Abstract
Primary open-angle glaucoma is associated with elevated intraocular pressure, which in turn is believed to result from impaired outflow of aqueous humour. Aqueous humour outflow passes mainly through the trabecular meshwork (TM) and then through pores formed in the endothelium of Schlemm's canal (SC), which experiences a basal-to-apical pressure gradient. This gradient dramatically deforms the SC endothelial cell and potentially contributes to the formation of those pores. However, mechanical properties of the SC cell are poorly defined. Using optical magnetic twisting cytometry and traction force microscopy, here we characterize the mechanical properties of primary cultures of the human SC cell, and for the first time, the scope of their changes in response to pharmacological agents that are known to modulate outflow resistance. Lysophosphatidic acid, sphingosine-1-phosphate (S1P) and thrombin caused an increase in cell stiffness by up to 200 per cent, whereas in most cell strains, exposure to latrunculin A, isoproterenol, dibutryl cyclic-AMP or Y-27632 caused a decrease in cell stiffness by up to 80 per cent, highlighting that SC cells possess a remarkably wide contractile scope. Drug responses were variable across donors. S1P, for example, caused 200 per cent stiffening in one donor strain but only 20 per cent stiffening in another. Isoproterenol caused dose-dependent softening in three donor strains but little or no response in two others, a finding mirrored by changes in traction forces and consistent with the level of expression of β2-adrenergic receptors. Despite donor variability, those drugs that typically increase outflow resistance systematically caused cell stiffness to increase, while in most cases, those drugs that typically decrease outflow resistance caused cell stiffness to decrease. These findings establish the endothelial cell of SC as a reactive but variable mechanical component of the aqueous humour outflow pathway. Although the mechanism and locus of increased outflow resistance remain unclear, these data suggest the SC endothelial cell to be a modulator of outflow resistance.
Keywords: aqueous humour, cell mechanics, mechanobiology, outflow resistance, primary open-angle glaucoma, Schlemm's canal
1. Introduction
Primary open-angle glaucoma (POAG) is a leading cause of irreversible blindness and afflicts tens of millions worldwide. In POAG, the primary risk factor and only proven therapeutic target is elevated intraocular pressure (IOP). Elevated IOP results from an increased resistance to the outflow of aqueous humour from the eye through the conventional outflow pathway [1]. While the precise locus of outflow resistance in the normal eye, or elevated resistance in a glaucomatous eye, is unknown, this resistance is thought to reside in the extracellular matrix (ECM) of the juxtacanalicular connective tissue (JCT), the inner wall endothelium of Schlemm's canal (SC) and/or its basement membrane [1,2].
The conventional pathway for aqueous humour outflow has a hydraulic conductivity of roughly 10−7 cm2 s−1 g−1, and thus sets a lower bound on the hydraulic conductivity attributable to the endothelial lining of SC. Remarkably, this value is two to five orders of magnitude greater than that of non-fenestrated endothelial barriers, the tightest of which is the endothelium of the brain [1]. To account for this high conductivity, it was suggested as early as in 1921 and subsequently confirmed that there exist micron-sized pores within the SC endothelium through which the aqueous humour passes [3–6]. The number density of these pores is decreased in POAG, moreover, and this finding thereby suggests the hypothesis that these pores and their changes might be implicated in the elevated IOP of POAG [2,7,8].
Although the mechanism is not understood, it has been suggested that pore formation is a mechanical response of the SC endothelial cell to the basal-to-apical pressure gradient to which it is exposed [1,9,10]. If so, it follows logically that mechanical properties of the SC cell and its cytoskeleton might play a role in pore formation, with fewer and/or smaller pores forming in stiffer cells. In the only prior study, Zeng et al. [11] used magnetic pulling cytometry to quantify the stiffness of SC cells cultured in vitro. In the SC cell, responsiveness of mechanics to pharmacological agents has not previously been studied, however, nor have differences in responsiveness across donors.
Here we report mechanical properties of the primary human SC cell, their changes in response to pharmacological agents that are known to modulate aqueous humour outflow resistance and donor-to-donor variability of these measures. We show that those agents that typically increase outflow resistance (sphingosine-1-phosphate (S1P) [12,13], thrombin [14] and lysophosphatidic acid (LPA) [13]) cause SC cells to stiffen, while in most cases those that typically decrease outflow resistance (latrunculin A [15], Y-27632 [14,16], DBcAMP [17–19] and isoproterenol [17,20–23]) cause SC cells to soften. These findings raise the question of the extent to which mechanical properties of the SC cell, and their changes, might be a modulator of aqueous humour outflow resistance. Although we cannot resolve these questions here, observations described in §2 illuminate these questions and provide a framework for their further investigation.
2. Results
2.1. Mechanical characterization of Schlemm's canal cells
Primary cultures of human SC cells were isolated from five different eye donors (table 1). Each such culture underwent rigorous characterization using criteria that were established previously [24].
Table 1.
Donor information and baseline stiffness of SC cells tested.
| SC strain | donor age | TEERa | dex testb | α6 testc | vecad testd | fibulin-2 teste | tissue source | cell stiffness (Pa)f | loss tangentf | r.m.s. tractionf |
|---|---|---|---|---|---|---|---|---|---|---|
| SC51 | 66 | 12 | − | + | + | + | corneal rim | 226.2 ± 2.7 | 0.285 ± 0.002 | 75.5 ± 12.9 |
| SC56 | 29 | 9 | − | n.a.g | + | + | corneal rim | 228.7 ± 2.9 | 0.284 ± 0.003 | 45.2 ± 6.7 |
| SC58 | 34 | 10 | − | + | + | + | corneal rim | 313.9 ± 3.8 | 0.235 ± 0.002 | 49.2 ± 4.8 |
| SC60 | 58 | 9 | − | + | + | + | corneal rim | 172.4 ± 2.3 | 0.241 ± 0.002 | 121.6 ± 14.4 |
| SC61 | 88 | 12 | − | + | − | + | whole eye | 340.1 ± 3.6 | 0.283 ± 0.002 | 85.9 ± 13.7 |
aTransendothelial electrical resistance (TEER) of a cell monolayer grown on a filter with filter resistance subtracted; units in Ω cm2.
bTest for dexamethasone-induced myocilin expression.
cPositive expression of integrin α-6 subunit.
dPositive expression of VE-cadherin.
ePositive expression of fibulin-2.
fData reported as median ± s.e.
gn.a., not available.
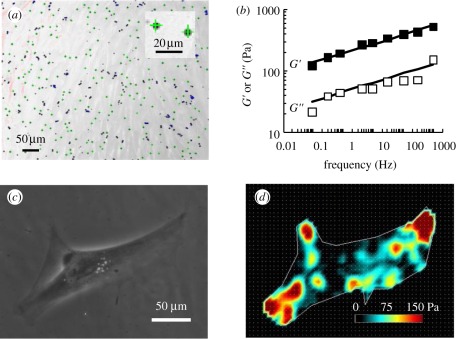
Using optical magnetic twisting cytometry (OMTC), we found that the SC cell exhibited power-law rheology with a good fit to the structural damping model (figure 1b). This behaviour is typical of all adherent eukaryotic cells [25,26]. At passage 2, the shear modulus in these donors at 0.77 Hz ranged from 172 to 340 Pa, and the loss tangent η ranged from 0.23 to 0.28 (table 1). The shear modulus was comparable with that reported previously for this cell type in culture using magnetic pull cytometry [11] and values measured using OMTC for other endothelial cell types [27].
Figure 1.
SC cells exhibit power-law rheology and exert traction forces on a deformable substrate. (a) A typical micrograph showing 4.5 µm magnetic beads adhered to a monolayer of SC cells during an OMTC measurement. The green crosses indicate real-time tracking of bead centroids. Inset: close-up of two beads. (b) The representative cell strain SC51 exhibits power-law rheology behaviour across four orders of magnitude in frequency. See §5 for definitions of G′ and G″; each data point is median over 598 magnetic beads; the error bars for standard errors are smaller than data symbols. The straight lines represent fitting using a two-parameter power-law rheology model (see text for details). (c,d) A representative SC cell from cell strain SC51 exerts traction when plated on a deformable substrate. Phase-contrast image is shown in (c) and traction intensity map in (d).
Using traction force microscopy (TFM), we found that the SC cell generated considerable traction forces (figure 1c,d), with a root mean square (r.m.s.) traction ranging from 45.2 ± 6.7 Pa (SC56) to 121.6 ± 14.4 Pa (SC60; table 1). This level of r.m.s. traction is higher than that generated by A549 epithelial cells [28] but comparable with that generated by human airway smooth muscle cells [29,30] or by human umbilical vein endothelial cells (R. Krishnan 2011, unpublished data).
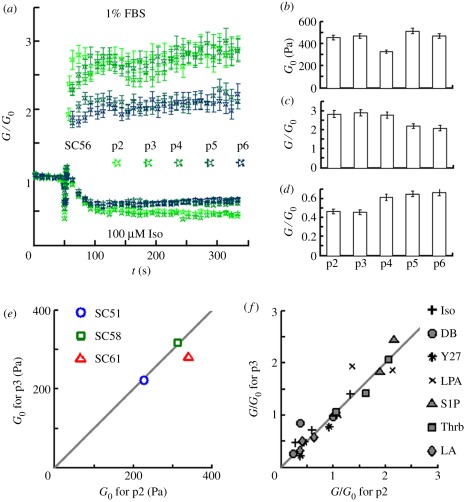
We evaluated the effects of cell passaging on SC cell stiffness, responses to serum (1% foetal bovine serum (FBS)) and responses to isoproterenol (100 µM). Although some changes were noted beyond passage 4, we found that baseline stiffness (figure 2b), contractile responses (figure 2a,c), and relaxing responses (figure 2a,d) were similar between passages 2 and 3. This finding was confirmed in three other cell strains where cell mechanical phenotype was shown to be stable between passages 2 and 3 (figure 2e,f). Thus, we limited all experiments to passages 2 and 3.
Figure 2.
SC cells exhibit stable mechanical phenotype up to passage 3. (a–d) Effect of passaging on the mechanical phenotype of SC56 cells. (a) From passage 2 to 6 (p2 , … , p6), SC cells stiffen in response to FBS (upper traces) and soften in response to isoproterenol (lower traces). G/G0: current cell stiffness (G) normalized by the baseline stiffness (G0). (b–d) The effect of in vitro passaging on baseline stiffness (G0; (b)), contractile response to FBS (G/G0 at 300 s; (c)), and relaxing response to isoproterenol (G/G0 at 300 s; (d)). Passaged once per week, confluently plated SC56 cells were grown in growth media for a week, serum starved for 4–6 h (instead of the overnight starvation, which was used for all other tests), incubated with poly-l-lysine (PLL)-coated beads and challenged with 1% FBS or 100 µM isoproterenol. In (a,c,d), each data point is median value of over 250 beads; in (b), each data point is median value of over 500 beads; the error bars are standard errors. (e,f) For three other donors, we tested their SC cells both at passages 2 and 3, and found that untreated cell stiffness (e) and all drug responses (f) were nearly identical. In (f), G/G0 was evaluated differently for different drugs: G/G0 for latrunculin A (LA), LPA and S1P was evaluated at 300 s at a single dose (cf. figure 3), and G/G0 for isoproterenol (Iso), DBcAMP (DB) and Y-27632 (Y27) was obtained after long-term treatment at doses of 10 µM, 5 mM and 100 µM, respectively (cf. figure 4).
2.2. Response to agents that increase outflow resistance
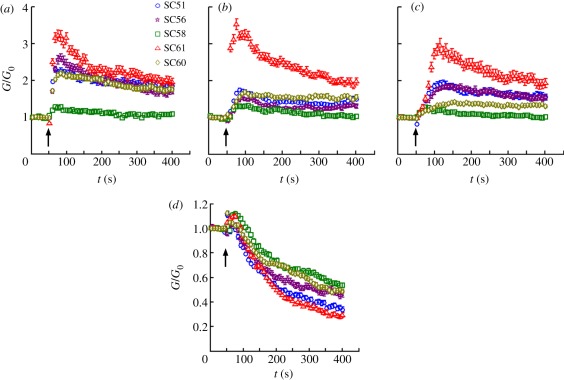
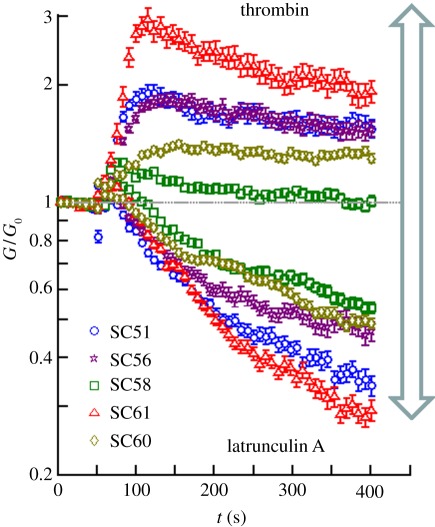
In response to S1P (0.1 µM), cell stiffness in all strains increased promptly, peaked after an average of 26 s and then slowly decayed over the course of several minutes (figure 3a). While the time course of this response was closely similar across donors, the amplitude of the response was highly variable across donors. For example, cell stiffness for donor SC58 increased at most by 1.2-fold (20% above baseline), whereas cell stiffness for the others increased by more than twofold (100% above baseline), with donor SC61 exhibiting the largest enhancement (3.2-fold, 220% above baseline). In response to LPA (0.1 µM), cell stiffness peaked after an average of 35 s (figure 3b). Finally, in response to thrombin (0.1 µ-NIH unit ml−1), cell stiffness peaked at an average of 103 s (figure 3c). Similar to S1P, LPA and thrombin induced highly variable magnitudes of stiffening across donors. For each of the three drugs, SC58 was the least contractile, while SC61 was the most contractile.
Figure 3.
SC cells stiffen in response to (a) S1P, (b) LPA, (c) thrombin, and (d) soften in response to latrunculin A. At concentrations of 0.1 µM for latrunculin A, LPA and S1P, and 0.1 µ-NIH unit ml−1 for thrombin, the treatments were applied at 50 s, indicated by the arrows. G/G0: current cell stiffness (G) normalized by the baseline stiffness (G0). All cells were tested at passage 2, except for the responses of SC58 and SC61 to thrombin, which were tested at passage 3. Each data series is median value of over 300 beads; the error bars are standard errors (which are often masked by the data symbols).
2.3. Response to agents that decrease aqueous outflow resistance
2.3.1. Changes in cell stiffness
In response to the actin-depolymerization drug latrunculin A (0.1 µM), cell stiffness in all strains decreased in a time-dependent fashion (figure 3d). Interestingly, this decrease was the greatest for SC61, the most contractile cell, and it was the least for SC58, the least contractile cell. The stiffness decrease was monotonic and a plateau was not reached at 400 s. Thus, while the peak stiffening response can be readily attained with a short stimulation, the peak softening response may be attainable only after prolonged treatment. Accordingly, for Y-27632, DBcAMP and isoproterenol, which were previously shown to soften other human cells [31,32], we decided to quantify the plateau response after prolonged treatment.
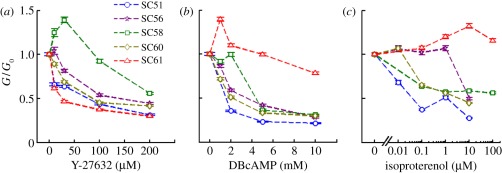
In response to Y-27632, which is a specific inhibitor of Rho-associated kinases, cell stiffness decreased in a dose-dependent fashion (figure 4a). In cells from four of the five donors, the responses were comparable, whereas the stiffness of cells from one donor (SC58) increased at low concentrations before decreasing at higher concentrations. In the case of DBcAMP, which is a cell-permeable analogue of cAMP (cyclic adenosine monophosphate), strong and consistent softening responses were observed, with the exception of donor SC61, which was an outlier that relaxed only minimally and only at the highest concentrations of DBcAMP (figure 4b). At the highest drug concentrations tested, latrunculin, Y-27632 and DBcAMP all caused a significant decrease in cell stiffness in all cell strains. This was not the case with isoproterenol, however, where responses were more varied across donors. While cells from three SC strains showed the expected softening in response to isoproterenol, cells from donor SC56 failed to relax except at the highest concentration, and cells from donor SC61 failed to relax even at the highest concentration studied (figure 4c).
Figure 4.
Most but not all SC cells relaxed dose-dependently in response to (a) Y-27632, (b) DBcAMP, and (c) isoproterenol. Cells were incubated with individual drugs at multiple doses for 30–0 mins before stiffness measurement. A G-to-G0 ratio smaller than 1 indicates cell softening; G is cell stiffness with drug treatment and G0 is cell stiffness with vehicle treatment. Each data point is median value of over 1000 beads at passage 2; the error bars are standard errors.
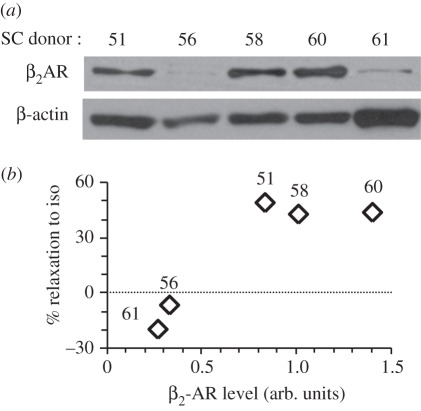
2.3.2. Expression levels of the β2-adrenergic receptor
Western blotting showed that donors SC56 and SC61 expressed diminished levels of β2-adrenergic receptor (β2AR; figure 5a). Across all donors, β2AR protein level positively correlated with the degree of relaxation triggered by isoproterenol (figure 5b). Because SC56 responded sensitively to DBcAMP but less so to isoproterenol, this pattern of response might be explained by low levels of the β2AR, whereas SC61 did not respond to DBcAMP, suggesting defective signalling downstream of cAMP.
Figure 5.
SC cells from different donors express variable levels of β2AR protein and the β2AR levels correlate with relaxation response to isoproterenol. (a) Western blot analysis of β2AR in SC cells from five donors (see table 1 for donor information). Nearly identical results were obtained with technical duplicates. (b) The extent of cell relaxation in response to 1 µM isoproterenol (iso) positively correlates with the β2AR levels (arb. units). % Relaxation was defined in §2, and relative β2AR protein amount was quantified by densitometry and normalized to β-actin.
2.3.3. Changes in cell traction
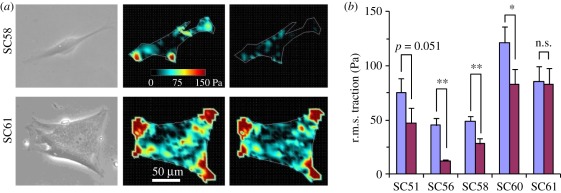
Treatment with 10 µM isoproterenol diminished traction forces generated by cells from donor SC58 (figure 6a, top), but did not alter that of cells from donor SC61 (figure 6a, bottom). Traction forces exerted by cells from the three other donors also decreased in response to isoproterenol, although the decrease for SC51 (p = 0.051) was not statistically significant (figure 6b). Overall, traction changes mirrored those of cell stiffness.
Figure 6.
Isoproterenol decreases the traction forces of SC cells from most but not all donors, consistent with its donor-dependent effects on cell stiffness. (a) Single SC cells exert traction when plated on a deformable substrate. Left: phase-contrast image; middle and right: traction intensity map before and 10 min after 10 µM isoproterenol (iso) treatment, respectively. While the SC58 cell diminished its traction in responses to isoproterenol (top), SC61 did not (bottom). (b) r.m.s. traction before and 10 min after 10 µM isoproterenol treatment for the five SC strains. Seven to 11 cells were tested for each donor. Error bars in (b) are standard errors, and statistical significance of the traction decrease is indicated as *p < 0.05, **p < 0.01, n.s. as p > 0.05 or p-value itself. Violet bars, baseline; purple, isoproterenol.
3. Discussion
We report here the first measurements of changes of SC cell mechanical properties with pharmacological challenge. The principal findings of this report are (i) stiffness and contractile force of the SC cell exhibited strong responses to pharmacological modulation, (ii) drugs known to increase outflow resistance increased SC cell stiffness, and conversely, drugs known to decrease outflow resistance decreased SC cell stiffness, and (iii) these drug responses were variable across cell strains obtained from different donor eye tissues. In what follows, we discuss the limitations of our study and the implications of our findings.
3.1. Methodological limitations
3.1.1. Measurement of cell stiffness
The methodological limitations and validation of OMTC have been discussed in detail elsewhere [25]. OMTC probes the cell through its apical surface, and thus it is possible that the findings do not apply to the whole cell. To address this potential limitation, we also probed the cell from the basal surface using TFM [33]. TFM and OMTC produced consistent results, thus lending support to the OMTC results. Furthermore, power-law responses observed here for the human SC cell (equation (5.1)) have been observed across all animal cells except for red blood cells [34]. Such power-law responses have been previously established across at least four orders of magnitude in frequency, and with experimental techniques including OMTC [25] as used here, atomic force microscopy [35], optical stretching [36], micropipette aspiration [37] and two-point microrheology [38].
3.1.2. Effects of cell passaging
Several key differences exist between SC cells in vivo versus those in vitro that might limit the physiological relevance of the current study. First, it is possible that passaging in vitro might cause the SC cell to dedifferentiate. While we did not have the opportunity to measure SC cell mechanics before passaging, our study on the effects of cell passaging suggests that this effect was perhaps modest up to passage 3.
3.1.3. Effects of culture substrate
Another noteworthy limitation is that these cells were grown on a plastic substrate that is functionally rigid, whereas cells in vivo grow on an ECM that is highly compliant; it has been shown in other cell types that matrix compliance can modulate cell phenotype [39–41] and that substrate compliance differs between normal versus glaucomatous eyes [42]. Nonetheless, the SC cell from different donors in this study exhibited different patterns of responsiveness, suggesting that certain key aspects of the phenotype persisted in vitro.
3.2. Donor-to-donor variability in baseline stiffness and responsiveness
In mesothelial cells from pleural effusions from seven donors, Cross et al. [43] showed that cellular shear moduli ranged from 583 to 700 Pa, but otherwise little is known about variability in cell mechanics across human donors. Similarly, we find here in SC cells a range of shear moduli from 172 to 340 Pa. In both instances, donor-to-donor variability in baseline cell stiffness did not exceed twofold. Responsiveness was more variable from donor-to-donor. For example, contractile agonists-induced stiffness increases varied from 20 per cent in SC58 to 200 per cent in SC61; isoproterenol-induced stiffness decreases varied from −20 per cent in SC61 to 49 per cent in SC51.
Although defining molecular mechanisms underlying the donor differences is beyond the scope of the current study, results indicate that SC56 and SC61 had diminished expression of β2AR compared with other donors. This low level of β2AR expression coincided with the lack of isoproterenol response for SC61 at all doses, and with the lack of isoproterenol response for SC56 at doses below 10 µM. Our data thus suggest that the donor variability in mechanical phenotype results from that at the molecular level.
Finally, IOP and outflow resistance also varies widely in humans [44,45]. Our study raises the question of the extent to which this variability in humans is associated with that of stiffness and/or responsiveness of SC cells.
3.3. Association between outflow resistance and cell stiffness
Aqueous humour outflow resistance can be increased by up to about 40 per cent by contractile agonists such as LPA and S1P [12,13], or decreased by up to about 80 per cent by relaxing agents such as Y-27632 [14]. If outflow facility is indeed controlled by SC cell stiffness, one would expect SC cell stiffness to possess similar scope of modulation by those agents. Indeed, our study revealed a dramatic contractile scope; cell stiffness increased by up to 200 per cent and decreased by up to 80 per cent (figure 7), which exceeds the scope of responsiveness of professional contractile cells such as the airway smooth muscle cell stimulated with histamine or relaxed with isoproterenol [46].
Figure 7.
The contractile scope of the SC cell is substantially variable across donors and generally quite large. Cells from any given donor could attain a wide range of stiffnesses, with the ratio of maximal to minimal stiffness denoting an effective contractile scope. In this example, cells from donor SC58 (green squares) attained a contractile scope of only 2.2-fold, whereas donor SC61 (red triangles) attained a contractile scope close to 10-fold.
While these data do not prove that SC cell stiffness modulates outflow facility, they suggest that it has the potential to do so. Indeed, for the seven drugs studied here, known effects on outflow resistance were seen to go hand-in-hand with their effects on SC cell stiffness. Interestingly, isoproterenol showed more variable effects on cell stiffness than did the other drugs studied, and, likewise, has been reported to have variable effects on outflow resistance [17,20–23].
Table 2 shows a list of drugs that have been shown to modulate outflow resistance in ocular perfusion studies, along with the effects of these drugs on cell stiffness and paracellular junctional resistance in a variety of cell types, including SC cells. One potential alternative explanation of how these drugs might affect outflow resistance is by changing of paracellular junctional resistance, but the effects of these drugs show little association between outflow resistance and paracellular resistance (table 2). For example, although bradykinin, histamine and thrombin are each associated with decreased paracellular resistance, each acts to increase outflow resistance. By contrast, for all 15 drugs, changes in outflow resistance went hand-in-hand with changes in cell stiffness (table 2); the cell types tested include fibroblasts, smooth muscle cells, epithelial cells, vascular endothelial cells and the current subject, the SC cells. Such a tight association between outflow resistance and cell stiffness is only correlative but is striking nonetheless.
Table 2.
Relationship between cell stiffness, junctional permeability and aqueous humour outflow resistance. Red arrows are measurements made on outflow pathway cells/tissues; blue arrows are measurements on vascular endothelial cells; black arrows are measurement on other cell types. Dash indicates no effect.
| agent | outflow resistancea | stiffness | junctional resistanceb | reference |
|---|---|---|---|---|
| colchicine | ↓ | ↓ | ? | [47–49] |
| cytochalasin D | ↓ | ↓ | ↓ | [31,47,50–53] |
| dibutyryl-cAMP | ↓ | ↓d | ↑ | [17–19,32,50,54] |
| forskolin | ↓− | ↓ | ↑↓ | [19,32,54–57] |
| isoproterenol | ↓− | ↓d | ↑↓ | [17,20–23,32,50,57] |
| latrunculin | ↓ | ↓d | ? | [15,31,58] |
| Y-27632 | ↓ | ↓d | ↓ | [14,16,31] |
| butanedione monoxime | ↓ | ↓ | ? | [59,60] |
| bradykinin | ↑ | ↑ | ↓ | [32,61–63] |
| histamine | ↑ | ↑ | ↓ | [32,52,61,63] |
| thrombin | ↑ | ↑d | ↓ | [14,54,61,64,65] |
| lysophosphatidic acid | ↑ | ↑d | ↑↓ | [13,52,66–68] |
| sphingosine 1-phosphate | ↑ | ↑d | ↑ | [12,13,69,70] |
| dexamethasone | ↑ | ↑ | ↑ | [71–74] |
| triamcinolone acetonide | ↑ | ↑ | ↑c | [71,75,76] |
aOutflow resistance is the inverse of outflow facility.
bJunctional resistance as determined from electrical resistance, hydraulic conductivity or permeability to solutes.
cResponse to corticosteroid.
dMeasurements on SC cells from this study.
Further support for this proposition is found in work examining the effects of cytoskeletal-active agents on outflow resistance, particularly those affecting Rho-kinase [13,14,77]. Based upon myosin light chain phosphorylation and fluorescent cytoskeletal imaging as a surrogate for cell relaxation/contraction, Rao et al. proposed that cell contraction is associated with increased outflow resistance, while cell relaxation is associated with decreased outflow resistance. However, these previous studies generally focused on the TM cells, on which quantitative mechanical/pharmacological measurement at the cellular level remains to be performed. The relative contribution to the modulation of outflow resistance by TM cells versus SC cells remains unknown.
How might SC cell stiffness modulate outflow resistance? It has been proposed that pore formation in the inner wall endothelium is driven by cell deformation induced by the transcellular pressure gradient across the cell [1,9,10]. Such a physical picture is fully consistent with the observation of giant-vacuole formation in the cultured SC cell perfused under physiological pressures [78]. Because a stiffer SC cell will tend to deform less under a pressure gradient, higher stiffness might act to reduce the size and number of giant vacuoles and pores. Previous studies [1,79] indicate that inner wall pores generate little flow resistance in and of themselves. However, if the pores cause a funnelling of flow through the adjacent JCT, as suggested by computational models [80,81], then these pathways could be the limiting factor in aqueous humour outflow resistance. If so, then stiffness of the SC cell could modulate outflow resistance through the process of pore formation. Further support is found in studies of Ethier et al. [82] who showed that latrunculin-B, an agent that decreases outflow resistance [15] and reduces cell stiffness in non-ocular cells [31], causes an increase in the density of pores in the inner wall SC endothelium.
4. Conclusion
Current literature emphasizes the role of the JCT in generating the bulk of outflow resistance [2,83,84], but the role of the endothelial lining of the inner wall of SC remains unclear. Here we have investigated the potential role of this endothelium by direct mechanical measurements at the cellular level. Specifically, we have shown that the SC cell is highly contractile and responsive to a range of pharmacological interventions but that responses are highly variable across donors. Moreover, drugs known to increase outflow resistance increased SC cell stiffness, and conversely, drugs known to decrease outflow resistance decreased SC cell stiffness. These findings, taken together, support the hypothesis that mechanical properties of the SC endothelium may contribute to aqueous humour outflow resistance, presumably through their effects upon modulation of pore formation [1,9,10,81].
5. Methods
5.1. Cell isolation and culture
SC cells were isolated from one enucleated eye that was obtained from NDRI and from four post-surgical corneoscleral remnants obtained from local cornea surgeons (Tucson, AZ, USA). Eye tissues were free of any known ocular disease (including glaucoma) and stored in a moist chamber or Optisol at 4°C until the time of dissection. Human SC cells were isolated using a cannulation technique as described in detail elsewhere [24]. Cells were characterized and distinguished from potential contaminants using previously established criteria. Thus, SC cells displayed characteristic ‘railroad track’ morphology and were contact-inhibited upon reaching confluence. Unlike their close neighbours—TM cells—expression of myocilin in SC cells was not induced upon treatment with corticosteroids (table 1). Moreover, SC cells after confluence for one week generated net transelectrical resistance between 9 and 18 Ω cm2; by comparison confluent TM cells attained 2–5 Ω cm2 [85]. Some cell strains (as were available) were also tested with more recent established protein markers for SC including integrin α6, fibulin-2 and VE-cadherin [86–88]. SC cell strains from five donors were used in the present study that satisfied all or the majority of these criteria (table 1).
As described below, cells were expanded and studied in passages 2–3. In one cell strain, cells were studied from passages 2 through 6 to examine the effect of passaging on cell mechanical properties. Cells were cryopreserved in liquid nitrogen until usage. In a 37°C, 5 per cent CO2 incubator, we grew cells in 25 cm2 culture flasks for expansion or in 96-well plates for mechanical testing or biochemical analysis. Growth media contained low-glucose DMEM (GIBCO 12320) supplemented with 10 per cent FBS (to promote cell proliferation), 0.25 µg ml−1 amphotericin B, 100 unit ml−1 penicillin, and 100 µg ml−1 streptomycin; serum-free media contained low-glucose DMEM supplemented with 100 unit ml−1 penicillin, 100 µg ml−1 streptomycin, and 1X ITS (10 µg ml−1 insulin from bovine pancreas, 5.5 µg ml−1 human transferrin (substantially iron-free) and 5 µg ml−1 sodium selenite; Sigma). We plated passages 2 and 3 SC cells in 96-well plates (coated with 5 µg ml−1 type I collagen from Inamed Biomaterials, Fremont, CA, USA) at confluent density, 6400 cells per well, for 7–8 days in the growth media to form a mature, confluent monolayer and then switched them to the serum-free media for overnight before mechanical testing or protein extraction. The serum-free media facilitates the study of drug responses.
5.2. Optical magnetic twisting cytometry
Detailed descriptions and validations of OMTC have been given elsewhere [25,89]. Briefly, to probe the rheology of the cytoskeleton, ferrimagnetic beads (4.5 µm diameter) were coated with poly-l-lysine (PLL) and allowed to adhere randomly to the apical cell surface. PLL was used by our group instead of RGD used in previous studies [25], as the latter has been shown to induce integrin clustering and focal adhesion formation that could possibly alter cell stiffness [90]. We have shown previously that cellular stiffness measured with beads coated with PLL is comparable to that measured with RGD [91–93]; however, such a stiffness is an order of magnitude higher than that probed through the lipid bilayer [94], suggesting that PLL is a suitable probe for cytoskeletal stiffness. To probe the rheology of the SC cell in preliminary studies, we twisted each bead at frequencies (f) ranging from 0.1 to 1000 Hz. Studies of drug effects were then done at an arbitrarily chosen frequency of 0.77 Hz that we have employed in our studies of other cell types [25,91].
OMTC measures the apparent stiffness of hundreds of cells individually but simultaneously (figure 2a). The complex ratio of the applied torque to the resulting complex bead displacement defines a complex apparent stiffness of the cell [25], g*(f) with units of Pa nm−1. With increasing frequency, the SC cell exhibited power-law rheology (figure 2b), which fits well to the structural damping model [25,37]:
| 5.1 |
Here, i2 = −1, g*(f) is the complex shear modulus, g′ is the stiffness modulus and g″ is the loss modulus. There are only two free parameters in the structural damping model, the prefactor A and the power-law exponent α. The loss tangent or hysteresivity (η = g″/g′) is related to α by the equation η = tan(απ/2) [25].
To convert the apparent stiffness, g*, to a shear modulus, G*(G* = G′ + iG″), we multiply by a length scale of 350 nm based upon previous finite-element simulations [95] together with a measured half immersion angle of 84° as determined for RGD-coated beads adherent to SC cells [11]. Then, if we denote |G*| at 0.77 Hz as G, using equation (5.1), we find G = A 0.77α × 350. We note that shear modulus of a cell is one-third of its Young's modulus, assuming the cell to be incompressible.
5.3. Protocols for measuring drug response
We added beads to cells and incubated for 30 min. Because the availability of human SC cells is quite limited, we used two protocols to optimize the utilization of this precious resource. In protocol A (time-response studies), baseline stiffness was measured by OMTC as described above at 0.77 Hz, a drug of interest was then added at a defined concentration, and the time course of resulting stiffness changes was recorded. In this protocol, each cell was used as its own control and we report the fractional change in cell stiffness relative to baseline. In protocol B (dose-response studies), we measured cell stiffness at 0.77 Hz and its responses to a drug of interest in a dose-response fashion, but related those changes to responses in a different cell that was treated with vehicle only and used as a time-control. Except where noted, reported values correspond to plateau responses that were typically recorded at 30–60 min following the addition of each drug. The degree of relaxation (% relaxation) was calculated as 1 − G/G0, where G is the cell stiffness with drug treatment and G0 is that without. All tests were carried out at 37°C.
5.4. Traction force microscopy
The details of TFM technique have been described elsewhere [30,33,96]. Briefly, 250 µl of a mixture consisting of 5 per cent acrylamide, 0.1 per cent bis-acrylamide (Bio-Rad, Hercules, CA, USA), 0.6 per cent fluorescent bead suspension (0.2 µm, yellow-green, Invitrogen, Eugene, OR, USA) and ultrapure water were induced to polymerize on a pre-treated glass-bottom dish by mixing with 0.5 per cent of ammonia persulphate and 0.05 per cent TEMED (Bio-Rad). These gels were surface-activated using 200 µl of 1 mM sulphosuccinimidyl-6-[4-azido-2-nitrophenylamino]hexanoate (Sulpho-SANPAH; Pierce, Rockford, IL, USA) under UV light, coated with 200 µl of type I collagen solution (0.1 mg ml−1; Inamed Biomaterials, Fremont, CA, USA) and stored overnight at 4°C. These gel substrates have a Young's modulus of 4 kPa. Cells were sparsely plated for 4–6 h on these substrates in the serum-free media described above. Using an inverted epifluorescence Leica microscope, the bead positions were recorded at the pre-treatment baseline, at several times points following drug treatment and after detachment of the cells by trypsinization at the end of the experiment. From these bead images, we computed the displacement field. From the displacement field, the substrate stiffness and knowledge of the cell's contour, we computed the constrained traction field [33]. From the constrained traction field, we extracted a scalar measure of cell contractility called the r.m.s. traction [29,33].
5.5. Drug preparations
For Protocol A, we used latrunculin A (0.1 µM with dimethylsulphoxide (DMSO) as its vehicle), LPA (0.1 µM with its vehicle being phosphate-buffered saline (PBS) with 1% bovine serum albumin (BSA)), S1P (0.1 µM with its vehicle being PBS with 1% BSA) and thrombin (0.1 µ-NIH unit ml−1 with water as its vehicle). For Protocol B, we used Y-27632 (10–200 µM with water as vehicle), dibutyryl-cAMP (DBcAMP, 1–10 mM freshly prepared in serum-free media) and isoproterenol (0.01–100 µM with water as its vehicle). All drugs were from Sigma–Aldrich unless otherwise stated. The maximum concentrations for the vehicles were 0.01 per cent DMSO, 0.01 per cent PBS plus 0.0001 per cent BSA or 1 per cent water; these vehicles had negligible influence on cell stiffness.
5.6. Further characterizations
In the special case of isoproterenol, we also quantified expression of the β2AR as well as traction forces. To measure the former, we used Western blotting described below and to measure the latter, we used TFM described above.
5.6.1. Western blotting
Cells were lysed in NP-40 lysis buffer (50 mM Tris–Cl, 150 mM NaCl, 0.5% NP-40) supplemented with protease inhibitors (Roche). Lysates were resolved on a Nupage 4–12 per cent Bis–Tris gel (Invitrogen), transferred onto a Hybond nitrocellulose membrane (Amersham) and probed with anti-β2AR (H20; Santa Cruz Biotech) or anti-β-actin (Santa Cruz Biotech) antibodies.
5.7. Statistical analysis
For traction changes after isoproterenol, we used a paired t-test with a significance level of 0.05. Data are reported as median ± s.e.
Acknowledgements
E.H.Z. thanks Joseph Brain for financial support. This research was supported by HL084224 (J.J.F.), EY017007 (W.D.S.), EY019696-01 (M.J., W.D.S. and J.J.F.) and Jere Mead Fellowship (E.H.Z.).
References
- 1.Johnson M. 2006. What controls aqueous humour outflow resistance? Exp. Eye Res. 82, 545–557 10.1016/j.exer.2005.10.011 (doi:10.1016/j.exer.2005.10.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamm E. R. 2009. The trabecular meshwork outflow pathways: structural and functional aspects. Exp. Eye Res. 88, 648–655 10.1016/j.exer.2009.02.007 (doi:10.1016/j.exer.2009.02.007) [DOI] [PubMed] [Google Scholar]
- 3.Seidel E. 1921. Weitere experimentelle Untersuchungen über die Quelle und den Verlauf der intraokularen Saftströmung. IX Mitteilung. Über den Abfluss des Kammerwassers aus der vorderen AugenKammer. Graefe's Arch. Clin. Exp. Ophthalmol. 104, 357–402 [Google Scholar]
- 4.Speakman J. S. 1959. Aqueous outflow channels in the trabecular meshwork in man. Br. J. Ophthalmol. 43, 129. 10.1136/bjo.43.3.129 (doi:10.1136/bjo.43.3.129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garron L. K., Fenney M. L., Hogan M. J., McEwen W. K. 1958. Electron microscopic studies of the human eye. Am. J. Ophthalmol. 46, 27–35 [DOI] [PubMed] [Google Scholar]
- 6.Holmberg A. 1959. The fine structure of the inner wall of Schlemm's canal. Arch. Ophthalmol. 62, 956–958 [Google Scholar]
- 7.Allingham R. R., de Kater A. W., Ethier C. R., Anderson P. J., Hertzmark E., Epstein D. L. 1992. The relationship between pore density and outflow facility in human eyes. Invest. Ophthalmol. Visual Sci. 33, 1661–1669 [PubMed] [Google Scholar]
- 8.Johnson M., Chan D., Read A. T., Christensen C., Sit A., Ethier C. R. 2002. The pore density in the inner wall endothelium of Schlemm's canal of glaucomatous eyes. Invest. Ophthalmol. Visual Sci. 43, 2950–2955 [PubMed] [Google Scholar]
- 9.Tripathi R. C. 1972. Aqueous outflow pathway in normal and glaucomatous eyes. Br. J. Ophthalmol. 56, 157. 10.1136/bjo.56.3.157 (doi:10.1136/bjo.56.3.157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee W. R., Grierson I. 1975. Pressure effects on the endothelium of the trabecular wall of Schlemm's canal: a study by scanning electron microscopy. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 196, 255–265 10.1007/BF00410037 (doi:10.1007/BF00410037) [DOI] [PubMed] [Google Scholar]
- 11.Zeng D., Juzkiw T., Read A. T., Chan D. W., Glucksberg M. R., Ethier C. R., Johnson M. 2010. Young's modulus of elasticity of Schlemm's canal endothelial cells. Biomech. Model Mechanobiol. 9, 19–33 10.1007/s10237-009-0156-3 (doi:10.1007/s10237-009-0156-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stamer W. D., Read A. T., Sumida G. M., Ethier C. R. 2009. Sphingosine-1–phosphate effects on the inner wall of Schlemm's canal and outflow facility in perfused human eyes. Exp. Eye Res. 89, 980–988 10.1016/j.exer.2009.08.008 (doi:10.1016/j.exer.2009.08.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mettu P. S., Deng P. F., Misra U. K., Gawdi G., Epstein D. L., Rao P. V. 2004. Role of lysophospholipid growth factors in the modulation of aqueous humor outflow facility. Invest. Ophthalmol. Visual Sci. 45, 2263–2271 10.1167/iovs.03-0960 (doi:10.1167/iovs.03-0960) [DOI] [PubMed] [Google Scholar]
- 14.Rao P. V., Deng P. F., Kumar J., Epstein D. L. 2001. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest. Ophthalmol. Visual Sci. 42, 1029–1037 [PubMed] [Google Scholar]
- 15.Peterson J. A., Tian B., Bershadsky A. D., Volberg T., Gangnon R. E., Spector I., Geiger B., Kaufman P. L. 1999. Latrunculin-A increases outflow facility in the monkey. Invest. Ophthalmol. Visual Sci. 40, 931–941 [PubMed] [Google Scholar]
- 16.Tian B., Kaufman P. L. 2005. Effects of the Rho kinase inhibitor Y-27632 and the phosphatase inhibitor calyculin A on outflow facility in monkeys. Exp. Eye Res. 80, 215–225 10.1016/j.exer.2004.09.002 (doi:10.1016/j.exer.2004.09.002) [DOI] [PubMed] [Google Scholar]
- 17.Neufeld A. H. 1978. Influences of cyclic nucleotides on outflow facility in the vervet monkey. Exp. Eye Res. 27, 387. 10.1016/0014-4835(78)90017-9 (doi:10.1016/0014-4835(78)90017-9) [DOI] [PubMed] [Google Scholar]
- 18.Kaufman P. L. 1987. Adenosine 3′,5′-cyclic-monophosphate and outflow facility in monkey eyes with intact and retrodisplaced ciliary muscle. Exp. Eye Res. 44, 415. 10.1016/S0014-4835(87)80175-6 (doi:10.1016/S0014-4835(87)80175-6) [DOI] [PubMed] [Google Scholar]
- 19.Gilabert R., Gasull X., Pales J., Belmonte C., Bergamini M. V., Gual A. 1997. Facility changes mediated by cAMP in the bovine anterior segment in vitro. Vision Res. 37, 9–15 10.1016/S0042-6989(96)00106-X (doi:10.1016/S0042-6989(96)00106-X) [DOI] [PubMed] [Google Scholar]
- 20.Kaufman P. L. 1986. Epinephrine, norepinephrine, and isoproterenol dose-outflow facility response relationships in cynomolgus monkey eyes with and without ciliary muscle retrodisplacement. Acta Ophthalmol. (Copenh) 64, 356–363 10.1111/j.1755-3768.1986.tb06933.x (doi:10.1111/j.1755-3768.1986.tb06933.x) [DOI] [PubMed] [Google Scholar]
- 21.Kaufman P. L. 1985. Isoproterenol dose-outflow facility response relationships in the vervet monkey. Curr. Eye Res. 4, 877–883 10.3109/02713688509095255 (doi:10.3109/02713688509095255) [DOI] [PubMed] [Google Scholar]
- 22.Gaasterland D., Kupfer C., Ross K., Gabelnick H. L. 1973. Studies of aqueous humor dynamics in man. III. Measurements in young normal subjects using norepinephrine and isoproterenol. Invest. Ophthalmol. 12, 267–279 [PubMed] [Google Scholar]
- 23.Brubaker R. F., Gaasterland D. 1984. The effect of isoproterenol on aqueous humor formation in humans. Invest. Ophthalmol. Visual Sci. 25, 357–359 [PubMed] [Google Scholar]
- 24.Stamer W. D., Roberts B. C., Howell D. N., Epstein D. L. 1998. Isolation, culture and characterization of endothelial cells from Schlemm's canal. Invest. Ophthalmol. Visual Sci. 39, 1804–1812 [PubMed] [Google Scholar]
- 25.Fabry B., Maksym G. N., Butler J. P., Glogauer M., Navajas D., Taback N. A., Millet E. J., Fredberg J. J. 2003. Time scale and other invariants of integrative mechanical behavior in living cells. Phys. Rev. E 68, 041914. 10.1103/PhysReve.68.041914 (doi:10.1103/PhysReve.68.041914) [DOI] [PubMed] [Google Scholar]
- 26.Fabry B., Maksym G. N., Butler J. P., Glogauer M., Navajas D., Fredberg J. J. 2001. Scaling the microrheology of living cells. Phys. Rev. Lett. 87, 148102. 10.1103/PhysRevLett.87.148102 (doi:10.1103/PhysRevLett.87.148102) [DOI] [PubMed] [Google Scholar]
- 27.An S. S., Pennella C. M., Gonnabathula A., Chen J., Wang N., Gaestel M., Hassoun P. M., Fredberg J. J., Kayyali U. S. 2005. Hypoxia alters biophysical properties of endothelial cells via p38 MAPK- and Rho kinase-dependent pathways. Am. J. Physiol. Cell Physiol. 289, C521–C530 10.1152/ajpcell.00429.2004 (doi:10.1152/ajpcell.00429.2004) [DOI] [PubMed] [Google Scholar]
- 28.Gavara N., Roca-Cusachs P., Sunyer R., Farre R., Navajas D. 2008. Mapping cell-matrix stresses during stretch reveals inelastic reorganization of the cytoskeleton. Biophys. J. 95, 464–471 10.1529/biophysj.107.124180 (doi:10.1529/biophysj.107.124180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang N., Tolic-Norrelykke I. M., Chen J., Mijailovich S. M., Butler J. P., Fredberg J. J., Stamenovic D. 2002. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am. J. Physiol. Cell Physiol. 282, C606–C616 [DOI] [PubMed] [Google Scholar]
- 30.Krishnan R., et al. 2009. Reinforcement versus fluidization in cytoskeletal mechanoresponsiveness. PLoS ONE 4, e5486. 10.1371/journal.pone.0005486 (doi:10.1371/journal.pone.0005486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.An S. S., Laudadio R. E., Lai J., Rogers R. A., Fredberg J. J. 2002. Stiffness changes in cultured airway smooth muscle cells. Am. J. Physiol. Cell Physiol. 283, C792–C801 [DOI] [PubMed] [Google Scholar]
- 32.Hubmayr R. D., Shore S. A., Fredberg J. J., Planus E., Panettieri R. A., Jr, Moller W., Heyder J., Wang N. 1996. Pharmacological activation changes stiffness of cultured human airway smooth muscle cells. Am. J. Physiol. 271, C1660–C1668 [DOI] [PubMed] [Google Scholar]
- 33.Butler J. P., Tolic-Norrelykke I. M., Fabry B., Fredberg J. J. 2002. Traction fields, moments, and strain energy that cells exert on their surroundings. Am. J. Physiol. Cell Physiol. 282, C595–C605 [DOI] [PubMed] [Google Scholar]
- 34.Puig-de-Morales-Marinkovic M., Turner K. T., Butler J. P., Fredberg J. J., Suresh S. 2007. Viscoelasticity of the human red blood cell. Am. J. Physiol. Cell Physiol. 293, C597–C605 10.1152/ajpcell.00562.2006 (doi:10.1152/ajpcell.00562.2006) [DOI] [PubMed] [Google Scholar]
- 35.Alcaraz J., Buscemi L., Grabulosa M., Trepat X., Fabry B., Farre R., Navajas D. 2003. Microrheology of human lung epithelial cells measured by atomic force microscopy. Biophys. J. 84, 2071–2079 10.1016/S0006-3495(03)75014-0 (doi:10.1016/S0006-3495(03)75014-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maloney J. M., Nikova D., Lautenschlager F., Clarke E., Langer R., Guck J., Van Vliet K. J. 2010. Mesenchymal stem cell mechanics from the attached to the suspended state. Biophys. J. 99, 2479–2487 10.1016/j.bpj.2010.08.052 (doi:10.1016/j.bpj.2010.08.052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou E. H., Quek S. T., Lim C. T. 2010. Power-law rheology analysis of cells undergoing micropipette aspiration. Biomech. Model Mechanobiol. 9, 563–572 10.1007/s10237-010-0197-7 (doi:10.1007/s10237-010-0197-7) [DOI] [PubMed] [Google Scholar]
- 38.Hoffman B. D., Massiera G., Van Citters K. M., Crocker J. C. 2006. The consensus mechanics of cultured mammalian cells. Proc. Natl Acad. Sci. USA 103, 10 259–10 264 10.1073/pnas.0510348103 (doi:10.1073/pnas.0510348103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engler A. J., Sen S., Sweeney H. L., Discher D. E. 2006. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 10.1016/j.cell.2006.06.044 (doi:10.1016/j.cell.2006.06.044) [DOI] [PubMed] [Google Scholar]
- 40.Dupont S., et al. 2011. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 10.1038/nature10137 (doi:10.1038/nature10137) [DOI] [PubMed] [Google Scholar]
- 41.McKee C. T., Wood J. A., Shah N. M., Fischer M. E., Reilly C. M., Murphy C. J., Russell P. 2011. The effect of biophysical attributes of the ocular trabecular meshwork associated with glaucoma on the cell response to therapeutic agents. Biomaterials 32, 2417–2423 10.1016/j.biomaterials.2010.11.071 (doi:10.1016/j.biomaterials.2010.11.071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Last J. A., Pan T., Ding Y., Reilly C. M., Keller K., Acott T. S., Fautsch M. P., Murphy C. J., Russell P. 2011. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest. Ophthalmol. Visual Sci. 52, 2147–2152 10.1167/iovs.10-6342 (doi:10.1167/iovs.10-6342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cross S. E., Jin Y. S., Rao J., Gimzewski J. K. 2007. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2, 780–783 10.1038/nnano.2007.388 (doi:10.1038/nnano.2007.388) [DOI] [PubMed] [Google Scholar]
- 44.Leske M. C., Connell A. M., Wu S. Y., Nemesure B., Li X., Schachat A., Hennis A. 2001. Incidence of open-angle glaucoma: the Barbados Eye Studies. The Barbados Eye Studies Group. Arch. Ophthalmol. 119, 89–95 [PubMed] [Google Scholar]
- 45.Toris C. B., Yablonski M. E., Wang Y. L., Camras C. B. 1999. Aqueous humor dynamics in the aging human eye. Am. J. Ophthalmol. 127, 407–412 10.1016/S0002-9394(98)00436-X (doi:10.1016/S0002-9394(98)00436-X) [DOI] [PubMed] [Google Scholar]
- 46.An S. S., et al. 2009. Cell stiffness, contractile stress and the role of extracellular matrix. Biochem. Biophys. Res. Commun. 382, 697–703 10.1016/j.bbrc.2009.03.118 (doi:10.1016/j.bbrc.2009.03.118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato M., Theret D. P., Wheeler L. T., Ohshima N., Nerem R. M. 1990. Application of the micropipette technique to the measurement of cultured porcine aortic endothelial cell viscoelastic properties. J. Biomech. Eng. 112, 263–268 10.1115/1.2891183 (doi:10.1115/1.2891183) [DOI] [PubMed] [Google Scholar]
- 48.Potard U. S., Butler J. P., Wang N. 1997. Cytoskeletal mechanics in confluent epithelial cells probed through integrins and E-cadherins. Am. J. Physiol. 272, C1654–C1663 [DOI] [PubMed] [Google Scholar]
- 49.Williams R. N., Cole D. F., Bhattacherjee P. 1983. The effect of colchicine on the rate of formation of aqueous humour and the gross outflow facility in the albino rabbit. Exp. Eye Res. 36, 711–717 10.1016/0014-4835(83)90108-2 (doi:10.1016/0014-4835(83)90108-2) [DOI] [PubMed] [Google Scholar]
- 50.Smith P. G., Deng L., Fredberg J. J., Maksym G. N. 2003. Mechanical strain increases cell stiffness through cytoskeletal filament reorganization. Am. J. Physiol. Lung Cell Mol. Physiol. 285, L456–L463 [DOI] [PubMed] [Google Scholar]
- 51.Laudadio R. E., Millet E. J., Fabry B., An S. S., Butler J. P., Fredberg J. J. 2005. Rat airway smooth muscle cell during actin modulation: rheology and glassy dynamics. Am. J. Physiol. Cell Physiol. 289, C1388–C1395 10.1152/ajpcell.00060.2005 (doi:10.1152/ajpcell.00060.2005) [DOI] [PubMed] [Google Scholar]
- 52.Hirase T., Kawashima S., Wong E. Y., Ueyama T., Rikitake Y., Tsukita S., Yokoyama M., Staddon J. M. 2001. Regulation of tight junction permeability and occludin phosphorylation by Rhoa-p160ROCK-dependent and -independent mechanisms. J. Biol. Chem. 276, 10 423–10 431 10.1074/jbc.M007136200 (doi:10.1074/jbc.M007136200) [DOI] [PubMed] [Google Scholar]
- 53.Kaufman P. L., Erickson K. 1982. Cytochalasin B and D dose-outflow facility response relationships in the cynomolgus monkey. Invest. Ophthalmol. Visual Sci. 23, 646–650 [PubMed] [Google Scholar]
- 54.Adamson R. H., Liu B., Fry G. N., Rubin L. L., Curry F. E. 1998. Microvascular permeability and number of tight junctions are modulated by cAMP. Am. J. Physiol. 274, H1885–H1894 [DOI] [PubMed] [Google Scholar]
- 55.Bartels S. P., Lee S. R., Neufeld A. H. 1982. Forskolin stimulates cyclic AMP synthesis, lowers intraocular pressure and increases outflow facility in rabbits. Curr. Eye Res. 2, 673–681 10.3109/02713688209019996 (doi:10.3109/02713688209019996) [DOI] [PubMed] [Google Scholar]
- 56.Lee P., Podos S., Mittag T., Severin C. 1984. Effect of topically applied forskolin on aqueous humor dynamics in cynomolgus monkey. Invest. Ophthalmol. Visual Sci. 25, 1206–1209 [PubMed] [Google Scholar]
- 57.Bindewald K., et al. 2004. Opposite effect of cAMP signaling in endothelial barriers of different origin. Am. J. Physiol. Cell Physiol. 287, C1246–C1255 10.1152/ajpcell.00132.2004 (doi:10.1152/ajpcell.00132.2004) [DOI] [PubMed] [Google Scholar]
- 58.Juzkiw T., Chan D., Dai W., Ethier C. R. 2005. Direct measurement of human trabecular meshwork stiffness. Invest. Ophthalmol. Vis. Sci. 46, E-Abstract 1346 [Google Scholar]
- 59.Epstein D. L., Rowlette L. L., Roberts B. C. 1999. Acto-myosin drug effects and aqueous outflow function. Invest. Ophthalmol. Visual Sci. 40, 74–81 [PubMed] [Google Scholar]
- 60.Matthews B. D., Overby D. R., Mannix R., Ingber D. E. 2006. Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J. Cell Sci. 119, 508–518 10.1242/jcs.02760 (doi:10.1242/jcs.02760) [DOI] [PubMed] [Google Scholar]
- 61.Ehringer W. D., Edwards M. J., Miller F. N. 1996. Mechanisms of α-thrombin, histamine, and bradykinin induced endothelial permeability. J. Cell. Physiol. 167, 562–569 (doi:10.1002/(SICI)1097-4652(199606)167:3<562::AID-JCP20>3.0.CO;2-4) [DOI] [PubMed] [Google Scholar]
- 62.Llobet A., Gual A., Pales J., Barraquer R., Tobias E., Nicolas J. M. 1999. Bradykinin decreases outflow facility in perfused anterior segments and induces shape changes in passaged BTM cells in vitro. Invest. Ophthalmol. Visual Sci. 40, 113–125 [PubMed] [Google Scholar]
- 63.Kaufman P. L., Barany E. H., Erickson K. A. 1982. Effect of serotonin, histamine and bradykinin on outflow facility following ciliary muscle retrodisplacement in the cynomolgus monkey. Exp. Eye Res. 35, 191–199 10.1016/S0014-4835(82)80066-3 (doi:10.1016/S0014-4835(82)80066-3) [DOI] [PubMed] [Google Scholar]
- 64.Trepat X., Grabulosa M., Buscemi L., Rico F., Farre R., Navajas D. 2005. Thrombin and histamine induce stiffening of alveolar epithelial cells. J. Appl. Physiol. 98, 1567–1574 10.1152/japplphysiol.00925.2004 (doi:10.1152/japplphysiol.00925.2004) [DOI] [PubMed] [Google Scholar]
- 65.Bausch A. R., Hellerer U., Essler M., Aepfelbacher M., Sackmann E. 2001. Rapid stiffening of integrin receptor-actin linkages in endothelial cells stimulated with thrombin: a magnetic bead microrheology study. Biophys. J. 80, 2649–2657 10.1016/S0006-3495(01)76234-0 (doi:10.1016/S0006-3495(01)76234-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kole T. P., Tseng Y., Huang L., Katz J. L., Wirtz D. 2004. Rho kinase regulates the intracellular micromechanical response of adherent cells to rho activation. Mol. Biol. Cell 15, 3475–3484 10.1091/mbc.E04-03-0218 (doi:10.1091/mbc.E04-03-0218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schulze C., Smales C., Rubin L. L., Staddon J. M. 1997. Lysophosphatidic acid increases tight junction permeability in cultured brain endothelial cells. J. Neurochem. 68, 991–1000 10.1046/j.1471-4159.1997.68030991.x (doi:10.1046/j.1471-4159.1997.68030991.x) [DOI] [PubMed] [Google Scholar]
- 68.Minnear F. L., Patil S., Bell D., Gainor J. P., Morton C. A. 2001. Platelet lipid(s) bound to albumin increases endothelial electrical resistance: mimicked by LPA. Am. J. Physiol. Lung Cell. Mol. Physiol. 281, L1337–L1344 [DOI] [PubMed] [Google Scholar]
- 69.Arce F. T., Whitlock J. L., Birukova A. A., Birukov K. G., Arnsdorf M. F., Lal R., Garcia J. G. N., Dudek S. M. 2008. Regulation of the micromechanical properties of pulmonary endothelium by S1P and thrombin: role of cortactin. Biophys. J. 95, 886–894 10.1529/biophysj.107.127167 (doi:10.1529/biophysj.107.127167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McVerry B. J., Garcia J. G. N. 2005. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell. Signall. 17, 131–139 10.1016/j.cellsig.2004.08.006 (doi:10.1016/j.cellsig.2004.08.006) [DOI] [PubMed] [Google Scholar]
- 71.Underwood J. L., Murphy C. G., Chen J., Franse-Carman L., Wood I., Epstein D. L., Alvarado J. A. 1999. Glucocorticoids regulate transendothelial fluid flow resistance and formation of intercellular junctions. Am. J. Physiol. 277, C330–C342 [DOI] [PubMed] [Google Scholar]
- 72.Romero I. A., Radewicz K., Jubin E., Michel C. C., Greenwood J., Couraud P. O., Adamson P. 2003. Changes in cytoskeletal and tight junctional proteins correlate with decreased permeability induced by dexamethasone in cultured rat brain endothelial cells. Neurosci. Lett. 344, 112–116 10.1016/S0304-3940(03)00348-3 (doi:10.1016/S0304-3940(03)00348-3) [DOI] [PubMed] [Google Scholar]
- 73.Clark A., Wilson K., de Kater A., Allingham R., McCartney M. 1995. Dexamethasone-induced ocular hypertension in perfusion-cultured human eyes. Invest. Ophthalmol. Visual Sci. 36, 478–489 [PubMed] [Google Scholar]
- 74.Puig F., Gavara N., Sunyer R., Carreras A., Farré R., Navajas D. 2009. Stiffening and contraction induced by dexamethasone in alveolar epithelial cells. Exp. Mech. 49, 47–55 10.1007/s11340-007-9072-6 (doi:10.1007/s11340-007-9072-6) [DOI] [Google Scholar]
- 75.Jermak C. M., Dellacroce J. T., Heffez J., Peyman G. A. 2007. Triamcinolone acetonide in ocular therapeutics. Survey Ophthalmol. 52, 503–522 10.1016/j.survophthal.2007.06.004 (doi:10.1016/j.survophthal.2007.06.004) [DOI] [PubMed] [Google Scholar]
- 76.Schrot S., Weidenfeller C., Schaffer T. E., Robenek H., Galla H. J. 2005. Influence of hydrocortisone on the mechanical properties of the cerebral endothelium in vitro. Biophys. J. 89, 3904–3910 10.1529/biophysj.104.058750 (doi:10.1529/biophysj.104.058750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rao V. P., Epstein D. L. 2007. Rho GTPase/Rho kinase inhibition as a novel target for the treatment of glaucoma. Biodrugs 21, 167–177 10.2165/00063030-200721030-00004 (doi:10.2165/00063030-200721030-00004) [DOI] [PubMed] [Google Scholar]
- 78.Pedrigi R. M., Simon D., Reed A., Stamer W. D., Overby D. R. 2011. A model of giant vacuole dynamics in human Schlemm's canal endothelial cells. Exp. Eye Res. 92, 57–66 10.1016/j.exer.2010.11.003 (doi:10.1016/j.exer.2010.11.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bill A., Svedbergh B. 1972. Scanning electron microscopic studies of the trabecular meshwork and the canal of Schlemm—an attempt to localize the main resistance to outflow of aqueous humor in man. Acta Ophthalmol. (Copenh) 50, 295–320 10.1111/j.1755-3768.1972.tb05954.x (doi:10.1111/j.1755-3768.1972.tb05954.x) [DOI] [PubMed] [Google Scholar]
- 80.Johnson M., Shapiro A., Ethier C. R., Kamm R. D. 1992. Modulation of outflow resistance by the pores of the inner wall endothelium. Invest. Ophthalmol. Visual Sci. 33, 1670–1675 [PubMed] [Google Scholar]
- 81.Overby D. R., Stamer W. D., Johnson M. 2009. The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endothelium. Exp. Eye Res. 88, 656–670 10.1016/j.exer.2008.11.033 (doi:10.1016/j.exer.2008.11.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ethier C. R., Read A. T., Chan D. W. 2006. Effects of latrunculin-B on outflow facility and trabecular meshwork structure in human eyes. Invest. Ophthalmol. Visual Sci. 47, 1991–1998 10.1167/iovs.05-0327 (doi:10.1167/iovs.05-0327) [DOI] [PubMed] [Google Scholar]
- 83.Tektas O. Y., Lutjen-Drecoll E. 2009. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp. Eye Res. 88, 769–775 10.1016/j.exer.2008.11.025 (doi:10.1016/j.exer.2008.11.025) [DOI] [PubMed] [Google Scholar]
- 84.Keller K. E., Aga M., Bradley J. M., Kelley M. J., Acott T. S. 2009. Extracellular matrix turnover and outflow resistance. Exp. Eye Res. 88, 676–682 10.1016/j.exer.2008.11.023 (doi:10.1016/j.exer.2008.11.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stamer W. D., Roberts B. C., Epstein D. L. 1999. Hydraulic pressure stimulates adenosine 3′,5′-cyclic monophosphate accumulation in endothelial cells from Schlemm's canal. Invest. Ophthalmol. Visual Sci. 40, 1983–1988 [PubMed] [Google Scholar]
- 86.VanderWyst S. S., Perkumas K. M., Read A. T., Overby D. R., Stamer W. D. 2011. Structural basement membrane components and corresponding integrins in Schlemm's canal endothelia. Mol. Vis. 17, 199–209 [PMC free article] [PubMed] [Google Scholar]
- 87.Heimark R. L., Kaochar S., Stamer W. D. 2002. Human Schlemm's canal cells express the endothelial adherens proteins, VE-cadherin and PECAM-1. Curr. Eye Res. 25, 299–308 10.1076/ceyr.25.5.299.13495 (doi:10.1076/ceyr.25.5.299.13495) [DOI] [PubMed] [Google Scholar]
- 88.Perkumas, Stamer In preparation. Protein markers and differentiation in culture for Schlemm's canal endothelial cells. [DOI] [PMC free article] [PubMed]
- 89.Fabry B., Maksym G. N., Shore S. A., Moore P. E., Panettieri R. A., Jr, Butler J. P., Fredberg J. J. 2001. Time course and heterogeneity of contractile responses in cultured human airway smooth muscle cells. J. Appl. Physiol. 91, 986–994 [DOI] [PubMed] [Google Scholar]
- 90.Price L. S., Leng J., Schwartz M. A., Bokoch G. M. 1998. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol. Biol. Cell 9, 1863–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou E. H., et al. 2009. Universal behavior of the osmotically compressed cell and its analogy to the colloidal glass transition. Proc. Natl Acad. Sci. USA 106, 10 632–10 637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bursac P., Fabry B., Trepat X., Lenormand G., Butler J. P., Wang N., Fredberg J. J., An S. S. 2007. Cytoskeleton dynamics: fluctuations within the network. Biochem. Biophys. Res. Commun. 355, 324–330 10.1016/j.bbrc.2007.01.191 (doi:10.1016/j.bbrc.2007.01.191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berntsen P., et al. 2010. Biomechanical effects of environmental and engineered particles on human airway smooth muscle cells. J. R. Soc. Interface 7(Suppl. 3), S331–S340 10.1098/rsif.2010.0068.focus (doi:10.1098/rsif.2010.0068.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Puig-De-Morales M., Millet E., Fabry B., Navajas D., Wang N., Butler J. P., Fredberg J. J. 2004. Cytoskeletal mechanics in the adherent human airway smooth muscle cell: probe specificity and scaling of protein–protein dynamics. Am. J. Physiol. Cell Physiol 287, C643–C654 10.1152/ajpcell.00070.2004 (doi:10.1152/ajpcell.00070.2004) [DOI] [PubMed] [Google Scholar]
- 95.Mijailovich S. M., Kojic M., Zivkovic M., Fabry B., Fredberg J. J. 2002. A finite element model of cell deformation during magnetic bead twisting. J. Appl. Physiol. 93, 1429–1436 [DOI] [PubMed] [Google Scholar]
- 96.Wang N., Naruse K., Stamenovic D., Fredberg J. J., Mijailovich S. M., Tolic-Norrelykke I. M., Polte T., Mannix R., Ingber D. E. 2001. Mechanical behavior in living cells consistent with the tensegrity model. Proc. Natl Acad. Sci. USA 98, 7765–7770 10.1073/pnas.141199598 (doi:10.1073/pnas.141199598) [DOI] [PMC free article] [PubMed] [Google Scholar]