Abstract
The ability of cells to adapt their mechanical properties to those of the surrounding microenvironment (tensional homeostasis) has been implicated in the progression of a variety of solid tumours, including the brain tumour glioblastoma multiforme (GBM). GBM tumour cells are highly sensitive to extracellular matrix (ECM) stiffness and overexpress a variety of focal adhesion proteins, such as talin. While talin has been shown to play critical early roles in integrin-based force-sensing in non-tumour cells, it remains unclear whether this protein contributes to tensional homeostasis in GBM cells. Here, we investigate the role of the talin isoform talin-1 in enabling human GBM cells to adapt to ECM stiffness. We show that human GBM cells express talin-1, and we use RNA interference to suppress talin-1 expression without affecting levels of talin-2, vinculin or phosphorylated focal adhesion kinase. Knockdown of talin-1 strongly reduces both cell spreading area and random migration speed but does not significantly affect overall focal adhesion size distributions. Most strikingly, atomic force microscopy indentation reveals that talin-1 suppression compromises adaptation of cell stiffness to changes in ECM stiffness. Together, these data support a role for talin-1 in the maintenance of tensional homeostasis in GBM and suggest a functional role for enriched talin expression in this tumour.
Keywords: talin-1, glioma, tensional homeostasis
1. Introduction
Tissue architecture reflects a force balance in which cells adapt their cytoskeletal tension to match forces generated by neighbouring cells and the surrounding extracellular matrix (ECM). This ‘tensional homeostasis’ is now recognized to play central roles in development, wound healing and tissue regeneration [1–3]; dysfunction in this process can contribute to a variety of diseases, including cancer [4–6]. For example, breast tumours are significantly stiffer than normal breast tissue, and malignant transformation may be promoted in vitro and in vivo by stiffening the ECM [7–9]. Similarly, the brain tumour glioblastoma multiforme (GBM), a malignancy of the central nervous system in which individual cells remodel and diffusely invade the surrounding ECM [10], is characterized by extensive tissue stiffening [11]. The proliferation, motility and mechanics of cultured GBM tumour cells are highly sensitive to changes in ECM stiffness [12,13], indicating that alterations in tensional homeostasis may play a significant role in GBM tumorigenesis and invasion.
The increasing appreciation of tensional homeostasis as a contributor to tumour progression has spurred interest in identifying molecular mediators of this process, with the goals of better understanding pathophysiology and developing novel drug targets. Focal adhesion proteins have emerged as natural candidates in this process, given their demonstrated importance in mediating integrin-based sensing of mechanical inputs from the ECM [14,15]. While focal adhesions are complex and dynamic structures with more than 80 known molecular components [16], the protein talin (specifically, its two human isoforms, talin-1 and talin-2) has garnered specific interest because of its abnormal regulation in several tumour types. For example, in oral squamous cell carcinoma, talin-1 overexpression has been correlated with a metastatic phenotype [17]. Similarly, in prostate cancer cells, talin-1 overexpression contributes to enhanced adhesion, migration and invasion through activation of survival signals and rendering resistance to anoikis [18]. Independent of its interactions with integrins, recent reports have also implicated talin-1 in regulating the expression of the cell–cell adhesion protein E-cadherin [19]. Given the close connection between GBM progression and aberrant cell adhesion and migration, focal adhesion proteins have begun to emerge as targets of interest in GBM. For example, the focal adhesion and actin crosslinking protein α-actinin has been shown to regulate the motility and mechanoadaptation of glioma cells [12,20]. Because both talin and α-actinin physically link the ECM to the cytoskeleton by binding simultaneously to integrins and actin, it is likely that talin plays a similarly important role in regulating glioma invasiveness. Consistent with this notion, heterogeneous high expression of talin across different glioma cell lines with different metastatic potential suggests that talin expression might be tied to the extent of invasiveness of glioma cells [21]. Together, these reports indicate that talin expression is closely tied to the invasive properties of multiple types of cancers, including potentially GBM, and may be used as a marker of tumour progression and metastasis.
The role of talin in tumour progression is particularly interesting when viewed in the context of its role in transducing mechanical signals from the ECM to the cytoskeleton through its engagement of integrins and actin. More specifically, the recruitment of talin to the cytoplasmic domains of integrins can facilitate ‘inside-out’ activation of integrins, which strongly increases the affinity of integrin extracellular domains for ECM proteins [22,23]. Moreover, talin is one of the first proteins recruited to integrin clusters in the early stages of focal adhesion formation and provides a binding site for vinculin, which can subsequently trigger further adhesion maturation [24]. Functionally, talin plays an important role during cell spreading and assembly of focal adhesions [25–27]. In cells expressing both talin isoforms, talin-1 deficiency can lead to compensatory upregulation of talin-2 and is sufficient for focal adhesion assembly and spreading [28,29]. However, in human umbilical vein endothelial cells (HUVECs) that express only talin-1, siRNA-mediated suppression of talin-1 has been shown to inhibit focal adhesion assembly and spreading [30]. The structural contributions of talin in focal adhesion organization have been more clearly elucidated by recent super-resolution imaging studies, which have revealed that talin interacts with integrins through its N-terminal head domain, and with focal adhesion kinase (FAK), paxillin and vinculin by means of its angled orientation at focal adhesions [31].
While talin is the key to transducing mechanical inputs from the ECM and has also been implicated in tumour progression, it is unclear whether, or to what extent, talin contributes to tensional homeostasis in tumours such as GBM, whose pathophysiology involves altered mechanobiological interactions with the ECM. In this paper, we address this open question by investigating the role of talin-1 in the regulation of spreading, motility and stiffness adaptation of human GBM cells. We show that talin-1 suppression reduces the spreading and motility of glioma cells without accompanying changes in the expression of talin-2, vinculin, phosphorylated FAK. Most importantly, we show that talin-1 suppression compromises the ability of glioma cells to adapt their intrinsic stiffness to changes in ECM stiffness, directly supporting a role for talin-1 in regulating tensional homeostasis in this tumour.
2. Material and methods
2.1. Cell culture
U373 MG human glioma cells were obtained from the Tissue Culture Facility at the University of California, Berkeley, CA, USA and cultured in DMEM (Invitrogen) supplemented with 10 per cent calf serum (J.R. Scientific), 1 per cent penicillin/streptomycin, 1 per cent non-essential amino acids and 1 per cent sodium pyruvate (all from Invitrogen). Motility, immunofluorescence and cell stiffness measurements were conducted with matrices of human plasma fibronectin (Millipore) adsorbed to glass coverslips, adsorbed to tissue culture plastic or conjugated to polyacrylamide (PA) hydrogels, respectively. PA hydrogels were synthesized by mixing varying amounts of acrylamide and bis-acrylamide to obtain gels of varying stiffness [32]. For cell culture experiments, these hydrogels were functionalized with fibronectin at a fixed density using the photoactivatable crosslinker sulpho-SANPAH as previously described [13]. For assessing the influence of talin-1 in glioma cells, cells were transfected with talin-1-specific siRNA (siTLN1) or scrambled sequence (siCTL; Dharmacon) according to manufacturer specifications. All experiments were carried out 3 days after the addition of siRNA.
2.2. Immunostaining and Western blots
For immunostaining, cells cultured on fibronectin-coated glass coverslips were rinsed with phosphate-buffered saline (PBS; Fisher Scientific) and fixed with 4 per cent para-formaldehyde solution for 10 min. Fixed cells were permeabilized with 0.5 per cent Triton X-100, and blocked in 5 per cent BSA for 1 h at room temperature and then incubated for 1 h at room temperature in one of the following primary antibody solutions in PBS at the specified dilutions: mouse anti-talin-1 (1 : 100, Sigma), chicken anti-talin-2 (1 : 100, Sigma), mouse anti-vinculin (1 : 200, Sigma) and rabbit anti-pY397FAK (1 : 200, Invitrogen). After incubation with primary antibody, cells were rinsed with PBS and then incubated for 1 h at room temperature with the relevant secondary antibodies (1 : 500, Invitrogen) and Alexa 488 phalloidin (1 : 200, Invitrogen). Finally, samples were mounted onto slides with Cytoseal 60 (Richard Allan Scientific). For Western blots, total cell lysates were obtained using radioimmunoprecipitation assay (RIPA)-buffer-containing protease inhibitors (both from Sigma), and the supernatant was collected after spinning at 10 000 r.p.m. for 10 min at 4°C. Immunoblots were performed per manufacturer protocol (Invitrogen) using the relevant buffers (Invitrogen), primary antibodies (talin-1, talin-2, vinculin, pY397FAK, GAPDH (glyceraldehyde 3-phosphate dehydrogenase; 1 : 20 000, Sigma)) and horseradish peroxidase (HRP)-conjugated secondary antibodies (Invitrogen). After colour development using tetramethyl benzidine substrates (Invitrogen), bands were scanned and intensities were measured using ImageJ.
2.3. Optical and atomic force microscopy
Phase contrast and fluorescence imaging were performed using an inverted Nikon TE2000-E2 microscope equipped with a motorized, programmable stage (Prior Scientific), and an incubator chamber to maintain constant temperature, humidity and CO2 levels (In Vivo Scientific). Images were captured with a CoolSNAP HQ2 camera (Roper Scientific/Photometrics) interfaced with SimplePCI software (Hamamatsu). Cell spreading areas were measured using ImageJ (NIH). For motility measurements, cells were imaged every 15 min at 10× magnification for at least 6 h and quantified using the manual tracking plugin in ImageJ. Stiffness of PA gels and cells were obtained with an Asylum Research MFP3D atomic force microscope mounted on a Nikon TE2000-U epifluorescence microscope, as described previously [33].
2.4. Quantification of size distribution of focal adhesions
To determine the distribution of focal adhesions, we acquired our Y397FAK immunofluorescence images under the same exposure and gain settings and then subjected them to four image-processing routines in ImageJ: First, using ‘Process—Subtract Background’, the background was subtracted from all images. Next, these processed images were converted to 8 bit images (Image—Type—8 bit) and then thresholded (Image—Adjust—Threshold) to the same extent. Thresholded images were then inverted (Edit—Invert) and then the number of black streaks (indicative of focal adhesions) were measured using the ‘Analyze—Measure Particles’ plugin. This provides a measure of the area and circularity of all the focal adhesions in a given image. This was repeated for all the images acquired for a given condition. The aggregate data were then quantified using Origin software to determine the distribution of adhesions based on their size.
3. Results
3.1. Suppression of talin-1 in human glioma cells
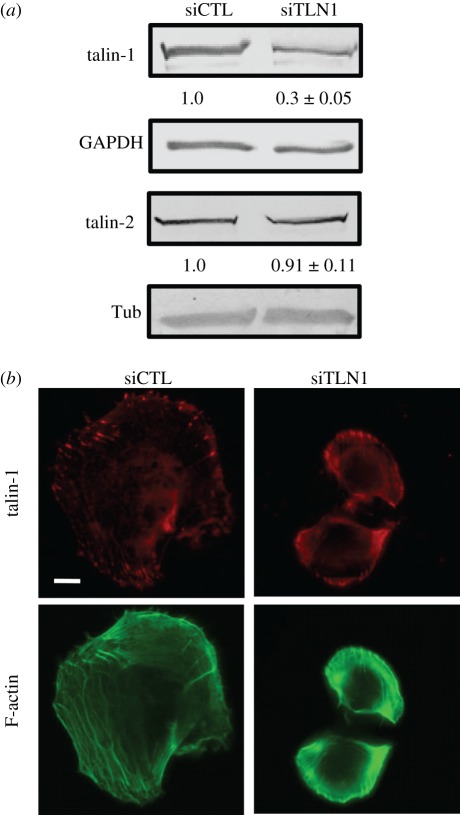
To investigate the role of talin-1 in a culture model of GBM, we used talin-1-specific siRNA (siTLN1) to suppress talin-1 expression in the U373 MG human glioma cell line and compared the results with cells treated with scrambled control siRNA (siCTL) 3 days after incubation with siRNA. Western blots of total cell lysates of control and talin-1-deficient samples revealed a approximately 70 per cent knockdown in talin-1 levels 3 days after transfection (figure 1a). Talin-1 suppression did not alter talin-2 levels in siTLN1 cells compared with controls. Consistent with the Western blots, talin-1-deficient cells stained weakly and non-specifically for talin-1 compared with control cells, which exhibited strong talin-1-positive focal adhesions (figure 1b).
Figure 1.
Suppression of talin-1 in glioma cells. (a) Western blots of cell lysates obtained from U373 MG glioma cells transfected with either scrambled control siRNA (siCTL) or siRNA directed against talin-1 (siTLN1) after 3 days in culture. The numbers below the talin-1 and talin-2 bands represent densitometric intensities normalized to the corresponding siCTL value (mean ± s.d. over n ≥ 3 blots). For example, the intensity of the talin-1/siTLN1 band normalized to the talin-1/siCTL band is 0.3, implying approximately 70% suppression of talin-1 expression. GAPDH and tubulin are loading controls used for internal normalization. (b) Immunofluorescence images of siCTL and siTLN1 cells stained for talin-1. Phalloidin labelling of F-actin shows stress fibres in these cells. Scale bar, 20 µm. (Online version in colour.)
3.2. Talin-1 contributes to the spreading and motility of glioma cells
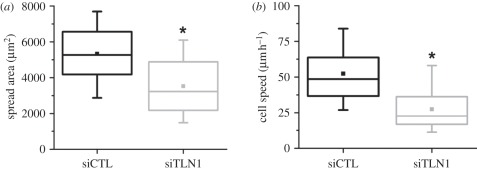
As described earlier, talin is known to play an important role in the formation and maturation of focal adhesions, and compromise of talin in non-glioma cells has been associated with altered adhesion and motility. Thus, we next asked whether knockdown of talin-1 altered these behaviours in our system. We compared the spreading of control and talin-1-deficient cells on fibronectin-coated tissue culture plastic after 3 days incubation with siRNA. At this time point, control cells were well spread with a mean spread area of approximately 5000 µm2. In contrast, talin-1-deficient cells were significantly less spread (*p < 0.001) with a mean spread area of approximately 3500 µm2 (figure 2a). To assess the effect of talin-1 knockdown on cell motility, we tracked random cell motility of control and talin-1-deficient cells for at least 6 h, using phase contrast time-lapse microscopy. Talin-1-deficient cells were substantially less motile than control cells, with talin-1 knockdown producing approximately 50 per cent reduction in mean cell speed from 52 to 27 µm h–1 (figure 2b). Collectively, these results demonstrate that talin-1 contributes strongly to both the spreading and motility of glioma cells.
Figure 2.
Contribution of talin-1 to spreading and motility of glioma cells. (a) Box–whisker plots of cell–ECM spreading area of siCTL and siTLN1 cells (subjected to RNAi treatment for 3 days) 24 h after trypsinization and plating on fibronectin-coated glass coverslips. (b) Box–whisker plots of mean speeds of siCTL and siTLN1 cells tracked over 6 h (*p < 0.001 in both cases).
3.3. Knockdown of talin-1 does not influence focal adhesion assembly in glioma cells
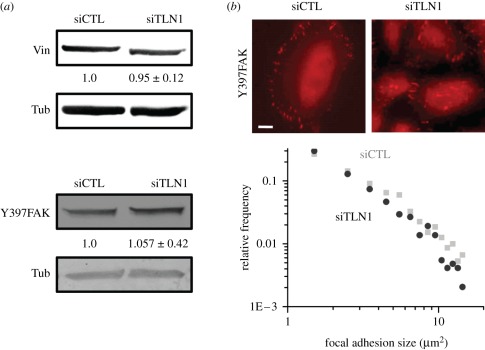
The previous results demonstrate that talin-1 deficiency leads to impaired spreading and motility in glioma cells. Such differences may arise owing to altered localization of other focal adhesion proteins, differences in the number and/or size of focal adhesions or to altered adhesion dynamics. To distinguish between these possibilities, we first compared levels of two well-characterized focal adhesion proteins that bind to talin—vinculin and FAK—in control and talin-1-deficient cells. While vinculin is involved in force-induced stabilization of focal adhesions, FAK is known to regulate migration through modulation of adhesion turnover. Interestingly, in glioma cells, neither vinculin expression nor FAK activation (i.e. pY397FAK levels) were affected by suppression of talin-1 levels (figure 3a). Further, quantitative image analysis of pY397FAK-labelled control and talin-1-deficient cells revealed no differences in either the number or the size distribution of pY397FAK-positive adhesions (figure 3b). Together, our findings suggest that the changes in cell adhesion and motility (figure 2) are not due strictly to altered adhesion assembly and maturation.
Figure 3.
Effect of talin-1 depletion on focal adhesion composition and size distribution. (a) Western blots of vinculin and pY397FAK in siCTL and siTLN1 cells revealed no significant alteration in the expression levels of these two proteins after treatment with RNAi for 3 days. Tubulin (Tub) levels are used as loading controls. As in figure 1, the numbers below the blots indicate densitometric band intensities (mean ± s.d. over n ≥ 3 blots) normalized to the corresponding siCTL value. (b) Localization of pY397FAK at focal adhesions in siCTL and siTLN1 cells. Scale bar, 20 µm. The plot depicts log–log histograms of focal adhesion areas for siCTL (black circles) and siTLN1 (grey squares), as determined from pY397FAK immunofluorescence images (see §2 for details on data acquisition and processing). The strong overlap between the two histograms implies similar focal adhesion size distributions for siCTL and siTLN1 cells. (Online version in colour.)
3.4. Talin-1 knockdown suppresses rigidity-sensing
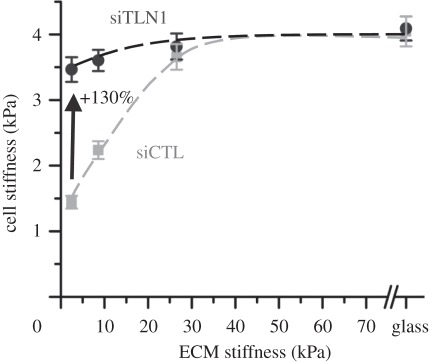
Impaired spreading and motility of talin-1-deficient cells indicate that talin-1 plays an important mechanosensory role in glioma cells. To determine whether talin-1 is also important in tensional homeostasis, we cultured control and talin-1-depleted cells on a series of fibronectin-coated PA hydrogels spanning a range of elasticities and compared the dependence of cortical stiffness on ECM elasticity (figure 4). In control cells, the cortical stiffness increased linearly from approximately 1.5 kPa on the soft substrates to approximately 2.0 kPa on 9 kPa gels and saturated to a value of approximately 4 kPa on ECMs of elasticities 26 kPa and higher. Depletion of talin-1 strongly suppressed this stiffness adaptation response. Irrespective of ECM stiffness, talin-1-depleted cells exhibited stiffnesses of approximately 4 kPa on all substrates, similar to the plateau stiffness of control cells observed on high stiffness substrates. These results demonstrate that talin-1 plays a critical role in the adaptation of glioma cells to micromechanical cues.
Figure 4.
Contributions of talin-1 to cell–ECM rigidity-sensing, as determined by atomic force microscopy (AFM) cortical stiffness measurements of siCTL cells (grey squares) and siTLN1 cells (black circles) cultured on defined-rigidity substrates 3 days after the corresponding siRNA treatment. Talin-1 suppression led to modest but statistically significant cell stiffening on the softest (2 kPa) substrates. On the stiffest substrates, no differences were observed.
4. Discussion
Our study identifies the focal adhesion protein talin-1 as a significant regulator of glioma cell spreading, motility and adaptation to ECM stiffness cues. Consistent with studies in other tumour systems, we find that loss of talin-1 significantly compromises cell motility. Interestingly, talin-1-deficient cells were unable to detect differences in ECM stiffness, which highlights the role of talin-1 in stiffness adapation. Collectively, our results support a role for talin-1 in modulating interactions of glioma cells with their ECM, which may in turn regulate glioma invasion. Repeating these studies in other cell types would help us to establish the generality of these findings.
Placing our results in context of previous functional studies of talin, it is important to note that the expression profiles and mutual crosstalk of talin-1 and talin-2 are highly cell-type-specific. While loss of talin-1 is embryonically lethal at gastrulation, mice are viable and healthy in the absence of the C-terminal half of talin-2 [34–36]. In contrast to HUVECs, which express only talin-1 [37], U373 MG glioma cells express both talin-1 and talin-2. However, in U373 MG glioma cells, we find no compensatory upregulation of talin-2 upon suppression of talin-1 (figure 1), suggesting the comparative absence of crosstalk between the two isoforms. Our findings also contrast somewhat with previous observations with embryonic stem-cell-derived fibroblasts, in which talin-1 suppression was found not to affect spreading [26]. The reduced motility observed in talin-1-deficient glioma cells is consistent with previous observations with prostate cancer and oral squamous carcinoma cells [17,18]. Cell motility involves polymerization of actin at the leading edge, stabilization of leading edge by formation of cell–ECM adhesions and myosin-dependent retraction of the trailing edge through disassembly of adhesions [38]. Because the size distribution of focal adhesions is not altered by talin-1 depletion, it is possible that in glioma cells either talin isoform is sufficient to mediate the assembly of adhesions. However, adhesion dynamics may be differentially regulated by talin-1 and talin-2. Additional experiments tracking the dynamics of focal adhesions would help us to clarify whether the reduced motility of talin-1-deficient cells is due to stronger adhesions, slower adhesion turnover or delay in formation of adhesions. Because talin-1 deficiency has been demonstrated to delay the formation of initial adhesions in fibroblasts [26], the reduced motility of talin-1-deficient glioma cells could potentially be attributed to a slow rate of formation of adhesions at the leading edge.
Our study also lends new insights into the role of talin-1 in mechanosensing, which is presumed to operate through talin-1's participation in integrin activation and reinforcement of integrin–cytoskeleton linkages. Using laser tweezers, Sheetz and co-workers [26] have demonstrated that talin-1 is directly involved in reinforcing initial integrin connections with the cytoskeleton and is associated with the recruitment of focal adhesion proteins vinculin and paxillin for stabilization of adhesions. Because the strength of integrin–cytoskeleton bonds depends on ECM rigidity [39], talin may help sense ECM rigidity through strengthening of cytoskeletal linkages in a force-dependent manner. Consistent with this, it was recently demonstrated that force-induced reinforcement in talin-1-deficient embryonic fibroblasts can be mediated through talin-2, and subsequent siRNA-induced depletion of talin-2 in these cells completely abolishes reinforcement [40]. In the context of studies on defined-stiffness ECMs, this phenomenon manifests itself as ‘stiffness adaptation’. For example, 3T3 fibroblasts tightly adapt their spreading area and cytoarchitecture to local variations in ECM stiffness [41] and can, within limits, match their intrinsic stiffness to that of the ECM [42]. We show here that glioma cells exhibit a similar mechanoadaptation response in which cell stiffness increases with ECM stiffness and saturates to a value of 4 kPa on ECMs of stiffness 26 kPa and higher. Strikingly, talin-1 depletion strongly suppresses this stiffness-sensing response as evidenced by the nearly flat profile of cell stiffness (figure 4), demonstrating a role for talin-1 in maintaining tensional homeostasis.
This strong reduction in stiffness adaptation is reminiscent of our previous study with α-actinin, in which we showed that suppression of either α-actinin-1 or -4 significantly restricted the ability of glioma cells to adaptively stiffen on highly rigid ECMs [12]. It is also consistent with the results of Byfield et al. [43], who showed that filamin-null melanoma cells remain relatively soft over a wide range of ECM stiffnesses. Unlike those earlier studies, however, we find here that talin-1-depleted cells are uniformly stiff on all ECMs and are in fact slightly stiffer than control cells on the most compliant substrates. It is important to note that this degree of stiffening (less than 1.5-fold) is quite modest compared with cell stiffness differences observed in some of these previous studies; so the functional importance of this result is uncertain. As one potential explanation, it is possible that talin-1-deficient cells redirect actin from basal stress fibres to the cortical cytoskeleton, producing high cell stiffnesses even on compliant ECMs. This concept is supported by the work of Solon et al. [42], who demonstrated that fibroblasts match their cortical stiffness to that of their underlying ECMs up to 5 kPa purely by reorganizing the cortical actin network and without forming additional stress fibres. Moreover, it is possible that talin-1 depletion may lead to enrichment of other focal adhesion proteins in the actin cortex, such as α-actinin and filamin A, which—in addition to crosslinking actin—also bind to β1 integrins, and can therefore act as mechanosensors [44,45]. Notably, it has been demonstrated that α-actinin and filamin can cooperatively enhance the stiffness of actin networks [46]. Given our previous results with α-actinin-depleted glioma cells [12], it is possible that cortical enrichment of α-actinin may lead to cell stiffening on soft matrices.
In conclusion, we have studied the role of talin-1 in regulating the tensional homeostasis of human glioma cells. While the association of talin with tumour invasiveness has been documented in some types of cancer, such as prostate and oral squamous cell carcinoma, our results demonstrate for the first time that talin-1 directly influences spreading, motility and rigidity-sensing in U373 MG human glioma cells. It remains to be seen if the effects of talin-1 suppression observed in U373 MG cells also holds true for other types of glioma cells or is dependent on tumour grade, and holds true in other types of cancers as well. Moreover, the expression profiles of talin-1 and talin-2 across different glioma cells need to be investigated, as does the degree to which talin-1 deficiency alters talin-2 levels. It would be interesting to study the function of talin-2 in talin-1-deficient cells and test if talin-2 mediates the assembly of focal adhesions in talin-1-deficient cells. Analogous experiments using talin-2-deficient cells will help us to understand the function of talin-1 in these cells and the nature of crosstalk between these two isoforms. Because both talin and actin crosslinking proteins such as α-actinin and filamin link integrins to the actin cytoskeleton but may have opposite effects on cell stiffness, it would also be interesting to explore whether the pattern of invasion of glioma cells is dependent on cytoskeletal mechanics by simultaneously manipulating expression of talin and actin crosslinking proteins.
Acknowledgements
S.K. gratefully acknowledges grant support from the NIH (1DP2OD00004213, Director's New Innovator Award, part of the NIH Roadmap for Medical Research; 1U54CA143836, NCI Physical Sciences-Oncology Centre Award) and a Young Investigator Award from the Arnold and Mabel Beckman Foundation.
References
- 1.Discher D., Dong C., Fredberg J. J., Guilak F., Ingber D., Janmey P., Kamm R. D., Schmid-Schonbein G. W., Weinbaum S. 2009. Biomechanics: cell research and applications for the next decade. Ann. Biomed. Eng. 37, 847–859 10.1007/s10439-009-9661-x (doi:10.1007/s10439-009-9661-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez J. I., Mouw J. K., Weaver V. M. 2008. Biomechanical regulation of cell orientation and fate. Oncogene 27, 6981–6993 10.1038/onc.2008.348 (doi:10.1038/onc.2008.348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wozniak M. A., Chen C. S. 2009. Mechanotransduction in development: a growing role for contractility. Nat. Rev. Mol. Cell Biol. 10, 34–43 10.1038/nrm2592 (doi:10.1038/nrm2592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butcher D. T., Alliston T., Weaver V. M. 2009. A tense situation: forcing tumour progression. Nat. Rev. Cancer 9, 108–122 10.1038/nrc2544 (doi:10.1038/nrc2544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaalouk D. E., Lammerding J. 2009. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 10, 63–73 10.1038/nrm2597 (doi:10.1038/nrm2597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S., Weaver V. M. 2009. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 28, 113–127 10.1007/s10555-008-9173-4 (doi:10.1007/s10555-008-9173-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gehler S., et al. 2009. Filamin A-β1 integrin complex tunes epithelial cell response to matrix tension. Mol. Biol. Cell 20, 3224–3238 10.1091/mbc.E08-12-1186 (doi:10.1091/mbc.E08-12-1186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levental K. R., et al. 2009. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 10.1016/j.cell.2009.10.027 (doi:10.1016/j.cell.2009.10.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paszek M. J., et al. 2005. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 10.1016/j.ccr.2005.08.010 (doi:10.1016/j.ccr.2005.08.010) [DOI] [PubMed] [Google Scholar]
- 10.Furnari F. B., et al. 2007. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 21, 2683–2710 10.1101/gad.1596707 (doi:10.1101/gad.1596707) [DOI] [PubMed] [Google Scholar]
- 11.Selbekk T., Bang J., Unsgaard G. 2005. Strain processing of intraoperative ultrasound images of brain tumours: initial results. Ultrasound Med. Biol. 31, 45–51 10.1016/j.ultrasmedbio.2004.09.011 (doi:10.1016/j.ultrasmedbio.2004.09.011) [DOI] [PubMed] [Google Scholar]
- 12.Sen S., Dong M., Kumar S. 2009. Isoform-specific contributions of α-actinin to glioma cell mechanobiology. PLoS ONE 4, e8427. 10.1371/journal.pone.0008427 (doi:10.1371/journal.pone.0008427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulrich T. A., de Juan Pardo E. M., Kumar S. 2009. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 69, 4167–4174 10.1158/0008-5472.CAN-08-4859 (doi:10.1158/0008-5472.CAN-08-4859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geiger B., Bershadsky A., Pankov R., Yamada K. M. 2001. Transmembrane crosstalk between the extracellular matrix and the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2, 793–805 10.1038/35099066 (doi:10.1038/35099066) [DOI] [PubMed] [Google Scholar]
- 15.Geiger B., Spatz J. P., Bershadsky A. D. 2009. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 10, 21–33 10.1038/nrm2593 (doi:10.1038/nrm2593) [DOI] [PubMed] [Google Scholar]
- 16.Wolfenson H., Henis Y. I., Geiger B., Bershadsky A. D. 2009. The heel and toe of the cell's foot: a multifaceted approach for understanding the structure and dynamics of focal adhesions. Cell Motil. Cytoskeleton 66, 1017–1029 10.1002/cm.20410 (doi:10.1002/cm.20410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai M. T., et al. 2010. Talin-1 overexpression defines high risk for aggressive oral squamous cell carcinoma and promotes cancer metastasis. J. Pathol. 224, 367–376 10.1002/path.2867 (doi:10.1002/path.2867) [DOI] [PubMed] [Google Scholar]
- 18.Sakamoto S., McCann R. O., Dhir R., Kyprianou N. 2010. Talin1 promotes tumor invasion and metastasis via focal adhesion signaling and anoikis resistance. Cancer Res. 70, 1885–1895 10.1158/0008-5472.CAN-09-2833 (doi:10.1158/0008-5472.CAN-09-2833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becam I. E., Tanentzapf G., Lepesant J. A., Brown N. H., Huynh J. R. 2005. Integrin-independent repression of cadherin transcription by talin during axis formation in Drosophila. Nat. Cell Biol. 7, 510–516 10.1038/ncb1253 (doi:10.1038/ncb1253) [DOI] [PubMed] [Google Scholar]
- 20.Quick Q., Skalli O. 2010. α-Actinin 1 and α-actinin 4: contrasting roles in the survival, motility, and RhoA signaling of astrocytoma cells. Exp. Cell Res. 316, 1137–1147 10.1016/j.yexcr.2010.02.011 (doi:10.1016/j.yexcr.2010.02.011) [DOI] [PubMed] [Google Scholar]
- 21.Belot N., et al. 2001. Molecular characterization of cell substratum attachments in human glial tumors relates to prognostic features. Glia 36, 375–390 10.1002/glia.1124 (doi:10.1002/glia.1124) [DOI] [PubMed] [Google Scholar]
- 22.Calderwood D. A. 2004. Talin controls integrin activation. Biochem. Soc. Trans. 32, 434–437 10.1042/BST0320434 (doi:10.1042/BST0320434) [DOI] [PubMed] [Google Scholar]
- 23.Calderwood D. A. 2004. Integrin activation. J. Cell Sci. 117, 657–666 10.1242/jcs.01014 (doi:10.1242/jcs.01014) [DOI] [PubMed] [Google Scholar]
- 24.Hu K., Ji L., Applegate K. T., Danuser G., Waterman-Storer C. M. 2007. Differential transmission of actin motion within focal adhesions. Science 315, 111–115 10.1126/science.1135085 (doi:10.1126/science.1135085) [DOI] [PubMed] [Google Scholar]
- 25.del Rio A., Perez-Jimenez R., Liu R., Roca-Cusachs P., Fernandez J. M., Sheetz M. P. 2009. Stretching single talin rod molecules activates vinculin binding. Science 323, 638–641 10.1126/science.1162912 (doi:10.1126/science.1162912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giannone G., Jiang G., Sutton D. H., Critchley D. R., Sheetz M. P. 2003. Talin1 is critical for force-dependent reinforcement of initial integrin–cytoskeleton bonds but not tyrosine kinase activation. J. Cell Biol. 163, 409–419 10.1083/jcb.200302001 (doi:10.1083/jcb.200302001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puklin-Faucher E., Sheetz M. P. 2009. The mechanical integrin cycle. J. Cell Sci. 122, 179–186 10.1242/jcs.042127 (doi:10.1242/jcs.042127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Priddle H., Hemmings L., Monkley S., Woods A., Patel B., Sutton D., Dunn G. A., Zicha D., Critchley D. R. 1998. Disruption of the talin gene compromises focal adhesion assembly in undifferentiated but not differentiated embryonic stem cells. J. Cell Biol. 142, 1121–1133 10.1083/jcb.142.4.1121 (doi:10.1083/jcb.142.4.1121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X., Jiang G., Cai Y., Monkley S. J., Critchley D. R., Sheetz M. P. 2008. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat. Cell Biol. 10, 1062–1068 10.1038/ncb1765 (doi:10.1038/ncb1765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopp P. M., et al. 2010. Studies on the morphology and spreading of human endothelial cells define key inter- and intramolecular interactions for talin1. Eur. J. Cell Biol. 89, 661–673 10.1016/j.ejcb.2010.05.003 (doi:10.1016/j.ejcb.2010.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanchanawong P., Shtengel G., Pasapera A. M., Ramko E. B., Davidson M. W., Hess H. F., Waterman C. M. 2011. Nanoscale architecture of integrin-based cell adhesions. Nature 468, 580–584 10.1038/nature09621 (doi:10.1038/nature09621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelham R. J., Jr, Wang Y. 1997. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl Acad. Sci. USA 94, 13 661–13 665 10.1073/pnas.94.25.13661 (doi:10.1073/pnas.94.25.13661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sen S., Kumar S. 2009. Cell–matrix de-adhesion dynamics reflect contractile mechanics. Cell. Mol. Bioeng. 2, 218–230 10.1007/s12195-009-0057-7 (doi:10.1007/s12195-009-0057-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monkley S. J., Pritchard C. A., Critchley D. R. 2001. Analysis of the mammalian talin2 gene TLN2. Biochem. Biophys. Res. Commun. 286, 880–885 10.1006/bbrc.2001.5497 (doi:10.1006/bbrc.2001.5497) [DOI] [PubMed] [Google Scholar]
- 35.Monkley S. J., et al. 2000. Disruption of the talin gene arrests mouse development at the gastrulation stage. Dev. Dyn. 219, 560–574 (doi:10.1002/1097-0177(2000)9999:9999<::AID-DVDY1079>3.0.CO;2-Y) [DOI] [PubMed] [Google Scholar]
- 36.Chen N. T., Lo S. H. 2005. The N-terminal half of talin2 is sufficient for mouse development and survival. Biochem. Biophys. Res. Commun. 337, 670–676 10.1016/j.bbrc.2005.09.100 (doi:10.1016/j.bbrc.2005.09.100) [DOI] [PubMed] [Google Scholar]
- 37.Kopp P. M., et al. 2010. Studies on the morphology and spreading of human endothelial cells define key inter- and intramolecular interactions for talin1. Eur. J. Cell Biol. 89, 661–673 10.1016/j.ejcb.2010.05.003 (doi:10.1016/j.ejcb.2010.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. 2003. Cell migration: integrating signals from front to back. Science 302, 1704–1709 10.1126/science.1092053 (doi:10.1126/science.1092053) [DOI] [PubMed] [Google Scholar]
- 39.Choquet D., Felsenfeld D. P., Sheetz M. P. 1997. Extracellular matrix rigidity causes strengthening of integrin–cytoskeleton linkages. Cell 88, 39–48 10.1016/S0092-8674(00)81856-5 (doi:10.1016/S0092-8674(00)81856-5) [DOI] [PubMed] [Google Scholar]
- 40.Roca-Cusachs P., Gauthier N. C., Del Rio A., Sheetz M. P. 2009. Clustering of α5β1 integrins determines adhesion strength whereas αvβ3 and talin enable mechanotransduction. Proc. Natl Acad. Sci. USA 106, 16 245–16 250 10.1073/pnas.0902818106 (doi:10.1073/pnas.0902818106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chou S. Y., Cheng C. M., LeDuc P. R. 2009. Composite polymer systems with control of local substrate elasticity and their effect on cytoskeletal and morphological characteristics of adherent cells. Biomaterials 30, 3136–3142 10.1016/j.biomaterials.2009.02.037 (doi:10.1016/j.biomaterials.2009.02.037) [DOI] [PubMed] [Google Scholar]
- 42.Solon J., Levental I., Sengupta K., Georges P. C., Janmey P. A. 2007. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys. J. 93, 4453–4461 10.1529/biophysj.106.101386 (doi:10.1529/biophysj.106.101386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Byfield F. J., Wen Q., Levental I., Nordstrom K., Arratia P. E., Miller R. T., Janmey P. A. 2009. Absence of filamin A prevents cells from responding to stiffness gradients on gels coated with collagen but not fibronectin. Biophys. J. 96, 5095–5102 10.1016/j.bpj.2009.03.046 (doi:10.1016/j.bpj.2009.03.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Otey C. A., Carpen O. 2004. α-Actinin revisited: a fresh look at an old player. Cell Motil. Cytoskeleton 58, 104–111 10.1002/cm.20007 (doi:10.1002/cm.20007) [DOI] [PubMed] [Google Scholar]
- 45.Stossel T. P., Condeelis J., Cooley L., Hartwig J. H., Noegel A., Schleicher M., Shapiro S. S. 2001. Filamins as integrators of cell mechanics and signalling. Nat. Rev. Mol. Cell Biol. 2, 138–145 10.1038/35052082 (doi:10.1038/35052082) [DOI] [PubMed] [Google Scholar]
- 46.Esue O., Tseng Y., Wirtz D. 2009. α-Actinin and filamin cooperatively enhance the stiffness of actin filament networks. PLoS ONE 4, e4411. 10.1371/journal.pone.0004411 (doi:10.1371/journal.pone.0004411) [DOI] [PMC free article] [PubMed] [Google Scholar]