statistics (Tobs) with its predicted values (Trep) and computed a pvalue:
| 2.5 |
A pvalue close to 0.5 flags a good fit (Trep ≈ Tobs), while an extreme pvalue (0 or 1) betrays a major model misfit. We chose three test-statistics (T) to assess which data regularities were captured by the model: minimum, maximum and kurtosis of the weaning mass distribution.
Model implementation with annotated BUGS code is detailed in the electronic supplementary material.
3. Results
(a). Stable isotopes
Carbon isotope ratios for pups ranged from −23.9 to −18.8‰ (mean = −21.1‰), while those of nitrogen ranged from 10.1 to 12.8‰ (mean = 11.4‰). Using regression coefficients estimated by Ducatez et al. [17], the mean predicted isotope ratios for females were −21.4 ± 0.1‰ and 10.1 ± 0.1‰ for carbon and nitrogen, respectively. Results of stable isotopes analyses are summarized in table 2 and in electronic supplementary material, figure S3. Variations in both carbon and nitrogen isotope values were small: the absolute coefficient of variation was less than 5 per cent for both isotopes. Across years, the distribution of δ15N values was very stable (except for 2007 owing to small sample size; electronic supplementary material, figure S3). Distributions of δ13C values were comparatively more variable (larger yearly deviations from the overall median; electronic supplementary material, figure S3) but deviations from the overall mean were small (<0.5‰).
Table 2.
Summary statistics for carbon and nitrogen isotopic composition of southern elephant seal pup blood on îles Kerguelen. The mean, standard deviation (s.d.) and coefficient of variation (CV) are reported.
|
δ13C |
δ15N |
||||||
|---|---|---|---|---|---|---|---|
| year | n | mean (‰) | s.d. (‰) | CV (%) | mean (‰) | s.d. (‰) | CV (%) |
| 2006 | 193 | −21.2 | 0.9 | 4.2 | 11.4 | 0.4 | 3.6 |
| 2007 | 57 | −20.9 | 0.9 | 4.3 | 11.1 | 0.4 | 3.9 |
| 2008 | 202 | −20.7 | 0.8 | 3.8 | 11.4 | 0.5 | 4.1 |
| 2009 | 234 | −21.3 | 0.9 | 4.1 | 11.4 | 0.5 | 4.0 |
| 2006–2009 | 686 | −21.1 | 0.9 | 4.2 | 11.4 | 0.5 | 4.0 |
(b). Model fitting and checking
The GLMM provided a worse fit to the data when compared with all HMMs (table 3 and figure 1c,d). Graphical inspection of observed versus predicted values (electronic supplementary material, figure S4) revealed little, if any, predictive power for the GLMM (table 3). In contrast, modelling weaning mass as a mixture of two Gaussian distributions accounted for about one-third of the observed variability (table 3). An HMM, either with or without an environmental covariate, provided an overall better fit (table 3). Neither the GLMM nor the HMMs managed to reproduce satisfactorily the observed maximum weaning mass: the GLMM tended to overestimate and the HMMS to underestimate it.
Table 3.
Posterior predictive checks (PPCs) of the fitted models (GLMM, generalized linear mixed model; HMM, hierarchical mixture model without environmental covariates; HMMChla, hierarchical mixture model with chlorophyll a covariate; HMMChla, SIE , hierarchical mixture model with chlorophyll a and sea ice extent covariates). PPCs are based on 1000 repetitions of the dataset. Reported p-values correspond to Pr(Trep > Tobs), where T is the chosen statistic, either the minimum, maximum or kurtosis of the weaning mass distribution. Extreme p-values (i.e. close to 0 or 1) betray misfit, while p-values around 0.5 indicate a good fit. A naive coefficient of determination R2 for the whole model (not just for the year level) is also reported. The selected model is in bold.
| statistic | GLMM | HMM | HMMChla | HMMChlaSIE |
|---|---|---|---|---|
| minimum | 0.45 | 0.42 | 0.48 | 0.46 |
| maximum | 0.67 | 0.38 | 0.32 | 0.25 |
| kurtosis | 0.80 | 0.57 | 0.53 | 0.58 |
| R2 | 0.07 | 0.31 | 0.33 | 0.33 |
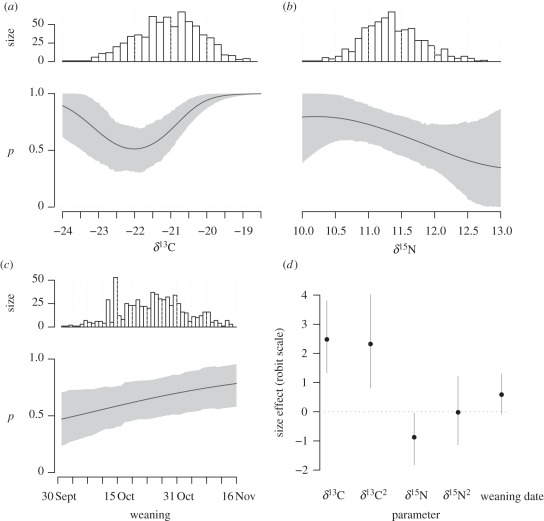
All predictors of group membership in HMMs were important (figure 2d). Carbon isotopic composition was a strong predictor of group membership, but in a non-monotonic way. Pups with either the largest or smallest blood δ13C values were very likely to have a large weaning mass, compared with pups with an intermediate value. In contrast, a greater δ15N value depressed, in a monotonic fashion, the probability of a pup to have a large weaning mass. Finally, weaning date had a modest positive effect: pups weaned later in the season were also more likely to have a larger weaning mass. There was no sign-reversal of coefficients between the GLMM and HMMs. Results from the HMMs are summarized in table 4.
Figure 2.
Graphical representation of the robit regression for group membership predictors of the HMM with environmental covariate. (a) Carbon and (b) nitrogen isotope values had a significant effect on predicting whether a pup's weaning mass was small (p = 0) or large (p = 1). (c) The effect of weaning date was also suggestive, although not statistically significant at the 95% level. (a–c) Histograms of the raw data are depicted above each plot. (d) Depicts the effect size of the estimated regression coefficients (on the robit scale). Reported estimates are for standardized variables. The grey envelope corresponds to a 95% credibility interval, and the black line to the posterior mean.
Table 4.
Results from the mixture model with environmental covariates. The difference in mass between the two groups is approximately 25 kg. A different ‘random’ year effect was fitted to each group. A ‘statistically significant’ difference between sex was found among large weanlings only. Reported estimates are for standardized variables: for example, a male in the second group is on average 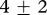 kg heavier than a female.
kg heavier than a female.
| parameter | mean | s.e. | lower bound | upper bound | |
|---|---|---|---|---|---|
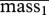 (kg) (kg) |
89 | 4 | 82 | 99 | |
| group 1: | sex (kg) | 2 | 4 | −5 | 9 |
| small weanlings | σyear1 (kg) | 4 | 4 | 0 | 11 |
| 1 − p (%) | 29 | 7 | 17 | 40 | |
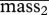 (kg) (kg) |
115 | 4 | 107 | 124 | |
| group 2: | sex (kg) | 4 | 2 | 1 | 8 |
| large weanlings | σyear2 (kg) | 7 | 4 | 2 | 16 |
| p (%) | 71 | 7 | 60 | 83 |
(c). Environmental covariates
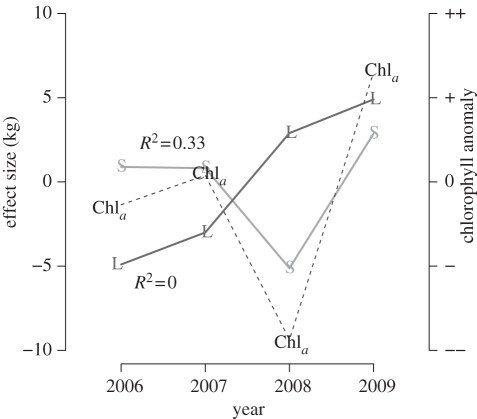
Inclusion of a proxy of global primary production in the interfrontal zone slighty improved model fit (table 3). Posterior predictive checks favoured the HMM with only chlorophyll a concentration at the interfrontal zone, but estimates from all HMMs were very similar, except for year-level variances. For small weanlings, chlorophyll a anomaly at the interfrontal zone accounted for one-third of the variability (figure 3). Antarctic sea ice extent accounted for 80 per cent of the variability of mass within large weanlings. Yet, adding this covariate to the model did not improve model fit judging from posterior predictive checks (table 4). Reported results are from the HMM with chlorophyll a anomaly.
Figure 3.
Year effect on the weaning mass of each estimated group of pups: S and L stand for small and large weanlings, respectively. The two groups differed in their response to environmental conditions. Yearly deviations from mean weaning mass for small pups, but not for large pups, positively covaried with cumulated chlorophyll a anomalies in the interfrontal zone. Solid lines correspond to posterior means.
(d). Mixture proportions
HMMs with environmental covariates (chlorophyll a only and chlorophyll a with sea ice extent) separated 29 per cent ([17 : 40]) of small pups from 71 per cent ([60 : 83]) of large pups (table 3). These proportions were relatively stable across years with 70 ([56 : 84]), 73 ([53 : 88]), 76 ([64 : 86]) and 67 ([53 : 81]) per cent of large pups in 2006, 2007, 2008 and 2009, respectively.
4. Discussion
(a). Pup weaning mass
Data on weaning mass from southern elephant seal pups at îles Kerguelen suggested that weanlings were a heterogeneous aggregate: about 30 per cent of weanlings had an average mass of 90 kg, while the remaining 70 per cent had an average mass of 115 kg (table 4). Given that an extra 5 kg may sustain a fasting pup for 10 days at sea [19], this difference is biologically significant. Females foraging at high (inferred from smaller δ13C values) or low (inferred from larger δ13C values) latitudes, had a greater probability of weaning a large pup than females foraging in the interfrontal zone (figure 2a and electronic supplementary material, figure S1). This non-monotonic relationship between a pup's blood carbon isotope ratio and its probability of a large weaning mass was surprising. We suspected a priori that the Antarctic strategy, because of the high productivity around Antarctica [34], was more profitable for females so that the smaller the δ13C value, the greater the probability for a pup to be large.
Stable isotope values actually suggested that females foraging in subtropical waters (high δ13C values) stored enough energy to wean a large pup. Although a temperate species, southern elephant seals may wander in low-latitude water masses (electronic supplementary material, figure S1), but we deem this strategy to be minor: only one tag-equipped female out of 44 went into sub-tropical waters (C. Guinet 2010, unpublished data).
Latitude is not the only factor affecting δ13C values. There is also an inshore/offshore gradient in carbon isotopes, whereby δ13C value is larger in neritic than in pelagic waters [35,36]. Îles Kerguelen are surrounded by a large and very productive plateau (bathymetry <1000 m) [37]. If females were to forage extensively on this plateau before hauling-out, a surge in the blood δ13C value of their pup would be expected. Females may be very opportunistic and feed also while transiting between foraging grounds [12], and particularly, on the Kerguelen plateau.
The Kerguelen plateau is an important foraging ground for adult males [8]. Breeding males are hauling-out before females so that, before pupping, females could forage on the Kerguelen plateau without competing with or being harassed by males [38]. Importantly, the majority of post-moulting tracks of females equipped with telemetric tags were usually incomplete for the last part of the homeward trip. This last part may be reflected in an increased blood δ13C value and could explain the observed positive relationship between a pup weaning mass and its blood carbon isotope ratio (see §4c).
Nitrogen isotope ratio negatively correlated with the probability for a pup to be large. This pattern may suggest that, within the Southern Ocean food web, lower trophic level preys (myctophid fish, δ15N values ε [7.6 : 10.2]‰ [39]) are of higher quality when compared with upper level ones (squids, δ15N values ε [10.0 : 10.9]‰ [39]). Traditional stomach content analyses concluded that southern elephant seals feed largely on squids [40]. Yet, these studies are biased by an over-representation of hard-to-digest items (cephalopod beaks) in the otherwise empty stomach of a fasting animal. With a stable isotope analysis, Cherel et al. [39] suggested that adult southern elephant seals may be feeding mainly on myctophid fish that represent the bulk of the mesopelagic fish biomass within the Southern Ocean [41]. Their high fat and protein contents [42] probably make them more profitable prey for female southern elephant seals when compared with squids. A non-exclusive alternative might be the occurrence of a gradient in δ15N values within the Southern Ocean owing to different baseline δ15N value in inshore [36] or sub-tropical [18] waters. Pup's blood δ13C and δ15N values were modestly correlated. However, there was no statistical support for a non-monotonic relationship between δ15N value and the probability of a pup to be large at weaning. Thus, δ13C and δ15N values are providing complementary information. Owing to logistic difficulties associated with (i) sampling the Southern Ocean and (ii) sampling the potential prey species of southern elephants seals, we assumed that differences in δ15N values stemmed from different diets rather than different baselines.
Weaning date, a crude proxy of maternal age [25], correlated positively with the probability for a pup to be large. This relationship was expected as older females are more experienced and, also being larger, can store more energy to transfer to their pup. For this interpretation to hold, we assumed that breeding females had a stable foraging strategy [43,44] and that younger females used the interfrontal zone as much as older females.
(b). Foraging strategies as maternal effects
Stable isotopes predicted pup weaning mass, and allowed us to infer maternal foraging strategies. With respect to a proxy of primary productivity in the interfontal zone, the two groups of pups we identified responded differently to environmental conditions. For a given year, weaning mass correlated positively with greater than average primary production in the interfrontal zone (figure 3) for small pups only. We inferred from the blood carbon isotope ratios of these pups that their mother would have foraged mainly in the interfrontal zone. Although we only have 4 years of data, we may speculate that the interfrontal zone is a safe bet for females: the observed positive correlation with current primary productivity may be interpreted as foraging success being more predictable there. Many top-predator species target fronts and mesoscale eddies to forage on mesopelagic fish whose spatial distribution is much more predictable close to frontal structures [45]. This decreased variability in resources of the interfrontal zone may also mean an increased competition, especially in bad years, such as in 2008 (figure 3). That there was no sex difference in mass among small pups in this highly dimorphic species, may also hint at the interfrontal zone being a safe, though poor, bet for breeding females.
Females adopting an Antarctic strategy managed to wean a larger pup (figure 2a). At the beginning of their post-moulting trip, females foraging in Antarctic waters started to exploit the Antarctic shelf (defined as the zone south from the Antarctic slope area with depths less than 500 m) but progressively retreated with the expansion of Antarctic sea ice [46]. Females stayed in the marginal sea-ice zone, and did not venture, as did males, into the pack ice [46]. This sexual segregation may result from distinct sex-specific constraints: females cannot afford to be blocked within the pack ice as they need to give birth on land. Antarctic foraging may thus be riskier for a female as she has to travel further away from îles Kerguelen, and her success will depend on Antarctic sea ice extent as she sticks to the marginal sea-ice zone. That females foraging at high latitudes still managed to wean a large pup may be testimony to a risky, yet very profitable, strategy.
Survival rates and breeding probabilities in relation to foraging strategies are to our knowledge unavailable, but adding stable isotope values as covariates in mark-capture-recapture analyses may help assessing the long-term life-history consequences of foraging strategies in this species. Burton et al. [47] cautioned that differences in weaning masses observed across colonies of the Southern Ocean may also be the result of local ‘natural selection due to differences in the importance of weaning mass for subsequent survival’. Yet, that we found such differences within a single colony begs an explanation.
The Kerguelen population of breeding southern elephant seal females crashed during the 1960–1980s [48]. Causes behind this decline are still unclear [49], although the hypothesis of an ecosystem regime shift affecting the Southern Ocean and impacting many species of upper marine predators is favoured [49,50]. Our results are consistent with this hypothesis: if foraging in the Antarctic zone allowed females to wean high-quality pups, a depressed biological productivity [50] would have decreased the larger fitness pay-offs enjoyed by those females when compared with others. As a result, all females would wean smaller pups with dimmer prospects of post-weaning survival. The large population decrease on îles Kerguelen could have stemmed from a small juvenile recruitment rate [51].
(c). Current limitations
Our data are largely observational by nature: pups were sampled at random yet knowledge of their mother's foraging strategy was missing. Accordingly, we conceptualized foraging strategy as missing data that we predicted from stable isotope values. Foraging strategy itself cannot be controlled for: it is not possible to assign pups to different ‘treatments’ (maternal foraging strategies) before sampling them. We acknowledge that strong causal claims derived from observational data are usually not warranted and some interpretations are speculative.
Latitude affects δ13C values in the Southern Ocean, but is not the only factor. We strongly suspect that southern elephant seal females may be feeding on the Kerguelen plateau prior to hauling-out, in which case the blood δ13C value of their pup could not be indicative of where they may have fed before reaching the Kerguelen peri-insular shelf. The probability for a pup to be large upon weaning reached a nadir of 0.5 (figure 2a), which translates as the maximum uncertainty possible. This nadir was observed for a carbon isotope value of pup blood of roughly −22‰, suggestive of a polar frontal signature. The group of large pups is most likely itself heterogeneous, aggregating pups whose mother foraged either (i) into the marginal Antarctic sea-ice zone or (ii) elsewhere but fed extensively on the plateau on the homeward trip to îles Kerguelen. Bio-logging studies and our isotopic investigation did not yield congruent proportions for the a priori strategies we considered (Antarctic versus interfrontal). This is unsurprising as females may be feeding on the homeward trip [12].
Using blood isotopic data from 26 seals that were sampled both upon tag application and retrieval, we found that carbon isotope values were strongly correlated with the mean latitude of a female's foraging trip [52]. Further analysis revealed that blood turn-over rate was at least four months [52]. Thus, blood carbon isotope ratio may in part reflect opportunistic feeding on the Kerguelen plateau, but seems nevertheless to reflect the whole foraging trip of females.
Our data encompassed only 4 years. We uncovered a small correlation between small weanlings and a proxy of productivity in the interfrontal zone. This correlation is biologically plausible both in sign and in effect size: southern elephant seals do not feed on chlorophyll, so a large effect would be unsound. Owing to data unavailability on chlorophyll a concentration around Antarctica, we assessed the effect of sea ice extent on the weaning mass of large pups and found a suspiciously large correlation. While the relationship is plausible [53], the effect size is probably an overestimate arising from the short time series we currently have, as corroborated by posterior predictive checks (table 3). Inclusion of proxy of productivity did slightly improve our HMMs (table 3). That the maximum weaning mass is underestimated suggests that an important predictor for large pups is still missing. Even a reasonably complex model only accounted for one-third of the observed variability in weaning mass, a figure typical of an observational study in ecology [54].
(d). A paradoxical result?
Nevertheless, a glance at figure 2a suggests a paradox: high and low δ13C values predicted with certainty a pup to be a large weanling. Yet, only 1 per cent of pups had a carbon signature lower than −23‰, suggestive of a true Antarctic maternal strategy. Likewise, only 3 per cent of pups had a carbon isotope ratio higher than −19.5‰, which we speculated reflects extensive feeding on the Kerguelen plateau. If both foraging in Antarctic oceanic waters and on the Kerguelen plateau are so profitable, why do most females still bother to forage in the interfrontal zone [8,55]?
Our analysis thus seems rather at odds with observed patterns from bio-logging studies as we identified two groups, one of which encompassed pups whose mother foraged in the interfrontal zone. These small pups represented about 30 per cent of our sample, which is not consistent with an estimated 73 per cent of females foraging in the interfrontal zone. However, the group of large pups we identified, probably being heterogeneous, may include pups whose mother foraged in the interfrontal zone and on the Kerguelen plateau. Our analysis suggested that the small pups were more likely to be from females that foraged exclusively in the interfrontal zone, but this relationship was not deterministic (figure 2a).
The paucity of signatures from the Kerguelen plateau also begs the question as to why adult males used it so much and females so little [8]. Predation by killer whales (Orcinus orca) or sleeper sharks (Somniosus antarcticus, [56]) may deter females from foraging on the Kerguelen plateau. Still, such predation may only be marginal and very difficult to evidence directly. Male harassment may also explain this pattern [38]. Isotopic analysis of archival tissues, such as whiskers or teeth, may prove useful to shed light on the ontogeny of this putative sexual segregation, a pattern documented in other seal species [57] and between juveniles of both sexes and adult females in southern elephant seals [58].
5. Conclusions
Southern elephant seals are astonishing swimmers and divers [7,8], turning the study of their foraging behaviour into a challenge. Using stable isotopes and a mixture modelling approach, we investigated maternal effects on offspring mass. Our analysis may suggest an important role for maternal foraging strategies in shaping population trends on îles Kerguelen [51]. Some evidence suggests that southern elephant seal females are faithful to their foraging grounds [43]. The relatively stable mixture proportions across years observed in this study could reflect a stable commitment of females to a foraging strategy. Yet, this may have adverse fitness consequences in both the short- and long-term. Females committed to a foraging strategy in the interfrontal zone may never contribute to the next generation, having weaned only small pups. This then begs the question of the ontogeny of foraging behaviour, which the relatively short integration time of blood precludes to address. In contrast, stable isotope analyses of metabolic inert tissues, such as teeth [44,59,60], may provide further insight into the ecology of southern elephant seals.
Acknowledgements
The ethics committee of the French Polar Institute (IPEV) approved this study. All animals in this study were cared for in accordance with its guidelines.
We thank all field workers who have collected data since 2006, in particular, Q. Delorme, S. Ducatez and S. Dalloyau. We are greatly indebted to Gaël Guillou for laboratory analyses of blood stable isotopes. We thank Luciano O. Valenzuela and two anonymous reviewers for critical comments on the manuscript. We thank Daniel Costa for his supportive comments. This study is part of a national research programme (no. 109, H. Weimerskirch and the observatory Mammifères Explorateurs du Milieu Océanique, MEMO SOERE CTD 02) supported by the French Polar Institute (Institut Paul Emile Victor, IPEV). The Territoire des Terres Australes et Antarctiques Françaises (TAAF) and ANR-VMC 07 IPSOS-SEAL programme contributed to this study.
References
- 1.Mousseau T., Fox T. 1998. Maternal effects as adaptations, 1st edn Oxford, UK: Oxford University Press [Google Scholar]
- 2.Inchausti P., Ginzburg L. 2009. Maternal effects mechanism of population cycling: a formidable competitor to the traditional predator–prey view. Phil. Trans. R. Soc. B 364, 1117–1124 10.1098/rstb.2008.0292 (doi:10.1098/rstb.2008.0292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown G., Shine R. 2009. Beyond size-number trade-offs: clutch size as a maternal effect. Phil. Trans. R. Soc. B 364, 1097–1106 10.1098/rstb.2008.0247 (doi:10.1098/rstb.2008.0247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAdam A. 2009. Maternal effects on evolutionary dynamics in wild small mammals. In Maternal effects in mammals (eds Maestripieri D., Mateo J. M.), pp. 64–82 Chicago, IL: The University of Chicago Press. [Google Scholar]
- 5.Bowen W. 2009. Maternal effects on offspring size and development in pinnipeds. In Maternal effects in mammals (eds Maestripieri D., Mateo J. M.), pp. 104–132 Chicago, IL: The University of Chicago Press. [Google Scholar]
- 6.Berta A., Sumich J., Kovacs K. 2006. Marine mammals evolutionary biology, 2nd edn The Netherlands: Academic Press, Elsevier [Google Scholar]
- 7.Biuw M., et al. 2007. Variations in behaviour and condition of a southern ocean top predator in relation to in situ oceanographic conditions. Proc. Natl Acad. Sci. USA 104, 13 705–13 710 10.1073/pnas.0701121104 (doi:10.1073/pnas.0701121104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailleul F., Authier M., Ducatez S., Roquet F., Charassin J.-B., Cherel Y., Guinet C. 2010. Looking at the unseen: combining bio-logging and stable isotopes to reveal a shift in the ecological niche of a deep-diving predator. Ecography 33, 709–719 10.1111/j.1600-0587.2009.06034.x (doi:10.1111/j.1600-0587.2009.06034.x) [DOI] [Google Scholar]
- 9.Hebblewhite M., Haydon D. 2010. Distinguishing technology from biology: a critical review of the use of GPS telemetry data in ecology. Phil. Trans. R. Soc. B 365, 2303–2312 10.1098/rstb.2010.0087 (doi:10.1098/rstb.2010.0087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kareiva P., Odell G. 1987. Swarms of predators exhibit ‘preytaxis’ if individual predators use area-restricted search. Am. Nat. 130, 233–270 10.1086/284707 (doi:10.1086/284707) [DOI] [Google Scholar]
- 11.Bailleul F., Pinaud D., Hindell M., Charassin J.-B., Guinet C. 2008. Assessment of scale-dependent foraging behaviour in southern elephant seals incorporating the vertical dimension: a development of the first passage time method. J. Anim. Ecol. 77, 948–957 10.1111/j.1365-2656.2008.01407.x (10.1111/j.1365-2656.2008.01407.x) [DOI] [PubMed] [Google Scholar]
- 12.Thums M., Bradshaw C., Hindell M. 2011. In situ measures of foraging success and prey encounter reveals marine habitat-dependent search strategies. Ecology 92, 1258–1270 10.1890/09-1299.1 (doi:10.1890/09-1299.1) [DOI] [PubMed] [Google Scholar]
- 13.West J., Bowen G., Cerling T., Ehleringer J. 2006. Stable isotopes as one of nature's ecological recorders. Trends Ecol. Evol. 21, 408–414 10.1016/j.tree.2006.04.002 (doi:10.1016/j.tree.2006.04.002) [DOI] [PubMed] [Google Scholar]
- 14.Kelly J. 2000. Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can. J. Zool. 78, 1–27 10.1139/z99-165 (doi:10.1139/z99-165) [DOI] [Google Scholar]
- 15.Hobson K., Piatt J., Pitocchelli J. 1994. Using stable isotopes to determine seabird trophic relationships. J. Anim. Ecol. 63, 786–798 10.2307/5256 (doi:10.2307/5256) [DOI] [Google Scholar]
- 16.Trull T., Armand L. 2001. Insight into southern ocean carbon export from the δ13C of particles and dissolved inorganic carbon using the SOIREE iron release experiment. Deep-Sea Res. Part II 48, 2655–2680 10.1016/S0967-0645(01)00013-3 (doi:10.1016/S0967-0645(01)00013-3) [DOI] [Google Scholar]
- 17.Ducatez S., Dalloyau S., Richard P., Guinet C., Cherel Y. 2008. Stable isotopes document winter trophic ecology and maternal investment of adult female southern elephant seals (Mirounga leonina) breeding at the Kerguelen islands. Mar. Biol. 155, 413–420 10.1007/s00227-008-1039-3 (doi:10.1007/s00227-008-1039-3) [DOI] [Google Scholar]
- 18.Jaeger A., Lecomte V., Weimerskirch H., Richard P., Cherel Y. 2010. Seabird satellite tracking validates the use of latitudinal isoscapes to depict predators' foraging areas in the southern ocean. Rapid Commun. Mass Spectrophotometry 24, 3456–3460 10.1002/rcm.4792 (doi:10.1002/rcm.4792) [DOI] [PubMed] [Google Scholar]
- 19.McMahon C., Burton H., Bester M. 2000. Weaning mass and the future survival of juvenile southern elephant seals, Mirounga leonina, at Macquarie Island. Antarctic Sci. 12, 149–153 10.1017/S0954102000000195 (doi:10.1017/S0954102000000195) [DOI] [Google Scholar]
- 20.Kim T.-H., White H. 2004. On more robust estimation of skewness and kurtosis. Financ. Res. Lett. 1, 56–73 10.1016/S1544-6123(03)00003-5 (doi:10.1016/S1544-6123(03)00003-5) [DOI] [Google Scholar]
- 21.An L., Ahmed E. 2008. Improving the performance of the kurtosis estimator. Comput. Stat. Data Anal. 52, 2669–2681 10.1016/j.csda.2007.09.024 (10.1016/j.csda.2007.09.024) [DOI] [Google Scholar]
- 22.DeNiro M., Epstein S. 1977. Mechanism of carbon isotope fractionation associated with lipid-synthesis. Science 197, 261–263 10.1126/science.327543 (doi:10.1126/science.327543) [DOI] [PubMed] [Google Scholar]
- 23.Darlington R. 1970. Is kurtosis really “peakedness”? Am. Stat. 24, 19–22 10.2307/2681925 (doi:10.2307/2681925) [DOI] [Google Scholar]
- 24.Hilderbrand D. 1971. Kurtosis measures bimodality? Am. Stat. 25, 42–43 10.2307/2682213 (doi:10.2307/2682213) [DOI] [Google Scholar]
- 25.Kirkman S., Bester M., Pistorius P., Hofmeyr G., Jonker F., Owen R., Strydom N. 2004. Variation in the timing of the breeding haulout in female southern elephant seals at Marion island. Aust. J. Zool. 52, 379–388 10.1071/ZO03038 (doi:10.1071/ZO03038) [DOI] [Google Scholar]
- 26.Green A. 2001. Mass length residuals: measures of body condition or generators of spurious results. Ecology 82, 1473–1483 10.1890/0012-9658(2001)082[1473:MLRMOB]2.0.CO;2 (doi:10.1890/0012-9658(2001)082[1473:MLRMOB]2.0.CO;2) [DOI] [Google Scholar]
- 27.Liu C. 2004. Robit regression: a simple robust alternative to logistic and probit regression. In Applied Bayesian modeling and causal inference from incomplete data perspectives (eds Gelman A., Meng X.-L.), ch. 21, pp 227–238 UK: John Wiley and Sons Ltd. [Google Scholar]
- 28.Field C., Behrenfeld M., Randerson J., Falkowski P. 1998. Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281, 237–240 10.1126/science.281.5374.237 (doi:10.1126/science.281.5374.237) [DOI] [PubMed] [Google Scholar]
- 29.Behrenfeld M., et al. 2001. Biospheric primary productivity during an ENSO transition. Science 291, 2594–2597 10.1126/science.1055071 (doi:10.1126/science.1055071) [DOI] [PubMed] [Google Scholar]
- 30.Mongin M., Molina E., Trull T. 2008. Seasonality and scale of Kerguelen plateau phytoplakton bloom: a remote sensing and modeling analysis of the influence of natural iron fertilization in the southern ocean. Deep-Sea Res. II 55, 880–892 10.1016/j.dsr2.2007.12.039 (doi:10.1016/j.dsr2.2007.12.039) [DOI] [Google Scholar]
- 31.Dragon A., Marchand S., Authier M., Cotté C., Blain S., Guinet C. 2011. Insights of the spatio-temporal productivity distribution in the indian southern ocean provided by satellite observations. In The Kerguelen plateau—marine ecosystem and fisheries (eds Duhamel G., Welsford D.), pp. 57–67 France: Société Française d'Ichtyologie. [Google Scholar]
- 32.Gelman A., Pardoe I. 2006. Bayesian measures of explained variance and pooling in multilevel (hierarchical) models. Technometrics 48, 241–251 10.1198/004017005000000517 (doi:10.1198/004017005000000517) [DOI] [Google Scholar]
- 33.Berkhof J., van Mechelen I., Gelman A. 2003. A Bayesian approach to the selection and testing of mixture models. Stat. Sin. 13, 423–442 [Google Scholar]
- 34.Ainley D., DeMaster D. 1990. Upper trophic levels in polar marine ecosystems. In Polar oceanography. Part B: chemistry, biology and geology (ed. Smith W. O.), pp. 599–630 New York, NY: Academic Press [Google Scholar]
- 35.Rau G., Sweeney R., Kaplan I. 1982. Plankton 13C/12C ratio changes with latitude: differences between northern and southern oceans. Deep Sea Res. I 29, 1035–1039 10.1016/0198-0149(82)90026-7 (doi:10.1016/0198-0149(82)90026-7) [DOI] [Google Scholar]
- 36.Cherel Y., Hobson K. 2007. Geographical variation in the carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the southern ocean. Mar. Ecol. Prog. Ser. 329, 281–287 10.3354/meps329281 (doi:10.3354/meps329281) [DOI] [Google Scholar]
- 37.Blain S., et al. 2001. A biogeographical study of the island mass effect in the context of the iron hypothesis: Kerguelen islands, southern ocean. Deep-Sea Res. I 48, 163–187 10.1016/S0967-0637(00)00047-9 (doi:10.1016/S0967-0637(00)00047-9) [DOI] [Google Scholar]
- 38.de Bruyn P., Tosh C., Bester M., Cameron E., McIntyre T., Wilkinson I. 2011. Sex at sea: alternative mating system in an extremely polygynous mammal. Anim. Behav. 82, 445–451 10.1016/j.anbehav.2011.06.006 (doi:10.1016/j.anbehav.2011.06.006) [DOI] [Google Scholar]
- 39.Cherel Y., Ducatez S., Fontaine C., Richard P., Guinet C. 2008. Stable isotopes reveals the trophic position and mesopelagic diet of female southern elephant seals breeding on the Kerguelen islands. Mar. Ecol. Prog. Ser. 370, 239–247 10.3354/meps07673 (doi:10.3354/meps07673) [DOI] [Google Scholar]
- 40.Rodhouse P., Arnbom T., Fedak M., Yeatman J., Murray A. 1992. Cephalopod prey of the southern elephant seal, Mirounga leonina l. Can. J. Zool. 70, 1007–1015 10.1139/z92-143 (doi:10.1139/z92-143) [DOI] [Google Scholar]
- 41.Cherel Y., Fontaine C., Richard P., Labat J. 2010. Isotopic niches and trophic levels of myctophid fishes and their predators in the southern ocean. Limnol. Oceanogr. 55, 324–332 10.4319/lo.2010.55.1.0324 (doi:10.4319/lo.2010.55.1.0324) [DOI] [Google Scholar]
- 42.Catul V., Gauns M., Karappasamy P. 2011. A review on mesopelagic fishes belonging to family myctophidae. Rev. Fish Biol. Fish. 21, 339–354 10.1007/s11160-010-9176-4 (doi:10.1007/s11160-010-9176-4) [DOI] [Google Scholar]
- 43.Bradshaw C., Hindell M., Sumner M., Michael K. 2004. Loyalty pays: potential life history consequences of fidelity to marine foraging regions by southern elephant seals. Anim. Behav. 68, 1349–1360 10.1016/j.anbehav.2003.12.013 (doi:10.1016/j.anbehav.2003.12.013) [DOI] [Google Scholar]
- 44.Martin C., Bentaleb I., Steedlandt S., Guinet C. 2011. Stable carbon and nitrogen isotope variations in canine dentin growth layers of Kerguelen southern elephant seals. Mar. Ecol. Prog. Ser. 439, 295–305 10.3354/meps09331 (doi:10.3354/meps09331) [DOI] [Google Scholar]
- 45.Bost C.-A., Cotté C., Bailleul F., Charassin B., Guinet C., Ainley D., Weimerskirch H. 2009. The importance of oceanographic fronts to marine birds and mammals of the southern oceans. J. Mar. Syst. 78, 363–376 10.1016/j.jmarsys.2008.11.022 (doi:10.1016/j.jmarsys.2008.11.022) [DOI] [Google Scholar]
- 46.Bailleul F., Charassin J.-B., Ezraty R., Girard-Ardhuin F., McMahon C., Field I., Guinet C. 2007. Southern elephant seals from Kerguelen islands confronted by antarctic sea ice. changes in movements and in diving behaviour. Deep Sea Res. II 54, 343–355 10.1016/j.dsr2.2006.11.005 (doi:10.1016/j.dsr2.2006.11.005) [DOI] [Google Scholar]
- 47.Burton H. R., Arnbom T., Boyd I. L., Bester M., Vergani D., Wilkinson I. 1997. Significant differences in weaning mass of southern elephant seals from five sub-Antarctic islands in relation to population declines. In Antarctic communities: species, structure and survival (eds Battaglia B., Valencia J., Walton D. W. H.), pp. 335–338 Cambridge, UK: Cambridge University Press [Google Scholar]
- 48.Guinet C., Jouventin P., Weimerskirch H. 1999. Recent population change of the southern elephant seal at îles Crozet and îles Kerguelen: the end of the decrease? Antarctic Sci. 11, 193–197 10.1017/S0954102099000255 (doi:10.1017/S0954102099000255) [DOI] [Google Scholar]
- 49.McMahon C., Bester M., Burton H., Hindell M., Bradshaw C. 2005. Population status, trends and a re-examination of the hypotheses explaining the recent declines in the southern elephant seal Mirounga leonina. Mamm. Rev. 35, 82–100 10.1111/j.1365-2907.2005.00055.x (doi:10.1111/j.1365-2907.2005.00055.x) [DOI] [Google Scholar]
- 50.Weimerskirch H., Inchausti P., Guinet C., Barbraud C. 2003. Trends in birds and seal populations as indicators of a system shift in the southern ocean. Antarctic Sci. 15, 249–256 10.1017/S0954102003001202 (doi:10.1017/S0954102003001202) [DOI] [Google Scholar]
- 51.McMahon C., Hindell M., Burton H., Bester M. 2005. Comparison of southern elephant seal populations, and observations of a population on a demographic knife-edge. Mar. Ecol. Prog. Ser. 288, 273–283 10.3354/meps288273 (doi:10.3354/meps288273) [DOI] [Google Scholar]
- 52.Authier M. 2011. Unveiling the at-sea ecology of southern elephant seals from indirect evidence. PhD thesis, Centre d'Études Biologiques de Chizè, Université de Poitiers, France [Google Scholar]
- 53.McMahon C., Burton H. 2005. Climate change and seal survival: evidence for environmentally mediated changes in elephant seal, Mirounga leonina, pup survival. Proc. R. Soc. B 272, 923–928 10.1098/rspb.2004.3038 (doi:10.1098/rspb.2004.3038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peek M., Leffler A., Flint S., Ryel R. 2003. How much variance is explained by ecologists? additional perspectives. Oecologia 137, 161–170 10.1007/s00442-003-1328-y (doi:10.1007/s00442-003-1328-y) [DOI] [PubMed] [Google Scholar]
- 55.Dragon A., Monestiez P., Bar-Hen A., Guinet C. 2010. Linking foraging behaviour to physical oceanographic structures: southern elephant seals and mesoscale eddies east of Kerguelen islands. Prog. Oceanogr. 87, 61–71 10.1016/j.pocean.2010.09.025 (doi:10.1016/j.pocean.2010.09.025) [DOI] [Google Scholar]
- 56.van den Hoff J., Morrice M. 2008. Sleeper sharks (Somniosus antarcticus) and other bite wounds observed on southern elephant seals (Mirounga leonina) at Macquarie island. Mar. Mamm. Sci. 24, 239–247 10.1111/j.1748-7692.2007.00181.x (doi:10.1111/j.1748-7692.2007.00181.x) [DOI] [Google Scholar]
- 57.Breed G., Bowen W., McMillan J., Leonard M. 2006. Sexual segregation of seasonal foraging habitats in a non-migratory marine mammal. Proc. R. Soc. B 273, 2319–2326 10.1098/rspb.2006.3581 (doi:10.1098/rspb.2006.3581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Newland C., Field I., Nichols P., Bradshaw C., Hindell M. 2009. Blubber fatty acid profiles indicate dietary resource partitioning between adult and juvenile elephant seals. Mar. Ecol. Prog. Ser. 384, 303–312 10.3354/meps08010 (doi:10.3354/meps08010) [DOI] [Google Scholar]
- 59.Hobson K., Sease J. 1998. Stable isotope analyses of tooth annuli reveal temporal dietary records: an example using steller sea lions. Mar. Mamm. Sci. 14, 116–129 10.1111/j.1748-7692.1998.tb00694.x (doi:10.1111/j.1748-7692.1998.tb00694.x) [DOI] [Google Scholar]
- 60.Mendes S., Newton J., Reid R., Zuur A., Pierce G. 2007. Stable carbon and nitrogen isotope ratio profiling of sperm whale teeth reveals ontogenetic movements and trophic ecology. Oecologia 151, 605–615 10.1007/s00442-006-0612-z (doi:10.1007/s00442-006-0612-z) [DOI] [PubMed] [Google Scholar]