Abstract
mRNA localization and localized translation is a common mechanism by which cellular asymmetry is achieved. In higher eukaryotes the mRNA transport machinery is required for such diverse processes as stem cell division and neuronal plasticity. Because mRNA localization in metazoans is highly complex, studies at the molecular level have proven to be cumbersome. However, active mRNA transport has also been reported in fungi including Saccharomyces cerevisiae, Ustilago maydis and Candida albicans, in which these events are less difficult to study. Amongst them, budding yeast S. cerevisiae has yielded mechanistic insights that exceed our understanding of other mRNA localization events to date. In contrast to most reviews, we refrain here from summarizing mRNA localization events from different organisms. Instead we give an in-depth account of ASH1 mRNA localization in budding yeast. This approach is particularly suited to providing a more holistic view of the interconnection between the individual steps of mRNA localization, from transcriptional events to cytoplasmic mRNA transport and localized translation. Because of our advanced mechanistic understanding of mRNA localization in yeast, the present review may also be informative for scientists working, for example, on mRNA localization in embryogenesis or in neurons.
Keywords: mRNA localization, She2p, She3p, ASH1 mRNA, Translational control, Myosin
Introduction
Regulation of protein expression at the level of translation is much faster than by transcriptional control. Often translational control is coupled to asymmetric localization of mRNAs, which also allows spatial gene regulation within a cell. Such coupled mRNA localization and localized translation happens in virtually all eukaryotic cells [1]. A prominent example is early embryogenesis of the fruit fly Drosophila melanogaster, which relies almost entirely on translational control of previously deposited mRNAs [2, 3]. This mechanism allows the initiation of embryogenesis before zygotic transcription is activated, and thus provides a competitive advantage over other species that lack such a mechanism. A recent study revealed the surprising insight that in Drosophila embryos about 70% of the expressed transcripts are subcellularly localized [4].
Such localization and asymmetric distribution of mRNAs is mainly achieved in three different ways. Transcripts can reach a certain destination by diffusion or cytoplasmic streaming and subsequent anchoring at particular sites [5]. A second mechanism is the asymmetric degradation of mRNAs in a cell, which only leaves mRNAs in a certain region of the cell intact for translation [5, 6]. The third possibility involves active transport processes. Here, transcripts are bound by dedicated RNA-binding proteins and incorporated into motor-protein containing particles [7]. Such messenger ribonucleoprotein particles (mRNPs) move their cargo along microtubules or actin filaments to distinct subcellular sites. There, they undergo reorganization and local protein synthesis is activated [5, 8]. Such active transport is not only used in the fruit fly, but also in a large range of species from vertebrates [5] to yeast [9]. For instance in neurons, mRNA transport ensures the availability of important factors in pre- and postsynaptic areas [10]. Diffusion alone would be insufficient to bring a particular set of proteins to these distant sites. Localized translation of predeposited mRNAs allows neurons to rapidly respond to extrinsic cues. It also helps to modulate synaptic activity and has been implicated in long-term potentiation for memory and learning [10].
In metazoans, the molecular machinery for mRNA localization is rather complex, involving several dozen proteins (for examples, see references [11–13]). Besides motor proteins and RNA-binding proteins, these complexes include translation factors, splicing factors, RNA helicases and additional proteins that might serve as adapters within mRNPs. Analyses of associated mRNAs by microarray or high-throughput sequencing approaches have identified hundreds to thousands of RNAs (for examples, see references [14, 15]). Such a rather high complexity of mRNPs renders mechanistic studies difficult. For example, it remains to be seen how many of the identified mRNAs are indeed localized, when and how these mRNAs are incorporated into transport complexes, and whether these mRNPs are homogeneous or if they constitute a blend of distinct complexes. For the majority of examples, it also remains unclear which molecular interactions mediate the association with the motor proteins and how such interactions activate directional transport. Thus, there is a need for a simple model system to elucidate the basic principles of mRNA transport.
In Saccharomyces cerevisiae, mRNA transport is less complex and relies on fewer factors than in higher eukaryotes [16]. In addition, budding yeast offers a range of advantages for mechanistic studies of cellular processes (a comprehensive overview is given in reference [17]). Its genome can be easily manipulated to generate knock-out strains, to perform rescue experiments, and to modify genes of interest. The modest size of the S. cerevisiae genome and recent advances in robotics allow saturating large-scale genetic screens in a relatively short time. Similarly, genome-wide expression and interaction studies as well as assessments of the intracellular concentration and localization of most yeast proteins have been performed. The result is an enormous accumulation of experimental data that are available in a curated database (www.yeastgenome.org) and an unparalleled collection of tools. Besides these advantages, yeast is unique with respect to the possibilities for combining directed genomic modifications and tagging strategies with live imaging and biochemical purification approaches. In particular, such combinations have helped advance our understanding of mRNA transport in S. cerevisiae.
Thanks to the contributions of a number of groups, we now understand the sequence of events that occurs from the cotranscriptional recruitment of localizing mRNAs to their anchoring at the sites of destination and their translational activation. Because such general information is missing for the localization of transcripts in higher eukaryotes, insights from yeast may be instructive for mRNA localization in general.
S. cerevisiae as a model system for mRNA localization and endoplasmic reticulum inheritance
During mitosis, S. cerevisiae undergoes unequal cell division, producing a larger mother cell and a smaller daughter cell [18]. The mother cell undergoes genomic recombination in the MAT gene locus, resulting in a conversion of its mating type from a to α or vice versa [18]. This recombination event is mediated by the enzymatic activity of the HO endonuclease [19]. In the daughter cell switching of the mating type is inhibited, ensuring different cell fates for the two progenies. About 15 years ago, it was reported that the protein Ash1p suppresses the HO endonuclease selectively in the daughter cell [19, 20]. This cell-specific activity is achieved by the directional transport and efficient localization of its transcript ASH1 mRNA into the daughter cell and the subsequent local translation of Ash1p at the bud tip [20–22]. A genetic screen identified the so-called SHE proteins as essential factors for this process [21]. In the following years, work from several groups helped to clarify their roles in mRNA localization [16, 23, 24]. Since then, more than 30 additional transcripts have been identified as targets for SHE machinery-specific transport into the daughter cell [25–28]. In addition to mRNA transport, concomitant inheritance of cortical endoplasmic reticulum (ER) by the SHE machinery has been reported [29, 30]. For this process, only a subset of the SHE proteins are required.
The localizing ASH1 mRNP: an overview
In the nucleus of the mother cell, ASH1 mRNA is bound by the RNA-binding protein She2p (Fig. 1) [31–34]. The crystal structure of She2p reveals a dimeric protein with an unusual type of nucleic acid binding domain, termed basic helical hairpin [35]. Further analyses showed that two She2p dimers form a tetrameric assembly in solution [36, 37]. One of the most striking structural features of this tetramer is the four small helices that protrude at right angles from the body of the structure into the solvent [35]. After nuclear export, the complex of ASH1 mRNA and She2p associates with a motor-containing subcomplex [38]. The protruding helices of She2p are required for the interaction with this subcomplex and the specific recognition of localizing RNAs [39].
Fig. 1.
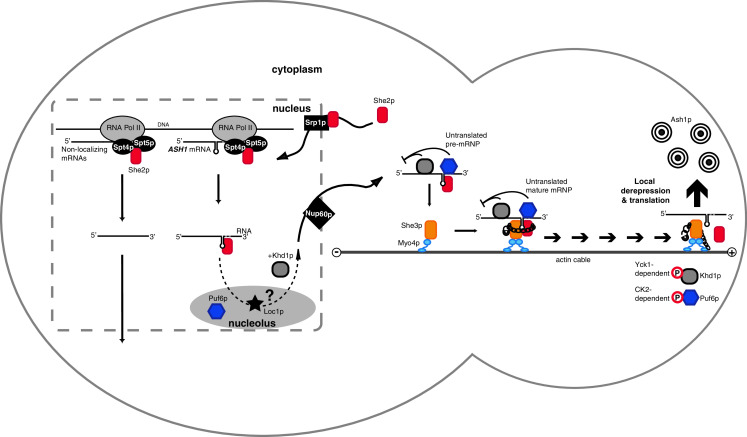
Model of ASH1 mRNA localization in S. cerevisiae. She2p is imported into the nucleus by the importin-alpha Srp1p. At sites of transcription, She2p binds to the elongating RNA polymerase II machinery and associates with nascent mRNAs. Before nuclear export, the ASH1 mRNA-She2p complex transits through the nucleolus, where Loc1p and Puf6p are present. The translational repressors Puf6p and Khd1p are thought to be loaded onto ASH1 mRNA in the nucleolus and in the nucleus, respectively. Efficient nuclear export of localizing mRNAs depends on the specific activity of the nucleoporin Nup60p. Once in the cytoplasm, the ASH1 mRNA-She2p cocomplex binds to Myo4p-associated She3p. Synergistic RNA binding by She2p and She3p dramatically increases the preference for zip-code containing RNAs and ensures that only localizing transcripts are transported. This important quality control step is illustrated by a chain with a padlock. Complex assembly also induces oligomerization of the monomeric Myo4p, which is required for continuous transport of the mRNP. Once the complex is localized at the bud tip, its cargo is translationally activated by phosphorylation of Khd1p and Puf6p. The result is Ash1p expression and inhibition of mating-type switching exclusively in the daughter cell. The mother cell is depicted on the left and the daughter cell on the right. Except for Myo4p, oligomeric states are not depicted. See Fig. 2 for details of ER inheritance
The molecular motor for the directional movement along actin filaments in the cytoplasm is the type V myosin Myo4p [20, 21], which is also referred to as She1p (Fig. 1). The myosin adapter She3p directly binds with its N-terminal half to the C-terminal tail of Myo4p [29, 31, 40, 41] to form a constitutive cytoplasmic cocomplex [29, 31, 40–42]. With its C-terminal half, She3p interacts with the RNA-binding protein She2p [31, 32, 39]. Recent actin-filament gliding assays with transport particles purified from yeast suggest that a minimal complex, consisting of Myo4p, She3p, She2p and RNA has motile activity [37]. It was long thought that the only function of She3p is to act as an adapter between She2p and Myo4p. More recently, however, it has been shown that cytoplasmic She3p also plays a central role in the specific recognition of localizing mRNAs for their incorporation into active transport particles (for details see below) [39].
For efficient mRNA localization in vivo, additional RNA-binding proteins are required (Table 1). Since the genomic deletion of such factors results in a reduction but not in a complete loss of ASH1 mRNA localization, we refer to them as accessory proteins. Two of them, termed Puf6p and Khd1p, are directly involved in translational repression during transport and de-repression at the destination (Fig. 1) [43–46]. One of the least-understood accessory factors is Loc1p. Long et al. showed that this protein is strictly localized in the nucleus [47] and enriched in the nucleolus (Fig. 1) [48]. Therefore, Loc1p is not directly involved in cytoplasmic events. Nevertheless, cells lacking loc1 show defective cytoplasmic ASH1 mRNA localization [47].
Table 1.
Proteins required for mRNA localization in yeast. Listed are proteins with a likely direct function in ASH1 mRNA localization. Excluded are proteins required for general cellular functions. Core components of the ASH1 mRNP are defined by a complete loss of ASH1 mRNA localization upon their genomic deletion
| Protein | Subcellular localization | Protein domain(s) | Core mRNP component? | Protein function | Defect upon mutation |
|---|---|---|---|---|---|
| She2p | Nucleus and cytoplasm | Unique | Yes | Cotranscriptional binding of localizing mRNAs and synergistic cytoplasmic recognition of transcripts with She3p; binds to zip-code elements and localizing mRNAs | Complete loss of mRNA localization |
| She3p | Cytoplasm | No known homology | Yes | Myosin-adapter and RNA-binding protein; acts synergistically with She2p for specific recognition of zip-code elements and localizing mRNAs; required for ER inheritance | Complete loss of mRNA localization |
| Myo4p/She1p | Cytoplasm | Type V myosin with myosin motor domain, IQ motifs, coiled-coil, and C-terminal cargo-binding domain | Yes | Motor protein; interacts with She3p and provides motile activity along actin filaments for mRNA and ER transport into the daughter cell | Complete loss of mRNA localization |
| She4p | Cytoplasm | UCS domain | Unclear | Binds to myosin motor domains and might regulate their function | Complete loss of mRNA localization |
| Puf6p | Mainly nuclear and nucleolar; some in cytoplasm | Seven pumilio-like repeats and CPL domain | No | Translational repression during mRNA transport to bud tip; binds to UUGU consensus motif | Reduced mRNA localization; premature translation |
| Khd1p | Nucleus and cytoplasm | Three K-homology domains | No | Translational repression during mRNA transport to bud tip; binds to CNN repeats and to the E1 element of ASH1 mRNA | Mildly reduced mRNA localization; premature translation |
| Loc1p | Nucleus, enriched in nucleolus | No known homology | No | Required for ASH1 mRNA localization and ribosome biogenesis; molecular function is unknown; binds to the E3 element of ASH1 mRNA and to double-stranded RNAs | Reduced mRNA localization; premature translation |
| Scp160 | Cytoplasm | Six K-homology domains | No | RNA-binding protein involved in telomere silencing and correct ASH1 mRNA localization; exact role in ASH1 mRNA localization is unknown | Reduced mRNA localization |
| Mtp5/Puf5p | Cytoplasm | Seven pumilio-like repeats | No | RNA-binding protein involved in longevity, cell wall integrity, mRNA degradation, and ASH1 mRNA localization; exact role in ASH1 mRNA localization is unknown | Reduced mRNA localization |
Bud-localizing mRNAs
In addition to ASH1 mRNA, She2p-dependent localization of more than 30 transcripts has been reported [25–28]. Interestingly, several of these mRNAs have a functional relationship. Almost half of them encode for membrane-associated proteins [27]. Several bud-localized transcripts have been investigated in more detail and in most of them at least one cis-acting localization element was found [31, 49–53]. These localization elements are also termed zip-code elements [54]. In yeast, zip-code elements are bound by the SHE machinery and thus serve as tethers for the incorporation of mRNAs into motile particles. Attempts to find common features for all of these zip-code elements have so far failed. However, for several well-defined zip-code elements a stem-loop structure with extensive bulged regions is necessary for binding [31, 49–51]. In a three-hybrid assay, Olivier et al. identified sequence motifs in a subset of zip-code elements that are required for binding of the She2p-She3p complex [53]. Most of these motifs consist of a CGA base triplet located in a loop and a single cytosine in a second loop, separated by a double-stranded RNA helix of four or five base pairs. Jambhekar et al. used a similar approach to show that this base triplet alone might not be sufficient for binding, and that the cytosine is not required in some cases [52]. It is interesting to note that in Drosophila the recognition of the K10 mRNA localization element relies to a great extent on unusual tertiary structural features [55]. This finding suggests that structural studies will be indispensable to unravel the defining features of zip-code RNAs in yeast and their recognition by the transport machinery.
With regard to the mRNA cargo, another interesting question is whether each mRNA has its own transport particle or whether they are localized together. To answer this question, Lange et al. used live cell imaging in combination with a dual tagging strategy [56]. They found that transport particles contain at least two different mRNA species that are indeed transported together. Such cotransport might provide an economical way for the cell to coordinate localization of different mRNAs.
Below we discuss in more detail the assembly and disassembly of transport complexes, the importance of nuclear events for the cytoplasmic fate of transcripts, and the central role of the mRNA cargo for the assembly of transport complexes.
The beginning: RNA binding in the nucleus
Kruse et al. showed that She2p is trapped in the nucleus when nuclear export is blocked in a temperature-sensitive mex67-5 mutant strain [38]. This observation indicates that She2p is a nuclear shuttling protein. The nuclear import of She2p is mediated by a nonclassical nuclear localization signal and binding of the importin-alpha Srp1p (Fig. 1) [34]. A recent chromatin immunoprecipitation study demonstrated that once in the nucleus, She2p is recruited to sites of active transcription [57]. Surprisingly, this recruitment does not show a strong preference for genes encoding localizing transcripts [39, 57]. It rather depends on an interaction of She2p with the RNA polymerase II-associated transcription elongation factors Spt4 and Spt5 [57]. RNase treatment prior to immunoprecipitation decreases the She2p occupancy on transcribed genes, suggesting that She2p binding to nascent mRNAs contributes to cotranscriptional recruitment [39, 57]. Whereas Shen et al. observed this effect specifically for genes encoding localized transcripts [57], our data suggest a rather nonspecific association with nascent mRNAs [39].
Recent in vitro studies have shown that She2p alone binds mRNAs rather indiscriminately [33, 36, 39]. A similarly low specificity for ASH1 mRNA has been reported for Puf6p alone and for a ternary complex with She2p and Puf6p [39]. In contrast, specific ASH1 mRNA binding is achieved when She2p forms a cocomplex with the strictly cytoplasmic cofactor She3p [39]. Although these observations indicate that nuclear events might be less specific than cytoplasmic events, it is beyond doubt that they are important. For instance, the deletion of the transcription elongation factor Spt4/5, to which She2p binds, results in impaired ASH1 mRNA localization to the bud tip [57]. Experiments involving artificial retention of She2p in the cytoplasm have provided additional information. Such cytoplasmic retention results in a reduced association of She2p, Puf6p and Loc1p with ASH1 mRNA [34] as well as impaired translational repression [33, 58]. In summary, these findings show that nuclear interactions are important for cytoplasmic ASH1 mRNA transport and localized translation. Such nuclear “priming” has also been described in Xenopus eggs, where it is important for the maturation of localizing mRNPs [59]. Moreover, the localization of oskar mRNA in Drosophila oocytes is dependent on nuclear splicing events [60]. On the other hand, mRNAs injected into the cytoplasm of Drosophila embryos or oligodendrocytes are localized correctly [61].
Thus, more experimental insights are required before we can comprehend why cotranscriptional loading of transport factors occurs and which mechanisms underlie the importance of nuclear assembly of immature mRNPs. Such studies will also help to clarify if highly specific ASH1 mRNA binding occurs in the nucleus or just during the final assembly of the cytoplasmic transport complex.
Nucleolar transition
After transcription, ASH1 mRNA has been suggested to pass through the nucleolus [33, 34]. Evidence comes from the observation that upon blocking nuclear export, She2p and ASH1 mRNA accumulate in the nucleolus [33]. Furthermore, a mutant version of She2p that fails to bind RNA shows nucleolar accumulation. These findings were surprising, as the nucleolus is primarily the site of ribosome biogenesis [62] and not part of the normal mRNA-export pathway. Although unlikely, these observations do not completely rule out the possibility that the nucleolus only functions as a depository for She2p and ASH1 mRNA that are artificially trapped in the nucleus. However, the nucleolar RNA-binding protein Loc1p also binds to ASH1 mRNA [47] as well as to She2p [34]. Deletion of loc1 results in less efficient localization of ASH1 mRNA [47] and a twofold upregulation of ASH1 mRNA levels [58]. Furthermore, the ASH1 mRNA binding protein Puf6p accumulates in the nucleolus even in wild-type cells [33]. Together, these observations indicate that nucleolar transition is an integral station on the path of an mRNA towards its cytoplasmic localization.
Puf6p and Loc1p have both been implicated in ribosome biogenesis and loc1 mutants exhibit defects in rRNA processing and nuclear export of 60S ribosomal subunits [48, 63, 64]. Consistently, Loc1p was found in cocomplexes with a range of ribosomal proteins [58, 65]. It remains particularly puzzling how the strictly nuclear Loc1p acts on such diverse processes as mRNA localization and ribosome biogenesis. A possibility is that Loc1p has a very general function, such as supporting modification or folding of RNAs entering the nucleolus.
It is interesting to note that nucleolar transition has also been reported for mRNA transport factors in higher eukaryotes. For instance in vertebrates, both isoforms of the mRNA localization core factor Staufen pass through the nucleolus [66, 67].
Nuclear export
As inferred from studies with nuclear export mutants, She2p is coexported with ASH1 mRNA [33, 38]. In addition to the dependence on the general export machinery, one non-essential nuclear pore component is important for the specific export of localizing mRNAs (Fig. 1) [68]. This nucleoporin, termed Nup60p, only affects export of localizing transcripts and not of the bulk of mRNAs. Interestingly, in a ∆nup60 strain ASH1 mRNA levels are also reduced, most likely by an increased degradation of transcripts retained in the nucleus over a longer time [68]. Furthermore, in the ∆nup60 strain the nuclear foci representing sites of active ASH1-mRNA transcription appear less located at the nuclear periphery than in wild-type cells [68]. These observations suggest that Nup60p might tether the ASH1 gene locus to the nuclear periphery, thereby coupling transcription to nuclear export. In a ∆nup60 strain the crescent-like ASH1-mRNA localization at the bud tip is also impaired. Thus, the specific nuclear export pathway even affects downstream events in the cytoplasm.
The mature cytoplasmic core complex
In the cytoplasm, the subcomplex of ASH1 mRNA and She2p meets the adapter protein She3p that is constitutively bound to the rod and globular tail of Myo4p (Fig. 1) [40–42, 69, 70]. Previous yeast two-hybrid experiments as well as pull-down and coimmunoprecipitation studies suggested that both subcomplexes interact by protein–protein interaction between She2p and She3p [31, 32]. More recently, it has been shown that She3p itself is also an RNA-binding protein [39, 71] and that the interaction between She2p and She3p in the absence of RNA is probably too weak to mediate stable complex formation [39]. Instead, both rather nonspecific RNA-binding proteins, She2p and She3p, have to jointly bind ASH1 mRNA to achieve high affinity and specificity for localizing transcripts (Fig. 1). The small protruding helices of She2p are essential for this synergistic interaction [39]. Interestingly, the zip-code RNA itself stabilizes the interaction between She2p and She3p [39]. Since Myo4p tightly binds to She3p [40, 42], this interaction also incorporates the motor into the mRNP. Thus, directional transport is directly coupled to specific recognition of localizing mRNAs. This mechanism constitutes an important quality control step to ensure that only zip-code-containing mRNAs are transported.
In distant fungal species such as Candida albicans, a She3p homolog but not a She2p homolog is present in the genome [72]. As in S. cerevisiae, ASH1 mRNA in C. albicans is transported in a She3p-dependent manner [72]. Surprisingly, the functional conservation between the two species is even strong enough to allow the localization of C. albicans ASH1 mRNA in S. cerevisiae [38]. The recent finding that She3p is an RNA-binding protein suggests that She3p mediates at least part of the ASH1 mRNA binding in C. albicans.
The oligomeric state of Myo4p
Myo4p is one of the two type V myosins (MyoV) found in yeast [73]. In contrast to MyoV from higher eukaryotes, Myo4p and its paralog Myo2p have been reported to be nonprocessive [42, 70, 74]. In agreement with this observation, three independent studies have shown that Myo4p by itself is monomeric [40, 42, 70]. Krementsova et al. have also demonstrated that a chimeric protein with the motor domain of yeast Myo4p fused to the neck and rod of mouse MyoV (Myo5a) is able to move processively [75]. This observation suggests that the motor domain of Myo4p is fully capable of processive movement. In related studies, chimeric motors consisting of motor and neck either from mouse Myo5a or from yeast Myo2p, and of the rod and tail of yeast Myo4p have been found to move their cargo nonprocessively [40, 42, 70]. These results indicate that the rod and globular tail domain of Myo4p might be responsible for its monomeric state and missing processivity.
One important feature of MyoV from higher eukaryotes is autoinhibition by its tail domain [76]. Increased ATPase activity upon deletion of the Myo4p C-terminal globular tail domain suggests that this protein might also be autoinhibited [42]. However, residues involved in MyoV autoinhibition are not conserved in Myo4p [41]. Autoinhibition of MyoVa is relieved by binding of the adapter protein melanophilin [77]. In contrast, binding of She3p to Myo4p neither increases its ATPase activity nor changes its motility properties [42, 70]. When considering the monomeric state of Myo4p, autoinhibition in the absence of cargo might not even be required.
The work of several groups suggests that oligomerization of Myo4p upon complex assembly might be required for sustained movement. Chung and Takizawa showed that one transport complex contains at least two Myo4p molecules [37]. Recently, Krementsova et al. confirmed the presence of two motors in complex with She2p by electron microscopy [78]. These complexes are capable of processive movement in a way that is consistent with the hand-over-hand walking model [78]. The authors further observed that this sustained transport occurs in the absence of RNA, which seems inconsistent with the previously described role of RNA in promoting complex assembly and transport [37, 39]. Thus, a main question to resolve in future is whether and how the binding of cargo RNA influences motility of the transport complex.
ASH1 mRNA with its four localization elements might be able to recruit several transport complexes. Such RNA-based oligomerization would increase the number of Myo4p motors in the complex. Indeed, Myo4p complexes purified from yeast only showed continuous movement in a single-molecule assay when at least three Myo4p molecules were present in one particle [70]. Moreover, artificial RNA-mediated multimerization of monomeric Myo4p demonstrated that the efficiency of mRNA localization in vivo increases with the motor copy number per RNA [37]. In summary, it appears likely that mRNA-mediated oligomerization of Myo4p enhances sustained transport of cargoes.
The UCS protein She4p interacts with the motor Myo4p and is required for Myo4p localization [79, 80]. It has been proposed that She4p binds to two Myo4p motor domains and helps to keep them at the correct distance for achieving a defined step size on actin filaments [81]. On the other hand, when ASH1 mRNPs were purified from yeast extracts, no She4p was found to be present in these particles [37].
The extended, translationally repressed transport complex
Like She2p, the two accessory RNA-binding proteins Puf6p and Khd1p shuttle into the nucleus and are thought to be exported together with ASH1 mRNA into the cytoplasm [33, 34]. However, only Puf6p is found together with She2p and ASH1 mRNA in the nucleolus [33], suggesting that Khd1p might join the complex only after the nucleolar transition but before its nuclear export (Fig. 1). While not being required for the formation of core motile particles [37], Puf6p and Khd1p are required for the asymmetric distribution of Ash1p by mediating translational repression of ASH1 mRNA during transport to the daughter cell [43–46]. Interestingly, deletion of Puf6p or Khd1p not only disturbs Ash1p sorting, but also reduces mRNA localization in budding yeast cells [45, 46]. Insertion of a stem-loop into the 5′-UTR of ASH1 mRNA to slow down translation partially rescued ASH1 mRNA and Ash1p localization in a puf6 strain [45]. Thus, repression of translation is required for efficient mRNA transport, possibly by preventing the ribosome from displacing transport complexes from the RNA. The functional similarities between Khd1p and Puf6p indicate that the translational repression by both proteins might be redundant. However, except for ASH1 mRNA, both proteins appear to associate with different subsets of localizing mRNAs [43–46, 82]. Thus they are likely to rather complement each other by repressing different pools of localizing mRNAs.
In the same systematic screen where Irie et al. identified Khd1p to be important for proper ASH1 mRNA localization [46] two other genes, mtp5 and scp160, were also found to impair ASH1 mRNA localization upon deletion. Like Khd1p and Puf6p, both proteins contain canonical RNA-binding domains (Table 1). However, their protein products do not localize with ASH1 mRNA at the bud tip [46]. This suggests that either they indirectly affect ASH1 mRNA localization or they are involved in processes such as remodelling of the mRNP at particular stages of assembly or disassembly.
Mechanisms of translational repression
The pumilio family (PUF) repeat containing RNA-binding protein Puf6p interacts with the PUF consensus sequence UUGU in the 3′ UTR of the ASH1 mRNA [45]. In addition, it binds to the general translation factor eIF5B to prevent assembly of 80S ribosomes on the mRNA [43]. This ensures translational repression of ASH1 mRNA during its transport. Khd1p contains three so-called K-homology domains, which are highly conserved and abundant RNA-binding domains [83, 84]. It has been suggested that Khd1p binds to CNN repeats in the coding region of transcripts [82]. In ASH1 mRNA, Khd1p binding has been mapped to the E1 element in the first half of its open reading frame [44, 46]. Moreover, Khd1p interacts with the C-terminal domain of the general translation initiation factor eIF4G1 [44]. This interaction might prevent recruitment of the preinitiation complex to the mRNA [8].
Anchoring and translational activation at the bud tip
Almost all models of mRNA localization suggest that there is a distinct anchoring step at the end of the transport process and a concomitant translational activation event. An exception is mRNA transport in the filamentous fungus Ustilago maedis, where definitely no anchoring occurs [9]. Whereas translational activation at the end of the transport process has indeed been clearly demonstrated in different species and is widely accepted as the final step of transport [8, 24], the picture is less clear for anchoring.
In budding yeast, the assumption of anchoring is based on the observation that transported ASH1 mRNA accumulates in a crescent-like shape close to the plasma membrane [85]. Certain mutations disrupt this crescent localization and produce a more fuzzy accumulation of mRNA in the daughter cell. This phenotype has been interpreted as loss of anchoring [38, 85, 86]. In this regard, one of the most interesting observations is that mutating the start codon of ASH1 mRNA strongly impairs its bud tip localization [46]. This finding suggests that active translation at the bud tip might be required for its correct localization. It is also consistent with a study showing that disruption of translational activation at the bud tip impairs ASH1 mRNA localization [43]. Thus, bud tip localization may not be a simple binding event to a membrane-associated factor, but instead appears to be closely linked to translation activation. This interpretation is also in agreement with the previous report that bud-tip localization involves reorganization of the core complex [86]. It will be interesting to see whether the molecular machinery can be identified that indeed mediates direct membrane tethering. The structural similarity between the cargo-binding tails of type V myosins and of the membrane-tethered exocyst components located at the bud tip might provide a structural suggestion [41]. Alternatively, the transport complex could be trapped in actin patches at the bud tip [87].
Once the ASH1 mRNP has reached its destination, Khd1p becomes phosphorylated by the membrane-associated kinase Yck1p [44]. This phosphorylation at the bud tip reduces its affinity for localizing transcripts and results in their release for local translation. Similarly, phosphorylation of the Puf6p N-terminus by the casein kinase II results in a decrease in Puf6p affinity for ASH1 mRNA and its subsequent translational activation [43]. Such a phosphorylation-dependent reduction in RNA affinity of transport core factors has also been described in vertebrates. Zip-code binding protein 1 (ZBP1) is the dedicated RNA-binding protein for β-actin mRNA localization in filopodia. Its phosphorylation at the leading edge by Src kinase reduces its affinity for β-actin mRNA and releases the transcript for translation [88]. Although the release mechanisms of translational repression by Khd1p, Puf6p and ZBP1 are intriguingly similar, ZBP1 also shows similarities to She2p. It recognizes zip-code elements for the transport of β-actin mRNA. Extending this analogy, it is reasonable to suggest posttranslational modifications in She2p or in She3p to help release ASH1 mRNA for more efficient activation of translation. Interestingly, phosphorylation sites in She3p that impact ASH1 mRNA localization have already been identified [71] and might potentially play a role in mRNA release at the bud tip.
ER inheritance by the SHE machinery
In addition to mRNA transport, the SHE machinery has been implicated in the inheritance of ER in small buds [29]. At a later stage, ER inheritance occurs independent of active transport. Myo4p and She3p, but not She2p, are required for ER inheritance (Fig. 2) [29, 30]. Nevertheless, She2p does cosegregate with ER in a She3p- and Myo4p-independent manner [30]. To date it is unknown how the ER is tethered to the transport machinery [89]. Since we still do not understand this interaction and its precise meaning, aspects of ER inheritance may be the focus of future research.
Fig. 2.
Inheritance of cortical ER in small bud cells. ER inheritance depends on the type V myosin Myo4p (blue) and the myosin adapter She3p (orange), but not on the RNA-binding protein She2p (red). Left diagram shows the stable core RNA-transport complex as in Fig. 1, with the exception that the N- and C-terminal domains of She3p are depicted separately and are marked N and C, respectively. Since it is still unknown how the motor complex associates with ER, an unknown “Factor X” might be involved in this interaction. Because She2p is not required for ER inheritance, but nevertheless interacts with ER in an unknown manner, different modes of ER binding can be envisaged
Conclusion
Although several questions remain to be answered, the studies on mRNA localization in yeast discussed above have already yielded detailed mechanistic insights. Given the enormous complexity of mRNA transport in higher eukaryotes, a knowledge of the mechanisms in yeast allows us to understand the general principles of mRNP assembly, transport, localization, and localized translation. In future, it will be important to assess why co- and posttranscriptional mRNP formation in the nucleus is required for ASH1 mRNA localization. Furthermore, the model system ASH1 mRNP might also allow us to reconstitute larger complexes with full-length RNAs and all involved cofactors to study their function under conditions more similar to complexes in vivo.
Since several ASH1 mRNA transport factors are not conserved in higher eukaryotes, the advantage of yeast is not to enable an understanding of the molecular details in higher eukaryotes by direct analogy, but rather to provide general principles and mechanisms of regulation, which will give the first examples and provide testable hypotheses for other model systems.
Acknowledgments
This work was supported by the Helmholtz Association (VG-NH 142) and Deutsche Forschungsgemeinschaft.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.St Johnston D. Moving messages: the intracellular localization of mRNAs. Nat Rev Mol Cell Biol. 2005;6:363–375. doi: 10.1038/nrm1643. [DOI] [PubMed] [Google Scholar]
- 2.Kugler JM, Lasko P. Localization, anchoring and translational control of oskar, gurken, bicoid and nanos mRNA during Drosophila oogenesis. Fly (Austin) 2009;3(1):15–28. doi: 10.4161/fly.3.1.7751. [DOI] [PubMed] [Google Scholar]
- 3.Becalska AN, Gavis ER. Lighting up mRNA localization in Drosophila oogenesis. Development. 2009;136(15):2493–2503. doi: 10.1242/dev.032391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lecuyer E, Yoshida H, Parthasarathy N, Alm C, Babak T, Cerovina T, Hughes TR, Tomancak P, Krause HM. Global analysis of mRNA localization reveals a prominent role in organizing cellular architecture and function. Cell. 2007;131(1):174–187. doi: 10.1016/j.cell.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Meignin C, Davis I. Transmitting the message: intracellular mRNA localization. Curr Opin Cell Biol. 2010;22(1):112–119. doi: 10.1016/j.ceb.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Holt CE, Bullock SL. Subcellular mRNA localization in animal cells and why it matters. Science. 2009;326(5957):1212–1216. doi: 10.1126/science.1176488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin KC, Ephrussi A. mRNA localization: gene expression in the spatial dimension. Cell. 2009;136(4):719–730. doi: 10.1016/j.cell.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Besse F, Ephrussi A. Translational control of localized mRNAs: restricting protein synthesis in space and time. Nat Rev Mol Cell Biol. 2008;9(12):971–980. doi: 10.1038/nrm2548. [DOI] [PubMed] [Google Scholar]
- 9.Zarnack K, Feldbrugge M. Microtubule-dependent mRNA transport in fungi. Eukaryot Cell. 2010;9(7):982–990. doi: 10.1128/EC.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redondo RL, Morris RG. Making memories last: the synaptic tagging and capture hypothesis. Nat Rev Neurosci. 2011;12(1):17–30. doi: 10.1038/nrn2963. [DOI] [PubMed] [Google Scholar]
- 11.Kanai Y, Dohmae N, Hirokawa N. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron. 2004;43(4):513–525. doi: 10.1016/j.neuron.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Ohashi S, Koike K, Omori A, Ichinose S, Ohara S, Kobayashi S, Sato TA, Anzai K. Identification of mRNA/protein (mRNP) complexes containing Pur-alpha, mStaufen, fragile X protein, and myosin Va and their association with rough endoplasmic reticulum equipped with a kinesin motor. J Biol Chem. 2002;277(40):37804–37810. doi: 10.1074/jbc.M203608200. [DOI] [PubMed] [Google Scholar]
- 13.Angenstein F, Evans AM, Ling SC, Settlage RE, Ficarro S, Carrero-Martinez FA, Shabanowitz J, Hunt DF, Greenough WT. Proteomic characterization of messenger ribonucleoprotein complexes bound to nontranslated or translated poly(A) mRNAs in the rat cerebral cortex. J Biol Chem. 2005;280(8):6496–6503. doi: 10.1074/jbc.M412742200. [DOI] [PubMed] [Google Scholar]
- 14.Furic L, Maher-Laporte M, DesGroseillers L. A genome-wide approach identifies distinct but overlapping subsets of cellular mRNAs associated with Staufen1- and Staufen2-containing ribonucleoprotein complexes. RNA. 2008;14(2):324–335. doi: 10.1261/rna.720308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB, Warren ST. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107(4):477–487. doi: 10.1016/S0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 16.Müller M, Heuck A, Niessing D. Directional mRNA transport in eukaryotes: lessons from yeast. Cell Mol Life Sci. 2007;64:171–180. doi: 10.1007/s00018-006-6286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Botstein D, Fink GR. Yeast: an experimental organism for 21st century biology. Genetics. 2011;189(3):695–704. doi: 10.1534/genetics.111.130765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosma MP. Daughter-specific repression of Saccharomyces cerevisiae HO: Ash1 is the commander. EMBO Rep. 2004;5(10):953–957. doi: 10.1038/sj.embor.7400251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sil A, Herskowitz I. Identification of asymmetrically localized determinant, Ash1p, required for lineage-specific transcription of the yeast HO gene. Cell. 1996;84(5):711–722. doi: 10.1016/S0092-8674(00)81049-1. [DOI] [PubMed] [Google Scholar]
- 20.Bobola N, Jansen RP, Shin TH, Nasmyth K. Asymmetric accumulation of Ash1p in postanaphase nuclei depends on a myosin and restricts yeast mating-type switching to mother cells. Cell. 1996;84(5):699–709. doi: 10.1016/S0092-8674(00)81048-X. [DOI] [PubMed] [Google Scholar]
- 21.Jansen RP, Dowzer C, Michaelis C, Galova M, Nasmyth K. Mother cell-specific HO expression in budding yeast depends on the unconventional myosin myo4p and other cytoplasmic proteins. Cell. 1996;84(5):687–697. doi: 10.1016/S0092-8674(00)81047-8. [DOI] [PubMed] [Google Scholar]
- 22.Long RM, Singer RH, Meng X, Gonzalez I, Nasmyth K, Jansen RP. Mating type switching in yeast controlled by asymmetric localization of ASH1 mRNA. Science. 1997;277(5324):383–387. doi: 10.1126/science.277.5324.383. [DOI] [PubMed] [Google Scholar]
- 23.Chartrand P, Singer RH, Long RM. RNP localization and transport in yeast. Annu Rev Cell Dev Biol. 2001;17:297–310. doi: 10.1146/annurev.cellbio.17.1.297. [DOI] [PubMed] [Google Scholar]
- 24.Paquin N, Chartrand P. Local regulation of mRNA translation: new insights from the bud. Trends Cell Biol. 2008;18(3):105–111. doi: 10.1016/j.tcb.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Hogan DJ, Riordan DP, Gerber AP, Herschlag D, Brown PO. Diverse RNA-binding proteins interact with functionally related sets of RNAs, suggesting an extensive regulatory system. PLoS Biol. 2008;6(10):e255. doi: 10.1371/journal.pbio.0060255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oeffinger M, Wei KE, Rogers R, Degrasse JA, Chait BT, Aitchison JD, Rout MP. Comprehensive analysis of diverse ribonucleoprotein complexes. Nat Methods. 2007;4(11):951–956. doi: 10.1038/nmeth1101. [DOI] [PubMed] [Google Scholar]
- 27.Shepard KA, Gerber AP, Jambhekar A, Takizawa PA, Brown PO, Herschlag D, DeRisi JL, Vale RD. Widespread cytoplasmic mRNA transport in yeast: identification of 22 bud-localized transcripts using DNA microarray analysis. Proc Natl Acad Sci U S A. 2003;100(20):11429–11434. doi: 10.1073/pnas.2033246100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takizawa PA, DeRisi JL, Wilhelm JE, Vale RD. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science. 2000;290(5490):341–344. doi: 10.1126/science.290.5490.341. [DOI] [PubMed] [Google Scholar]
- 29.Estrada P, Kim J, Coleman J, Walker L, Dunn B, Takizawa P, Novick P, Ferro-Novick S. Myo4p and She3p are required for cortical ER inheritance in Saccharomyces cerevisiae. J Cell Biol. 2003;163(6):1255–1266. doi: 10.1083/jcb.200304030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmid M, Jaedicke A, Du T-D, Jansen R-P. Coordination of endoplasmic reticulum and mRNA localization to the yeast bud. Curr Biol. 2006;16(15):1538–1543. doi: 10.1016/j.cub.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 31.Böhl F, Kruse C, Frank A, Ferring D, Jansen RP. She2p, a novel RNA-binding protein tethers ASH1 mRNA to the Myo4p myosin motor via She3p. EMBO J. 2000;19(20):5514–5524. doi: 10.1093/emboj/19.20.5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long RM, Gu W, Lorimer E, Singer RH, Chartrand P. She2p is a novel RNA-binding protein that recruits the Myo4p-She3p complex to ASH1 mRNA. EMBO J. 2000;19(23):6592–6601. doi: 10.1093/emboj/19.23.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du TG, Jellbauer S, Müller M, Schmid M, Niessing D, Jansen RP. Nuclear transit of the RNA-binding protein She2p is required for translational control of localized ASH1 mRNA. EMBO Rep. 2008;9:781–787. doi: 10.1038/embor.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen Z, Paquin N, Forget A, Chartrand P. Nuclear shuttling of She2p couples ASH1 mRNA localization to its translational repression by recruiting Loc1p and Puf6p. Mol Biol Cell. 2009;20(8):2265–2275. doi: 10.1091/mbc.E08-11-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niessing D, Hüttelmaier S, Zenklusen D, Singer RH, Burley SK. She2p is a novel RNA-binding protein with a basic helical hairpin motif. Cell. 2004;119:491–502. doi: 10.1016/j.cell.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Müller M, Richter K, Heuck A, Kremmer E, Buchner J, Jansen RP, Niessing D. Formation of She2p tetramers is required for mRNA binding, mRNP assembly, and localization. RNA. 2009;15(11):2002–2012. doi: 10.1261/rna.1753309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung S, Takizawa PA. Multiple Myo4 motors enhance ASH1 mRNA transport in Saccharomyces cerevisiae. J Cell Biol. 2010;189(4):755–767. doi: 10.1083/jcb.200912011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kruse C, Jaedicke A, Beaudouin J, Böhl F, Ferring D, Güttler T, Ellenberg J, Jansen RP. Ribonucleoprotein-dependent localization of the yeast class V myosin Myo4p. J Cell Biol. 2002;159(6):971–982. doi: 10.1083/jcb.200207101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Müller M, Heym RG, Mayer A, Kramer K, Schmid M, Cramer P, Urlaub H, Jansen RP, Niessing D. A cytoplasmic complex mediates specific mRNA recognition and localization in yeast. PLoS Biol. 2011;9(4):e1000611. doi: 10.1371/journal.pbio.1000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heuck A, Du TG, Jellbauer S, Richter K, Kruse C, Jaklin S, Muller M, Buchner J, Jansen RP, Niessing D. Monomeric myosin V uses two binding regions for the assembly of stable translocation complexes. Proc Natl Acad Sci U S A. 2007;104(50):19778–19783. doi: 10.1073/pnas.0706780104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heuck A, Fetka I, Brewer DN, Huls D, Munson M, Jansen RP, Niessing D. The structure of the Myo4p globular tail and its function in ASH1 mRNA localization. J Cell Biol. 2010;189(3):497–510. doi: 10.1083/jcb.201002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hodges AR, Krementsova EB, Trybus KM. She3p binds to the rod of yeast myosin V and prevents it from dimerizing, forming a single-headed motor complex. J Biol Chem. 2008;283(11):6906–6914. doi: 10.1074/jbc.M708865200. [DOI] [PubMed] [Google Scholar]
- 43.Deng Y, Singer RH, Gu W. Translation of ASH1 mRNA is repressed by Puf6p-Fun12p/eIF5B interaction and released by CK2 phosphorylation. Genes Dev. 2008;22(8):1037–1050. doi: 10.1101/gad.1611308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paquin N, Menade M, Poirier G, Donato D, Drouet E, Chartrand P. Local activation of yeast ASH1 mRNA translation through phosphorylation of Khd1p by the casein kinase Yck1p. Mol Cell. 2007;26(6):795–809. doi: 10.1016/j.molcel.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 45.Gu W, Deng Y, Zenklusen D, Singer RH. A new yeast PUF family protein, Puf6p, represses ASH1 mRNA translation and is required for its localization. Genes Dev. 2004;18(12):1452–1465. doi: 10.1101/gad.1189004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irie K, Tadauchi T, Takizawa PA, Vale RD, Matsumoto K, Herskowitz I. The Khd1 protein, which has three KH RNA-binding motifs, is required for proper localization of ASH1 mRNA in yeast. EMBO J. 2002;21(5):1158–1167. doi: 10.1093/emboj/21.5.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Long RM, Gu W, Meng X, Gonsalvez G, Singer RH, Chartrand P. An exclusively nuclear RNA-binding protein affects asymmetric localization of ASH1 mRNA and Ash1p in yeast. J Cell Biol. 2001;153(2):307–318. doi: 10.1083/jcb.153.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urbinati CR, Gonsalvez GB, Aris JP, Long RM. Loc1p is required for efficient assembly and nuclear export of the 60S ribosomal subunit. Mol Genet Genomics. 2006;276(4):369–377. doi: 10.1007/s00438-006-0151-7. [DOI] [PubMed] [Google Scholar]
- 49.Chartrand P, Meng XH, Hüttelmaier S, Donato D, Singer RH. Asymmetric sorting of ash1p in yeast results from inhibition of translation by localization elements in the mRNA. Mol Cell. 2002;10(6):1319–1330. doi: 10.1016/S1097-2765(02)00694-9. [DOI] [PubMed] [Google Scholar]
- 50.Chartrand P, Meng XH, Singer RH, Long RM. Structural elements required for the localization of ASH1 mRNA and of a green fluorescent protein reporter particle in vivo. Curr Biol. 1999;9(6):333–336. doi: 10.1016/S0960-9822(99)80144-4. [DOI] [PubMed] [Google Scholar]
- 51.Gonzalez I, Buonomo SB, Nasmyth K, von Ahsen U. ASH1 mRNA localization in yeast involves multiple secondary structural elements and Ash1 protein translation. Curr Biol. 1999;9(6):337–340. doi: 10.1016/S0960-9822(99)80145-6. [DOI] [PubMed] [Google Scholar]
- 52.Jambhekar A, McDermott K, Sorber K, Shepard KA, Vale RD, Takizawa PA, DeRisi JL. Unbiased selection of localization elements reveals cis-acting determinants of mRNA bud localization in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2005;102:18005–18010. doi: 10.1073/pnas.0509229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olivier C, Poirier G, Gendron P, Boisgontier A, Major F, Chartrand P. Identification of a conserved RNA motif essential for She2p recognition and mRNA localization to the yeast bud. Mol Cell Biol. 2005;25(11):4752–4766. doi: 10.1128/MCB.25.11.4752-4766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kislauskis EH, Zhu X, Singer RH. Sequences responsible for intracellular localization of beta-actin messenger RNA also affect cell phenotype. J Cell Biol. 1994;127(2):441–451. doi: 10.1083/jcb.127.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bullock SL, Ringel I, Ish-Horowicz D, Lukavsky PJ. A’-form RNA helices are required for cytoplasmic mRNA transport in Drosophila. Nat Struct Mol Biol. 2010;17(6):703–709. doi: 10.1038/nsmb.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lange S, Katayama Y, Schmid M, Burkacky O, Brauchle C, Lamb DC, Jansen RP. Simultaneous transport of different localized mRNA species revealed by live-cell imaging. Traffic. 2008;9(8):1256–1267. doi: 10.1111/j.1600-0854.2008.00763.x. [DOI] [PubMed] [Google Scholar]
- 57.Shen Z, St-Denis A, Chartrand P. Cotranscriptional recruitment of She2p by RNA pol II elongation factor Spt4-Spt5/DSIF promotes mRNA localization to the yeast bud. Genes Dev. 2010;24(17):1914–1926. doi: 10.1101/gad.1937510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Komili S, Farny NG, Roth FP, Silver PA. Functional specificity among ribosomal proteins regulates gene expression. Cell. 2007;131(3):557–571. doi: 10.1016/j.cell.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kress TL, Yoon YJ, Mowry KL. Nuclear RNP complex assembly initiates cytoplasmic RNA localization. J Cell Biol. 2004;165(2):203–211. doi: 10.1083/jcb.200309145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hachet O, Ephrussi A. Splicing of oskar RNA in the nucleus is coupled to its cytoplasmic localization. Nature. 2004;428(6986):959–963. doi: 10.1038/nature02521. [DOI] [PubMed] [Google Scholar]
- 61.Bullock SL. Messengers, motors and mysteries: sorting of eukaryotic mRNAs by cytoskeletal transport. Biochem Soc Trans. 2011;39(5):1161–1165. doi: 10.1042/BST0391161. [DOI] [PubMed] [Google Scholar]
- 62.Kressler D, Hurt E, Bassler J. Driving ribosome assembly. Biochim Biophys Acta. 2010;1803(6):673–683. doi: 10.1016/j.bbamcr.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 63.Nissan TA, Bassler J, Petfalski E, Tollervey D, Hurt E. 60S pre-ribosome formation viewed from assembly in the nucleolus until export to the cytoplasm. EMBO J. 2002;21(20):5539–5547. doi: 10.1093/emboj/cdf547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harnpicharnchai P, Jakovljevic J, Horsey E, Miles T, Roman J, Rout M, Meagher D, Imai B, Guo Y, Brame CJ, Shabanowitz J, Hunt DF, Woolford JL., Jr Composition and functional characterization of yeast 66S ribosome assembly intermediates. Mol Cell. 2001;8(3):505–515. doi: 10.1016/S1097-2765(01)00344-6. [DOI] [PubMed] [Google Scholar]
- 65.Collins SR, Kemmeren P, Zhao XC, Greenblatt JF, Spencer F, Holstege FC, Weissman JS, Krogan NJ. Toward a comprehensive atlas of the physical interactome of Saccharomyces cerevisiae. Mol Cell Proteomics. 2007;6(3):439–450. doi: 10.1074/mcp.M600381-MCP200. [DOI] [PubMed] [Google Scholar]
- 66.Macchi P, Brownawell AM, Grunewald B, DesGroseillers L, Macara IG, Kiebler MA. The brain-specific double-stranded RNA-binding protein Staufen2: nucleolar accumulation and isoform-specific exportin-5-dependent export. J Biol Chem. 2004;279(30):31440–31444. doi: 10.1074/jbc.C400226200. [DOI] [PubMed] [Google Scholar]
- 67.Martel C, Macchi P, Furic L, Kiebler MA, Desgroseillers L. Staufen1 is imported into the nucleolus via a bipartite nuclear localization signal and several modulatory determinants. Biochem J. 2006;393(Pt 1):245–254. doi: 10.1042/BJ20050694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Powrie EA, Zenklusen D, Singer RH. A nucleoporin, Nup60p, affects the nuclear and cytoplasmic localization of ASH1 mRNA in S. cerevisiae. RNA. 2011;17(1):134–144. doi: 10.1261/rna.1210411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bookwalter CS, Lord M, Trybus KM. Essential features of the class V myosin from budding yeast for ASH1 mRNA transport. Mol Biol Cell. 2009;20(14):3414–3421. doi: 10.1091/mbc.E08-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dunn BD, Sakamoto T, Hong MS, Sellers JR, Takizawa PA. Myo4p is a monomeric myosin with motility uniquely adapted to transport mRNA. J Cell Biol. 2007;178(7):1193–1206. doi: 10.1083/jcb.200707080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Landers SM, Gallas MR, Little J, Long RM. She3p possesses a novel activity required for ASH1 mRNA localization in Saccharomyces cerevisiae. Eukaryot Cell. 2009;8(7):1072–1083. doi: 10.1128/EC.00084-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elson SL, Noble SM, Solis NV, Filler SG, Johnson AD. An RNA transport system in Candida albicans regulates hyphal morphology and invasive growth. PLoS Genet. 2009;5(9):e1000664. doi: 10.1371/journal.pgen.1000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haarer BK, Petzold A, Lillie SH, Brown SS. Identification of MYO4, a second class V myosin gene in yeast. J Cell Sci. 1994;107(Pt 4):1055–1064. doi: 10.1242/jcs.107.4.1055. [DOI] [PubMed] [Google Scholar]
- 74.Reck-Peterson SL, Tyska MJ, Novick PJ, Mooseker MS. The yeast class V myosins, Myo2p and Myo4p, are nonprocessive actin-based motors. J Cell Biol. 2001;153(5):1121–1126. doi: 10.1083/jcb.153.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krementsova EB, Hodges AR, Lu H, Trybus KM. Processivity of chimeric class V myosins. J Biol Chem. 2006;281(9):6079–6086. doi: 10.1074/jbc.M510041200. [DOI] [PubMed] [Google Scholar]
- 76.Trybus KM. Myosin V from head to tail. Cell Mol Life Sci. 2008;65(9):1378–1389. doi: 10.1007/s00018-008-7507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li XD, Ikebe R, Ikebe M. Activation of myosin Va function by melanophilin, a specific docking partner of myosin Va. J Biol Chem. 2005;280(18):17815–17822. doi: 10.1074/jbc.M413295200. [DOI] [PubMed] [Google Scholar]
- 78.Krementsova EB, Hodges AR, Bookwalter CS, Sladewski TE, Travaglia M, Sweeney HL, Trybus KM. Two single-headed myosin V motors bound to a tetrameric adapter protein form a processive complex. J Cell Biol. 2011;195(4):631–641. doi: 10.1083/jcb.201106146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Toi H, Fujimura-Kamada K, Irie K, Takai Y, Todo S, Tanaka K. She4p/Dim1p interacts with the motor domain of unconventional myosins in the budding yeast, Saccharomyces cerevisiae. Mol Biol Cell. 2003;14(6):2237–2249. doi: 10.1091/mbc.E02-09-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wesche S, Arnold M, Jansen RP. The UCS domain protein She4p binds to myosin motor domains and is essential for class I and class V myosin function. Curr Biol. 2003;13(9):715–724. doi: 10.1016/S0960-9822(03)00264-1. [DOI] [PubMed] [Google Scholar]
- 81.Shi H, Blobel G. UNC-45/CRO1/She4p (UCS) protein forms elongated dimer and joins two myosin heads near their actin binding region. Proc Natl Acad Sci U S A. 2010;107(50):21382–21387. doi: 10.1073/pnas.1013038107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hasegawa Y, Irie K, Gerber AP. Distinct roles for Khd1p in the localization and expression of bud-localized mRNAs in yeast. RNA. 2008;14(11):2333–2347. doi: 10.1261/rna.1016508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Messias AC, Sattler M. Structural basis of single-stranded RNA recognition. Acc Chem Res. 2004;37(5):279–287. doi: 10.1021/ar030034m. [DOI] [PubMed] [Google Scholar]
- 84.Auweter SD, Oberstrass FC, Allain FH. Sequence-specific binding of single-stranded RNA: is there a code for recognition? Nucleic Acids Res. 2006;34(17):4943–4959. doi: 10.1093/nar/gkl620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beach DL, Salmon ED, Bloom K. Localization and anchoring of mRNA in budding yeast. Curr Biol. 1999;9(11):569–578. doi: 10.1016/S0960-9822(99)80260-7. [DOI] [PubMed] [Google Scholar]
- 86.Gonsalvez GB, Little JL, Long RM. ASH1 mRNA anchoring requires reorganization of the Myo4p/She3p/She2p transport complex. J Biol Chem. 2004;279:46286–46294. doi: 10.1074/jbc.M406086200. [DOI] [PubMed] [Google Scholar]
- 87.Revenu C, Athman R, Robine S, Louvard D. The co-workers of actin filaments: from cell structures to signals. Nat Rev Mol Cell Biol. 2004;5(8):635–646. doi: 10.1038/nrm1437. [DOI] [PubMed] [Google Scholar]
- 88.Hüttelmaier S, Zenklusen D, Lederer M, Dictenberg J, Lorenz M, Meng X, Bassell GJ, Condeelis J, Singer RH. Spatial regulation of beta-actin translation by Src-dependent phosphorylation of ZBP1. Nature. 2005;438(7067):512–515. doi: 10.1038/nature04115. [DOI] [PubMed] [Google Scholar]
- 89.Gerst JE. Message on the web: mRNA and ER co-trafficking. Trends Cell Biol. 2008;18(2):68–76. doi: 10.1016/j.tcb.2007.11.005. [DOI] [PubMed] [Google Scholar]



