Abstract
Physical exercise is thought to hold promise as a non-invasive countermeasure against skeletal fragility arising from post-menopausal and age-related osteoporosis. Importantly, mechanical loading and exercise are capable of increasing bone size via periosteal expansion, which by far, is the most effective means of strengthening the structure of a given bone. The focus of this review was to therefore explore whether exercise has the potential to increase periosteal modeling and bone size in the senescent skeleton. A survey of exercise trials in humans suggests that exercise interventions that enhance periosteal modeling in the young skeleton fail to do the same in the elderly skeleton. Underlying this ineffectiveness, in vitro studies indicate that aging lowers basal levels of cell function and degrades bone mechanotransduction at a variety of levels from altered second messenger signaling to gene expression driving proliferation and/or differentiation. Given these age-related alterations, the ultimate efficacy of an exercise intervention may depend upon concurrent supplementation that directly address deficits in signaling and/or cell function. In this context, in vivo animal models of mechanical loading that simulate the muted periosteal adaptation in the elderly hold potential to examine the efficacy of countermeasures. Preliminary in vivo experiments suggest that pharmacologically counteracting age-related deficits in cellular function can restore exercise induced periosteal modeling in the senescent skeleton to levels observed in young animals. If the safety and efficacy of this strategy were to be confirmed for human use, it would enable the utilization of exercise as a viable countermeasure against skeletal fragility at senescence.
Keywords: Age-related, Periosteal Modeling, Exercise, Mechanotransduction, Pharmaceutical Augmentation
1.1 Introduction
Skeletal loading induced by physical activity has long been identified as capable of substantially enhancing bone morphology and structural properties. However, most reports of profound bone size augmentation in response to physical activity have been associated with individuals that have initiated exercise during growth and development (Greene et al. 2005; Hind et al. 2011; Kontulainen et al. 2003). Additionally, exercise trials in humans, particularly in the elderly, have produced extremely modest gains in bone mass and morphology, which confer minimal structural benefit (Forwood et al. 1993; Nikander et al. 2010). This review initially focuses on assessment of relevant human studies that summarize the potential of mechanical stimuli to enhance bone size via periosteal modeling in the senescent skeleton. Given the apparent inability of current exercise regimens to enhance structural adaptation in aged bone, we next reviewed cellular mechanisms that may underlie this deficit. Finally, we reviewed relevant pre-clinical models describing effects of mechanical loading in the aged skeleton and propose a strategy that will enable the use of exercise as an effective intervention to augment bone structure in the elderly.
1.2 Using Exercise to Enhance Bone Morphology
From a structural mechanics perspective, the bending and torsional loads borne by the appendicular skeleton during normal functional activity create maximal strains on the periosteal surface. As such, strategies to reduce peak strains (and thus diminish fracture risk) during normal functional activity are most effectively achieved by expanding the outer surface of bone (Currey 1984). In this context, mechanical loading of bone occurring during exercise can robustly enhance periosteal adaptation and bone structural properties such as total area and moments of inertia. For instance, a number of cross-sectional studies suggest that physical activity that incorporates high strain magnitude and rates (such as racquet sports) are positively associated with focally increased trabecular and cortical bone morphology (Greene et al. 2005; Hind et al. 2011; Kontulainen et al. 2003). While these observations suggest the possibility that exercise can serve as a means to potently augment bone strength, direct examination of exercise interventions in randomized control trials have led to mixed results.
Focusing upon prospective randomized trials that have reported morphologic alterations as outcomes measures revealed a series of studies in which exercise was implemented across a wide age-range (3 – 75 yrs; Table 1). As observed in a recent meta-analysis (Nikander et al. 2010), the ability of exercise to influence bone structural properties is highly dependent upon the age at which exercise is initiated. High-intensity /impact /resistance exercise is capable of increasing bone structure and strength via periosteal expansion in the young, and possibly in the young adult population (3 – 35 yrs) (MacKelvie et al. 2004; Specker et al. 2003; Vainionpaa et al. 2007). Interestingly, while the efficacy of exercise in influencing bone mass and strength is markedly diminished, it is not completely abolished in the elderly. Specifically, it appears that exercise in the elderly can enhance BMD and even cross-sectional areas at the femoral neck. However, these increases have primarily resulted from a slowing of the rate of endocortical bone loss via suppression of osteoclast activity (secondary to menopause or aging) and/or exercise induced increases in endocortical bone formation (Karinkanta et al. 2007; Kukuljan et al. 2011; Uusi-Rasi et al. 2003). To our knowledge, exercise trials in the elderly have not reported changes in periosteal expansion (e.g., increases in total cross-sectional area or diameter; Table 1).
Table 1.
Summary of recent randomized control exercise trials focusing on periosteal adaptation.
| Population | Exercise | Measure/Design | Results | Comments |
|---|---|---|---|---|
| Pre-school children (M/F), US, Caucasian (3–5 yrs)(Specker et al. 2003) | Fine motor (seated activity) vs. gross motor (jump, skip, hops), 30 mins/d, 5 d/wk, 12-Mo | DXA based total body, arm, leg BMC, area. pQCT for periosteal, endosteal circumference, cortical thickness, area at 20% distal tibia. ANCOVA with gender, age, pre-term birth history, height, weight, childcare center, total body fat as co-variates. Childcare-based intervention. Ca supplementation interaction with activity type. | 9.7% increase in leg BMC for gross vs. fine motor when Ca supplementation included. Periosteal and endocortical circumference of 20% tibia shaft increased significantly in gross vs. fine motor activity. Gross motor and Ca supplements interact to increase cortical area and thickness. | Overall participation/compliance (72–75%). Gross motor exercise increases bone size and Ca supplementation (with exercise) increases area and thickness of cortical bone. |
| Pre-pubertal boys, Canada, Caucasian, Asian, mixed race (8.8 – 12.1 yrs)(MacKelvie et al. 2004) | Progressive , circuit training, plyometric/alternating foot/obstacle jumps, 12 min/d, 3 d/wk for 20 Mo. | DXA based BMC, aBMD for total body, lumbar spine, proximal femur, and HSA for narrow neck (NN), intertrochanter, and femoral shaft. School based intervention. ANCOVA controlling for baseline, change in height and final Tanner stage. | 12.4% increase in CSMI and 7.4% increase in Z-modulus at NN region vs. controls. | Trends for periosteal expansion (2.6 – 2.9%, p = 0.1) underlying the CSMI and Z-modulus changes. |
| Premenopaus al women, UK (33.6 ± 11.1 yrs) (Bailey et al. 2010) | 50 hops/d, 0, 2, 4 or 7 d/wk for 6-Mo. Peak GRF increased from 2.5 to 2.8 times BW. | DXA at femoral neck. Unilateral exercise design. Home based, activity questionnaire. RMMANOVA with contralateral and baseline as covariates | BMD adjusted for contralateral and baseline −0.3%, 0.0%, 0.9% and 1.8% for 0, 2, 4, 7d/wk. No effect on section modulus. | Compliance at 86.7% and independent of exercise frequency. BMD increases independent of geometric changes. |
| Pre-menopaus al women, Caucasian, Finland, (35 – 40 Yrs) (Vainionpaa et al. 2007) | Progressive impact activities, step patterns, stamping, jumping, running, walking. 60 min/d, 3 d/wk + 10 min/d, daily at home, 12 Mo. | Spiral QCT of femur (50%), tibia (67%, 5%). Bone circumference, CSA, other structural measures. Daily accelerometerbased human body movement monitoring and binning from 0.3g – 9.9g. ANCOVA with weight, weight change and CT baselines as covariates. Stepwise regressions for acceleration vs. bone structure associations. ITT analysis. | 0.2% increase in mid-femur bone circumference in exercise vs. control. In highest compliers (> 66 sessions/yr), 1.2% increase in circumference, 0.5% in CSA and 2.5% gain in CSMI vs. lowest quartile (< 19 sessions/yr). In the pooled and exercise only groups, relative daily counts of impacts ≥ 1.1 g associated with mid-femur attenuation and with CSMI, ≥ 2.5g with circumference, and 3.9g – 5.3g with cortical thickness. | Average compliance for supervised activity − 0.9 times/wk (~30% of weekly load). Count and intensity at levels > 1.1g influenced bone structure. Geometric alterations indicative of periosteal apposition. |
| Postmenop ausal women, Caucasian, Finland (>1 and <5 yrs postmenopaus e; ~ 53 + 2.2 yrs)(Uusi-Rasi et al. 2003) | Progressive multidirectio nal jumping and calisthenics, 2.1 – 5.6 BW during jumping, 1 hr (20 min jumping), 3 d/wk, 12 Mo. | BMC at lumber spine, proximal femur, distal radius and z-section modulus (femoral neck) via DXA and structural parameters at mid-shaft and distal tibia via pQCT. | No BMC effects from exercise. Exercise increased distal tibia section modulus (3.6%) and CoA/ToA (3.7%) and tibia shaft CoA (1%) vs. control. | Mean compliance with exercise was 1.6 + 0.9 times/wk (53% of weekly load). No effect of exercise on total area (ToA) indicating a lack of periosteal adaptation. |
| Elderly women, Caucasian, Finland (72 years ± 2.5 yrs) (Karinkant a et al. 2007) | Resistance, Balance-jump training, Combined, ~1Hr/d, 3d/wk for 12-Mo. | Proximal femur BMD, BMC via DXA, and Z-modulus and periosteal diameter. pQCT for TrD, BSI, CoA and CoD in distal and mid-shaft tibia. Bilateral, supervised exercise. ANCOVA with baseline, age and time interval as covariates | No BMC differences. Per-protocol analysis − 2% less decrease in tibia mid-shaft BSI for COMB vs. Controls. | Overall compliance (67%). No effect on bone diameter indicating lack of periosteal expansion. |
| Elderly Caucasian men, Australia (50 – 79 yrs) (Kukuljan et al. 2011) | Multicomponent, progressive resistance training and moderate impact exercise, 60–75 mins/d, 3 d/wk for 18 Mo. | DXA and QCT, BMD and bone structure at lumbar spine, proximal femur, mid-femur and mid-tibia. Community based. ANOVA and pooled time series regression analysis. | 1.9% increase in femoral neck BMD, 1.8% in CsA, and 2.1% in Z-modulus and 2.2% increase in lumbar spine vBMD for exercise vs. no-exercise. | Overall compliance (63%). No change in femoral neck diameter, indicating that structural benefits due to endocortical adaptation. |
It is possible that the relatively short trial durations of these studies (< 18 months) did not permit detection of modest periosteal modeling (Nikander et al. 2010). However, given that bone formation initiated by mechanical stimuli (i.e., exercise) clearly diminishes with repeated exposure to the same stimulus (Schriefer et al. 2005), it is likely that extending the duration of trials would not alter this conclusion. It is also possible that the inability of exercise to increase bone size arises, in part, because the elderly are unable to comply with the types of exercise most associated with bone size augmentation. Notwithstanding this possibility, the compliance rate for these interventions appears to be equivalently poor in the elderly and in the very young (~30 – 60%), yet the ability of exercise to influence periosteal adaptation is markedly reduced in the elderly but not in the young (Table 1). Taken together, these observations suggest that the ineffectiveness of exercise emerges from other factors, such as age-related alterations at the cellular level that impair the ability of the periosteum to respond to an otherwise osteogenic stimulus.
1.3 Age-Related Cellular Alterations
Aging is accompanied by numerous alterations within the cellular milieu that, in sum, result in declines in bone mass beyond the third decade of life (Riggs et al. 1986). The primary factor underlying age-related bone loss is thought to be an imbalance in bone remodeling (specifically, the inability of osteoblastic bone formation to keep pace with osteoclastic resorption (Parfitt 1984)). Underlying this imbalance, there is a marked age-related increase in marrow adiposity (Rozman et al. 1989) that reduces the pool of osteoblast progenitors (Bergman et al. 1996). Further, aging is accompanied by a decline in periosteal lining cell numbers (Silbermann et al. 1987) and in osteoblast life-span due to increased propensity for apoptosis (Jilka et al. 1998). These age-related alterations decrease both osteoblast numbers and the duration over which osteoblasts are involved in biosynthetic activity. Additionally, degradations in the basal activity (Liang et al. 1992) and/or functional response (Donahue et al. 2001; Pfeilschifter et al. 1993) of osteoblasts mediated by age-related alterations in extracellular milieu (Gazit et al. 1998; Nicolas et al. 1994) could diminish biosynthetic activity in the aged skeleton. Age-related increases in osteocyte apoptosis is also observed (Dunstan et al. 1993) and holds potential to alter intercellular signaling and the function of the bone cell syncytium (Vashishth et al. 2000). These alterations in osteocytic function have more recently been implicated as being an important factor in the decline in bone strength and integrity at senescence (Manolagas et al. 2010). In terms of bone resorption, age-associated alterations in the potential for osteoclastogenesis and osteoclast activity appear to be more controversial. While precursor pools and osteoclastogenesis have been reported as both increasing and declining with age (Cao et al. 2005; Pietschmann et al. 2007), a marked increase in osteoclastic resorption during the perimenopausal period is well established. Alterations in neuroendocrine systems that potentially control and/or are related to these bone cellular changes are also likely to modulate bone homeostasis and impact the bone remodeling disequilibrium underlying age-related bone loss (Amling et al. 2000; Elefteriou 2008)
Given these cellular alterations, the age-related suppression of bone formation elicited by mechanical stimuli at the periosteal surface is not entirely surprising. With respect to mechanotransduction, age-related alterations in cellular function would additionally include decreased biophysical stimulation of bone cells and/or alterations in the signaling pathways activated in response to mechanical stimuli.
It has been suggested that attenuated biophysical stimulation could arise in part from decreases in the surface to volume ratio of bone mineral matrix and increased viscosity of interstitial fluids (Bennett et al. 1981; Bonar et al. 1983; Rubin et al. 1992). If these physical mechanisms were to occur in vivo in bone, the magnitudes of fluid flow induced secondary to skeletal loading could be reduced with age. Considering that fluid flow induced during mechanical loading is thought to be a major component of the biophysical stimuli perceived by bone cells (Burger et al. 1999), age-related reduction in fluid flows could underlie observations that the loading magnitudes to initiate bone adaptation are increased in aged vs. younger animals (Rubin et al. 1992; Turner et al. 1995).
The ability of bone cells to transduce mechanical stimuli into biochemical signals may also be impaired with age. In terms of acute signals and activation of second messengers by mechanical stimuli, PGE2 release has been found to be substantially increased in cells derived from aged vs. young human donors (Klein-Nulend et al. 2002). In contrast, the number of cells displaying spontaneous Ca2+ oscillations and fluid flow induced oscillations were found to be significantly lower in primary cells derived from aged animals, while the amplitudes of Ca2+ oscillations themselves were equivalent (Donahue et al. 2001). These studies suggest that age-related alterations, at least at the level of second messengers relevant to mechanotransduction, are multifaceted with some aspects being decreased (number of cells responding), unchanged (e.g., Ca2+ amplitudes), while others can even be enhanced (PGE2 release). Interestingly, activation of phosphatases and kinases downstream of second messenger signaling including Calcineurin, CAMK, and MAPK are decreased with age (Pahlavani et al. 1999; Pahlavani et al. 2000). Further downstream (of the phosphatases and kinases), activation and DNA binding by a variety of transcription factors such as NFAT, AP-1, and Wnt/β-catenin also display age-related deficits or suppression (Manolagas et al. 2007; Modder et al. 2011; Pahlavani et al. 1997; Whisler et al. 1996).
Given age-related pathway alterations and interactions between pathways, the resulting alterations in gene expression downstream of these signaling pathways are multifactorial and complex. For instance, our preliminary studies in primary cells isolated from young and senescent mice reveals that relative increases in Cox-2 and c-fos gene expression levels in response to fluid flow (vs. no flow controls) were not altered with age (Worton et al. 2011). Interestingly, the absolute Cox-2 but not c-fos levels displayed age-related declines in large part due to lower baseline levels of Cox-2 expression in cells derived from aged animals. However, given that pathways that regulate gene expression partner with, or antagonize each other, in specific contexts (e.g., co-regulation of c-fos and Cox-2 by NFAT and AP-1 pathways; (MacDonnell et al. 2009; Macian et al. 2001)), the interactions between signaling pathways in their age-altered states are likely to cause complex downstream adaptive phenotypes. Nevertheless, exploitation of age-related alterations in pathways activated during mechanotransduction and/or consideration of means to counteract diminished cellular functionality may yield therapeutic strategies that enable exercise to enhance bone structural strength at senescence.
1.4 Pre-Clinical Animal Studies
Evaluation of therapeutic, exercise-based strategies requires appropriate pre-clinical models that simulate the human condition of muted periosteal adaptation. In this context, animal studies have substantiated the ability of mechanical stimuli to induce periosteal modeling. However, controversy exists as to whether aging degrades this adaptive response. Animal studies using externally controlled bone loading, which enables matching of load induced periosteal strains across age, report that aging markedly diminishes the periosteal response to mechanical stimuli (Rubin et al. 1992; Srinivasan et al. 2010; Turner et al. 1995). For example, bone adaptation to mechanical loading regimens that are robustly osteogenic in the young skeleton are completely ineffective in the senescent skeleton (Rubin et al. 1992). Further, the strain magnitude required to initiate periosteal bone formation in aged animals is significantly greater than in younger animals (Turner et al. 1995). Similarly, we have observed that the periosteal surface of senescent mice is capable of modestly responding to external loading only when exposed to a potent osteogenic stimulus (Srinivasan et al. 2003). However, strategies that enhance the periosteal response in young animals (e.g., increasing strain magnitudes, inserting rest-intervals, increasing loading cycle numbers (Srinivasan et al. 2007)) were not successful in senescent animals. More recent studies that were strain magnitude equilibrated and protocol matched further confirmed a substantially decreased rate of loading induced periosteal bone formation in senescent vs. young animals (Fig 1 a) (Srinivasan et al. 2010). Consistent with human studies, mechanical signals that are capable of eliciting dose-dependent responses in young adult animals instead induce a muted, binary on-off periosteal adaptation in the senescent skeleton (Fig 1 b).
Figure 1. Loading induces a muted periosteal adaptive response in the senescent skeleton.
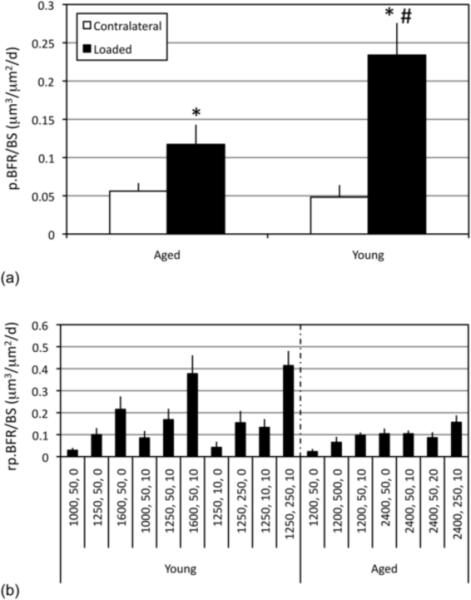
In experiments where loading induced strains were explicitly equilibrated between young and aged animals, periosteal bone formation rates (p.BFR/BS; mean + s.e.; a) were significantly increased in experimentally loaded bones compared with contralateral controls in both young (4 Mo) and aged female C57BL/6 mice (22 Mo; *). However, p.BFR/BS was significantly lowered in experimentally loaded aged vs. young animals (#). Mean relative periosteal bone formation rates (rp.BFR/BS) induced by protocols in young animals varies according to the potency of the protocol but is muted and binary (with very little variation) despite attempts to increase the osteogenic potential of loading protocols for aged animals (b; protocols are denoted by peak strain amplitude, number of cycles, and seconds of rest inserted between each load cycle). Adapted from (Srinivasan et al. 2010).
In contrast to studies demonstrating an age-related decline in periosteal modeling due to mechanical stimulation, other animal studies have reported no difference (Brodt et al. 2010; Buhl et al. 2001; Raab et al. 1990; Umemura et al. 1995) or an increased periosteal response (Leppanen et al. 2008). While two studies report that exercise training increased structural adaptation equivalently in young and aged animals (Raab et al. 1990; Umemura et al. 1995), the methodology used does not permit clear conclusions regarding whether this adaptation was achieved via periosteal expansion or inhibition of endocortical resorption. We therefore focused on studies published within the past decade that permit interpretations regarding whether exercise induced bone adaptation involves periosteal modeling in aged animals (Brodt et al. 2010; Buhl et al. 2001; Leppanen et al. 2008). First, in a study examining age-related response of bone to a progressively increasing overload training exercise, periosteal adaptation was not induced in young (4 Mo), adult (12 Mo) or aged rats (22 Mo), but medullary areas were decreased in exercised, aged rats compared to their age-matched controls (Buhl et al. 2001). In a second study, age-related mechano-responsiveness was examined in an experimental model in which the tibiae of BALB/c mice were exposed to axial compression (Brodt et al. 2010). In assays of early osteoblastic response, loading induced equivalent increases in periosteal bone formation in both senescent (22 Mo) and young adult mice (7 Mo) compared to contralateral control bones. Following loading, senescent mice in this study also demonstrated significantly increased endocortical bone formation vs. young adult animals. Finally, a 14 wk treadmill exercise regimen was shown to induce modest periosteal responses in senescent (19–22 Mo) rats but not in adult rats (12 Mo) (Leppanen et al. 2008). However, direct comparison and confirmation of these beneficial effects (which were interpreted from changes in total cross-sectional area) with dynamic histomorphometry data in the previous studies is challenging (Brodt et al. 2010; Buhl et al. 2001).
A number of contributing factors underlie these disparate observations. For instance, bone adaptation and alterations in structural properties that arise via decreases in marrow area, particularly in the absence of alterations in dynamic histomorphometry parameters (Buhl et al. 2001), are most likely to emerge via suppression of increased osteoclastic activity normally associated with aging (Bar-Shira-Maymon et al. 1989; Cao et al. 2005; Rubin et al. 1999; You et al. 2008). Furthermore, animal studies utilizing involuntary exercise (e.g., (Leppanen et al. 2008)) are confounded by the possibility that exercise may induce larger deformations/ strains in senescent vs. mature animals (as bones in senescent animals are typically weaker and display features of age-related osteoporosis at baseline (Brodt et al. 2010; Leppanen et al. 2008)). Interestingly, Brodt and Silva (Brodt et al. 2010) implemented an external loading model to explicitly control for these variables and found that the periosteum of aged and young adult mice equivalently responded to mechanical loading. However, it is clear that the response of the mouse skeleton to mechanical loading is influenced by interactions between age and genotype (Judex et al. 2002; Poliachik et al. 2008). Thus, it is possible that BALBc mice used in these studies do not suffer age-related suppression of periosteal response to loading as has been observed in other mouse strains and species (Rubin et al. 1992; Srinivasan et al. 2003; Srinivasan et al. 2010; Turner et al. 1995). In summary, there currently exists controversy within the literature regarding whether aging suppresses the periosteal response to mechanical loading in animal models. These discrepancies will only be resolved by studies that expose animals to equivalently calibrated mechanical stimuli and are broad enough in scope to delineate genetic contributions to the observed responses.
1.5 Reversing the Age-Related Decline in Periosteal Bone Formation
Despite the above controversy, animal models that mimic the human condition of muted periosteal adaptation could be used to examine if supplemental use of anabolic agents can beneficially amplify the impact of mechanically stimulating the senescent skeleton (Jee et al. 2005). An increasing number of anabolic agents have been identified that hold potential to increase BMD and also enhance bone quality and strength (Sibai et al. 2011). For example, recombinant human PTH has been approved for clinical use in the treatment of osteoporosis in the US and is an anabolic agent that increases bone mass (Neer et al. 2001). Importantly, PTH is reported to enhance periosteal adaptation by increasing the numbers of osteoblasts (via pro-differentiation and pro-survival effects) and may be particularly useful in the senescent skeleton (Jilka et al. 2010; Jilka et al. 2009; Ma et al. 2011). Similarly, strontium ranelate (approved for use in Europe) enhances bone mass (Meunier et al. 2002), in part, by increasing the mineralizing surface of bone (Bain et al. 2009). However, the impacts of skeletal incorporation of strontium on bone quality over the long-term remains to be clarified (Boivin et al. 2010). Given that mechanical stimuli interacts (sometimes synergistically) with a number of these and other known anabolic agents (Gross et al. 2002; Ma et al. 1999; Mo et al. 2002; Robling et al. 2008; Sugiyama et al. 2008), supplemental use of pharmacologic agents in conjunction with physical exercise could rescue periosteal adaptation in the aged, thereby enabling a viable strategy to improve bone strength.
In this context, an improved understanding of systemic age-related alterations and their effects upon specific mechanotransduction signaling pathways may also yield novel therapeutic targets for augmenting periosteal bone formation in the senescent skeleton. For example, suppressed Wnt signaling (Manolagas et al. 2007), a pathway known to be involved in bone mechanotransduction (Case et al. 2008; Robinson et al. 2006), may be caused by age-related increases in circulating levels of sclerostin (Modder et al. 2011). Reports of substantial increases in bone mass following a single treatment of AMG 785 (Padhi et al. 2011), a sclerostin antibody, is one such agent that could be evaluated for its potential to enhance the effects of exercise at senescence (Lin et al. 2009).
Targeting of specific mechanotransduction pathways is yet another therapeutic strategy to counteract the age-related decline in the skeleton's response to exercise. For example, guided by the critical importance of Ca2+/NFAT signaling in the bone's adaptive response to mechanical stimuli (Celil Aydemir et al. 2007; Riddle et al. 2006), we developed a computational framework to explore pathway activation in response to mechanical stimuli and its age-related alterations (Srinivasan et al. 2010). Our simulations predicted that deficits in NFAT transcription factor activation and DNA binding downstream of Ca2+ signaling (but not other aspects of Ca2+ signaling per se) were the primary mechanisms underlying the muted periosteal response observed in senescent animals (Srinivasan et al. 2003). While we initially focused on NFAT pathway activation as a surrogate for real-time cellular signaling, this modeling framework could be readily applied to explore activation of other pathways (e.g., CAMK/AP-1 pathway) and their age-related deficits (Pahlavani et al. 2000; Whisler et al. 1996). Given the model-identified deficits in transcription factor activation and DNA binding, a survey of the literature suggested that Cyclosporin A (CsA), at low-dosages, could be a candidate agent to address age-related deficits in NFAT (or AP-1) activation and transcription (Fig 2). In subsequent in vivo experiments, we found that when senescent mice (22 Mo) were subjected to mechanical loading in conjunction with low-dose CsA treatment, the resulting periosteal bone formation was significantly increased compared to vehicle-treated aged matched controls subject to loading alone. Even more surprising, we found that periosteal bone formation in aged mice in response to mechanical loading plus low-dose CsA was restored to levels observed in young adult mice (4 Mo; Fig 2; (Srinivasan et al. 2010)). While we continue to investigate the mechanisms underlying this interaction, our observation that CsA supplementation can reverse the age-related deficit in periosteal modeling in senescent animals is suggestive of a promising, cost-effective, therapeutic strategy for restoring the bone anabolic benefits of exercise in the elderly.
Figure 2. Restoring loading induced periosteal adaptation at senescence.
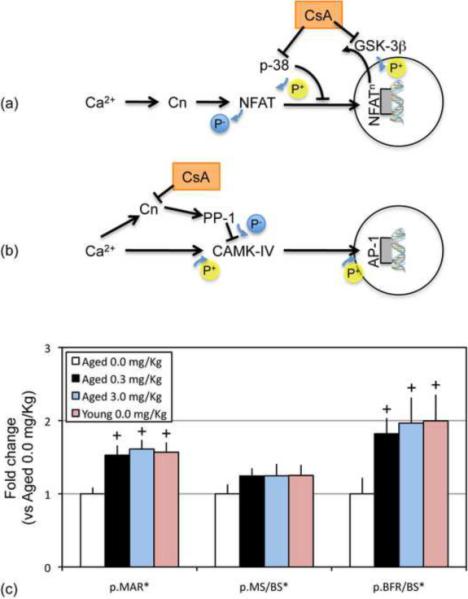
Low-dose CsA can potentially enhance activation of NFAT signaling (via inhibition of p38 and GSK-3β proteins that negatively regulate NFAT signaling; a) (Kreideweiss et al. 1999; Matsuda et al. 2000). Alternately, CsA supplementation could enhance activation of the AP-1 pathway and downstream phenotypes (via competitive inhibition of the Calcineurin/NFAT signaling axis; b) (Blitzer et al. 1998; Kasahara et al. 1999; Yeo et al. 2007). When loading was supplemented with low-dose CsA (0.3, 3.0 mg/Kg), periosteal mineral apposition rates (p.MAR*), and bone formation rates (p.BFR/BS*) induced in senescent animals (22 Mo) was significantly increased vs. vehicle treated aged animals (+), but were not different than that induced in young animals (4 Mo; c; mean + s.e.). Periosteal response measures in experimentally loaded bones are presented after normalization by the respective aged animal mean. Adapted, in part, from (Srinivasan et al. 2010).
1.6 Conclusions and Future Directions
Skeletal fragility associated with osteoporosis and related co-morbidities represent a significant public health issue in the rapidly aging western world. Upon diagnosis, anti-resorptive therapies are typically implemented to prevent any further loss of bone mass (Hosking et al. 1998). Additionally, measures such as whole body vibration or Tai Chi that improve postural stability and potentially combat muscle wasting and decreased proprioception have recently been explored in initial trials (Rubin et al., 2007, Cheung, et al., 2007, Leung, et al., 2011). In addition to their potential utility in enhancing bone morphology, these interventions may also combat sarcopenia. This is especially relevant given that sarcopenia is a significant co-morbidity of senile osteoporosis in the frail elderly (Di Monaco et al. 2011; Frisoli et al. 2011) and increases susceptibility to falls (Moreland et al. 2004). Interestingly, general exercise based countermeasures may also serve to counteract sarcopenia and could provide a means to reduce falls and thus, the occurrence of some types of fractures (Weatherall 2004).
While preventive strategies are useful, concomitant interventions that buttress weakened skeletal structures are still required. The anabolic potential of mechanical loading and exercise continues to hold promise in this realm. From our review of the literature, it appears that vigorous exercise can influence bone mass at senescence by slowing the rate of bone loss. While this is of benefit within and outside the skeletal system, vigorous activity has not been shown to influence periosteal expansion in the elderly. We consider this to be a critical shortcoming, as periosteal expansion is the morphologic adaptation that can most effectively enhance bone strength and resistance to bending and torsion accompanying normal functional activity (e.g., our estimates indicate that a 10% increase in bone area could result in over a 50% increase in bending strength if adaptation occurred on the periosteal vs. endocortical surface). As such, in the absence of periosteal expansion, it remains unclear whether the modest endocortical adaptation induced by exercise in the elderly is sufficient to prevent skeletal fractures.
Translation of the benefits of exercise observed in pre-clinical studies has been hampered by a variety of potential limitations. First, the elderly are unable to comply with the types of activity most capable of periosteal expansion. This is especially evident for interventions that have incorporated lengthy exercise sessions (~ 1 hr/d) and utilize progressive training approaches (increased intensity/effort over successive days). In fact, progressive training does not have a clear efficacy in pre-clinical models, given that bone cells become rapidly desensitized to mechanical stimuli and the osteogenic responses to mechanical loading decline over time despite progressive increases in loading potency (Rubin et al. 1984; Schriefer et al. 2005; Umemura et al. 1997). Given these challenges, there is a clear need for exercise regimens that are mild in duration and vigor, yet still capable of significantly enhancing bone quality and quantity. In this context, incorporation of recent concepts such as inclusion of rest periods between load cycles, separation of exercise sessions into shorter duration bouts, or consideration of low-magnitude, high frequency regimens that augment the potency of mechanical stimuli without requiring increases in loading effort may be more efficacious (Robling et al. 2002; Rubin et al. 2001; Srinivasan et al. 2007). For instance, incorporation of such strategies into activities such as Tai Chi (Lui et al. 2008) may permit the deployment of less vigorous exercise designs, thereby mitigating poor compliance by the elderly, while maintaining or even enhancing the potency of the intervention.
While opportunities to optimize exercise regimens clearly remain, such strategies may prove insufficient to overcome age-induced muting of bone mechanotransduction that arises from the cellular to tissue levels. As such, it may simply not be possible to define an exercise regimen for the elderly that is sufficiently osteogenic at the periosteal surface without explicit interventions that address at least some of the age-related cell function and signaling pathway deficits. Instead, targeting and promoting periosteal adaptation via therapeutic adjuvants may more readily permit the consideration of less vigorous exercise than those that have been currently implemented. This important additional benefit to targeting periosteal adaptation via adjuvants could be achieved considering that current vigorous exercise designs induce endocortical, not periosteal adaptation, despite the bending and torsional loading induced deformations (and strains) being significantly higher at the periosteal surface. In these contexts, it may be possible to supplement exercise with anabolics that influence periosteal adaptation by broadly addressing cell function deficits (e.g., PTH, strontium ranelate) (Meunier et al. 2002; Neer et al. 2001) or specifically address signaling pathway deficits (e.g., AMG 785, low-dose CsA)(Padhi et al. 2011; Srinivasan et al. 2010). However, supplementation of exercise with anabolics would require substantial optimization in both preclinical models and in randomized control trials, neither of which have been undertaken to date. In this context, given the parametric possibilities involved in any such optimization, initial screening using computational approaches may enable testing of a substantial range of drug/exercise combinations prior to preclinical studies and trials (Pivonka et al. 2010; Srinivasan et al. 2010). Such a combined strategy would require less vigorous exercise and lowered anabolics dosage, thereby improving compliance and minimizing toxicity and costs, while targeting periosteal adaptation to specific skeletal sites most at risk for fracture.
Highlights
Exercise increases bone size and periosteal modeling in young but not in elderly.
Muted periosteal modeling most likely arises from cell function/signaling deficits.
Animal models of muted periosteal modeling permit evaluation of countermeasures.
Pharmaceutical augmentation of loading can rescue periosteal modeling in vivo.
Adjuvants that restore mechanotransduction deficits can strengthen bone in elderly.
Acknowledgements
This review was supported, in part, by funding from NIAMS AR56235 (SS), AR56652 (TSG), the Sigvard T. Hansen, Jr. Endowed Chair (TSG), and the Zimmer Fracture Biology Professorship (SDB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Amling M, Takeda S, Karsenty G. A neuro (endo)crine regulation of bone remodeling. Bioessays. 2000;22(11):970–975. doi: 10.1002/1521-1878(200011)22:11<970::AID-BIES3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Bailey CA, Brooke-Wavell K. Optimum frequency of exercise for bone health: randomised controlled trial of a high-impact unilateral intervention. Bone. 2010;46(4):1043–1049. doi: 10.1016/j.bone.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Bain SD, Jerome C, Shen V, Dupin-Roger I, Ammann P. Strontium ranelate improves bone strength in ovariectomized rat by positively influencing bone resistance determinants. Osteoporos Int. 2009;20(8):1417–1428. doi: 10.1007/s00198-008-0815-8. [DOI] [PubMed] [Google Scholar]
- Bar-Shira-Maymon B, Coleman R, Steinhagen-Thiessen E, Silbermann M. Correlation between alkaline and acid phosphatase activities and age-related osteopenia in murine vertebrae. Calcif Tissue Int. 1989;44(2):99–107. doi: 10.1007/BF02556468. [DOI] [PubMed] [Google Scholar]
- Bennett GD, Kay MM. Homeostatic removal of senescent murine erythrocytes by splenic macrophages. Exp Hematol. 1981;9(3):297–307. [PubMed] [Google Scholar]
- Bergman RJ, Gazit D, Kahn AJ, Gruber H, McDougall S, Hahn TJ. Age-related changes in osteogenic stem cells in mice. J Bone Miner Res. 1996;11(5):568–577. doi: 10.1002/jbmr.5650110504. [DOI] [PubMed] [Google Scholar]
- Blitzer RD, Connor JH, Brown GP, Wong T, Shenolikar S, Iyengar R, Landau EM. Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science. 1998;280(5371):1940–1942. doi: 10.1126/science.280.5371.1940. [DOI] [PubMed] [Google Scholar]
- Boivin G, Farlay D, Khebbab MT, Jaurand X, Delmas PD, Meunier PJ. In osteoporotic women treated with strontium ranelate, strontium is located in bone formed during treatment with a maintained degree of ineralization. Osteoporos Int. 2010;21(4):667–677. doi: 10.1007/s00198-009-1005-z. [DOI] [PubMed] [Google Scholar]
- Bonar LC, Roufosse AH, Sabine WK, Grynpas MD, Glimcher MJ. X-ray diffraction studies of the crystallinity of bone mineral in newly synthesized and density fractionated bone. Calcif Tissue Int. 1983;35(2):202–209. doi: 10.1007/BF02405032. [DOI] [PubMed] [Google Scholar]
- Brodt MD, Silva MJ. Aged mice have enhanced endocortical response and normal periosteal response compared with young-adult mice following 1 week of axial tibial compression. J Bone Miner Res. 2010;25(9):2006–2015. doi: 10.1002/jbmr.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl KM, Jacobs CR, Turner RT, Evans GL, Farrell PA, Donahue HJ. Aged bone displays an increased responsiveness to low-intensity resistance exercise. J Appl Physiol. 2001;90(4):1359–1364. doi: 10.1152/jappl.2001.90.4.1359. [DOI] [PubMed] [Google Scholar]
- Burger EH, Klein-Nulend J. Mechanotransduction in bone--role of the lacuno-canalicular network. Faseb J. 1999;13(Suppl):S101–112. [PubMed] [Google Scholar]
- Cao JJ, Wronski TJ, Iwaniec U, Phleger L, Kurimoto P, Boudignon B, Halloran BP. Aging increases stromal/osteoblastic cell-induced osteoclastogenesis and alters the osteoclast precursor pool in the mouse. J Bone Miner Res. 2005;20(9):1659–1668. doi: 10.1359/JBMR.050503. [DOI] [PubMed] [Google Scholar]
- Case N, Ma M, Sen B, Xie Z, Gross TS, Rubin J. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008;283(43):29196–29205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celil Aydemir AB, Lee S, Kim DW, Gardner TR, Prince D, Ahn JM, Lee FY. Nuclear Factor Of Activated T Cell Mediates Proinflammatory Gene Expression In Response To Mechanotransduction. Ann N Y Acad Sci. 2007 doi: 10.1196/annals.1402.004. [DOI] [PubMed] [Google Scholar]
- Currey JD. The mechanical adaptations of bones. Princeton University Press; Princeton, NJ: 1984. [Google Scholar]
- Di Monaco M, Vallero F, Di Monaco R, Tappero R. Prevalence of sarcopenia and its association with osteoporosis in 313 older women following a hip fracture. Arch Gerontol Geriatr. 2011;52(1):71–74. doi: 10.1016/j.archger.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Donahue SW, Jacobs CR, Donahue HJ. Flow-induced calcium oscillations in rat osteoblasts are age, loading frequency, and shear stress dependent. Am J Physiol Cell Physiol. 2001;281(5):C1635–1641. doi: 10.1152/ajpcell.2001.281.5.C1635. [DOI] [PubMed] [Google Scholar]
- Dunstan CR, Somers NM, Evans RA. Osteocyte death and hip fracture. Calcif Tissue Int. 1993;53(Suppl 1):S113–116. doi: 10.1007/BF01673417. discussion S116–117. [DOI] [PubMed] [Google Scholar]
- Elefteriou F. Regulation of bone remodeling by the central and peripheral nervous system. Archives of biochemistry and biophysics. 2008;473(2):231–236. doi: 10.1016/j.abb.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forwood MR, Burr DB. Physical activity and bone mass: exercises in futility? Bone Miner. 1993;21(2):89–112. doi: 10.1016/s0169-6009(08)80012-8. [DOI] [PubMed] [Google Scholar]
- Frisoli A, Jr., Chaves PH, Ingham SJ, Fried LP. Severe osteopenia and osteoporosis, sarcopenia, and frailty status in community-dwelling older women: results from the Women's Health and Aging Study (WHAS) II. Bone. 2011;48(4):952–957. doi: 10.1016/j.bone.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Gazit D, Zilberman Y, Ebner R, Kahn A. Bone loss (osteopenia) in old male mice results from diminished activity and availability of TGF-beta. J Cell Biochem. 1998;70(4):478–488. doi: 10.1002/(sici)1097-4644(19980915)70:4<478::aid-jcb5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Greene DA, Naughton GA, Briody JN, Kemp A, Woodhead H, Corrigan L. Bone strength index in adolescent girls: does physical activity make a difference? Br J Sports Med. 2005;39(9):622–627. doi: 10.1136/bjsm.2004.014498. discussion 627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross TS, Srinivasan S, Liu CC, Clemens TL, Bain SD. Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction. J Bone Miner Res. 2002;17(3):493–501. doi: 10.1359/jbmr.2002.17.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind K, Gannon L, Whatley E, Cooke C, Truscott J. Bone cross-sectional geometry in male runners, gymnasts, swimmers and non-athletic controls: a hip-structural analysis study. Eur J Appl Physiol. 2011 doi: 10.1007/s00421-011-2008-y. [DOI] [PubMed] [Google Scholar]
- Hosking D, Chilvers C, Christiansen C, Ravn P, Wasnich R, Ross P, McClung M, Balske A, Thompson D, Daley M, Yates A. Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. Early Postmenopausal Intervention Cohort Study Group. N Engl J Med. 1998;338(Feb 19):485–492. doi: 10.1056/NEJM199802193380801. [DOI] [PubMed] [Google Scholar]
- Jee WS, Tian XY. The benefit of combining non-mechanical agents with mechanical loading: a perspective based on the Utah Paradigm of Skeletal Physiology. J Musculoskel Neuron Interact. 2005;5(2):110–118. [PubMed] [Google Scholar]
- Jilka RL, Almeida M, Ambrogini E, Han L, Roberson PK, Weinstein RS, Manolagas SC. Decreased oxidative stress and greater bone anabolism in the aged, when compared to the young, murine skeleton with parathyroid hormone administration. Aging Cell. 2010;9(5):851–867. doi: 10.1111/j.1474-9726.2010.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, O'Brien CA, Ali AA, Roberson PK, Weinstein RS, Manolagas SC. Intermittent PTH stimulates periosteal bone formation by actions on post-mitotic preosteoblasts. Bone. 2009;44(2):275–286. doi: 10.1016/j.bone.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res. 1998;13(5):793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- Judex S, Donahue LR, Rubin C. Genetic predisposition to low bone mass is paralleled by an enhanced sensitivity to signals anabolic to the skeleton. Faseb J. 2002;16(10):1280–1282. doi: 10.1096/fj.01-0913fje. [DOI] [PubMed] [Google Scholar]
- Karinkanta S, Heinonen A, Sievanen H, Uusi-Rasi K, Pasanen M, Ojala K, Fogelholm M, Kannus P. A multi-component exercise regimen to prevent functional decline and bone fragility in home-dwelling elderly women: randomized, controlled trial. Osteoporos Int. 2007;18(4):453–462. doi: 10.1007/s00198-006-0256-1. [DOI] [PubMed] [Google Scholar]
- Kasahara J, Fukunaga K, Miyamoto E. Differential effects of a calcineurin inhibitor on glutamate-induced phosphorylation of Ca2+/calmodulin-dependent protein kinases in cultured rat hippocampal neurons. J Biol Chem. 1999;274(13):9061–9067. doi: 10.1074/jbc.274.13.9061. [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J, Sterck JG, Semeins CM, Lips P, Joldersma M, Baart JA, Burger EH. Donor age and mechanosensitivity of human bone cells. Osteoporos Int. 2002;13(2):137–146. doi: 10.1007/s001980200005. [DOI] [PubMed] [Google Scholar]
- Kontulainen S, Sievanen H, Kannus P, Pasanen M, Vuori I. Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: a peripheral quantitative computed tomography study between young and old starters and controls. J Bone Miner Res. 2003;18(2):352–359. doi: 10.1359/jbmr.2003.18.2.352. [DOI] [PubMed] [Google Scholar]
- Kreideweiss S, Ahlers C, Nordheim A, Ruhlmann A. Ca2+-induced p38/SAPK signalling inhibited by the immunosuppressant cyclosporin A in human peripheral blood mononuclear cells. Eur J Biochem. 1999;265(3):1075–1084. doi: 10.1046/j.1432-1327.1999.00830.x. [DOI] [PubMed] [Google Scholar]
- Kukuljan S, Nowson CA, Sanders KM, Nicholson GC, Seibel MJ, Salmon J, Daly RM. Independent and Combined Effects of Calcium-Vitamin D3 and Exercise on Bone Structure and Strength in Older Men: An 18-Month Factorial Design Randomized Controlled Trial. J Clin Endocrinol Metab. 2011 doi: 10.1210/jc.2010-2284. [DOI] [PubMed] [Google Scholar]
- Leppanen OV, Sievanen H, Jokihaara J, Pajamaki I, Kannus P, Jarvinen TL. Pathogenesis of age-related osteoporosis: impaired mechano-responsiveness of bone is not the culprit. PLoS One. 2008;3(7):e2540. doi: 10.1371/journal.pone.0002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang CT, Barnes J, Seedor JG, Quartuccio HA, Bolander M, Jeffrey JJ, Rodan GA. Impaired bone activity in aged rats: alterations at the cellular and molecular levels. Bone. 1992;13(6):435–441. doi: 10.1016/8756-3282(92)90087-d. [DOI] [PubMed] [Google Scholar]
- Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24(10):1651–1661. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- Lui PP, Qin L, Chan KM. Tai Chi Chuan exercises in enhancing bone mineral density in active seniors. Clin Sports Med. 2008;27(1):75–86. viii. doi: 10.1016/j.csm.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Ma Y, Jee WS, Yuan Z, Wei W, Chen H, Pun S, Liang H, Lin C. Parathyroid hormone and mechanical usage have a synergistic effect in rat tibial diaphyseal cortical bone. J Bone Miner Res. 1999;14(3):439–448. doi: 10.1359/jbmr.1999.14.3.439. [DOI] [PubMed] [Google Scholar]
- Ma YL, Marin F, Stepan J, Ish-Shalom S, Moricke R, Hawkins F, Kapetanos G, de la Pena MP, Kekow J, Martinez G, Malouf J, Zeng QQ, Wan X, Recker RR. Comparative effects of teriparatide and strontium ranelate in the periosteum of iliac crest biopsies in postmenopausal women with osteoporosis. Bone. 2011;48(5):972–978. doi: 10.1016/j.bone.2011.01.012. [DOI] [PubMed] [Google Scholar]
- MacDonnell SM, Weisser-Thomas J, Kubo H, Hanscome M, Liu Q, Jaleel N, Berretta R, Chen X, Brown JH, Sabri AK, Molkentin JD, Houser SR. CaMKII negatively regulates calcineurin-NFAT signaling in cardiac myocytes. Circ Res. 2009;105(4):316–325. doi: 10.1161/CIRCRESAHA.109.194035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macian F, Lopez-Rodriguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20(19):2476–2489. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- MacKelvie KJ, Petit MA, Khan KM, Beck TJ, McKay HA. Bone mass and structure are enhanced following a 2-year randomized controlled trial of exercise in prepubertal boys. Bone. 2004;34(4):755–764. doi: 10.1016/j.bone.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Almeida M. Gone with the Wnts: {beta}-catenin, TCF, FOXO, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol Endocrinol. 2007 doi: 10.1210/me.2007-0259. [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Parfitt AM. What old means to bone. Trends Endocrinol Metab. 2010;21(6):369–374. doi: 10.1016/j.tem.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47(2–3):119–125. doi: 10.1016/s0162-3109(00)00192-2. [DOI] [PubMed] [Google Scholar]
- Meunier PJ, Slosman DO, Delmas PD, Sebert JL, Brandi ML, Albanese C, Lorenc R, Pors-Nielsen S, De Vernejoul MC, Roces A, Reginster JY. Strontium ranelate: dose-dependent effects in established postmenopausal vertebral osteoporosis--a 2-year randomized placebo controlled trial. J Clin Endocrinol Metab. 2002;87(5):2060–2066. doi: 10.1210/jcem.87.5.8507. [DOI] [PubMed] [Google Scholar]
- Mo A, Yao W, Li C, Tian X, Su M, Ling Y, Zhang Q, Setterberg R, Jee W. Bipedal stance exercise and prostaglandin E2 (PGE(2)) and its synergistic effect in increasing bone mass and in lowering the PGE(2) dose required to prevent ovariectomized-induced cancellous bone loss in aged rats. Bone. 2002;31(3):402. doi: 10.1016/s8756-3282(02)00835-9. [DOI] [PubMed] [Google Scholar]
- Modder UI, Hoey KA, Amin S, McCready LK, Achenbach SJ, Riggs BL, Melton LJ, 3rd, Khosla S. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res. 2011;26(2):373–379. doi: 10.1002/jbmr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2004;52(7):1121–1129. doi: 10.1111/j.1532-5415.2004.52310.x. [DOI] [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- Nicolas V, Prewett A, Bettica P, Mohan S, Finkelman RD, Baylink DJ, Farley JR. Age-related decreases in insulin-like growth factor-I and transforming growth factor-beta in femoral cortical bone from both men and women: implications for bone loss with aging. J Clin Endocrinol Metab. 1994;78(5):1011–1016. doi: 10.1210/jcem.78.5.8175953. [DOI] [PubMed] [Google Scholar]
- Nikander R, Sievanen H, Heinonen A, Daly RM, Uusi-Rasi K, Kannus P. Targeted exercise against osteoporosis: A systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2010;8:47. doi: 10.1186/1741-7015-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26(1):19–26. doi: 10.1002/jbmr.173. [DOI] [PubMed] [Google Scholar]
- Pahlavani MA, Harris MD, Richardson A. The increase in the induction of IL-2 expression with caloric restriction is correlated to changes in the transcription factor NFAT. Cell Immunol. 1997;180(1):10–19. doi: 10.1006/cimm.1997.1155. [DOI] [PubMed] [Google Scholar]
- Pahlavani MA, Vargas DM. Age-related decline in activation of calcium/calmodulin-dependent phosphatase calcineurin and kinase CaMK-IV in rat T cells. Mech Ageing Dev. 1999;112(1):59–74. doi: 10.1016/s0047-6374(99)00077-9. [DOI] [PubMed] [Google Scholar]
- Pahlavani MA, Vargas DM. Influence of aging and caloric restriction on activation of Ras/MAPK, calcineurin, and CaMK-IV activities in rat T cells. Proc Soc Exp Biol Med. 2000;223(2):163–169. doi: 10.1046/j.1525-1373.2000.22322.x. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. Age-related structural changes in trabecular and cortical bone: cellular mechanisms and biomechanical consequences. Calcif Tissue Int. 1984;36(Suppl 1):S123–128. doi: 10.1007/BF02406145. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J, Diel I, Pilz U, Brunotte K, Naumann A, Ziegler R. Mitogenic responsiveness of human bone cells in vitro to hormones and growth factors decreases with age. J Bone Miner Res. 1993;8(6):707–717. doi: 10.1002/jbmr.5650080609. [DOI] [PubMed] [Google Scholar]
- Pietschmann P, Skalicky M, Kneissel M, Rauner M, Hofbauer G, Stupphann D, Viidik A. Bone structure and metabolism in a rodent model of male senile osteoporosis. Exp Gerontol. 2007;42(11):1099–1108. doi: 10.1016/j.exger.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Pivonka P, Komarova SV. Mathematical modeling in bone biology: from intracellular signaling to tissue mechanics. Bone. 2010;47(2):181–189. doi: 10.1016/j.bone.2010.04.601. [DOI] [PubMed] [Google Scholar]
- Poliachik SL, Threet D, Srinivasan S, Gross TS. 32 wk old C3H/HeJ mice actively respond to mechanical loading. Bone. 2008;42(4):653–659. doi: 10.1016/j.bone.2007.12.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab DM, Smith EL, Crenshaw TD, Thomas DP. Bone mechanical properties after exercise training in young and old rats. J Appl Physiol. 1990;68(1):130–134. doi: 10.1152/jappl.1990.68.1.130. [DOI] [PubMed] [Google Scholar]
- Riddle RC, Taylor AF, Genetos DC, Donahue HJ. MAP kinase and calcium signaling mediate fluid flow-induced human mesenchymal stem cell proliferation. Am J Physiol Cell Physiol. 2006;290(3):C776–784. doi: 10.1152/ajpcell.00082.2005. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ., 3rd Involutional osteoporosis. N Engl J Med. 1986;314(26):1676–1686. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281(42):31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- Robling AG, Hinant FM, Burr DB, Turner CH. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res. 2002;17(8):1545–1554. doi: 10.1359/jbmr.2002.17.8.1545. [DOI] [PubMed] [Google Scholar]
- Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283(9):5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- Rozman C, Feliu E, Berga L, Reverter JC, Climent C, Ferran MJ. Age-related variations of fat tissue fraction in normal human bone marrow depend both on size and number of adipocytes: a stereological study. Exp Hematol. 1989;17(1):34–37. [PubMed] [Google Scholar]
- Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001;412(6847):603–604. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- Rubin CT, Bain SD, McLeod KJ. Suppression of the osteogenic response in the aging skeleton. Calcif Tissue Int. 1992;50(4):306–313. doi: 10.1007/BF00301627. [DOI] [PubMed] [Google Scholar]
- Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am. 1984;66(3):397–402. [PubMed] [Google Scholar]
- Rubin J, Fan X, Biskobing DM, Taylor WR, Rubin CT. Osteoclastogenesis is repressed by mechanical strain in an in vitro model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1999;17(5):639–645. doi: 10.1002/jor.1100170504. [DOI] [PubMed] [Google Scholar]
- Schriefer JL, Warden SJ, Saxon LK, Robling AG, Turner CH. Cellular accommodation and the response of bone to mechanical loading. J Biomech. 2005;38(9):1838–1845. doi: 10.1016/j.jbiomech.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Sibai T, Morgan EF, Einhorn TA. Anabolic agents and bone quality. Clin Orthop Relat Res. 2011;469(8):2215–2224. doi: 10.1007/s11999-010-1722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbermann M, Weiss A, Reznick AZ, Eilam Y, Szydel N, Gershon D. Age-related trend for osteopenia in femurs of female C57BL/6 mice. Compr Gerontol [A] 1987;1(1):45–51. [PubMed] [Google Scholar]
- Specker B, Binkley T. Randomized trial of physical activity and calcium supplementation on bone mineral content in 3- to 5-year-old children. J Bone Miner Res. 2003;18(5):885–892. doi: 10.1359/jbmr.2003.18.5.885. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Agans SC, King KA, Moy NY, Poliachik SL, Gross TS. Enabling bone formation in the aged skeleton via rest-inserted mechanical loading. Bone. 2003;33(6):946–955. doi: 10.1016/j.bone.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Ausk BJ, Poliachik SL, Warner SE, Richardson TS, Gross TS. Rest-inserted loading rapidly amplifies the response of bone to small increases in strain and load cycles. J Appl Physiol. 2007;102(5):1945–1952. doi: 10.1152/japplphysiol.00507.2006. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Ausk BJ, Prasad J, Threet D, Bain SD, Richardson TS, Gross TS. Rescuing loading induced bone formation at senescence. PLoS Comput Biol. 2010;6(9):e1000924. doi: 10.1371/journal.pcbi.1000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Saxon LK, Zaman G, Moustafa A, Sunters A, Price JS, Lanyon LE. Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1–34) on trabecular and cortical bone in mice. Bone. 2008;43(2):238–248. doi: 10.1016/j.bone.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Turner CH, Takano Y, Owan I. Aging changes mechanical loading thresholds for bone formation in rats. J Bone Miner Res. 1995;10(10):1544–1549. doi: 10.1002/jbmr.5650101016. [DOI] [PubMed] [Google Scholar]
- Umemura Y, Ishiko T, Tsujimoto H, Miura H, Mokushi N, Suzuki H. Effects of jump training on bone hypertrophy in young and old rats. Int J Sports Med. 1995;16(6):364–367. doi: 10.1055/s-2007-973021. [DOI] [PubMed] [Google Scholar]
- Umemura Y, Ishiko T, Yamauchi T, Kurono M, Mashiko S. Five jumps per day increase bone mass and breaking force in rats. J Bone Miner Res. 1997;12(9):1480–1485. doi: 10.1359/jbmr.1997.12.9.1480. [DOI] [PubMed] [Google Scholar]
- Uusi-Rasi K, Kannus P, Cheng S, Sievanen H, Pasanen M, Heinonen A, Nenonen A, Halleen J, Fuerst T, Genant H, Vuori I. Effect of alendronate and exercise on bone and physical performance of postmenopausal women: a randomized controlled trial. Bone. 2003;33(1):132–143. doi: 10.1016/s8756-3282(03)00082-6. [DOI] [PubMed] [Google Scholar]
- Vainionpaa A, Korpelainen R, Sievanen H, Vihriala E, Leppaluoto J, Jamsa T. Effect of impact exercise and its intensity on bone geometry at weight-bearing tibia and femur. Bone. 2007;40(3):604–611. doi: 10.1016/j.bone.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Vashishth D, Verborgt O, Divine G, Schaffler MB, Fyhrie DP. Decline in osteocyte lacunar density in human cortical bone is associated with accumulation of microcracks with age. Bone. 2000;26(4):375–380. doi: 10.1016/S8756-3282(00)00236-2. [DOI] [PubMed] [Google Scholar]
- Weatherall M. Prevention of falls and fall-related fractures in community-dwelling older adults: a meta-analysis of estimates of effectiveness based on recent guidelines. Intern Med J. 2004;34(3):102–108. doi: 10.1111/j.1444-0903.2004.t01-15-.x. [DOI] [PubMed] [Google Scholar]
- Whisler RL, Beiqing L, Chen M. Age-related decreases in IL-2 production by human T cells are associated with impaired activation of nuclear transcriptional factors AP-1 and NF-AT. Cell Immunol. 1996;169(2):185–195. doi: 10.1006/cimm.1996.0109. [DOI] [PubMed] [Google Scholar]
- Worton L, Kwon RY, Gardiner EM, Gross TS, Srinivasan S. 33rd Am Soc Bone Miner Res. San Diego, CA: 2011. Fluid flow induced early response genes are selectively altered by age. [Google Scholar]
- Yeo H, Beck LH, McDonald JM, Zayzafoon M. Cyclosporin A elicits dose-dependent biphasic effects on osteoblast differentiation and bone formation. Bone. 2007;40(6):1502–1516. doi: 10.1016/j.bone.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You L, Temiyasathit S, Lee P, Kim CH, Tummala P, Yao W, Kingery W, Malone AM, Kwon RY, Jacobs CR. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2008;42(1):172–179. doi: 10.1016/j.bone.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]


