Abstract
It is likely that neuroinflammation begins well before detectable cognitive impairment in Alzheimer’s disease (AD) occurs. Clarifying the alterations occurring prior to the clinical manifestation of overt AD dementia may provide valuable insight into the early diagnosis and management of AD. Herein, to address the issue that neuroinflammation precedes development of AD pathology, we analyzed cytokine expression profiles of the brain, with focus on non-demented control patients with increasing AD pathology, referred to as high pathology control (HPC) cases, who provide an intermediate subset between AD and normal control cases referred to as low pathology control (LPC) cases. With a semi-quantitative analysis of cytokine mRNA, among 15 cytokines and their related molecules tested, we found the involvement of eight: interleukin-1(IL-1) receptor antagonist (IL-1ra), IL-1 converting enzyme (ICE), IL-2, IL-6, IL-8, tumor necrosis factor (TNF) α, macrophage-colony stimulating factor (M-CSF) and transforming growth factor (TGF) β1 during the development from LPC to HPC, while decreases in IL-1ra, IL-8, MCP-1 and TNFα, and an increase in TACE were implicated in the later development from HPC to AD. These findings indicate that neuroinflammation precedes the clinical manifestation of overt dementia, rather than being involved at the later stages of AD.
Keywords: neuroinflammation, cytokines, Alzheimer’s disease (AD), RT-PCR, AD pathology
Introduction
Determination of the biological and molecular alterations occurring prior to the clinical manifestation of overt AD dementia may provide valuable insight into the early diagnosis and management of AD1). Recent gene ontology analyses have reveled that the significant group of differentially expressed genes between control and AD brains partly relate to genes involved in inflammation and immunological signaling, suggesting the involvement of immune cells and various inflammatory mediators in AD pathological process1, 2). So our major focus was placed on clarifying the role of inflammation in AD pathogenesis, particularly prior to the manifestation of overt dementia. In fact, there have been increasing reports demonstrating peripheral and neural alterations in inflammatory factors in subjects with mild cognitive impairment (MCI)3–6). MCI describes a preclinical stage of AD, applied to a transitional period between normal aging and early AD3, 7). The alterations in the immune response in MCI suggest that inflammatory events may precede the clinical development of AD3, 4)and that a higher proinflammatorystate in patients is at risk for the manifestation of overt AD dementia8).
We previously reported cytokine expression profiles in the autopsied brains of AD patients9) compared to brains from normal healthy elderly (referred to as LPC) and non-demented patients with increasing AD pathology (referred to as HPC)10). MCI is defined entirely according to clinical criteria7). But the pathological backgrounds of patients having MCI actually vary and are not restricted to AD changes11), although the term MCI was first applied by Petersen7) to a transitional period between normal aging and early AD7). HPC, on the other hand, is defined clinico-pathologically. Lue et al. 12, 13) isolated HPC cases that were non-demented (CDR<0.5) but exhibited sufficient amyloid β (Aβ) deposits and neurofibrillary tangles (NFT) to otherwise qualify for the diagnosis of AD. HPC cases provide an intermediate subset between AD and LPC cases. To elucidate the role of inflammation and also aid in the design of therapeutics for early AD, collection of data from HPC cases is particularly important.
However, for our previous study9) we used autopsied brain samples fixed with paraformaldehyde. Generally, with fixed tissues, it should be difficult to obtain polymerase chain reaction (PCR) products longer than approximately 400 base pairs (bp)14, 15). In obtaining enough PCR products to be detected on electrophoretic gels, we found that the success rates between PCR procedures using fixed and frozen tissues were very different9). Data from fixed brain samples were not satisfactory. Therefore, in the present study, we used brain samples that were frozen immediately at autopsy with a short postmortem intervals (PMI) (<4 hr) that clearly allows optimal use of the resource16).
Next, although in our previous study9) we used glyceraldehyde-3-phosphate dehydrogenase (G3PDH) as a reference, we added β-actin as another reference to normalize expression levels of the mRNA of interest. To evaluate the expression levels more accurately for each cytokine among cases, normalization by multiple housekeeping genes instead of a single gene should be required17).
Using these methods improved, in this study we performed a semi-quantitative analysis of cytokine mRNA expression profiles in the autopsied brains from LPC, HPC and AD cases. Our investigations of what inflammatory mechanisms or mediators are earlier or more significantly involved in the developmental process of AD pathology prior to the manifestation of overt dementia are expected to provide more accurate and useful information for the establishment of therapeutic strategies for early AD pathological progression.
Materials and methods
Patient samples
From among the 100 routine brain autopsies per year of patients who had prospectively enrolled in the Sun Health Research Institute Tissue Donation Program and given premortem consent, five to nine typical cases each were selected from AD, HPC and LPC patients, based on prior medical records with antemortem neuropsychological test scores, including data of the Folstein Mini Mental Status Examination (MMSE) and the Clinical Dementia Rating Scale (CDR), and on postmortem neuropathological records with the Consortium to establish a registry for AD (CERAD) pathological criteria18) and the Braak staging19). In addition, our study was approved by the ethical committees of both Shiga University of Medical Science and the National Hospital Organization Tottori Medical Center.
Tissue preparation
Briefly, brain tissue was removed within four hours of death, sectioned coronally at 1 cm intervals, immediately frozen with dry ice, and stored at −80°C until use.
Extraction of total RNA
The frozen tissue slices from the superior temporal cortex were mildly thawed. Approximately 100 mg of the tissue block was cut from the softened tissue slice per case. The block was immediately transferred to a 15 ml centrifuge tube, and dissolved in 1ml of Trizol reagent (Gibco, Invitrogen). Then, total RNA was extracted with Trizol reagent, according to the manufacturer’s instructions. The quality of obtained total RNA was checked by spectrophotometric measurements at 240, 260, 280 and 300 nm, and the amount of the total RNA was quantified with the peak value at 260 nm.
Reverse Transcriptase (RT)-polymerase chain reaction (PCR)
We reverse-transcribed 2.5 μg of total RNA with Superscript II reverse transcriptase (Gibco, Invitrogen), and the reverse-transcribed first-stranded cDNA was amplified with AmpliTag Gold (Applied Biosystems), as described in our previous report9). The same sense and antisense primers, and the same profile of the thermal cycle were selected for amplifying each cytokine mRNA as done in our previous study9), to compare them with the data reported.
As an additional reference, a PCR for β-actin was conducted. The sequences of the sense and antisense primers were 5′-TGGTGGGCATGGGTCAGAAGG ATTC-3′ and 5′-CATGGCTGGGGTGTTGAAGGTC TCA-3′, respectively. The profile of the thermal cycle used was denaturation at 95°C for 30 sec, annealing at 56 °C for 30 sec, and extension at 72 °C for 60 sec, following the first hot start at 95 °C for 10 min. On the last amplification cycle, the mixture was incubated for an additional 8 min at 72 °C. The number of cycles was 30.
PCR amplification of the samples from each case was repeated three times for each cytokine and data were expressed as the ratio of the specific mRNA of interest normalized to the mRNA expressed from the housekeeping genes G3PDH and β-actin. Data were statistically compared between two pairs: LPC and HPC, HPC and AD, and LPC and AD, pulled from the LPC, HPC, and AD groups with the Mann-Whitney test, because we focused on the cytokine expression profiles of the HPC cases.
Determination of the reliability of G3PDH and β-actin as references
We used two housekeeping genes, G3PDH and β-actin, as references to normalize cytokine mRNA expression levels obtained with our present semi-quantitative RT-PCR analysis. To test the reliability of these two genes as references, we conducted a real-time PCR (LightCycler 480 Real-Time PCR System, Roche) of ten housekeeping genes, including G3PDH (GAPDH), β-actin (ACTB), porphobilinogen deaminase (PBGD), hypoxanthine phosphoribosyl-transferase 1 (HPRT), phosphoglycerate kinase 1 (PGK1), glucose-6-phosphate dehyderogenase (G6PD), peptidyl-prolyl isomerase A (PPIA), TATA box binding protein (TBP), β2-microglobulin (B2M) and β-glucuronidase (GUSB) (Universal Probe Library Reference Gene Assays, Roche), with the same set of the brain samples used for our present semi-quantitative RT-PCR. Then, we identified the most stably expressed genes in a given set of the samples, according to the averaged expression stability values calculated with a geNorm analysis17).
Results
Patient demographics
The demographics of the patients studied are summarized in Table 1. Histochemistry
Table 1.
Demographics of the patients studied
| Patient condition |
|||
|---|---|---|---|
| LPC | HPC | AD | |
| Case serial number | #1–6 | #7, 9, 16, 19, 20 | #8, 10–15, 17, 18 |
| The number of cases | 6 | 5(4)† | 9 (8) (7)‡ |
| Age (years)* | 83.8 ± 4.9§ | 83.4 + 3.2§ (84.8 + 1.9)§ | 85.0 ± 3.9§ (84.8 ± 4.1)§ (84.7 ± 4.4)§ |
| Gender (F/M) | 4/2 | 5/0 (4/0) | 7/2 (6/2) (5/2) |
| Postmortem interval (hours)* | 2.5 ± 0.4¶ | 2.7 + 0.4¶ (2.7 + 0.5)¶ | 2.6 ± 0.5 ¶(2.5 ± 0.5)¶ (2.5 ± 0.5)¶ |
| Brain weight (grams)* | 1178 ± 1311, 3 | 1111 + 731, 2(1115 + 811, 2) | 991 ± 992, 3 (981 ± 1002, 3) (972 ± 1042, 3) |
| CERAD neuropathological diagnosis | not AD | possible AD | definite AD |
| Braak staging | II – III | III – IV | V – VI |
Values are expressed as means ± SEM.
As described in the Results and Table 2, data on case #19 were removed from our statistical analysis, resulting in n = 4 as an HPC group.
As shown in Table 2, the amount of the sample volume was not enough in cases #13 and 15, resulting in n = 9, 8 or 7, as an AD group.
No significant differences between any pairs from the LPC, HPC and AD groups (Mann-Whitney test).
No significant differences between any pairs from the LPC, HPC and AD groups (Mann-Whitney test).
no significant differences between any pairs from the data numbered 1 (Mann-Whitney test).
p<0.05, comparison between any pairs from the data numbered 2 (Mann-Whitney test)
p<0.01, comparison between any pairs from the data numbered 3 (Mann-Whitney test)
For histological diagnosis of our cases, Campbell-Switzer silver staining to reveal senile plaques and NFT was performed (data not shown).
Qualification and Quantification of total RNA extracted
The total RNA samples obtained were qualified by a peak value around 260 nm among absorbance values from 240 to 300 nm.
The reliability of G3PDH and β-actin as references
Among the ten housekeeping genes tested, our real-time PCR followed by the geNorm analysis17) showed that G3PDH, β-actin and GUSB produced the lowest values of the averaged expression stability, indicating the suitability and reliability of G3PDH and β-actin as references. As a result, for our following semi-quantitative RT-PCR analysis of cytokine mRNA, we used these two genes as references.
G3PDH and β-actin mRNA expression
With our electrophoretic analysis of the RT-PCR products, a single band corresponding each to the expected size (294 bp) of G3PDH mRNA and that (290 bp) of β-actin was constantly observed in all cases, although the intensity of the band varied somewhat among cases (Fig. 1).
Fig. 1. Representative gels of the electrophoretic analysis of the PCR products for cytokine mRNA tested. The numbers indicating the cases are identical to those in Table 2.
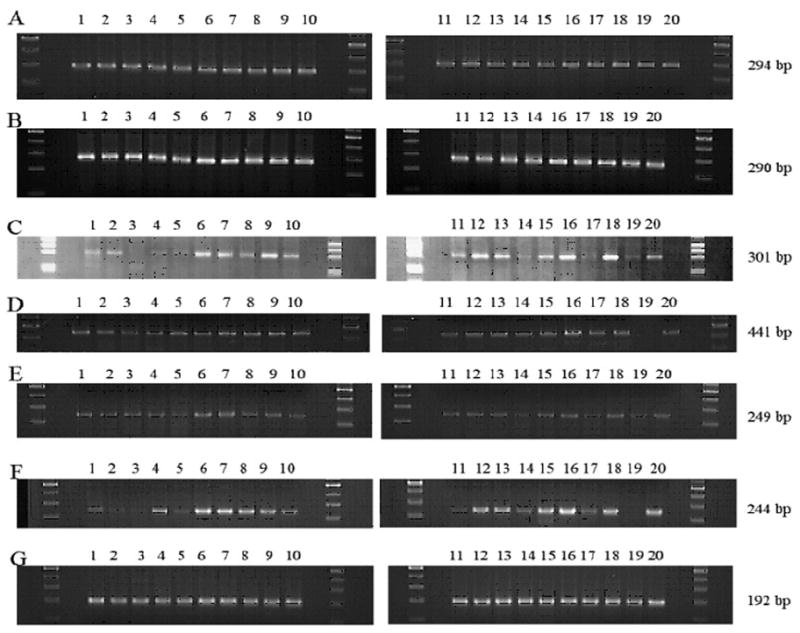
A: G3PDH (294 bp), B: β-actin (290 bp), C: IL-1 receptor antagonist (IL-1ra), secretory form (301bp), D: IL-2 (441bp), E: IL-8 (249 bp), F: TNFα (244 bp), G: M-CSF (192 bp)
Cytokine mRNA expression
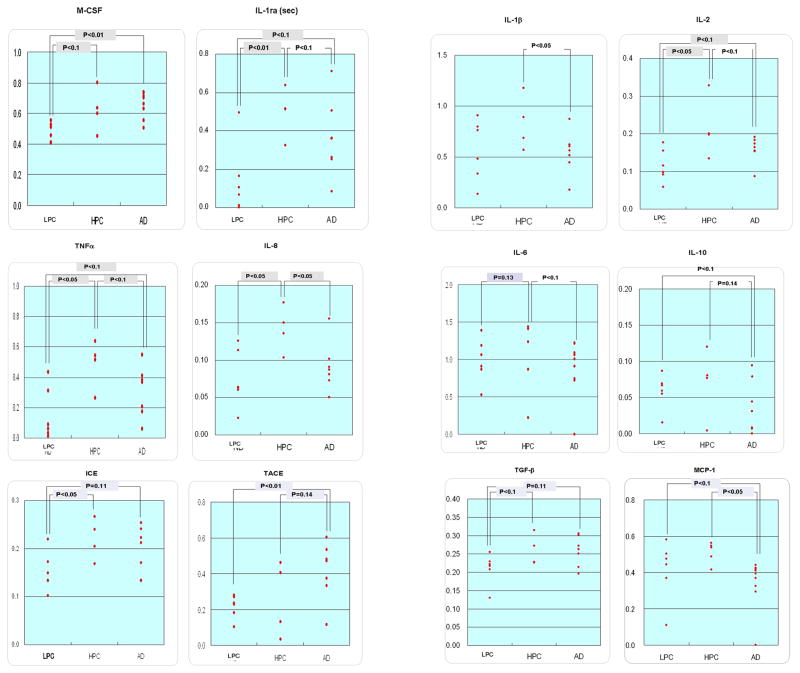
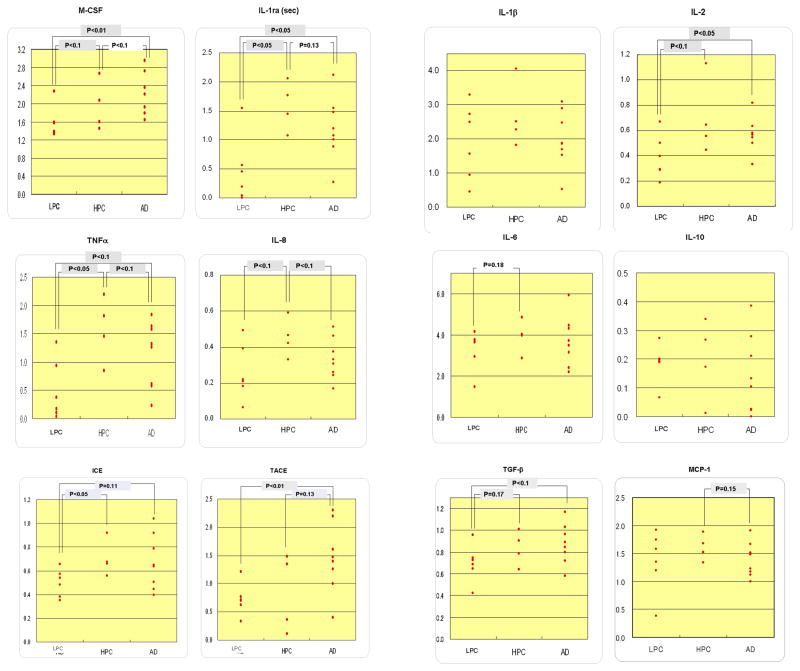
Expression levels of the specific mRNA of interest were determined by three independent PCR amplifications, each of which was conducted using a total of 18, 19 or 20 samples consisting of 6 LPC, 5 HPC, and 7, 8 or 9 AD cases. Fig. 1 shows the representative gels of our electrophoretic analysis of the PCR products. In Table 2, data on the mRNA expression levels of each cytokine in each case are expressed as the ratios to the G3PDH and β-actin mRNA levels. Each of the data in Table 2 is an averaged value obtained from the results of three independent experiments, each of which consisted of a series of 6 LPC, 5 HPC, and 7, 8 or 9 AD cases. Thus, the value of the ratio in each case in each cytokine is shown in Table 2. The ratio of an undetectable band was calculated as zero (Table 2). Table 3 shows the averaged values in each of three groups which consist of 6 LPC, 5 HPC and 7, 8 or 9 AD cases, and the results of statistical analysis with the Mann-Whitney test. As Table 2 shows, the values of the ratios in case #19 (HPC) widely shifted from those in the other four HPC cases. Therefore, we decided that case #19 was not suitable as a representative of HPC, and the data of case #19 were removed from our statistical analysis. In the AD cases, the amount of the sample volume in cases #13 and 15 was not enough for completing the PCR of all the mRNA of interest studied here. Therefore, RT-PCR analyses could not be conducted on the intracellular form of IL-1ra that is referred to as IL-1ra (int), and GM-CSF for cases #13 and 15; or on β-actin for case #15. Fig. 2 and 3 represent scatter grams of the ratios shown in Table 2, with exclusion of all the data of case #19; the data on IL-1ra (int) and GM-CSF of case #13, normalized to G3PDH and β-actin; the data on IL-1ra (int) and GM-CSF of case #15, normalized to G3PDH; and the data of case #15 on all the cytokines tested here, normalized to β-actin.
Table 2.
Semi-quantitative data of mRNA expression levels on each cytokine in the temporal cortex of each LPC, HPC and AD case.
| Patient condition | LPC | HPC | AD | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| case serial number | #1 | #2 | #3 | #4 | #5 | #6 | #9 | #16 | #19¶ | #20 | #7 | #8 | #10 | #11 | #12 | #13† | #14 | #15† | #17 | #18 |
| GM-CSF (G)* | 0.121 | 0.148 | 0.248 | 0.117 | 0.201 | 0.230 | 0.116 | 0.214 | 0.060 | 0.133 | 0.416 | 0.191 | 0.310 | 0.343 | 0.189 | ND | 0.206 | ND | 0.189 | 0.205 |
| GM-CSF (a)** | 0.028 | 0.043 | 0.076 | 0.039 | 0.071 | 0.073 | 0.036 | 0.062 | 0.018 | 0.040 | 0.147 | 0.054 | 0.104 | 0.090 | 0.062 | ND | 0.052 | ND | 0.056 | 0.069 |
| IL-1ra (int) (G) | 0.679 | 0.064 | 0.374 | 0.000 | 0.573 | 0.566 | 0.025 | 0.582 | 0.113 | 0.814 | 0.822 | 0.042 | 0.278 | 0.721 | 0.318 | ND | 0.115 | ND | 0.052 | 1.183 |
| IL-1ra (int) (a) | 0.156 | 0.019 | 0.115 | 0.000 | 0.202 | 0.181 | 0.008 | 0.169 | 0.034 | 0.244 | 0.291 | 0.012 | 0.094 | 0.189 | 0.104 | ND | 0.029 | ND | 0.016 | 0.397 |
| IL-1ra (sec) (G) | 0.452 | 0.559 | 0.000 | 0.036 | 0.190 | 1.546 | 2.054 | 1.772 | 0.265 | 1.072 | 1.450 | 0.888 | 1.075 | 1.000 | 1.546 | 1.472 | 0.148 | 1.191 | 0.276 | 2.115 |
| IL-1ra (sec) (a) | 0.104 | 0.163 | 0.000 | 0.012 | 0.067 | 0.494 | 0.638 | 0.514 | 0.079 | 0.322 | 0.513 | 0.250 | 0.362 | 0.261 | 0.505 | 0.359 | 0.037 | ND | 0.082 | 0.709 |
| IL-1β (G) | 3.298 | 0.453 | 1.569 | 2.732 | 0.948 | 2.501 | 1.821 | 4.069 | 1.149 | 2.276 | 2.520 | 3.102 | 1.534 | 1.700 | 0.533 | 2.483 | 0.620 | 2.903 | 1.883 | 1.856 |
| IL-1β (a) | 0.761 | 0.132 | 0.483 | 0.910 | 0.334 | 0.799 | 0.566 | 1.179 | 0.341 | 0.683 | 0.892 | 0.874 | 0.517 | 0.445 | 0.174 | 0.606 | 0.156 | ND | 0.561 | 0.622 |
| IL-2 (G) | 0.670 | 0.396 | 0.188 | 0.292 | 0.499 | 0.288 | 0.643 | 1.133 | 0.000 | 0.447 | 0.556 | 0.544 | 0.564 | 0.330 | 0.500 | 0.635 | 0.627 | 0.820 | 0.582 | 0.547 |
| IL-2 (a) | 0.155 | 0.115 | 0.058 | 0.097 | 0.176 | 0.092 | 0.200 | 0.328 | 0.000 | 0.134 | 0.197 | 0.153 | 0.190 | 0.086 | 0.163 | 0.155 | 0.158 | ND | 0.174 | 0.183 |
| IL-10 (G) | 0.067 | 0.189 | 0.191 | 0.200 | 0.197 | 0.274 | 0.173 | 0.267 | 0.123 | 0.013 | 0.340 | 0.280 | 0.022 | 0.000 | 0.026 | 0.385 | 0.000 | 0.210 | 0.105 | 0.133 |
| IL-10 (a) | 0.016 | 0.055 | 0.059 | 0.067 | 0.069 | 0.087 | 0.081 | 0.077 | 0.036 | 0.004 | 0.120 | 0.079 | 0.007 | 0.000 | 0.008 | 0.094 | 0.000 | ND | 0.031 | 0.044 |
| IL-8 (G) | 0.492 | 0.218 | 0.208 | 0.181 | 0.063 | 0.393 | 0.330 | 0.465 | 0.236 | 0.591 | 0.423 | 0.259 | 0.242 | 0.332 | 0.308 | 0.373 | 0.107 | 0.512 | 0.168 | 0.464 |
| IL-8 (a) | 0.113 | 0.063 | 0.064 | 0.060 | 0.022 | 0.126 | 0.103 | 0.135 | 0.070 | 0.177 | 0.150 | 0.073 | 0.081 | 0.087 | 0.101 | 0.091 | 0.027 | ND | 0.050 | 0.155 |
| MCP-1 (G) | 1.923 | 0.379 | 1.200 | 1.740 | 1.345 | 1.575 | 1.335 | 1.677 | 1.889 | 1.518 | 1.523 | 1.507 | 1.169 | 1.121 | 0.993 | 1.513 | 1.667 | 1.909 | 1.481 | 1.224 |
| MCP-1 (a) | 0.443 | 0.110 | 0.369 | 0.580 | 0.474 | 0.503 | 0.415 | 0.486 | 0.561 | 0.546 | 0.539 | 0.425 | 0.394 | 0.293 | 0.324 | 0.369 | 0.420 | ND | 0.441 | 0.410 |
| TGF-β1 (G) | 0.957 | 0.746 | 0.423 | 0.688 | 0.724 | 0.649 | 1.012 | 0.783 | 0.603 | 0.904 | 0.639 | 0.963 | 0.893 | 1.171 | 0.801 | 1.029 | 0.836 | 0.846 | 0.717 | 0.580 |
| TGF-β1 (a) | 0.221 | 0.217 | 0.130 | 0.229 | 0.255 | 0.208 | 0.315 | 0.227 | 0.179 | 0.271 | 0.226 | 0.271 | 0.301 | 0.306 | 0.262 | 0.251 | 0.211 | ND | 0.214 | 0.195 |
| IL-6 (G) | 3.777 | 3.660 | 2.947 | 4.161 | 1.495 | 3.710 | 3.985 | 4.863 | 0.741 | 2.885 | 4.051 | 4.319 | 3.153 | 3.492 | 3.739 | 4.467 | 4.007 | 5.942 | 2.416 | 2.208 |
| IL-6 (a) | 0.871 | 1.065 | 0.907 | 1.386 | 0.526 | 1.185 | 1.239 | 1.410 | 0.220 | 0.866 | 1.435 | 1.217 | 1.063 | 0.913 | 1.221 | 1.089 | 1.010 | ND | 0.720 | 0.740 |
| ICE (G) | 0.576 | 0.353 | 0.484 | 0.659 | 0.385 | 0.542 | 0.661 | 0.920 | 0.230 | 0.562 | 0.677 | 0.791 | 0.508 | 0.921 | 0.649 | 1.043 | 0.501 | 0.639 | 0.446 | 0.399 |
| ICE (a) | 0.133 | 0.103 | 0.149 | 0.220 | 0.135 | 0.173 | 0.205 | 0.267 | 0.068 | 0.169 | 0.240 | 0.223 | 0.171 | 0.241 | 0.212 | 0.254 | 0.126 | ND | 0.133 | 0.134 |
| TACE (G) | 1.214 | 0.627 | 0.341 | 0.706 | 0.768 | 0.719 | 1.491 | 0.120 | 0.624 | 1.353 | 0.369 | 0.409 | 1.398 | 2.311 | 1.477 | 2.199 | 1.326 | 1.608 | 1.263 | 1.001 |
| TACE (a) | 0.280 | 0.183 | 0.105 | 0.235 | 0.270 | 0.230 | 0.463 | 0.035 | 0.186 | 0.406 | 0.131 | 0.115 | 0.471 | 0.604 | 0.483 | 0.536 | 0.334 | ND | 0.376 | 0.335 |
| TNFα (G) | 0.381 | 0.108 | 0.046 | 0.946 | 0.176 | 1.360 | 0.849 | 2.201 | 0.030 | 1.816 | 1.458 | 1.318 | 0.617 | 0.234 | 1.262 | 1.577 | 0.700 | 1.837 | 0.583 | 1.636 |
| TNFα (a) | 0.088 | 0.031 | 0.014 | 0.315 | 0.062 | 0.434 | 0.264 | 0.638 | 0.009 | 0.545 | 0.516 | 0.371 | 0.208 | 0.061 | 0.412 | 0.385 | 0.176 | ND | 0.174 | 0.548 |
| M-CSF (G) | 2.285 | 1.396 | 1.337 | 1.368 | 1.576 | 1.595 | 1.462 | 2.082 | 2.141 | 2.674 | 1.603 | 1.798 | 1.647 | 2.730 | 1.931 | 2.727 | 2.916 | 2.955 | 2.366 | 2.210 |
| M-CSF (a) | 0.527 | 0.406 | 0.412 | 0.456 | 0.555 | 0.510 | 0.454 | 0.603 | 0.636 | 0.803 | 0.568 | 0.507 | 0.555 | 0.714 | 0.631 | 0.665 | 0.735 | ND | 0.705 | 0.741 |
| IL-3 (G) | 2.219 | 1.933 | 1.679 | 1.693 | 1.930 | 1.523 | 1.795 | 2.133 | 2.067 | 2.290 | 1.671 | 2.043 | 1.667 | 2.806 | 1.880 | 2.803 | 2.809 | 2.823 | 2.095 | 1.931 |
| IL-3 (a) | 0.512 | 0.562 | 0.517 | 0.564 | 0.680 | 0.487 | 0.558 | 0.618 | 0.614 | 0.687 | 0.592 | 0.576 | 0.562 | 0.734 | 0.614 | 0.684 | 0.708 | ND | 0.624 | 0.647 |
Data are expressed as ratios to the G3PDH and β-actin mRNA levels. Each value is an average of three independent experiments using six LPC, five HPC and nine, eight or seven AD cases. The ratio of an undetectable band was calculated as zero.
G: normalized with G3PDH mRNA expression levels
a: normalized with β-actin mRNA expression levels
Values of the ratios in case #19 (HPC) widely shifted from those in the other four HPC cases. Therefore, we have decided that case #19 was not suitable as a representative of HPC, and all the data from case #19 were removed from our statistical analysis.
ND: The amount of the sample volume was not enough in cases #13 and 15. Therefore, RT-PCR analyses were not conducted on IL-1ra (int) and GM-CSF for cases #13 and 15, or on β-actin for case #15.
Table 3.
Averaged data on cytokine mRNA expression levels in each of LPC, HPC and AD groups
| Reference gene | G3PDH | (p value) | β-actin | (p value) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient condition | LPC | HPC¶ | AD† | LPC-HPC | HPC-AD | LPC-AD | LPC | HPC¶ | AD†† | LPC-HPC | HPC-AD | LPC-AD |
| The number of cases | 6 | 4 | 9 or 7 | 6 | 4 | 8 or 7 | ||||||
| Case number | #1–6 | #7, 9, 16, 20 | #8, 10–15, 17, 18 | #1–6 | #7, 9, 16, 20 | #8, 10–14, 17, 18 | ||||||
| GM-CSF | 0.18 ± 0.06 | 0.22 ± 0.12 | 0.26 ± 0.09 | 0.06 ± 0.02 | 0.07 ± 0.04 | 0.08 ± 0.03 | ||||||
| IL-lra | ||||||||||||
| (intracellular) | 0.38 ± 0.29 | 0.56 ± 0.32 | 0.44 ± 0.42 | 0.11 ± 0.09 | 0.18 ± 0.11 | 0.14 ± 0.14 | ||||||
| (secreted) | 0.46 ± 0.58 | 1.59 ± 0.37 | 1.16 ± 0.59 | <0.05 | 0.13 | <0.05 | 0.14 ± 0.18 | 0.51 ± 0.11 | 0.34 ± 0.21 | <0.01 | <0.1 | <0.1 |
| IL-1β | 1.92 ± 1.11 | 2.67 ± 0.84 | 1.19 ± 0.87 | 0.57 ± 0.30 | 0.83 ± 0.23 | 0.54 ± 0.26 | <0.05 | |||||
| IL-2 | 0.39 ± 0.17 | 0.69 ± 0.26 | 0.57 ± 0.12 | <0.1 | <0.05 | 0.12 ± 0.04 | 0.21 ± 0.07 | 0.16 ± 0.03 | <0.05 | <0.1 | <0.1 | |
| IL-10 | 0.19 ± 0.07 | 0.20 ± 0.12 | 0.15 ± 0.15 | 0.06 ± 0.02 | 0.07 ± 0.04 | 0.04 ± 0.05 | 0.14 | <0.1 | ||||
| IL-8 | 0.26 ± 0.16 | 0.45 ± 0.10 | 0.32 ± 0.13 | <0.1 | <0.1 | 0.08 ± 0.04 | 0.14 ± 0.03 | 0.09 ± 0.04 | <0.05 | <0.05 | ||
| MCP-1 | 1.36 ± 0.55 | 1.51 ± 0.12 | 1.41 ± 0.28 | 0.15 | 0.41 ± 0.16 | 0.50 ± 0.05 | 0.40 ± 0.07 | <0.05 | <0.1 | |||
| TGF-β1 | 0.70 ± 0.17 | 0.86 ± 0.17 | 0.85 ± 0.18 | 0.17 | <0.1 | 0.21 ± 0.04 | 0.26 ± 0.04 | 0.25 ± 0.04 | <0.1 | 0.11 | ||
| IL-6 | 3.29 ± 0.96 | 3.95 ± 0.70 | 3.78 ± 1.07 | 0.18 | 0.99 ± 0.29 | 1.24 ± 0.23 | 1.05 ± 0.23 | 0.13 | <0.1 | |||
| ICE | 0.50 ± 0.12 | 0.71 ± 0.13 | 0.66 ± 0.21 | <0.05 | 0.11 | 0.15 ± 0.04 | 0.22 ± 0.04 | 0.19 ± 0.05 | <0.05 | 0.11 | ||
| TACE | 0.73 ± 0.28 | 0.83 ± 0.64 | 1.34 ± 0.64 | 0.13 | <0.01 | 0.22 ± 0.07 | 0.26 ± 0.18 | 0.38 ± 0.17 | 0.14 | <0.01 | ||
| TNFα | 0.50 ± 0.53 | 1.58 ± 0.50 | 1.12 ± 0.54 | <0.05 | <0.1 | <0.1 | 0.16 ± 0.17 | 0.49 ± 0.14 | 0.32 ± 0.17 | <0.05 | <0.1 | <0.1 |
| M-CSF(α, β, γ) | 1.59 ± 0.36 | 1.96 ± 0.47 | 2.29 ± 0.53 | <0.1 | <0.1 | <0.01 | 0.48 ± 0.06 | 0.61 ± 0.13 | 0.65 ± 0.09 | <0.1 | <0.01 | |
| IL-3 | 1.83 ± 0.25 | 1.97 ± 0.25 | 2.25 ± 0.50 | <0.1 | 0.55 ± 0.07 | 0.61 ± 0.05 | 0.19 ± 0.05 | <0.1 | <0.05 | |||
Data are expressed as ratios to the G3PDH and β-actin mRNA levels. Each value is an average in each of three groups: LPC, HPC, and AD groups.
Values of the HPC group are from four cases with exclusion of data of case #19.
Values of the AD group normalized with G3PDH are from nine or seven cases. The averaged data of the cytokines, except IL-1ra (intracellular) and GM-CSF, are from nine cases. The averaged data of IL-1ra (intracellular) and GM-CSF are from seven cases, because of lack of data in cases #13 and 15 for these two cytokines.
Values of the AD group normalized with β-actin are from eight or seven cases. The averaged data of IL-1ra (intracellular) and GM-CSF are from seven cases, because of lack of data in cases #13 and 15 for these two cytokines. The averaged data of the cytokines, except IL-1ra (intracellular) and GM-CSF, are from eight not nine cases, because of lack of data on β-actin for case #15.
p value: Comparison between the LPC and HPC, the HPC and AD, and the LPC and AD groups, with the Mann-Whitney test. Blank means p>0.2.
Fig. 2.
Scattergrams on the ratios of the expression levels of cytokine mRNA, normalized with G3PDH mRNA expression levels. In the HPC group, the data of case #19 have been excluded. The p values were obtained with the Mann-Whitney test.
Fig. 3.
Scattergrams on the ratios of the expression levels of cytokine mRNA, normalized with β-actin mRNA expression levels. In the HPC group, the data of case #19 have been excluded. In the AD group, there are no data of case #15. The p values were obtained with the Mann-Whitney test.
In the ratios of the expression levels of the mRNA of interest to the G3PDH mRNA levels (Table 3, 4; Fig. 2), we found statistical differences, including a tendency (p<0.2), in the secretory form of IL-1ra that is referred to as IL-1ra (sec), ICE, IL-2, IL-6, IL-8, TNFα, M-CSF and TGFβ1 mRNA expression levels between the LPC and HPC groups, in IL-1 ra (sec), IL-8, MCP-1, TNFα, TACE and M-CSF mRNA expression levels between the HPC and AD groups, and in IL-1ra (sec), ICE, IL-2, IL-3, TNFα, TACE, M-CSF and TGFβ1 mRNA expression levels between the LPC and AD groups. In the ratios of the expression levels of the mRNA of interest to the β-actin mRNA levels (Table 3, Fig. 3), we observed statistical differences, including a tendency (p<0.2), in IL-1ra (sec), ICE, IL-2, IL-3, IL-6, IL-8, TNFα, M-CSF and TGFβ1 mRNA expression levels between the LPC and HPC groups, in IL-1β, IL-1ra (sec), IL-2, IL-6, IL-8, IL-10, MCP-1, TNFα and TACE mRNA expression levels between the HPC and AD groups, and in IL-1ra (sec), IL-2, IL-3, IL-10, MCP-1, TNFα TACE, M-CSF and TGFβ1 mRNA expression levels between the LPC and AD groups.
In summary, cytokine mRNAs showing the similar direction of changes (increases or decreases), including a tendency (p<0.2), between the data using G3PDH and β-actin as references were as follows (Table 3): IL-1ra (sec), ICE, IL-2, IL-6, IL-8, TNFα, M-CSF and TGFβ1 increased in HPC, compared to LPC; IL-1ra (sec), ICE, IL-2, IL-3, TNFα, TACE, M-CSF and TGFβ1 increased finally in AD in comparison to LPC; IL-1ra (sec), IL-8, MCP-1 and TNFα decreased, albeit increased in TACE, in AD in comparison to HPC.
Regarding housekeeping genes, G3PDH and β-actin were utilized here as references to normalize cytokine mRNA expression levels. There was somewhat of a higher percentage in calculating p<0.1 using β-actin than in calculating using G3PDH as a reference, when the LPC and HPC cases, and the HPC and AD cases were compared (Table 3).
Discussion
In the present study, we examined the role of inflammatory cytokines in the development of AD pathology, particularly prior to the clinical manifestation of overt AD dementia. To address this issue, we performed a semi-quantitative analysis of cytokine mRNA expression in the brain, with focus on HPC cases, which provide an intermediate subset between LPC and AD cases. As a result, we successfully found that the mRNAs encoding IL-1ra (sec), ICE, IL-2, IL-6, IL-8, TNFα, M-CSF and TGFβ1 increased in the HPC group, compared to the LPC group. Actually, 8 of the 15 cytokines and their related molecules tested here were shown to increase in the HPC group, compared to the LPC group, while only four: IL-1ra (sec), MCP-1, IL-8 and TNFα; and only TACE represented a decrease and an increase, respectively, in the AD group, compared to the HPC group. These findings may suggest that the mobilization of inflammatory mediators is more implicated in the developmental course of AD pathology prior to the manifestation of overt dementia than in the later AD stage after significant cognitive impairment occurs.
In the present study, we have newly shown participation of IL-2, IL-8, M-CSF and TGFβ1 in the early AD pathology before the manifestation of overt dementia. With fixed tissues in the previous study9), it was completely impossible to obtain detectable bands on electrophoresis for the PCR product for IL-2. In the present study, with freshly frozen tissues, we were able to detect a small amount of the mRNA encoding cytokines like IL-2 as well as to obtain PCR products longer than approximately 400 bp14, 15).
In addition, we used two housekeeping genes, G3PDH and β-actin, as references to normalize cytokine mRNA expression levels in the present study. The expression levels of β-actin were much higher than those of G3PDH, and the expression levels of G3PDH were closer to the cytokine mRNA expression levels tested here. This resulted in more limited variations in the ratios of the expression levels of the mRNA of interest normalized to the β-actin mRNA levels in each of the LPC, HPC and AD groups than the variations in the ratios to the G3PDH mRNA levels (Fig. 2, 3). However, G3PDH, for which the mRNA expression levels were closer to the expression levels of mRNA of cytokines tested, should be more adequate as a reference, although there have been numerous reports demonstrating some extent of inducible changes in G3PDH as well as β-actin mRNA expression levels in non-steady states20). Gutala and Reddy21) reported that G3PDH is more suitable than β-actin as a reference for comparative gene expression analysis using postmortem AD brains. However, it is also true that there have been many studies demonstrating influence of Aβ on G3PDH activity and metabolism22). Selection of the best references should be one of the major concerns in a quantitative PCR analysis. In fact, for accurate gene quantification, it is essential to normalize real-time PCR data to the most stably expressed housekeeping genes in each individual experimental condition. For this reason, we tested the reliability of G3PDH and β-actin as references, using the data obtained with a real-time PCR analysis of the same set of the brain samples examined in the present study, and identified G3PDH and β-actin as the most stably expressed genes in a given set of the samples. As a result, we determined that it was reasonable to select G3PDH and β-actin as the best references in our present study.
Our present study also indicated involvement of the IL-1 family in the early events leading later to the development of AD pathology, as did our previous study9). Here, we will first discuss the meaningful roles of chemokines, M-CSF and IL-2 in the AD pathogenesis. We previously showed that IL-6, IL-10 and TNFα, expressed under normal conditions, had a tendency to decrease in the HPC9). In the present study, however, it was observed that IL-6 and TNFα mRNA expression levels increased in the HPC group, while no significant changes in the mRNA expressions in IL-10 occurred compared to those in the LPC group. Then we will discuss the discrepancy in changes in the mRNA expression levels of IL-6, IL-10 and TNFα.
Recent studies have begun to focus attention on the role of chemokines and chemokine receptors in neuroinflammatory diseases of the central nervous system (CNS), including multiple sclerosis and human immunodeficiency virus infection, in which the blood brain barrier is compromised and leukocyte infiltration is found at the lesion sites23, 24). In AD, unlike the aforementioned neuroinflammatory diseases in the CNS23, 24), abnormal or excessive migration of inflammatory cells into the CNS has not definitely been shown to occur23). Nonetheless, there is growing evidence that chemokines and chemokine receptors are upregulated in AD brains, and that chemokines may contribute to plaque-associated inflammation and neurodegeneration23–25). This evidence may offer support for an inflammatory hypothesis of AD, which would portray chronically activated microglia producing inflammatory mediators as harmful cellular elements24, 25). In the present study, we demonstrated that IL-8 mRNA expression levels increased in the HPC group, compared to the LPC group. This observation is consistent with the role of chemokines in neuroinflammation. In fact, IL-8 has been reported to be upregulated in AD1, 2, 4, 24–26).
Production of M-CSF from the neurons activated by tau and Aβ through the receptor for advanced glycation end products (RAGE) can cause the attraction, activation, and proliferation of surrounding microglia23). Following the activation of microglia with M-CSF, the microglia are subject to the activation of astrocytes and additional microglia, which can magnify the production of inflammatory mediators, leading to neuroinflammation or direct injuries of neurons, and in turn intensify the AD pathology27–30). As Mitrasinovic et al.30) pointed out in their report; however, microglia may have a neuroprotective function. Whether microglia play a major role in exacerbation or clearance of amyloid plaques seems to be controversial31–33).
Unlike multiple sclerosis and experimental allergic encephalomyelitis, in the pathogenesis of which IL-2 and its receptors play an important role34), fewer reports on IL-2 involvement in AD pathogenesis are known1, 23, 25, 26, 35, 36). In the peripheral immune system of AD patients, the serum levels of IL-2 decrease as AD dementia progresses, suggesting a counter-regulatory mechanism involved in the underlying immune process throughout the AD progression37). In addition, IL-2 production from peripheral mononuclear cells and splenocytes has been reported to be reduced in AD patients38, 39). These results may be consistent with the fact that the safer and more effective outcome of immunotherapy for AD using active immunization with Aβ requires induction of the T helper (Th) type 2 (Th2)-polarized immune response, consisting of increased Th2 cytokines (IL-4 and IL-10) and decreased Th1 cytokine (IL-2 and IFN-γ)40). The inhibition of IL-2, which occurs in the later stages of AD, is thought to be a reciprocal change to protect the CNS from detrimental effects of neuroinflammation in AD37). In the present study, the mRNA expression levels of not only IL-2, but also IL-10 decreased in the AD group in comparison to the HPC group, but only while normalized with β-actin for IL-10. According to the scatter grams on IL-10 (Fig. 2, 3), in the HPC cases in comparison to the LPC cases, IL-10 mRNA expression levels showed somewhat of a tendency to increase. We, of course, demonstrated herein that, in the HPC cases in comparison to the LPC cases, IL-2 mRNA expression levels significantly increased. Therefore, there may be an earlier mobilization of IL-10, which is expected to down-regulate cytokines including IL-2. IL-10 is thought to increase in the preclinical stages of AD (HPC) to protect against undesirable, destructive effects of the brain immune response. As a result, we assume that, at the later AD stages, not only that IL-2 decreases due to the inhibitory effect of IL-10, but also that IL-10 decreases after cessation of its role against IL-2. In addition, since the increase in cytokines in the HPC may partly have beneficial effects on the CNS, changes in IL-2 and IL-10 in the AD progression need further clarification.
Like IL-10, IL-6 mRNA expression decreased in the AD cases in comparison to the HPC cases, but only while normalized with β-actin. In addition, IL-10 mRNA expression levels finally somewhat decreased in the AD, compared also to the LPC. These results may indicate down regulation of IL-6 and IL-10 not only in the later AD stages but also throughout the total AD pathological course, and their possession of physiological function in the steady state. Therefore, our present data on IL-6 and IL-10 are partly consistent with our previous data9), although the discrepancy between the previous9) and present data were observed in the changes in the HPC in comparison to the LPC.
Another discrepancy found in the changes in TNFα mRNA expression levels between our present and previous9) studies needs further clarification. TNFα expression in AD brains generally seems to be upregulated41), although Lanzrein et al.42) reported its decline in AD. To clarify the role of TNFα in the AD pathogenesis, we should examine the two TNF signaling pathways mediated by two receptors, p55 and p7543), which respectively exert detrimental and beneficial effects in target cells44).
Considering the role of M-CSF mentioned above, the production and functioning of M-CSF should be exerted in the early stage of AD27). Similarly, chemokines have been reported to play roles in a very early event in the AD pathogenesis4–6). Involvement of TNFα and IL-10 in the early events of AD progression has already been demonstrated elsewhere3, 45). As mentioned above, it is likely that IL-10 is mobilized in the early stages of AD. Our present study is the first to show the involvement of IL-2 in the early AD stages, as far as we have been able to determine. One possible explanation for IL-2 is that the present study using frozen brain tissues may provide more precise information on cytokine expression profiles during development of the AD pathology. The significant participation of TNFα, IL-2, IL-6 and IL-10 in the AD progression prior to the manifestation of overt dementia needs further clarification.
In conclusion, in this study, we demonstrated the temporal expression of cytokines: IL-1ra (sec), ICE, IL-2, IL-8, TNFα, M-CSF and TGFβ1 in the HPC brain compared to the LPC brain. Eight of the 15 cytokines and their related molecules were tested here. Our results suggest that significant alterations in the immune response, including cytokine mobilization, precede the clinical AD stage manifesting overt dementia.
Acknowledgments
We are grateful to the Sun Health Research Institute Brain Donation Program of Sun City, Arizona, U.S.A. for the provision of postmortem human brain tissues. The Brain Donation Program is partially supported by a National Institute on Aging grant (P30 AG19610) Arizona Alzheimer’s Disease Core Center).
References
- 1.Weeraratna AT, Kalehua A, DeLeon I, Bertak D, Maher G, Wade MS, Lustig A, Becker KG, Wood W, III, Walker DG, Beach TG, Taub DD. Alterations in immunological and neurological gene expression patterns in Alzheimer’s disease tissues. Exp Cell Res. 2007;313:450–461. doi: 10.1016/j.yexcr.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galimberti D, Schoonenboom N, Scarpini E, Sceltens P. Chemokines in serum and cerebrospinal fluid of Alzheimer’s disease patients. Ann Neurol. 2003;53:547–548. doi: 10.1002/ana.10531. [DOI] [PubMed] [Google Scholar]
- 3.Magaki S, Mueller C, Dickson C, Kirsch W. Increased production of inflammatory cytokines in mild cognitive impairment. Exp Gerontol. 2007;42:233–240. doi: 10.1016/j.exger.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galimberti D, Schoonenboom N, Scheltens P, Fenoglio C, Bouwman F, Venturelli E, Guidi I, Blankenstein MA, Bresolin N, Scarpini E. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2006;63:538–543. doi: 10.1001/archneur.63.4.538. [DOI] [PubMed] [Google Scholar]
- 5.Galimberti D, Fenoglio C, Lovati C, Venturelli E, Guidi I, Corra B, Scalabrini D, Clerici F, Mariani C, Bresolin N, Scarpini E. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer’s disease. Neurobiol Aging. 2006;27:1763–1768. doi: 10.1016/j.neurobiolaging.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Kim TS, Lim HK, Lee JY, Kim DJ, Park S, Lee C, Lee CU. Changes in the levels of plasma soluble fractalkine in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2008;436:196–200. doi: 10.1016/j.neulet.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 8.Tarkowski E, Andreasen N, Tarkowski A, Blennow K. Intrathecal inflammation precedes development of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:1200–1205. doi: 10.1136/jnnp.74.9.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konishi Y, Satoh H, Harano K, Harano T, Tooyama I. Cytokine expression profiles in the brain of non-demented control patients with increasing Alzheimer’s disease pathology, in comparison with normal control and Alzheimer’s disease patients. Tottori J Clin Res. 2008;1:152–168. [Google Scholar]
- 10.Konishi Y, Beach T, Sue LI, Hampel H, Lindholm K, Shen Y. The temporal localization of frame-shift ubiquitin-B and amyloid precursor protein, and complement proteins in the brain of non-demented control patients with increasing Alzheimer’s disease pathology. Neurosci Lett. 2003;348:46–50. doi: 10.1016/s0304-3940(03)00567-6. [DOI] [PubMed] [Google Scholar]
- 11.Saito Y, Murayama S. Neuropathology of mild cognitive impairment. Neuropathology. 2007;27:578–584. doi: 10.1111/j.1440-1789.2007.00806.x. [DOI] [PubMed] [Google Scholar]
- 12.Lue LF, Brachova L, Civin WH, Rogers J. Inflammation, Aβ deposition, and neurofibrillary tangle formation as correlates of Alzheimer’s disease neurodegeneration. J Neuropathol Exp Neurol. 1996;55:1083–1088. [PubMed] [Google Scholar]
- 13.Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid β peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macabeo-Ong M, Ginzinger DG, Dekker N, McMillan A, Regezi JA, Wong DTW, Jordan RCK. Effect of duration of fixation on quantitative reverse transcription polymerase chain reaction analyses. Mod Pathol. 2001;15:979–987. doi: 10.1097/01.MP.0000026054.62220.FC. [DOI] [PubMed] [Google Scholar]
- 15.Bereczki L, Kis G, Bagdi E, Krenacs L. Optimization of PCR amplification for B- and T-cell clonality analysis on formalin-fixed and paraffin-embedded samples. Pathol Oncol Res. 2007;13:209–214. doi: 10.1007/BF02893501. [DOI] [PubMed] [Google Scholar]
- 16.Beach TG, Sue LI, Walker DG, Roher AE, Lue LF, Vedders L, Connor DJ, Sabbagh MN, Rogers J. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Banking. 2008;9:229–245. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology. 2002;3:research0034.1–0034.11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to establish a registry for Alzheimer’s disease (CERAD). Part II Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 19.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 20.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 21.Gutala RV, Reddy PH. The use of real-time PCR analysis in a gene expression study of Alzheimer’s disease post-mortem brains. J Neurosci Methods. 2004;132:101–107. doi: 10.1016/j.jneumeth.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Cumming RC, Schubert D. Amyloid-β induces disulfide bounding and aggregation of GAPDH in Alzheimer’s disease. FASEB J. 2005;19:2060–2062. doi: 10.1096/fj.05-4195fje. [DOI] [PubMed] [Google Scholar]
- 23.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikalenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WST, Hampel H, Hull M, Landreth G, Lue L-F, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyama I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Streit WJ, Conde JR, Harrison JK. Chemokines and Alzheimer’s disease. Neurobiology. 2001;22:909–913. doi: 10.1016/s0197-4580(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 25.Farfara D, Lifshitz V, Frenkel D. Neuroprotective and neurotoxic properties of glial cells in the pathogenesis of Alzheimer’s disease. J Cell Mol Med. 2008;12:762–780. doi: 10.1111/j.1582-4934.2008.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGeel PL, McGeer EG. Inflammation of the brain in Alzheimer’s disease: implications for therapy. J Leukoc Biol. 1999;65:409–415. doi: 10.1002/jlb.65.4.409. [DOI] [PubMed] [Google Scholar]
- 27.Yan SD, Zhu H, Fu J, Yan SF, Roher A, Tourtellotte WW, Rajavashisth T, Shen X, Godman GC, Stern D, Schmidt AM. Amiloid-β peptide-receptor for advanced glycation endproduct interaction elicits neuronal expression of macrophage-colony stimulating factor: A proinflammatory pathway in Alzheimer disease. Proc Natl Acad Sci USA. 1997;94:5296–5301. doi: 10.1073/pnas.94.10.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy GM, Jr, Yang L, Cordell B. Macrophage colony-stimulating factor augments β-amiloid-induced Interleukin-1, Interleukin-6, and nitric oxide production by microglial cells. J Biol Chem. 1998;273:20967–20971. doi: 10.1074/jbc.273.33.20967. [DOI] [PubMed] [Google Scholar]
- 29.Murphy GM, Jr, Zhao F, Yang L, Cordell B. Expression of macrophage colony-stimulating factor receptor is increased in the AβPPV717F transgenic mouse model of Alzheimer’s disease. Am J Pathol. 2000;157:895–904. doi: 10.1016/s0002-9440(10)64603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitrasinobic OM, Perez GV, Zhao FF, Lee YL, Poon C, Murphy GM., Jr Over expression of macrophage colony-stimulating factor receptor on microglial cells induces an inflammatory response. J Biol Chem. 2001;276:30142–30149. doi: 10.1074/jbc.M104265200. [DOI] [PubMed] [Google Scholar]
- 31.Kawata T, Tsutsui K, Kohno S, Kaku M, Fujita T, Tenjou K, Ohtani J, Motokawa M, Shigekawa M, Tohma Y, Tanne K. Amiloid β protein deposition in osteopetrotic (op/op) mice is reduced by injections of macrophage colony stimulating factor. J Int Med Res. 2005;33:654–660. doi: 10.1177/147323000503300607. [DOI] [PubMed] [Google Scholar]
- 32.Majumdar A, Cruz D, Asamoah N, Buxbaum A, Sohar I, Lobel P, Maxfield FR. Activation of microglia acidifies lysosomes and leads to degradation of Alzheimer amyloid fibrils. Mol Biol Cell. 2007;18:1490–1496. doi: 10.1091/mbc.E06-10-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kondo Y, Lemere CA, Seabrook TJ. Osteopetrotic (op/op) mice have reduced microglia, no Aβ deposition, and no changes in dopaminergic neurons. J Neuroinflammation. 2007;10:1742–2094. doi: 10.1186/1742-2094-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ransohoff RM, Lars B. Cytokines in CNS inflammation: Status of experimental autoimmune encephalomyelitis and multiple sclerosis as cytokine-regulated delayed-type hypersensitivity reactions. In: Ransohoff RM, Benveniste EN, editors. Cytokines and the CNS . CRC Press; Boca Raton: 1996. pp. 221–237. [Google Scholar]
- 35.Szelényi J. Cytokines and the central nervous system. Brain Res Bull. 2001;54:329–338. doi: 10.1016/s0361-9230(01)00428-2. [DOI] [PubMed] [Google Scholar]
- 36.Skaper SD. The brain as a target for inflammatory processes and neuroprotective strategies. Ann N Y Acad Sci. 2007;1122:23–34. doi: 10.1196/annals.1403.002. [DOI] [PubMed] [Google Scholar]
- 37.Bonotis K, Krikki E, Holeva V, Aggouridaki C, Costa V, Baloyannis S. Systemic immune aberrations in Alzheimer’s disease patients. J Neuroimmunol. 2008;193:183–187. doi: 10.1016/j.jneuroim.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 38.Beloosesky Y, Salman H, Bergman M, Bessler H, Djaldetti M. Cytokine levels and phagocytic activity in patients with Alzheimer’s disease. Gerontology. 2002;48:128–132. doi: 10.1159/000052830. [DOI] [PubMed] [Google Scholar]
- 39.Town T, Vendrame M, Patel A, Poetter D, DelleDonne A, Mori T, Smeed R, Crawford F, Klein T, Tan J, Mullan M. Reduced Th1 and enhanced Th2 immunity after immunization with Alzheimer’s beta-amyloid(1–42) J Neuroimmunol. 2002;132:49–59. doi: 10.1016/s0165-5728(02)00307-7. [DOI] [PubMed] [Google Scholar]
- 40.Kim HD, Jin JJ, Maxwell JA, Fukuchi K. Enhancing Th2 immune responses against amyloid protein by a DNA prime-adenovirus boost regimen for Alzheimer’s disease. Immunol Lett. 2007;112:30–38. doi: 10.1016/j.imlet.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry RT, Collins JS, Wiener H, Acton R, Go RC. The role of TNF and its receptors in Alzheimer’s disease. Neurobiol Aging. 2001;22:873–883. doi: 10.1016/s0197-4580(01)00291-3. [DOI] [PubMed] [Google Scholar]
- 42.Lanzrein AS, Johnston CM, Perry VH, Jobst KA, King EM, Smith AD. Longitudinal study of inflammatory factors in serum, cerebrospinal fluid, and brain tissue in Alzheimer disease: interleukin-1β, interleukin-6, interleukin-1 receptor antagonist, tumor necrosis factor-α, the soluble tumor necrosis factor receptors I and II, and α1-Antichymotrypsin. Alzheimer Dis Assoc Disord. 1998;12:215–227. doi: 10.1097/00002093-199809000-00016. [DOI] [PubMed] [Google Scholar]
- 43.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147:232–240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li R, Yang LB, Lindholm K, Konishi Y, Yue X, Hampel H, Zhang D, Shen Y. Tumor necrosis factor death receptor signaling cascade is required for amyloid-β protein-induced neuron death. J Neurosci. 2004;24:1760–1771. doi: 10.1523/JNEUROSCI.4580-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarkowski E, Andreasen N, Tarkowski A, Blennow K. Intrathecal inflammation precedes development of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:1200–1205. doi: 10.1136/jnnp.74.9.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]