Abstract
To clarify the effect of concentration polarization of oxidative modification of low-density lipoproteins (ox-LDLs) on human smooth muscle cells (SMCs), the proliferation, ox-LDL uptake and apoptosis with SMCs cultured on permeable (the permeable group) or non-permeable membranes (the non-permeable group) were analysed by 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) assay, spectrofluorometry and flow cytometry using a parallel-plate flow chamber technique. The concentration polarization of ox-LDLs at the surface of the cultured cell monolayer was assessed by confocal laser microscopy. The results showed that concentration polarization of ox-LDLs could indeed occur at the cultured cell monolayer surface of the permeable group, leading to an enhanced wall concentration of ox-LDLs that was over 15 per cent higher than the bulk concentration of the perfusion solution at a pressure of 100 mmHg. When concentration of ox-LDLs in the perfusion solution was less than or equal to 100 µg ml–1, SMCs' proliferation was induced, while cell apoptosis was induced when its concentration was above 150 µg ml–1. The uptake of ox-LDLs by the cultured cells was significantly higher for the permeable group than for the non-permeable group. In addition, the ox-LDL-induced cell death and apoptosis were much more severe in the permeable group than that in the non-permeable group. Therefore, the experimental study suggests that concentration polarization of ox-LDLs plays an adverse role in the vascular system owing to its toxicity to vascular cells, in turn enhance ox-LDL infiltration into the arterial wall and accelerate SMC apoptosis.
Keywords: atherosclerosis, concentration polarization, oxidized low-density lipoprotein, wall shear stress
1. Introduction
Oxidized low-density lipoprotein (ox-LDL) is believed to play a key role in the initiation and progression of atherogenesis characterized by chronic inflammation, accumulation of lipids and vascular cell modifications in the arterial wall [1,2]. Unlike native LDLs, ox-LDLs are not recognized by the LDL receptors, but are taken up in a non-regulated manner by the scavenger receptors in vascular cells [3]. Moreover, in pathological conditions such as atherosclerosis, the endothelium barrier covering the blood vessel wall becomes more permeable to atherogenic lipids when subjected to ox-LDLs [4,5]. This process leads to the accumulation of cholesterol in the vascular cells, forming foam cells—the hallmark of the atherosclerosis lesion [6,7].
Owing to the fact that the early event leading to the genesis of atherosclerosis is the accumulation of cholesterol and other lipids within the arterial wall, Deng et al. [8] has theoretically predicted a mass transport phenomenon of concentration polarization of atherogenic LDLs and verified it by experiments in vitro [9]. They believe that it is the concentration of atherogenic lipoproteins at the luminal surface of blood vessels, not the lipid bulk concentration in blood, that plays important roles in atherogenesis [10].
Recent studies have shown that ox-LDL induces SMCs' proliferation associated with the ability of ox-LDL to simultaneously (i) increase the expression of specific cell cycle-activating proteins (e.g. CDC2, Cdk2, Cdk4, cyclin B1, D1 and PCNA1) and cell cycle-inhibiting proteins (e.g. p21 and p27) and (ii) augment intracellular signalling pathways (e.g. PI3K and PLC pathways) involved in the mitogenic response [11,12]. Okura et al. [13] found that ox-LDLs were cytotoxic at higher concentrations, causing apoptosis in intimal vascular SMCs and increasing plaque instability and rupture in acute coronary syndromes. In the process of ox-LDL-induced apoptosis, it involves both Fas (apoptosis stimulating fragment) and tumor necrosis factor (TNF) receptors I and II signalling pathways, which leads to the downregulation of antiapoptotic proteins of the Bcl-2 family, upregulation of apoptotic proteins including caspase 3, and the activation of MAP and Jun kinase-dependent transcription factors (e.g. STAT, NFkB, p53, ATF-2, ELK-1, CREB and AP-1) [14]. Ox-LDLs can be taken up by monocytes, smooth muscle cells and endothelial cells via several known scavenger receptors such as scavenger receptor class AI and II, CD36, and CD68 and lectin-like ox-LDL receptor (LOX-1) [15,16].
Nevertheless, the SMCs used in the aforementioned studies were cultured on non-permeable membranes across which no filtration flow could pass the cultured SMCs. Therefore, these studies could not reveal the effect of wall concentration of ox-LDLs on SMCs from a mass transport viewpoint. To the best of our knowledge, no study has been reported on the effect of ox-LDL concentration polarization on the proliferation, cycle and apoptosis of human SMCs cultured on a permeable membrane. The present study was therefore designed to investigate the role of ox-LDL concentration polarization in the proliferation of SMCs and its cytotoxic effect on SMCs under a shear flow condition.
Owing to the toxicity of ox-LDLs to vascular cells, we hypothesize that if SMCs were exposed directly to blood flow streams for some reasons (for instance, denudation of endothelial cells in angioplasty or arterial stenting procedures), concentration polarization of ox-LDLs might severely affect proliferation, cycle, apoptosis and ox-LDL uptake in SMCs. To verify this hypothesis, in the present article, using a parallel-plate flow chamber technique, we investigated whether concentration polarization of ox-LDLs could also occur on the surface of SMCs and its effect on human SMCs in terms of cell viability, cell cycle, ox-LDL uptake and cell apoptosis.
2. Material and methods
2.1. Lipoprotein isolation, modification and labelling
Native LDLs were prepared and purified from fresh plasma obtained from healthy volunteers by gradient ultracentrifugation according to the method of Redgrave et al. [17]. Ox-LDLs were prepared by incubation of EDTA-free LDL with 5 µM CuSO4 in phosphate-buffered saline (PBS) for 18 h at 37°C. Then 0.24 mM ethylene diamine tetraacetic acid (EDTA) was added to stop oxidation. Ox-LDL was concentrated with Centriflo Cones (Bio Rad) (2200 r.p.m., 20 min, 4°C) and washed twice with PBS buffer [18]. Labelling of ox-LDL with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; Molecular Probes, Eugene, OR, USA) was performed as previously described [19].
2.2. Smooth muscle cells monolayer culture preparations
Human umbilical cords' SMCs were isolated from human umbilical cords by ‘explanting pieces of tissue’ with a Dulbecco's modified Eagle's medium (DMEM) cell culture medium. SMCs at a density of 1 × 106 cells cm–2 were seeded on: (i) non-permeable group: a glass slide which was non-permeable to plasma; (ii) permeable group: a Millicell-CM membrane (PICM 03050; Millipore Corp., Bedford, MA, USA) which was permeable to plasma with pores of 0.4 µm in diameter. The state of attachment of cells onto the membrane and confluence of cells on the membranes were monitored by a phase-contrast microscope from time to time.
2.3. Experimental set-up
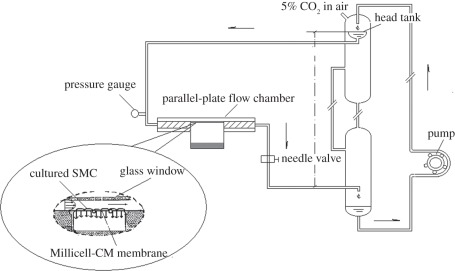
The same experimental perfusion system as the one described previously by Ding et al. [20] was used in the present study (figure 1). It consisted of a head tank, a downstream collecting reservoir, a modified parallel-plate flow chamber with a height of 0.5 × 10−3 m, a peristaltic flow pump to circulate the perfusion fluid (DMEM) and a blender of air and CO2 with a constant temperature (37°C ± 1°C). All the components of the perfusion system were connected using tygon tubing. The flow rate was controlled by adjusting the height of the overflow head tank and the resistance of the needle valve so that both the desired flow rate and a perfusion pressure could be achieved simultaneously. The SMC side of the cell culture insert was exposed to the flow. The abluminal side of the cell culture insert was supported with a handmade membrane support so that the culture did not sag even when perfusion pressure was applied to it. A pressure transducer and a flow meter were used to monitor the perfusion pressure and the flow rate through the flow chamber, respectively. During the experiment, the flow chamber was enclosed in a container to keep it at a constant temperature of 37 ± 1°C.
Figure 1.
Schematic of the experimental perfusion system. The overflow head tank provided a steady flow to the parallel-plate flow chamber. The filtration rate measurement cell on the abluminal side of the culture was filled with the same fluid as the perfusate.
2.4. Measurement of filtration rate and DiI-oxidized low-density lipoprotein wall concentration
During this measurement, steady wall shear stress and constant perfusion pressure within the flow chamber was kept at 1.3 Pa and 100 mmHg, while the DiI-ox-LDL concentration of the perfusion solution was varied at 10, 50, 100, 150 and 200 µg ml–1, respectively. The perfusion flow was maintained at 24 h. For each experiment, the filtration rate across the wall of the cell culture insert was measured following the same procedure described by Deng et al. [8] with the help of the calibrated pipette on the dish support, which had an inner diameter of 1 mm (figure 1).
The concentration of DiI-ox-LDL at the luminal surface of SMC layer was assessed by measuring the fluorescence intensity of the DiI-ox-LDL with a confocal laser microscope (SPII, Leica, Heidelberg, Germany).
2.5. MTT colorimetric assay
After 24 h incubation, the effect of different concentrations (10–200 µg ml–1) of ox-LDL on cell proliferation was determined by MTT assay as described by Yan et al. [21]. Briefly, cells (1 × 105 cells ml–1) in their exponential growth phase were seeded into each well (200 ml media per well) of a 96-well plate and incubated for 24 h at 37°C in a CO2 incubator. Different concentrations of ox-LDL were added to the wells, and the cells were grown at 37°C for 24 h. Control cells were maintained in an identical medium without adding ox-LDL for the same period of time. After removing the supernatants, 20 ml of MTT and 180 ml of PBS were added and incubated at 37°C for 4 h. Again the supernatant was carefully removed and 150 ml of dimethylsulphoxide (DMSO) was added into each well to dissolve the MTT formazan at the bottom of the wells. After 10 min, the absorbance was read at 492 nm using enzyme-linked immunosorbent assay (ELISA) plate reader.
2.6. Measurement of DiI-oxidized low-density lipoprotein uptake
During this measurement, wall shear stress and the perfusion pressure within the flow chamber were kept at 1.3 Pa and 100 mmHg, while the DiI-ox-LDL concentration of the perfusion solution was varied from 10 to 200 µg ml–1. Wall shear stress was calculated using the formula as follows: τ = 6μQ/wh2, where μ is the viscosity of the medium and Q is the flow rate, h and w are the width and height of the parallel-plate flow chamber.
After perfusion flow, the cell culture insert was disassembled from the flow chamber and washed three times with cold PBS. These cells were lysed with 1 ml of 0.4 per cent Triton-X in PBS for 10 min and then detached from the membrane using a cell scraper. Complete lysis of the cells was achieved by gentle pipetting of the lysate, followed by removal of cell debris by centrifugation. The fluorescence intensity of the samples was measured with a spectrofluorometer (Cary Eclipse, Varian, USA) at excitation and emission wavelengths of 514 and 550 nm, respectively. The fluorescence intensity was then converted to the concentration of lipoproteins with a calibration curve of DiI-ox-LDL. The amount of lipoprotein uptake by the arterial wall was expressed as the weight of lipoproteins taken up per effective surface area per hour (ng cm–2 h–1).
A standard procedure was followed throughout the entire experiment. Prior to the exposure of the cultured cells to DiI-ox-LDL, the cells were first subjected to steady laminar shear flow of 1.2 Pa for 24 h to precondition the SMCs.
2.7. Cell cycle analysis
The conditions in this part of experiment were the same as those described in §2.6. Cells were trypsinized after treatment with ox-LDL, fixed and treated with 500 µl RNase A (100 µg ml–1) for 30 min at 37°C. Followed by staining with 100 µl propidium iodide (50 µg ml–1) at 4°C for 5 min and then analysed by flow cytometry (FCM; Becton Dickinson, USA). The fractions of the cells in G0/G1 and S phase were analysed using the cell cycle analysis software—Multicycle (Phoenix Flow System, USA).
2.8. Flow cytometry analysis of apoptosis
The conditions in this part of experiment were the same as those described in §2.6. After perfusion flow, cells were collected at a concentration of 1 × 106 cells ml–1 and washed three times with PBS (4°C pH 7.4), and centrifuged at 1000 r.p.m. for 5 min. Then the cells were resuspended by adding 200 µl binding buffer, and 10 µl annexin V-FITC (Bender MedSystems, Vienna, Austria) was added to the cell suspension and the mixture was incubated for 15 min in dark at room temperature. After adding 300 µl binding buffer and 5 µl PI to the cells, the apoptosis was analysed by FCM (Becton Dickinson) and the results were analysed with the software Lysish.
2.9. Statistical analysis
Data from at least three sets of samples were used for statistical analysis. Results are shown as mean ± s.d. Multiple means were compared using a one-way analysis of variance (ANOVA). Student's paired t-test was used to assess the significant differences between the two groups. p < 0.05 was considered significant.
3. Results
3.1. Water-filtration rate and wall concentration of DiI-oxidized low-density lipoproteins, cw
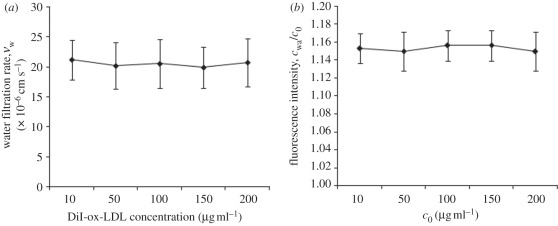
Figure 2a shows the water-filtration rate (vw) across the cell monolayer for the permeable group with various concentrations of DiI-ox-LDLs. As shown from the figure, the average vw was about 20.5 × 10−6 cm s–1. For the non-permeable group, the water-filtration rate was zero (data not shown in the figure).
Figure 2.
(a) Water-filtration rate for the permeable group (diamonds) with concentration of DiI-ox-LDL varied from 10 to 200 µg ml–1. (b) Wall concentration of DiI-ox-LDLs in permeable group (diamonds) was assessed by measuring the fluorescence intensity of DiI-ox-LDL using a confocal laser microscope. All of the data were normalized with the bulk concentration, c0. The results are expressed as means ± s.d. (n = 5).
Figure 2b shows wall concentration of DiI-ox-LDLs in the permeable group. As shown from the figure, the relative wall concentration for the permeable group, cw/c0, is higher than 1.0, indicating that the concentration polarization of ox-LDLs occurred at the cultured SMCs layer surface. The measurement showed that cw was about 15.3 per cent higher than c0 in all conditions in the permeable group. Different from the permeable group, owing to no filtration flow across the SMC layer, cw/c0 was always 1.0 for the non-permeable group (data not shown in the figure).
3.2. Effect of oxidized low-density lipoproteins on cellular proliferation
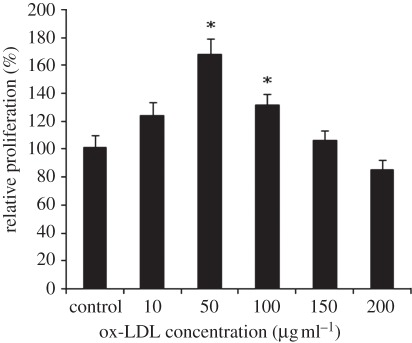
Figure 3 shows the effect of ox-LDL concentration (10–200 µg ml–1) on cellular proliferation by MTT assay. As shown in figure 3, treatment with ox-LDL (10–100 µg ml–1) for 24 h resulted in increased cell proliferation, and reached its highest value at 50 µg ml–1. However, doses beyond 200 mg ml–1 had an obvious cytotoxic effect.
Figure 3.
Effect of different concentrations of ox-LDL on human SMCs proliferation assessed by MTT assay. The results are expressed as means ± s.d. (n = 5). Asterisks denote significant different between experimental and control groups (p < 0.05).
3.3. DiI-oxidized low-density lipoprotein uptake
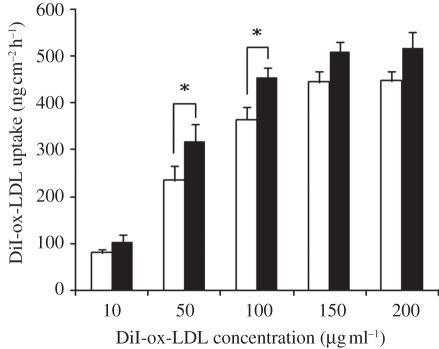
Figure 4 illustrates DiI-ox-LDL uptake by the SMCs cultured on the permeable and the non-permeable membranes. The SMCs were treated with ox-LDL suspensions of different concentrations for 24 h. As evident from the figure, the DiI-ox-LDL uptake by SMCs was positively correlated with the concentration of DiI-ox-LDLs for both the groups, but the correlation was highly nonlinear. At low concentrations (10–150 µg ml–1), the DiI-ox-LDL uptake increased gradually with increasing concentration. Beyond 150 µg ml–1, the uptake curve reached a plateau. The experimental results also showed that DiI-ox-LDL uptakes in the permeable group were obviously higher than those in the non-permeable group.
Figure 4.
DiI-ox-LDL uptake by SMCs cultured on the permeable (filled bars) and non-permeable (open bars) membranes treated with varied ox-LDL suspensions for 24 h. The wall shear stress and the perfusion pressure were kept at 1.3 Pa and 100 mmHg, respectively. The results are expressed as means ± s.d. (n = 5). Asterisks denote significant difference between permeable and non-permeable groups (p < 0.05).
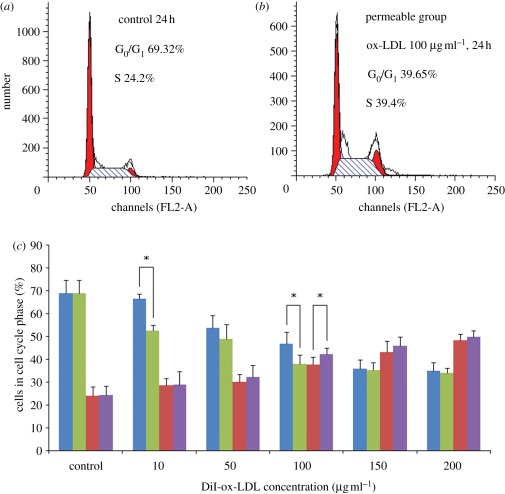
3.4. Oxidized low-density lipoprotein-induced cell cycle
Effect of different concentrations of ox-LDL on the cell cycle phases was analysed by FCM with the SMCs cultured on the permeable and the non-permeable membranes (figure 5). Control SMCs demonstrated normal cell cycle characteristics. When treated with 10–200 µg ml–1 ox-LDL in both the experimental groups, SMCs had gradual substantial decreases in the proportion of cells in G0/G1 phase, contrarily the proportion of cells in S phase increased. Nevertheless, the proportion of cells in S phase increased very sharply with ox-LDL concentrations beyond 100 µg ml–1 compared with the control group. The results suggest that low concentrations of ox-LDL induce SMCs proliferation by shortening G0/G1 phase, while high concentrations of ox-LDL restrain SMCs proliferation by prolonging the duration of the S phase of their cell cycle.
Figure 5.
Effect of different concentrations of ox-LDL on SMCs' cell cycle phase analysed by flow cytometry. (a) Control group, not treated with ox-LDL; (b) permeable group, treated with 100 µg ml–1 ox-LDL; (c) permeable group (green bars, G0/G1 phase; purple bars, S phase), treated with 10–200 µg ml–1 ox-LDL; non-permeable group (blue bars, G0/G1 phase; red bars, S phase), treated with 10–200 µg ml–1 ox-LDL. The results are expressed as means ± s.d. (n = 5). Asterisks denote significant difference between permeable and non-permeable groups (p < 0.05).
3.5. Oxidized low-density lipoprotein-induced apoptosis
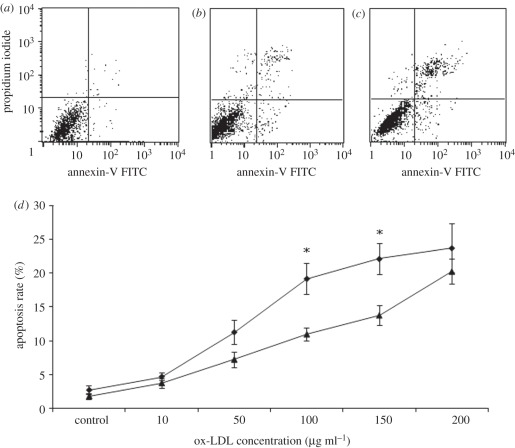
Figure 6a–c gives examples of ox-LDL-induced apoptosis in SMCs cultured on the permeable and the non-permeable membranes treated with ox-LDL suspensions of 100 µg ml–1 for 24 h. Figure 6d shows the apoptosis rate of SMCs. As shown in the figure, the apoptosis rate increased with increasing concentration of ox-LDLs in both two experimental groups. Nevertheless, the apoptosis rates in the permeable group were always higher than that in non-permeable group, especially when the concentration of ox-LDLs was at 100 and 150 µg ml–1.
Figure 6.
Effect of different concentrations of ox-LDL on SMCs' apoptosis rate. The percentage of apoptotic cell population was determined by flow cytometry. (a) Control; (b) non-permeable group: treated with 100 µg ml–1 ox-LDL; (c) permeable group treated with 100 µg ml–1 ox-LDL; (d) apoptosis rate in SMCs cultured on the permeable (diamonds) and non-permeable (triangles) membranes. The results are expressed as means ± s.d. (n = 5). Asterisks denote significant difference between permeable and non-permeable groups (p < 0.05).
4. Discussion
Ox-LDL is believed to play a key role in cellular dysfunction, as well as cholesterol accumulation and subsequent foam-cell transformation in macrophages and SMCs [12–24]. Because the endothelium of the artery displays low permeability to plasma proteins, it has been suggested that the filtration flow across the artery wall may cause concentration polarization of LDLs with the LDLs increasing in concentration from bulk value towards interface within the arterial system [8]. In the present study, to study the effect of ox-LDLs' concentration polarization on SMCs, we investigated the water-filtration rate and wall concentration of ox-LDLs in the vascular SMC culture setting in vitro. We compared cell viability, ox-LDL uptake, cell cycle and cell apoptosis by the SMCs cultured on a permeable membrane with those cultured on a non-permeable membrane.
The experimental results showed that for the permeable group, owing to the presence of filtration flow, the wall concentration of ox-LDLs (cw) was about 15.3 per cent higher than the bulk concentration (c0), indicating that concentration polarization of ox-LDLs occurred at the surface of the cultured cell layers. For the non-permeable group, because the water-filtration rate across the cell monolayer was zero, the value of cw remained the same as that of c0. Consequently, the DiI-ox-LDL uptake was obviously higher for the permeable group than that for the non-permeable group. As a result, the permeable group showed an obviously elevated SMC apoptosis compared with the non-permeable group.
Abnormal proliferation of SMC in the subendothelial space of the arterial wall is a major feature of atherosclerotic lesions [25,26]. Cellular proliferation is dependent on the cell cycle progression, in which cells transit through the G0/G1 phase to the S phase [27]. Data from this report demonstrated that cells treated with 10–100 µg ml–1 ox-LDL had obvious decreases in the proportion of cells in G0/G1 for 24 h circulation. In contrast, cells maintained in high ox-LDL concentration (100–200 µg ml–1) remained 39–49% S-arrested. Consistent with MTT colorimetric assay, treatment with at low concentration of ox-LDL (10–100 µg ml–1) resulted in increased cell proliferation in a dose-dependent manner; however, ox-LDL at doses of 200 µg ml–1 began to elicit a cytotoxic effect. This finding of ox-LDL exhibiting a biphasic effect on SMC is similar to that reported by Auge et al. [28] and Chatterjee [25].
Experimental results by Galle et al. [29] and Essler et al. [30] found that high concentrations of ox-LDLs could induce the formation of intercellular gaps and vascular lesions, which would most probably lead to increased filtration flow across the arterial wall, hence a significant increase in ox-LDL uptake by the arterial wall. In turn, this may accelerate apoptosis in vascular cells. The present study revealed that DiI-ox-LDL uptake by cultured SMCs was positively but nonlinearly correlated with the concentration of DiI-ox-LDLs. At low concentration (10–50 µg ml–1), the DiI-ox-LDL uptake in this stage increased drastically as a result of the increase in cell number. With the increase in concentration (100–150 µg ml–1), toxicity of DiI-ox-LDLs started to take effect, leading to the formation of intercellular gaps, hence increased filtration flow across the cultured SMC monolayer. The slight toxicity of DiI-ox-LDLs-induced SMC death and apoptosis, resulting in the ox-LDL uptake increasing gradually in this stage. At high concentrations (150–200 µg ml–1), the toxicity of DiI-ox-LDLs led to significantly increased non-viable cells and a high apoptosis rate. Therefore, DiI-ox-LDL uptake by the cells stopped and the uptake curve levelled out.
Under certain conditions SMCs would be exposed directly to blood flow streams, e.g. denudation of endothelial cells in angioplasty or arterial stenting procedures, which might result in severe concentration polarization of atherogenic lipoproteins, accelerating atherogenesis. Artery bypass graft surgery with autologous vein grafting is widely performed to relieve arterial occlusions, but the re-occlusion problem that contributes to poor long-term patency rates remains unresolved. It has been suggested that arteriosclerosis plays a major role in late vein graft occlusion and yields lesions [31]. Experimental results by Vlodaver & Edwards [32] showed that vein grafts become occluded when abnormal cell proliferation in the SMC layer produces extra tissue in the inner lining of the vessel, a process called neointimal hyperplasia. In addition, neointimal cells also express abnormal adhesion molecules that attract atherosclerotic deposits [33,34]. The present study showed that concentration polarization of ox-LDL will occur at the surface of the SMC monolayer that was directly exposed to blood flow, leading to increasing ox-LDL uptake and cell apoptosis, which plays a crucial role in atherosclerosis. If we intentionally reduce the incidence of atherogenic lipoproteins concentration polarization in autologous vein grafts, such as inducing swirling flow pattern at some critical areas [35,36], it might lower the concentration of atherogenic lipoproteins near the wall and suppress the interaction of atherogenic lipoproteins with the wall of the autologous vein grafts.
More interestingly, it was noted that when studying the effects of ox-LDLs on SMCs' proliferation, uptake, cell cycle and apoptosis rate by SMCs cultured on the permeable or non-permeable membranes, a significant difference between the two groups was only evident at a concentration of 100 µg ml−1 ox-LDL (p < 0.05). It could be concluded that concentration polarization of ox-LDLs has its maximum effect only in a limited area.
It should be noted that the present findings were obtained under conditions of steady wall shear stress and constant perfusion pressure within the flow chamber. Further studies will focus on the effects of pulsatile flow with different levels of shear stress and pressure on concentration polarization of LDL/ox-LDL. From the results of the present study and others [37], we speculate that low level steady and pulsatile shear stress with high blood pressure would aggravate polarization of LDL/ox-LDL at the surface of the arterial wall and accelerate the development of atherosclerosis.
5. Conclusion
The present study has provided evidence to support that the existence of concentration polarization of ox-LDLs may cause increased death and apoptosis of SMCs. Subsequently, it would enhance the rate of ox-LDL infiltration/accumulation within the arterial wall, leading to atherogenesis.
Acknowledgements
This work is supported by Grants-in-Aid from the National Natural Science Foundation of China (nos. 31170904 and 11072023) and the School Doctoral Foundation of Zhengzhou University of Light Industry.
References
- 1.Ross R. 1993. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362, 801–809 10.1038/362801a0 (doi:10.1038/362801a0) [DOI] [PubMed] [Google Scholar]
- 2.Tabas I. 1999. Nonoxidative modifications of lipoproteins in atherogenesis. Annu. Rev. Nutr. 19, 123–139 10.1146/annurev.nutr.19.1.123 (doi:10.1146/annurev.nutr.19.1.123) [DOI] [PubMed] [Google Scholar]
- 3.Adachi H., Tsujimotoa M. 2006. Endothelial scavenger receptors. Prog. Lipid. Res. 45, 379–404 10.1016/j.plipres.2006.03.002 (doi:10.1016/j.plipres.2006.03.002) [DOI] [PubMed] [Google Scholar]
- 4.Cobbold C. A., Sherratt J. A., Maxwell S. R. J. 2002. Lipoprotein oxidation and its significance for atherosclerosis: a mathematical approach. Bull. Math. Biol. 64, 65–95 10.1006/bulm.2001.0267 (doi:10.1006/bulm.2001.0267) [DOI] [PubMed] [Google Scholar]
- 5.Colles S. M., Maxson J. M., Carlson S. G., Chisolm G. M. 2001. Oxidized LDL-induced injury and apoptosis in atherosclerosis: potential roles for oxysterols. Trends. Cardiovasc. Med. 11, 131–138 10.1016/S1050-1738(01)00106-2 (doi:10.1016/S1050-1738(01)00106-2) [DOI] [PubMed] [Google Scholar]
- 6.Hoff H. F., O'Neil J. A. 1991. Oxidation of LDL: role in atherogenesis. Wien. Klin. Wochenschr. 69, 1032–1038 10.1007/BF01645153 (doi:10.1007/BF01645153) [DOI] [PubMed] [Google Scholar]
- 7.Lusis A. J. 2000. Atherosclerosis. Nature 407, 233–241 10.1038/35025203 (doi:10.1038/35025203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng X. Y., Marois Y., How T., Merhi Y., King M., Guidoin R. 1995. Luminal surface concentration of lipoprotein (LDL) and its effect on the wall uptake of cholesterol by canine carotid arteries. J. Vasc. Surg 21, 135–145 10.1016/S0741-5214(95)70252-0 (doi:10.1016/S0741-5214(95)70252-0) [DOI] [PubMed] [Google Scholar]
- 9.Wang G. X., Deng X. Y., Guidoin R. 2004. Concentration polarization of macromolecules in canine carotid arteries and its implication for the localization of atherogenesis. J. Biomech. 36, 45–51 10.1016/S0021-9290(02)00277-4 (doi:10.1016/S0021-9290(02)00277-4) [DOI] [PubMed] [Google Scholar]
- 10.Ding Z. F., Fan Y. B., Deng X. Y., Sun A. Q., Kang H. Y. 2010. 3,3′-Dioctadecylindocarbocyanine-low-density lipoprotein uptake and flow patterns in the rabbit aorta–iliac bifurcation under three perfusion flow conditions. Exp. Biol. Med. 235, 1062–1071 10.1258/ebm.2010.010035 (doi:10.1258/ebm.2010.010035) [DOI] [PubMed] [Google Scholar]
- 11.Joe M., James J. Y., Rosanne B., St 2010. Microarray analysis of ox-LDL (oxidized low-density lipoprotein)-regulated genes in human coronary artery smooth muscle cells. Cell. Biol. Int. Rep. 17, 33–45 10.1042/CBR20100006 (doi:10.1042/CBR20100006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zettler M. E., Prociuk M. A., Austria J. A., Massaeli H., Zhong G., Pierce G. N. 2003. OxLDL stimulates cell proliferation through a general induction of cell cycle proteins. Am. J. Physiol. Heart. Circ. Physiol. 284, H644–H653 10.1152/ajpheart.00494.2001 (doi:10.1152/ajpheart.00494.2001) [DOI] [PubMed] [Google Scholar]
- 13.Okura Y., Brink M., Itabe H., Scheidegger K. J., Kalangos A., Delafontaine P. 2000. Oxidized low-density lipoprotein is associated with apoptosis of vascular smooth muscle cells in human atherosclerotic plaques. Circulation 102, 2680–2686 10.1161/01.CIR.102.22.2680 (doi:10.1161/01.CIR.102.22.2680) [DOI] [PubMed] [Google Scholar]
- 14.Napoli C., Quehenberger O., De Nigris F., Abete P., Glass C. K., Palinski W. 2000. Mildly oxidized low density lipoprotein activates multiple apoptotic signaling pathways in human coronary cells. FASEB. J. 14, 1996–2007 10.1096/fj.99-0986com (doi:10.1096/fj.99-0986com) [DOI] [PubMed] [Google Scholar]
- 15.Mehta J. L., Chen J., Hermonat P. L., Romeo F., Novelli G. 2006. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc. Res. 69, 36–45 10.1016/j.cardiores.2005.09.006 (doi:10.1016/j.cardiores.2005.09.006) [DOI] [PubMed] [Google Scholar]
- 16.Kunjathoor V. V., et al. 2002. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 277, 49982–49988 10.1074/jbc.M209649200 (doi:10.1074/jbc.M209649200) [DOI] [PubMed] [Google Scholar]
- 17.Redgrave T. G., Roberts D. C. K., West C. E. 1974. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal. Biochem. 65, 42–49 10.1016/0003-2697(75)90488-1 (doi:10.1016/0003-2697(75)90488-1) [DOI] [PubMed] [Google Scholar]
- 18.Zhang A. Q., Vertommena J., Gaala V. L., Leeuwa I. D. 1994. A rapid and simple method for measuring the susceptibility of low-density-lipoprotein and very-low-density-lipoprotein to copper-catalyzed oxidation. Clin. Chim. Acta. 227, 159–173 10.1016/0009-8981(94)90144-9 (doi:10.1016/0009-8981(94)90144-9) [DOI] [PubMed] [Google Scholar]
- 19.Hamilton C. A. 1997. Low-density lipoprotein and oxidised low-density lipoprotein: their role in the development of atherosclerosis. Pharmacol. Ther. 74, 55–72 10.1016/S0163-7258(96)00202-1 (doi:10.1016/S0163-7258(96)00202-1) [DOI] [PubMed] [Google Scholar]
- 20.Ding Z. F., Fan Y. B., Deng X. Y. 2009. Effect of LDL concentration polarization on the uptake of LDL by human endothelial cells and smooth muscle cells co-cultured. Acta. Biochim. Biophys. Sin. 41, 146–153 10.1093/abbs/gmn017 (doi:10.1093/abbs/gmn017) [DOI] [PubMed] [Google Scholar]
- 21.Yan Q. J., Li Y. X., Jiang Z. Q., Sun Y., Zhu L. F., Ding Z. F. 2009. Antiproliferation and apoptosis of human tumor cell lines by a lectin (AMML) of Astragalus mongholicus. Phytomedicine 16, 586–593 10.1016/j.phymed.2008.12.024 (doi:10.1016/j.phymed.2008.12.024) [DOI] [PubMed] [Google Scholar]
- 22.Ling W. H., Peng G. Y., Ma J. 2000. OX-LDL induced apoptosis of arterial vascular smooth muscle cells of rats. Atherosclerosis 151, 306. 10.1016/S0021-9150(00)81390-8 (doi:10.1016/S0021-9150(00)81390-8) [DOI] [Google Scholar]
- 23.Mayr M., Xu Q. 2001. Smooth muscle cell apoptosis in arteriosclerosis. Exp. Gerontol. 36, 969–987 10.1016/S0531-5565(01)00090-0 (doi:10.1016/S0531-5565(01)00090-0) [DOI] [PubMed] [Google Scholar]
- 24.Ross R. 1999. Atherosclerosis: an inflammatory disease. N. Engl. J. Med. 340, 115–126 10.1016/S1053-0770(99)90233-1 (doi:10.1016/S1053-0770(99)90233-1) [DOI] [PubMed] [Google Scholar]
- 25.Chatterjee S. 1992. Role of oxidized human plasma low-density lipoproteins in atherosclerosis: effects on smooth muscle cell proliferation. Mol. Cell. Biochem. 111, 143–147 10.1007/BF00229586 (doi:10.1007/BF00229586) [DOI] [PubMed] [Google Scholar]
- 26.Dzau V. J., Braun-Dullaeus R. C., Sedding D. G. 2002. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat. Med. 8, 1249–1256 10.1038/nm1102-1249 (doi:10.1038/nm1102-1249) [DOI] [PubMed] [Google Scholar]
- 27.Norbury C., Nurse P. 1992. Animal cell cycles and their control. Annu. Rev. Biochem. 61, 441–468 10.1146/annurev.bi.61.070192.002301 (doi:10.1146/annurev.bi.61.070192.002301) [DOI] [PubMed] [Google Scholar]
- 28.Auge N., Pieraggi M. T., Thiers J., Negre-Salvayre R. 1995. Proliferation and cytotoxic effects of mildly oxidized low-density lipoproteins on vascular smooth muscle cells. Biochem. J. 309, 1015–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galle J., Hansen-Hagge T., Wanner C., Seibold S. 2006. Impact of oxidized low density lipoprotein on vascular cells. Atherosclerosis 185, 219–226 10.1016/j.atherosclerosis.2005.10.005 (doi:10.1016/j.atherosclerosis.2005.10.005) [DOI] [PubMed] [Google Scholar]
- 30.Essler M., Retzer M., Bauer M., Heemskerk J. W., Aepfelbacher M., Siess W. 1999. Mildly oxidized low density lipoprotein induces contraction of human endothelial cells through activation of Rho/Rho kinase and inhibition of myosin light chain phosphatase. J. Biol. Chem. 274, 30 361–30 364 10.1074/jbc.274.43.30361 (doi:10.1074/jbc.274.43.30361) [DOI] [PubMed] [Google Scholar]
- 31.Mayr M., Li C., Zou Y., Huemer U., Hu Y., Xu Q. 2000. Biomechanical stress-induced apoptosis in vein grafts involves p38 mitogen-activated protein kinases. FASEB. J. 15, 261–270 [DOI] [PubMed] [Google Scholar]
- 32.Vlodaver A., Edwards G. E. 1971. Pathologic changes in aortic-coronary arterial saphenous vein grafts. Circulation 44, 719–728 [DOI] [PubMed] [Google Scholar]
- 33.Motwani J. G., Topol E. J. 1998. Aortocoronary saphenous vein graft disease: pathogenesis predisposition prevention. Circulation 97, 916–931 [DOI] [PubMed] [Google Scholar]
- 34.Salvayre R., Auge N., Benoist H., Negre-Salvayre A. 2002. Oxidized low-density lipoprotein-induced apoptosis. Biochim. Biophys. Acta. 1585, 213–221 [DOI] [PubMed] [Google Scholar]
- 35.Fan Y. B., Xu Z. P., Jiang W. T., Deng X. Y., Wan K., Sun A. Q. 2008. S-type bypass can improve the hemodynamics in the bypassed arteries and suppress intimal hyperplasia along the host artery floor. J. Biomech. 41, 2498–2505 10.1016/j.jbiomech.2008.05.008 (doi:10.1016/j.jbiomech.2008.05.008) [DOI] [PubMed] [Google Scholar]
- 36.Ding Z. F., Fan Y. B., Deng X. Y., Zhan F., Kang H. Y. 2010. Effect of swirling flow on the uptakes of native and oxidized LDLs in a straight segment of the rabbit thoracic aorta. Exp. Biol. Med. 235, 506–513 10.1258/ebm.2009.009245 (doi:10.1258/ebm.2009.009245) [DOI] [PubMed] [Google Scholar]
- 37.Wootton D. M., Ku D. N. 1999. Fluid mechanics of vascular systems, diseases, and thrombosis. Annu. Rev. Biomed. Eng. 1, 299–329 10.1146/annurev.bioeng.1.1.299 (doi:10.1146/annurev.bioeng.1.1.299) [DOI] [PubMed] [Google Scholar]