SUMMARY
Sleep consolidates experience-dependent brain plasticity, but the precise cellular mechanisms mediating this process are unknown [1]. De novo cortical protein synthesis is one possible mechanism. In support of this hypothesis, sleep is associated with increased brain protein synthesis [2, 3] and transcription of mRNAs involved in protein synthesis regulation [4, 5]. Protein synthesis in turn is critical for memory consolidation and persistent forms of plasticity in vitro and in vivo [6, 7]. However, it is unknown if cortical protein synthesis in sleep serves similar functions. We investigated the role of protein synthesis in the sleep-dependent consolidation of a classic form of cortical plasticity in vivo (ocular dominance plasticity: ODP [8, 9]) in the cat visual cortex. We show that intracortical inhibition of mammalian target of rapamycin (mTOR)-dependent protein synthesis during sleep abolishes consolidation, but has no effect on plasticity induced during wakefulness. Sleep also promotes phosphorylation of protein synthesis regulators (i.e. 4E-BP1 and eEF2) and the translation (but not transcription) of key plasticity-related mRNAs (ARC and BDNF). These findings show that sleep promotes cortical mRNA translation. Interruption of this process has functional consequences, as it abolishes the consolidation of experience in the cortex.
RESULTS AND DISCUSSION
Protein synthesis during sleep, but not wake, is important for ocular dominance plasticity (ODP)
ODP refers to plastic changes in visual cortical circuits triggered by transiently blocking patterned vision in one eye (monocular deprivation: MD)[8, 9]. ODP appears to involve Hebbian and non-Hebbian forms of synaptic plasticity [10], many of which require de novo protein synthesis to be consolidated [11]. We have previously shown that ODP can be divided into an induction phase (during waking) and a consolidation phase (during sleep) [9, 12]. What is not known, however, is the relative role of protein synthesis in the waking and sleeping phases of ODP.
To address this issue, we inhibited protein synthesis in visual cortex (V1) with rapamycin (RAPA) which interferes with the Raptor/mTOR complex (mTORC1)[13], preventing mTORC1-mediated cap-dependent translation initiation [14]. mTORC1 is crucial for consolidation of several forms of plasticity [15], but its role in ODP has not been investigated. We first determined if mTORC1-mediated translation was required for the sleep-dependent consolidation of ODP. These animals underwent 6 hours of MD (while awake) and then were allowed to sleep ad lib for 6 hours during which time RAPA or vehicle (VEH) were intracortically infused in V1 (“MD + sleep” in Figure 1A). The animals were then immediately assessed for changes in ODP using micro-electrode recording of single V1 neurons (see Supplemental Experimental Procedures).
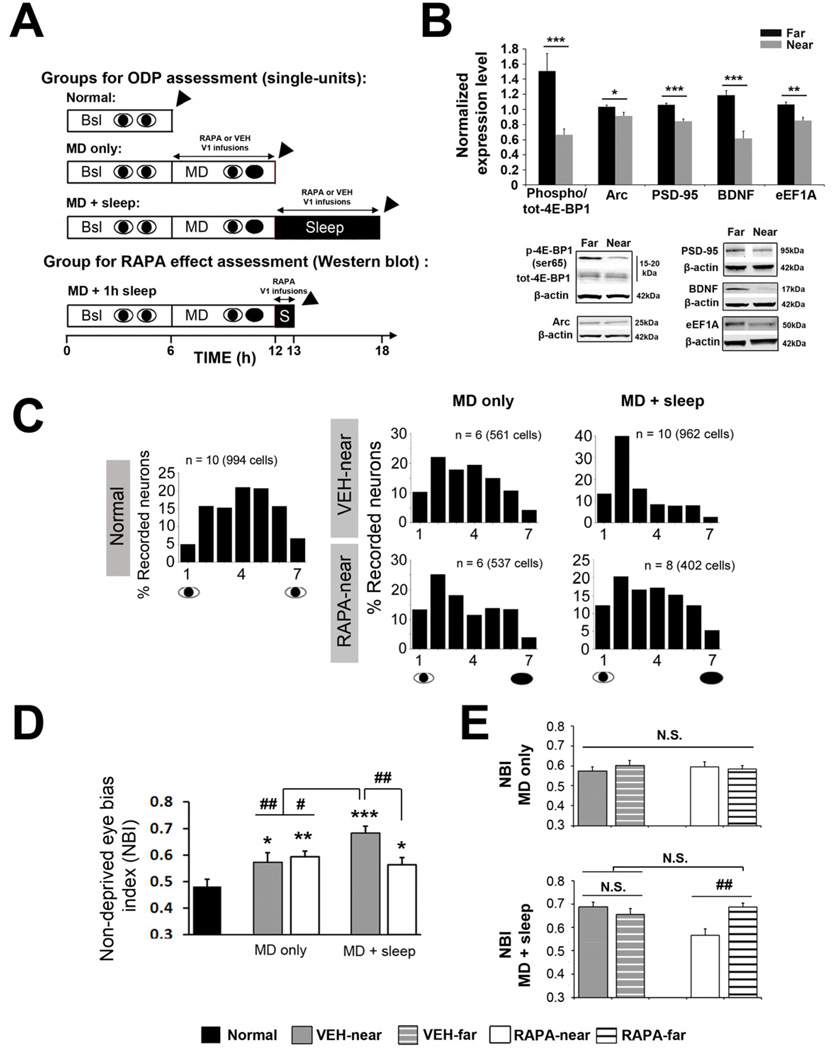
Figure 1. Cortical plasticity during sleep, but not wake, requires protein synthesis via mTORC1.
(A) Experimental designs for the main groups. V1 was continuously infused for 6 hours with vehicle (VEH) or rapamycin (RAPA) in the “MD + sleep” and “MD only” groups during sleep or wake, respectively. In an additional group, RAPA was infused during 1h of post-MD sleep to measure the efficacy of RAPA on cortical mRNA translation (“MD + 1h sleep”). Arrowhead = ODP assessment (single-units) or tissue harvesting (Western Blot).
(B) RAPA infusion during 1h post-MD sleep inhibits phosphorylation of the direct downstream target of mTORC1, 4E-BP1 and expression of several proteins implicated in synaptic plasticity (Arc, PSD-95, and BDNF) or translation regulator (eEF1A) (represented as normalized phospho/tot or expression level relative to “far” site, see Supplemental Experimental Procedures) near the infusion site (near vs. far, ***p < 0.001, **p < 0.01, *p < 0.05 t-test, n = 10 hemispheres). Representative Western blots are shown.
(C) Ocular dominance (OD) histograms near the infusion site in “MD only” and “MD + sleep” groups infused with RAPA or VEH. OD histogram from “Normal” animals is on the left. N = Number of hemispheres (total number of neurons).
(D) Quantitative scalar measure of OD. Non-deprived eye bias index (NBI) values calculated near the infusion site show that MD only induced a shift in ODP in favor of the NDE compared to animals with normal vision (Normal); this was unaffected by RAPA. A subsequent 6 hour ad lib sleep period further increases ODP in VEH infused animals but this was blocked in the RAPA group. 1-way ANOVA: F = 9.13, p < 0.001, vs. Normal: *** p < 0.001, ** p < 0.01, * p < 0.05; vs. MD + sleep VEH: ## p < 0.01, # p < 0.05, Holm-Sidak test.
(E) NBI values near the RAPA infusion sites were reduced compared to far sites only in the “MD + sleep group” (1-way ANOVA: F = 5.43, p = 0.004, ## p < 0.01, Holm-Sidak test). No differences were found between near and far sites for VEH-and RAPA-infused hemispheres after “MD only infusion” (N.S.). All values are represented as means ± s.e.m.
When infused during post-MD sleep, RAPA inhibited mTORC1 signaling as measured by phosphorylation of its direct downstream target; eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1) (Figure 1B and see Supplemental Experimental Procedures: Validation of RAPA efficacy). RAPA also reduced cortical expression of several plasticity-related proteins (ARC, BDNF, PSD-95) and the translation factor eukaryote elongation factor 1A (eEF1A) (Figure 1B). RAPA also completely abolished ODP consolidation. Micro-electrode recording and corresponding ocular dominance (OD) histograms ([16]) showed that the normal sleep-dependent shift in visual responses toward the non-deprived eye (NDE) did not occur in neurons infused with RAPA. Sleep following MD in the VEH-infused animals increased the proportion of cells more strongly activated by stimulation of the NDE (OD scores of 1–3); this did not occur in cells infused with RAPA (Figure 1C). This was confirmed using the non-deprived bias index (NBI), a weighted average of the OD histogram [9, 12, 16] (Supplemental Experimental Procedures). The NBI, which ranges from 0.5 (equal dominance of both eyes) to 1 (total dominance by the NDE), showed that the effects of sleep on ODP were abolished in RAPA infused neurons (Figure 1D, E). As shown in Table S1, RAPA prevented the normal sleep-dependent potentiation of NDE circuits and depression of deprived-eye (DE) circuits [12]. This indicated that both plastic changes require sleep-dependent protein synthesis.
These results are unlikely explained by non-specific effects of RAPA. First, RAPA is highly selective for mTORC1 rather than mTORC2 (the mTOR complex which influences cell survival and cytoskeletal organization [14]). However, because prolonged exposure to RAPA might also alter mTORC2 function [17] we inactivated a downstream mediator of mTORC2 function (Akt [18]). The selective Akt inhibitor (LY294002) had no effect on ODP consolidation (Figure S1D, E). This result is consistent with previous findings indicating that downstream targets of mTORC2 (i.e. PKC) are not essential for ODP [19]. Second, RAPA had no effect on ongoing neuronal (EEG) activity or sleep behavior and did not produce abnormalities in sensory processing in V1 neurons (Figure S1A, C and Table S1).
To further examine the role of protein synthesis in ODP consolidation, we next infused cycloheximide (CHX) during post-MD sleep. CHX disrupts the translocation-elongation step of protein synthesis and globally reduce cortical protein synthesis in vivo [20]. CHX also completely blocked ODP consolidation (n = 3 hemispheres, NBI: RAPA, 0.56 ± 0.03; CHX, 0.59 ± 0.04, p > 0.05, t-test). In contrast to RAPA, CHX produced abnormalities in cat V1 responses compared to control animals with normal binocular vision (Table S1). This may be related to neurotoxic effects of CHX which not only globally reduces protein synthesis throughout the cell but can also causes DNA damage and synthesis inhibition [21]. We therefore used RAPA for all protein-synthesis inhibition experiments.
We next determined if mTORC1 was also required for the induction of ODP during wakefulness. These animals underwent 6 hours of MD while awake combined with V1 infusion of VEH or RAPA (“MD only” in Figure 1A). Consistent with previous findings [9, 12], 6 hours of MD in the awake cat induced a small, but significant shift in OD in favor of the NDE. This form of plasticity, however, was unaffected by RAPA (Figure 1C-E). These results demonstrate that, in contrast to sleep-dependent consolidation, protein synthesis is not required for the induction of ODP during wakefulness.
Sleep promotes translation initiation
To further explore the relationship between sleep and mRNA translation, we measured sleep-dependent changes in translation regulation at the initiation (4E-BP1) and elongation (eukaryotic translation elongation factor 2; eEF2) steps. Phosphorylation of 4E-BP1 by mTORC1 relieves its inhibition on eIF4E; the first and crucial step in cap-dependent translation initiation [14]. 4E-BP1 and eEF2 have also been directly implicated in persistent forms of synaptic plasticity and memory [6, 11]. However, their roles in sleep-dependent cortical plasticity in vivo have not been explored.
Using Western blot, we measured changes in the phosphorylation state of both translation factors in synaptoneurosomal (SN: enriched in synaptic proteins, Figure 2B) and total (TOT: whole cell extract) protein fractions (see Supplemental Experimental Procedures) from V1. SN fractions were examined because rapid translation of pre-existing pools of synaptic mRNAs mediates several forms of persistent plasticity in vitro [22], but this has not been explored in sleep-dependent plasticity. Using an experimental design similar to the one used for RAPA infusion, cats were divided into the following groups: 6 hours of MD only (in awake cats), or 6 hours of MD combined with 1, 2 or 6 hours of ad lib sleep in complete darkness (Figure 2A). Control groups consisted of animals treated exactly the same except that binocular vision was left intact (noMD). This group would determine if molecular changes observed after MD were specifically due to synaptic remodeling or, instead, processes generally promoted either by wake or sleep (see Supplementary Tables S2-S4 for details on group formation)[12]. Following these procedures the animals were sacrificed and V1 cortices harvested.
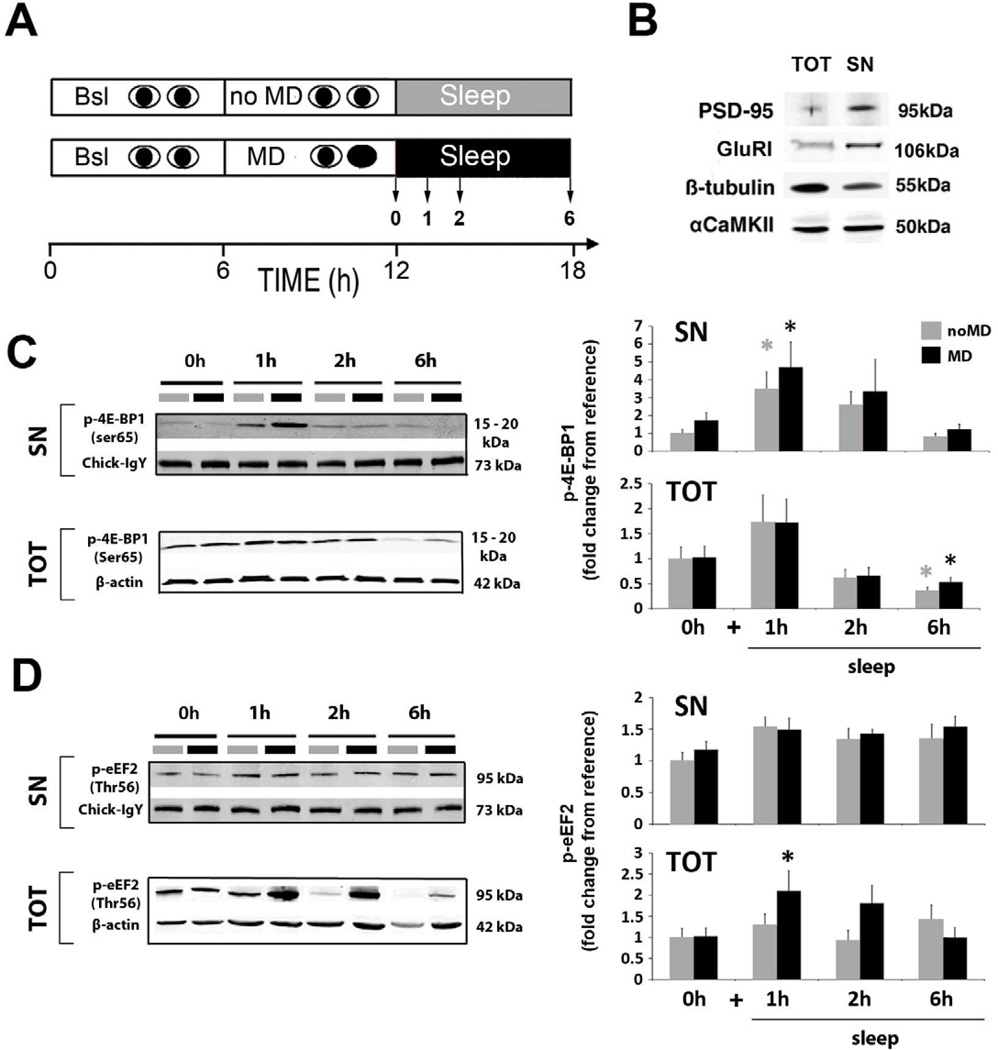
Figure 2. Sleep and waking experience affect phosphorylation of translation factors.
(A) Experimental design for the main groups: MD (black bars) or normal binocular vision (noMD control: grey bars) was combined with 0 (i.e. MD only and noMD only) or 1, 2 or 6 hours ad lib sleep. V1 was then harvested and processed separately for total mRNA extraction (see Figure 3A) and total (TOT) / synaptoneurosome (SN) protein extraction.
(B) Validation of synaptoneurosome enrichment. Representative immunoblots showing enrichment of PSD-95 and GluRI protein level [37], decreased ß-tubulin [38] and unchanged αCaMKII [39] expression in the SN preparation compared to total protein extract in the same V1 sample. Equal amounts (40μg) of protein were loaded for both fractions.
(C,D) Representative Western bots (left panels) and quantification of pooled data (right graphs) showing changes in phosphorylation state for 4E-BP1 (C) and eEF2 (D) in SN and TOT protein fractions.
(C) Translation initiation, via 4E-BP1 phosphorylation (Ser65), increased in the first hour of sleep in both noMD and MD groups, but this was only significant in the SN fraction (1-Way ANOVA: noMD groups: H = 15.33, p = 0.002; MD groups, H = 12.75, p = 0.005, * p < 0.05 Dunn’s test). In the TOT fraction there was a significant decrease in p-4E-BP1 after 6 hours of sleep compared to wake in both noMD and MD groups (1-way ANOVA: noMD groups, H = 11.69, p = 0.009; MD groups, H = 10.24, p = 0.017, * p < 0.05 Dunn’s test).
(D) Translation elongation arrest, via eEF2 phosphorylation (Thr56), was enhanced after MD+1h sleep, in the TOT fraction, but not in synaptic enriched fractions (1-Way ANOVA: MD groups, H = 11.29, p = 0.01, * p < 0.05, Dunn’s test).
Normalizing procedures are described in Supplemental Experimental Procedure. Between 8–20 samples were used per condition (Table S3 for details). All values are represented as means ± s.e.m.
We found a simultaneous increase in phosphorylation of 4E-BP1 and eEF2 after 1–2 hours of sleep. These changes appeared to be compartmentalized; increased translation initiation (i.e. increased p-4E-BP1) occurred preferentially in SN fractions (Figure 2C) and decreased protein elongation (i.e. increased peEF2) occurred preferentially in TOT protein fractions and specifically in the MD group (Figure 2D). We also found a 36.5% increase in the phosphorylation (at Ser209) of the key cap-dependent initiation factor eIF4E [14] after 1 hour of sleep in the MD group, but this did not reach significance (data not shown). When the samples shown in Figure 1B were tested for eEF2, we found no effect of RAPA on eEF2 phosphorylation (RAPA far site: 1.05 ± 0.05 vs. RAPA near site: 1.18 ± 0.11, p = 0.3, n = 10, t-test). This suggests that in the remodeling visual cortex, eEF2 is not a downstream target of mTORC1 [23] and is probably activated by another pathway. ERK for example is also activated in post-MD sleep [12] and can also phosphorylate eEF2 [24].
These results indicate that sleep promotes translation initiation (i.e. p-4E-BP1) and when V1 is triggered to remodel, this is accompanied by a decrease in protein elongation (i.e. p-eEF2). Similar co-regulation and compartmentalization of initiation and elongation factors are also reported following in vivo BDNF-mediated LTP [25] and during long-term facilitation in Aplysia [26], suggesting that this is a conserved mechanism in persistent forms of plasticity. In addition this may also enhance the translation of specific pools of mRNAs [27]. This is because decreasing the elongation step (via eEF2 phosphorylation) may shift the rate limiting step in protein synthesis away from initiation towards elongation, which increases the translation of what are termed ‘poorly initiated proteins’ (e.g. ARC) [27]. Our findings indicate that these latter events may be specifically promoted by sleep.
Translation and transcription of ARC and BDNF are divided across wake and sleep
Our results suggest that mRNAs important for plasticity are translated during sleep. We therefore examined sleep-dependent changes in the translation (and for comparison, transcription) of two mRNAs centrally involved in Hebbian and non-Hebbian forms of plasticity [28, 29] and which were reduced (as proteins) by RAPA during sleep (Figure 1B): ARC [also known as Arg3.1] and BDNF. These mRNAs are also translated in LTP and LTD protocols that trigger phosphorylation of mTORC1 and/or eEF2 [30, 31] and are important for ODP [32, 33]. We also examined the translation and transcription of αCaMKII (i.e. CAMK2A) and GLURI (i.e. GRIA); which, at a protein level, were unaffected by intracortical RAPA infusion (Figure S3A). All 4 mRNAs have also been shown to be trafficked to synapses where they remain untranslated until synaptic plasticity is induced [22]. Using Western Blot and quantitative PCR (qPCR), we measured sleep-dependent changes in the transcription and translation of ARC, BDNF, αCaMKII and GLURI from cortices used in Figure 2.
In agreement with studies in adult rodents [4, 34], ARC and BDNF mRNA transcript levels were reduced after sleep (in both noMD and MD groups, Figure 3A). In contrast to these studies, the corresponding proteins were transiently, but significantly upregulated in the first (BDNF: MD+ sleep, Figure 3C) and second hour (ARC: noMD + sleep and MD+ sleep, Figure 3B) of sleep, declining only after 6 hours (Figure 3B,C). This corresponded to the period of enhanced translation initiation in sleep (Figure 2) These findings indicate that the transcription and translation of ARC and BDNF do not always occur in parallel during sleep and that the first few hours of sleep may be a time of accelerated protein synthesis. In contrast, there were only modest changes in αCaMKII and GLURI transcription and translation across sleep and wake (Figure S3B). These latter results indicate that not all transcripts are regulated in a statedependent fashion and further suggest that only a subset of proteins is actively translated during sleep.
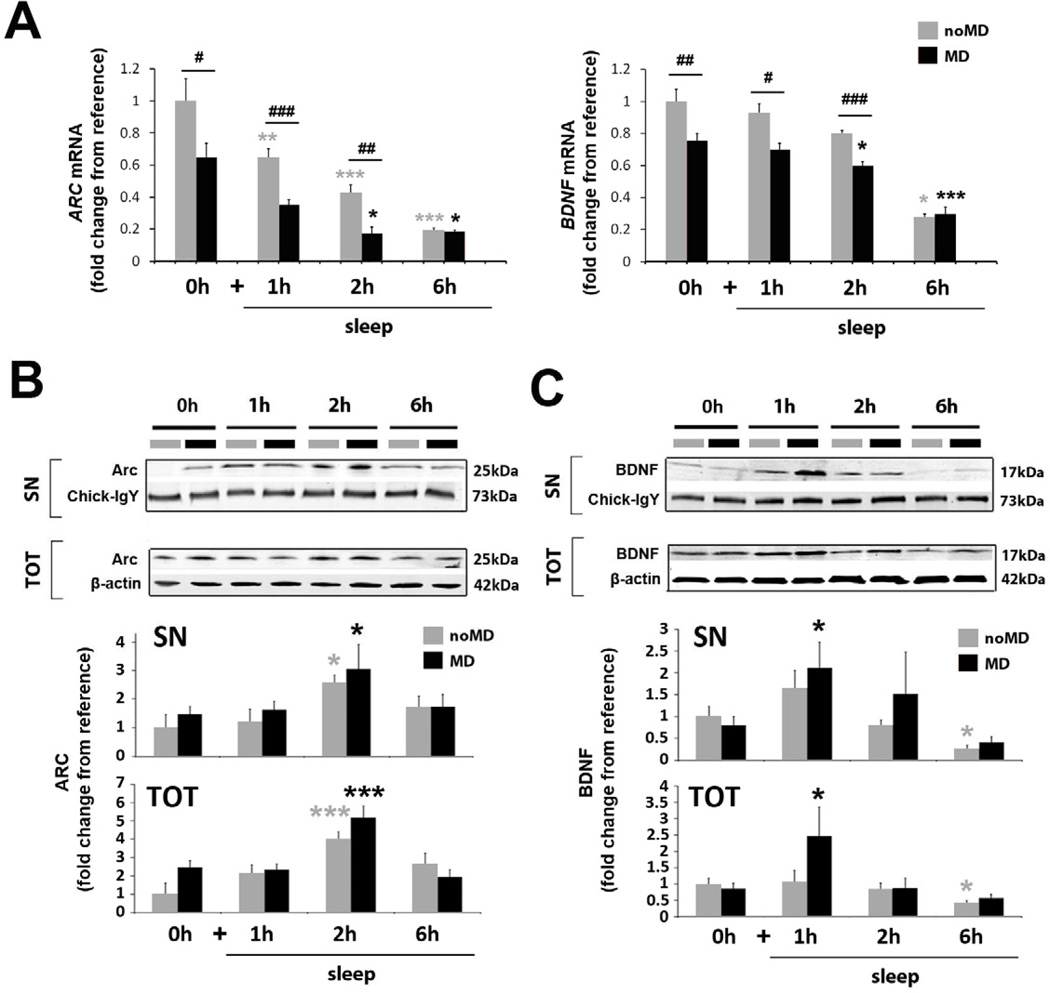
Figure 3. Sleep promotes translation, but not transcription, of ARC and BDNF.
(A) Quantitative PCR for ARC and BDNF (experimental designs shown in Figure 2A). ARC and BDNF expression are reduced during sleep in both noMD and MD animals compared to wake (1-Way ANOVA: noMD groups, H = 24.60, p < 0.001 and H = 21.64, p < 0.001 for ARC and BDNF respectively; MD groups, H = 25.89, p < 0.001 and F = 17.63, p < 0.001 for ARC and BDNF respectively; *** p < 0.001, ** p < 0.01, * p < 0.05, Holm-Sidak or Dunn’s test where appropriate). ARC and BDNF expression are reduced by MD compared to noMD animals during wakefulness and in hours 1 and 2 of the ad lib sleep period (### p < 0.001, ## p < 0.01, #p < 0.05; t-test or Mann-Whitney test where appropriate).
(B, C) Representative Western blots and quantification of pooled data for ARC (B) and BDNF (C) in SN and TOT protein fractions.
(B) ARC protein in both SN and TOT fractions significantly increased in the second hour of sleep (1-way ANOVA, noMD group: TOT: F = 5.17, p = 0.005, SN: H = 8.97, p = 0.003, MD group: TOT: F = 10.17, p ≤ 0.001, SN: H = 9.2, p = 0.34, *** p < 0.001, * p < 0.05, Holm-Sidak or Dunn’s test where appropriate).
(C) BDNF protein in both SN and TOT fractions significantly increased in the first hour of post-MD sleep (1-way ANOVA, TOT: H = 12.08, p = 0.007, SN: H = 12.45, p = 0.006, * p < 0.05, Dunn’s test), and declined after 6h of sleep in both groups-significantly in the noMD group relative to waking (1-way ANOVA, TOT: H = 8.14, p = 0.043, SN: H = 15.87, p = 0.001, * p < 0.05, Dunn’s test).
Normalizing procedures are described in Supplemental Experimental Procedures. Between 8–16 samples were used per condition (Table S4 for details). All values are represented as means ± s.e.m.
Translational events are promoted by sleep; transcription by patterned vision during wakefulness
We next determined if the reduction in mRNAs observed after sleep was merely an indirect effect of reducing visual input to V1 rather than an active repression of transcription. An indirect effect was suggested by the observation that ARC and BDNF mRNAs were reduced by MD in awake animals (relative to noMD animals, Figure 3A). Because MD reduces visual drive to V1 and reducing visual input decreases mRNA transcription in V1 [35], we hypothesized that decreases observed after sleep might also reflect a decrease in visual input during sleep. To explore this possibility, we examined the effects of 6 hours of binocular deprivation (BD only, Figure 4A and Supplemental Experimental Procedures) in awake cats, which does not trigger ODP [8], on ARC and BDNF mRNAs. We also examined FOS (c-fos) mRNA, as expression of this immediate early gene is a widely-used marker of activity-dependent transcription [36] and because FOS also decreases during sleep (Figure S4A). Consistent with our hypothesis, 6 hours of BD in the awake cat reduced transcription of ARC, BDNF and FOS to a level observed after 6 hours of sleep (Figures 4B and S4B). This suggests that sleep-related decreases in ARC, BDNF and FOS (at least within the visual cortex) may be passive epiphenomena of sleep unrelated to experience-dependent plasticity.
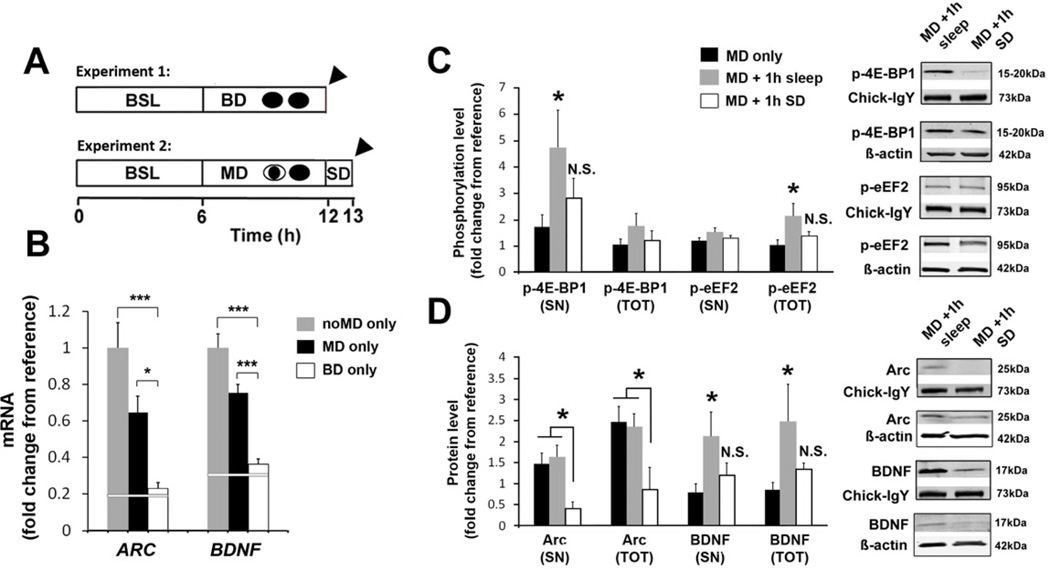
Figure 4. Visual experience triggers mRNA transcription but translation requires sleep.
(A) Experimental designs. In experiment 1, the role of patterned visual input in ARC and BDNF transcription was assessed. Instead of MD, animals underwent 6 hours of binocular deprivation (BD only) while awake. In experiment 2, the necessity of sleep in cortical protein synthesis was assessed. The MD period was followed by 1hour of sleep deprivation (SD) in complete darkness (to prevent any additional visual input) prior to sacrifice (MD+1h SD). Arrowheads = V1 tissue harvest for mRNA (BD) or protein (SN/TOT) extraction (MD + 1h SD).
(B) ARC and BDNF mRNA levels were reduced in the BD only group compared to the MD only and noMD only group (values are reproduced from Figure 3) (1-way ANOVA, ARC: F = 7.67, p = 0.002; BDNF: F = 18.57, p< 0.001; *** p< 0.001, * p< 0.05, Holm-Sidak test). White reference line represents mean values from animals that slept 6 hours after the waking period (averaged from the noMD+6h sleep and MD+6h sleep groups shown in Figure 3).
(C) Phosphorylation of 4E-BP1 in SN and eEF2 in TOT fraction is prevented by SD (white bars) (1-way ANOVA, p-4E-BP1-SN: H = 8.76, p = 0.013; p-eEF2-TOT: H = 8.29, p = 0.016, * p < 0.05 Dunn’s test).
(D) Increases in SN and TOT BDNF protein observed after sleep do not occur after SD (white bars) (1-way ANOVA, BDNF-SN: H = 6.93, p = 0.031; BDNF-TOT: H = 8.39, p = 0.015, * p < 0.05 Dunn’s test). ARC protein in TOT and SN fractions was also decreased in the MD + 1h SD group compared to MD only and MD+1h sleep (1-way ANOVA, TOT: F = 3.56, p = 0.041; SN: F = 3.74, p = 0.036, * p < 0.05 Holm-Sidak test).
For (C) and (D), MD only group and MD+1 h sleep values are reproduced from Figures 2 and 3. Next to each graph are representative corresponding Western blots. ARC and BDNF mRNA levels were not significantly different in the MD + 1h SD group to levels observed after 1h of sleep (Figure S4C).
Normalizing procedures for changes in mRNA and protein expression (represented as means ± s.e.m.) are described in Supplemental Experimental Procedures. Between 6–16 samples were used per condition (see Tables S3 and S4 for details). All values are represented as means ± s.e.m.
We then determined if the protein changes shown in Figures 2 and 3 were sleep-dependent or merely time-dependent. We found that the largest changes in proteins observed after sleep did not occur when animals were instead kept awake 1 hour after MD (‘MD+1h SD’, Figure 4A,C,D). Increases in eEF2 and SN 4E-BP1 phosphorylation observed after sleep did not occur in the MD+1h SD group (Figure 4C). This was also true for BDNF, where increases in TOT and SN fractions were no longer significant compared to wakefulness (Figure 4D). 1 hour of SD also significantly decreased ARC protein amounts compared to both the MD+1h sleep and MD only group (Figure 4C). Because eEF1A and PSD-95 were also reduced by RAPA during post-MD sleep (Figure 1B), we examined a subset of these samples for these proteins. While there were no differences between MD and noMD conditions, when grouped together as ‘wake only’ (MD only+ noMD only), “sleep” (MD +1h sleep + noMD+1h sleep), and ‘sleep-deprived’ (MD+1h SD), there was a significant increase (80.34%) in PSD-95 synaptic content after sleep, which was significantly reduced by 55.4% in the ‘sleep-deprived’ group (Figure S4D).There was no effect of vigilance state on eEIF1A synaptic content. Taken together, these results indicated that protein changes observed after sleep in Figures 2 and 3 were sleep-dependent.
In summary, we show that sleep promotes cortical mRNA translation and interruption of this process prevents the consolidation of a canonical form of cortical plasticity in vivo. These findings are novel for the following reasons. First, although protein synthesis has been previously shown to be necessary for ODP [20], we now show that this process specifically occurs during sleep. Second, while it is known that sleep is associated with heightened brain protein synthesis [2, 3, 5], ours is the first demonstration that this serves an important cortical function (i.e. consolidating experience). Third, our findings suggest that the induction of ODP during wakefulness and its subsequent consolidation during sleep involve different cellular mechanisms. Protein synthesis is essential for plastic changes that occur during sleep, but unnecessary for plasticity induced during wake. Lastly, our findings demonstrate that while experience is required for the transcription of key plasticity-related mRNAs, their translation into protein requires sleep. This may represent a sleep-dependent mechanism that converts labile plastic changes into more permanent forms.
EXPERIMENTAL PROCEDURES
Intracortical infusion experiments and drugs
All cats underwent a standard design to induce ODP as described previously [9]. Drugs (rapamycin [RAPA, 150μM]; cycloheximide [CHX, 6mM] or LY294002 [LY29, 5mM]) and Vehicle (VEH) were infused intracortically (@ 0.25μl/min) in V1 by mean of an indwelling cannula. See Table S2 and Supplemental Experimental Procedures for details on group formation and single-unit recordings.
Sleep analyses
Polysomnography was used to verify that animals in all groups were equally awake during the enforced waking period and equally asleep during the subsequent ad lib sleep period. See Figures S1 and S2 and Supplemental Experimental Procedures for additional details on sleep analysis.
Protein and mRNA measures
Control (“no MD”) or animal undergoing MD were sacrificed after 0, 1, 2 or 6 h of sleep (Figure 2A). V1 was rapidly removed bilaterally, immediately frozen on dry ice, and stored at −80°C until use. See Supplementary Experimental Procedures and Tables S2–4 for details on experimental groups and biochemistry techniques and analysis.
HIGHLIGHTS.
mTORC1 inhibition selectively impairs sleep-dependent cortical plasticity
Sleep promotes translation factors regulation.
ARC and BDNF transcription and translation are divided across wake and sleep
Transcription occurs in response to waking experience but translation requires sleep.
Supplementary Material
ACKNOWLEDGMENTS
We thank D. Raizen and J.J. Remy for helpful comments on the manuscript. We thank members of the Pack laboratory (University of Pennsylvania Center for Sleep and Respiratory Neurobiology) for assistance with biochemical experiences. We especially thank M. Mackiewicz and L. Peixoto for assistance with qPCR and comparative genomics, respectively. We also thank C. Cirelli and G. Tononi for advice with the synaptoneurosomal protein preparation. The authors are grateful to C.J. Richard and C. Broussard for providing data-analysis software and M. Haskins (University of Pennsylvania Veterinary Medicine) for providing animals. This work was supported by the University of Pennsylvania, the National Institutes of Health (R01-EY 019002 to M.G.F., F32-EY017766 to S.J.A, T32-GM07517 to A.W. and M.C.D, and F31-NS067935 to M. C. D), the Pickwick postdoctoral fellowships (to J.S.) from the National Sleep Foundation, and a L'Oréal USA For Women in Science fellowship (to S.J.A).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frank MG, Benington JH. The role of sleep in memory consolidation and brain plasticity: dream or reality? Neuroscientist. 2006;12:477–488. doi: 10.1177/1073858406293552. [DOI] [PubMed] [Google Scholar]
- 2.Ramm P, Smith CT. Rates of cerebral protein synthesis are linked to slow wave sleep in the rat. Physiol. Behav. 1990;48:749–753. doi: 10.1016/0031-9384(90)90220-x. [DOI] [PubMed] [Google Scholar]
- 3.Nakanishi H, Sun Y, Nakamura RK, Mori K, Ito M, Suda S, Namba H, Storch FI, Dang TP, Mendelson TW, et al. Positive correlations between cerebral protein synthesis rates and deep sleep in Macaca mulatta. Eur. J. Neurosci. 1997;9:271–279. doi: 10.1111/j.1460-9568.1997.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 4.Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- 5.Mackiewicz M, Shockley KR, Romer MA, Galante RJ, Zimmerman JE, Naidoo N, Baldwin DA, Jensen ST, Churchill GA, Pack AI. Macromolecule biosynthesis: a key function of sleep. Physiol. Genomics. 2007;31:441–457. doi: 10.1152/physiolgenomics.00275.2006. [DOI] [PubMed] [Google Scholar]
- 6.Sossin WS, Lacaille J-C. Mechanisms of translational regulation in synaptic plasticity. Curr. Opin. Neurobiol. 2010;20:450–456. doi: 10.1016/j.conb.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez PJ, Abel T. The role of protein synthesis in memory consolidation: progress amid decades of debate. Neurobiol Learn Mem. 2008;89:293–311. doi: 10.1016/j.nlm.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J. Neurophysiol. 1965;28:1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- 9.Frank MG, Issa NP, Stryker MP. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30:275–287. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- 10.Tropea D, Van Wart A, Sur M. Molecular mechanisms of experience-dependent plasticity in visual cortex. Philos. Trans. R. Soc. Lond. B, Biol. Sci. 2009;364:341–355. doi: 10.1098/rstb.2008.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aton SJ, Seibt J, Dumoulin M, Jha SK, Steinmetz N, Coleman T, Naidoo N, Frank MG. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron. 2009;61:454–466. doi: 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou H, Luo Y, Huang S. Updates of mTOR inhibitors. Anticancer Agents Med Chem. 2010;10:571–581. doi: 10.2174/187152010793498663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 15.Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jha SK, Jones BE, Coleman T, Steinmetz N, Law C-T, Griffin G, Hawk J, Dabbish N, Kalatsky VA, Frank MG. Sleep-dependent plasticity requires cortical activity. J. Neurosci. 2005;25:9266–9274. doi: 10.1523/JNEUROSCI.2722-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarbassov DD, Ali SM, Sengupta S, Sheen J-H, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 18.Hresko RC, Mueckler M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J. Biol. Chem. 2005;280:40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 19.Daw NW, Beaver CJ. Developmental changes and ocular dominance plasticity in the visual cortex. Keio J Med. 2001;50:192–197. doi: 10.2302/kjm.50.192. [DOI] [PubMed] [Google Scholar]
- 20.Taha S, Stryker MP. Rapid ocular dominance plasticity requires cortical but not geniculate protein synthesis. Neuron. 2002;34:425–436. doi: 10.1016/s0896-6273(02)00673-6. [DOI] [PubMed] [Google Scholar]
- 21.Basić-Zaninović T, Papes D, Franekić J. Cycloheximide genotoxicity in in vitro and in vivo test systems. Mutat. Res. 1991;263:203–210. doi: 10.1016/0165-7992(91)90002-l. [DOI] [PubMed] [Google Scholar]
- 22.Wang DO, Martin KC, Zukin RS. Spatially restricting gene expression by local translation at synapses. Trends Neurosci. 2010;33:173–182. doi: 10.1016/j.tins.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Browne GJ, Proud CG. A novel mTOR-regulated phosphorylation site in elongation factor 2 kinase modulates the activity of the kinase and its binding to calmodulin. Mol. Cell. Biol. 2004;24:2986–2997. doi: 10.1128/MCB.24.7.2986-2997.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO. J. 2001;20:4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanhema T, Dagestad G, Panja D, Tiron A, Messaoudi E, Håvik B, Ying S-W, Nairn AC, Sonenberg N, Bramham CR. Dual regulation of translation initiation and peptide chain elongation during BDNF-induced LTP in vivo: evidence for compartment-specific translation control. J. Neurochem. 2006;99:1328–1337. doi: 10.1111/j.1471-4159.2006.04158.x. [DOI] [PubMed] [Google Scholar]
- 26.Weatherill DB, McCamphill PK, Pethoukov E, Dunn TW, Fan X, Sossin WS. Compartment-specific, differential regulation of eukaryotic elongation factor 2 and its kinase within Aplysia sensory neurons. J. Neurochem. 2011;117:841–855. doi: 10.1111/j.1471-4159.2011.07251.x. [DOI] [PubMed] [Google Scholar]
- 27.Belelovsky K, Elkobi A, Kaphzan H, Nairn AC, Rosenblum K. A molecular switch for translational control in taste memory consolidation. Eur. J. Neurosci. 2005;22:2560–2568. doi: 10.1111/j.1460-9568.2005.04428.x. [DOI] [PubMed] [Google Scholar]
- 28.Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Front Mol Neurosci. 2010;3:1. doi: 10.3389/neuro.02.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat. Neurosci. 2011;14:279–284. doi: 10.1038/nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verpelli C, Piccoli G, Zanchi A, Gardoni F, Huang K, Brambilla D, Di Luca M, Battaglioli E, Sala C. Synaptic activity controls dendritic spine morphology by modulating eEF2-dependent BDNF synthesis. J. Neurosci. 2010;30:5830–5842. doi: 10.1523/JNEUROSCI.0119-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park S, Park JM, Kim S, Kim J-A, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCurry CL, Shepherd JD, Tropea D, Wang KH, Bear MF, Sur M. Loss of Arc renders the visual cortex impervious to the effects of sensory experience or deprivation. Nat. Neurosci. 2010;13:450–457. doi: 10.1038/nn.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galuske RA, Kim DS, Castrén E, Singer W. Differential effects of neurotrophins on ocular dominance plasticity in developing and adult cat visual cortex. Eur. J. Neurosci. 2000;12:3315–3330. doi: 10.1046/j.1460-9568.2000.00213.x. [DOI] [PubMed] [Google Scholar]
- 34.Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J. Neurosci. 2000;20:9187–9194. doi: 10.1523/JNEUROSCI.20-24-09187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosen KM, McCormack MA, Villa-Komaroff L, Mower GD. Brief visual experience induces immediate early gene expression in the cat visual cortex. Proc. Natl. Acad. Sci. U.S.A. 1992;89:5437–5441. doi: 10.1073/pnas.89.12.5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu. Rev. Cell Dev. Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okabe S. Molecular anatomy of the postsynaptic density. Mol. Cell. Neurosci. 2007;34:503–518. doi: 10.1016/j.mcn.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Cohen RS, Blomberg F, Berzins K, Siekevitz P. The structure of postsynaptic densities isolated from dog cerebral cortex. I. Overall morphology and protein composition. J. Cell Biol. 1977;74:181–203. doi: 10.1083/jcb.74.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villasana LE, Klann E, Tejada-Simon MV. Rapid isolation of synaptoneurosomes and postsynaptic densities from adult mouse hippocampus. J. Neurosci. Methods. 2006;158:30–36. doi: 10.1016/j.jneumeth.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.