Abstract
Widespread use of 7-valent pneumococcal conjugate vaccine (PCV7) has led to significant reductions in disease while changing pneumococcal population dynamics via herd immunity and serotype replacement. We performed multilocus sequence typing (MLST) on 590 pneumococcal isolates obtained during the American Indian clinical trial of PCV7, in which communities were randomized for eligible children to receive either PCV7 or a meningococcal conjugate vaccine (MCV). Sequence types (STs) were analyzed to determine the impact of the vaccine on pneumococcal population structure and to assess the possible impact of pneumococcal genetic background on vaccine effects. One hundred forty-three STs were obtained, the most frequent being ST199, the only one that included vaccine serotypes (VTs), non–vaccine-associated nonvaccine serotypes (NVA/NVTs), and vaccine-associated serotypes (VATs). Serotype replacement observed in the PCV communities was due to a diverse population of STs, most of which also existed in the MCV communities. Possible capsular switching to create novel ST associations with NVA/NVTs was detected only once. Reductions in VTs and changes in VATs in PCV communities did not show evidence of variation by ST, after accounting for lower vaccine effectiveness against serotype 19F. These observations suggest the hypothesis that the vaccine acts as a “serotype filter”: its effect on a particular strain can be predicted on the basis of the serotype of the strain, with little effect of genetic background (as assessed by MLST) over and above capsule. If sustained, such patterns provide some cause for optimism that rapid evolution of PCV escape strains with drug resistance or high virulence is unlikely.
Pneumococcal conjugate vaccine (PCV) prevents invasive pneumococcal disease, pneumonia, and even otitis media (OM) caused by capsular serotypes included in the vaccine (vaccine serotypes [VTs]) [1–4]. Such vaccines reduce nasopharyngeal (NP) carriage of VT Streptococcus pneumoniae in vaccinated individuals and their contacts [5, 6]. Consequently, VT invasive disease has declined even in age groups not targeted for vaccination [7].
These benefits of PCV have occurred despite the fact that, in clinical trials, vaccination reduces carriage of VT S. pneumoniae by only ~50% [5], that vaccine efficacy and effectiveness vary by serotype [1, 2, 4], and that serotype replacement has occurred, whereby the carriage of serotypes not included in the vaccine and not in the same serogroup as a VT (non–vaccine-associated nonvaccine serotypes [NVA/NVTs]) increases in prevalence [5], as does the incidence of NVA/NVT disease [7]. Mechanistic explanations of these potential limitations of the effectiveness of conjugate vaccine remain incomplete. In particular, the role played by bacterial genotype in each of these phenomena is not fully understood. By analyzing changes in the pneumococcal population in an area in which 7-valent PCV (PCV7) was introduced in comparison to those in the population in an area in which a control vaccine was introduced during the same period, we sought to assess the impact of PCV on the genetic composition of the pneumococcal population.
The effectiveness of PCV7 against NP colonization by particular S. pneumoniae strains depends on the capsular serotype: VT pneumococci are inhibited (although 19F is less inhibited than other VTs), most vaccine-associated serotypes (VAT; i.e., those not included in the vaccine but part of the same serogroup as a VT) are also inhibited, and NVA/NVT pneumococci are promoted by the vaccine’s effectiveness against competing VT pneumococci. Besides the capsule, many other variable loci are implicated in virulence and in interaction with the immune system. Genetic variation at such loci may play a role in determining the effectiveness of PCV7 vaccination.
The American Indian trial of PCV7, conducted in Navajo and White Mountain Apache communities in the Southwest of the United States between April 1997 and October 2000, has been described elsewhere [8, 9]. This was a group-randomized controlled efficacy trial of a PCV7 (PnCRM7; Wyeth Vaccines), with a study of NP colonization nested within the trial [6, 10]. Herd immunity effects, as well as serotype replacement, depend on the presence of a high proportion of immunized individuals within a community [11, 12]. Hence, the cluster-randomized design of this trial offered an excellent setting for studying these phenomena [13], in that it simulated the large-scale use of vaccine within a community that occurs in routine use settings while offering a contemporaneous control group that is not present in routine use settings.
We sought to characterize the relationship between genetic variation in pneumococci and the effects of conjugate vaccination. We addressed the following questions about pneumococcal population biology. First, how did the pneumococcal population change to generate serotype replacement? Specifically, did the NVA/NVT pneumococci that increased in frequency in PCV7-vaccinated communities reflect the full diversity of NVA/NVT strains existing in control communities or a limited subset of clones? Moreover, did serotype replacement mainly involve expansion of existing NVA/NVT clones, or was capsular switching—acquisition of novel (in this case, NVA/NVT) capsular serotypes by previously VT pneumococcal clones—an important contributor to serotype replacement [14]? Second, was the genetic background of a strain—other than capsular serotype—associated with the ability of PnCRM7 to inhibit colonization?
METHODS
Study population
The PnCRM7 study was conducted among Navajo and White Mountain Apache children <2 years of age. The Navajo and White Mountain Apache reservations are located in the Southwest of the United States. Depending on the randomization unit in which the child lived, enrolled children received either PnCRM7 vaccine (“PCV communities”) or Neisseria meningitidis group C protein conjugate vaccine (MnCC; Wyeth Vaccines) as a control vaccine (“MCV communities”). PnCRM7 contains 2 mg each of serotypes 4, 9V, 14, 19F, and 23F polysaccharides; 2 µg of serotype 18C oligosaccharide; and 4 µg of serotype 6B polysaccharide, each conjugated to the protein CRM197, a nontoxic variant of diphtheria toxin. MnCC contains group C oligosaccharides coupled to CRM197 by reductive amination. Each dose of study vaccine also contained 0.5 mg of aluminum phosphate as an adjuvant. Infants who were enrolled between 6 weeks and 7 months of age received 3 doses of vaccine 2 months apart and a booster dose at 12–15 months of age. A subset of efficacy trial participants was concurrently enrolled in a carriage study to evaluate the impact of PnCRM7 on carriage. NP swabs were collected from efficacy trial participants at ~7, 12–15, and 18–24 months of age. The carriage study took place between February 1998 and May 2000.
The parent study was approved by the institutional review boards of Johns Hopkins University, the Centers for Disease Control and Prevention (CDC), the Navajo Nation, the Phoenix Area Indian Health Service, and the National Indian Health Service. Tribal approval was given by the Navajo Nation and the White Mountain Apache tribe. Parents or guardians of study children signed a written informed consent document after reading the document or having the consent document read and explained to them in English or in their native language. The analyses reported here were further approved by the institutional review board of the Harvard School of Public Health.
Pneumococcal isolates
Trained nurses and field workers collected NP specimens. A small, flexible calcium alginate swab was inserted through the nostril until resistance was encountered. The swabs were inoculated into STGG transport medium (consisting of skim milk, tryptone, glucose, and glycerin) [15], frozen at −70°C, and transported to the CDC for pneumococcal culture, isolation, and serotyping. Specimens were streaked onto gentamicin–tryptic soy 5% sheep blood agar plates (Becton Dickinson) and incubated overnight at 37°C in 5% CO2. Phenotypic characteristics (morphology and α-hemolysis) were used for the presumptive identification of pneumococci. Pneumococcal identification was confirmed by optochin susceptibility and bile solubility assays. Pneumococci were serotyped by a novel immunoblot method to detect multiple pneumococcal serotypes in NP specimens [16].
Invasive disease isolates from Navajo and White Mountain Apache of all ages were collected throughout the study period. Isolates from spontaneously draining OM episodes were also collected. These pneumococcal isolates were collected and serotyped at the Arctic Investigations Program of the CDC in Anchorage, Alaska.
Selection of isolates for analysis
The last 400 isolates from the nested NP study, excluding strains from follow-up visits to avoid multiple isolations from the same carriage episode [10], were chosen for the present analysis. Of these, 375 were successfully reisolated from frozen specimens. Selection of NP isolates from the end of the trial ensured that these isolates reflected a population with high vaccine coverage; during the period from March 1999 to April 2000, when isolates were collected, the proportion of children in specific community units who had received 3 doses by 1 year of age ranged from 44% to 54%. When multiple serotypes were isolated from a subject’s specimen, we included in the present analysis the isolate listed first in the database.
Invasive disease and OM isolates were collected regardless of the age or vaccination status of the patient. All invasive disease isolates collected from June 1997 through February 2001 and all OM isolates collected from January 1998 through August 2001 from the study communities were included in our analysis.
Multilocus sequence typing (MLST)
All isolates were subjected to MLST, using 2 forward and 2 reverse sequences for every gene. The term “sequence type” (ST) is used throughout to indicate a distinct MLST pattern. The allelic profile information has been submitted to the MLST database at http://spneumoniae.mlst.net. Relatedness analyses and comparisons between MCV and PCV communities were performed and displayed using eBURST (version 3; http://eburst.mlst.net). To ensure against contamination or other errors in interlaboratory transfer, the serotype (determined before the present study commenced) and ST (determined at the end of the chain of custody) were checked against the MLST database; any strain whose ST was in the database but was not associated in the database with that ST was reserotyped to confirm identity. Strains with an ST not in the database were reserotyped to exclude such errors.
Statistical analysis
To determine whether PCV use preferentially inhibited some STs more than others, random-effects logistic regression was applied to all VT isolates, with isolation from a PCV community as the outcome (vs. isolation from an MCV community) and ST as a random effect. The hypothesis of a nonzero variance between STs in their tendency to appear in PCV (vs. MCV) communities was tested. Equivalently, to test for evidence that particular STs were more strongly implicated in serotype replacement, the same procedure was applied to NVA/NVT isolates. P <.05 for the null hypothesis of zero variance was taken as evidence of heterogeneity.
RESULTS
Isolates analyzed and overall population structure
Five hundred ninety isolates were subjected to MLST, of which 375 (64%) were from NP carriage, 89 (14%) were from OM, and 126 (22%) were from invasive disease. The characteristics of these isolates are shown in table 1.
Table 1.
No. of pneumococcal isolates analyzed, by site of isolation, community of vaccine randomization, and serotype.
| Community of randomization |
NP carriage |
Otitis media |
Invasive disease |
All |
|---|---|---|---|---|
| MCV | ||||
| NVA/NVT | 61 | 25 | 22 | 108 |
| VT | 64 | 19 | 34 | 117 |
| VAT | 53 | 15 | 10 | 78 |
| Total | 178 | 59 | 66 | 303 |
| PCV | ||||
| NVA/NVT | 115 | 14 | 22 | 151 |
| VT | 27 | 13 | 29 | 69 |
| VAT | 55 | 3 | 9 | 67 |
| Total | 197 | 30 | 60 | 287 |
| All | 375 | 89 | 126 | 590 |
NOTE. Specific sequence types for each cell are given in table 2. MCV, meningococcal conjugate vaccine; NP, nasopharyngeal;NVA/NVT, non–vaccine-associated nonvaccine serotype; PCV, pneumococcal conjugate vaccine; VAT, vaccine-associated serotype; VT, vaccine serotype.
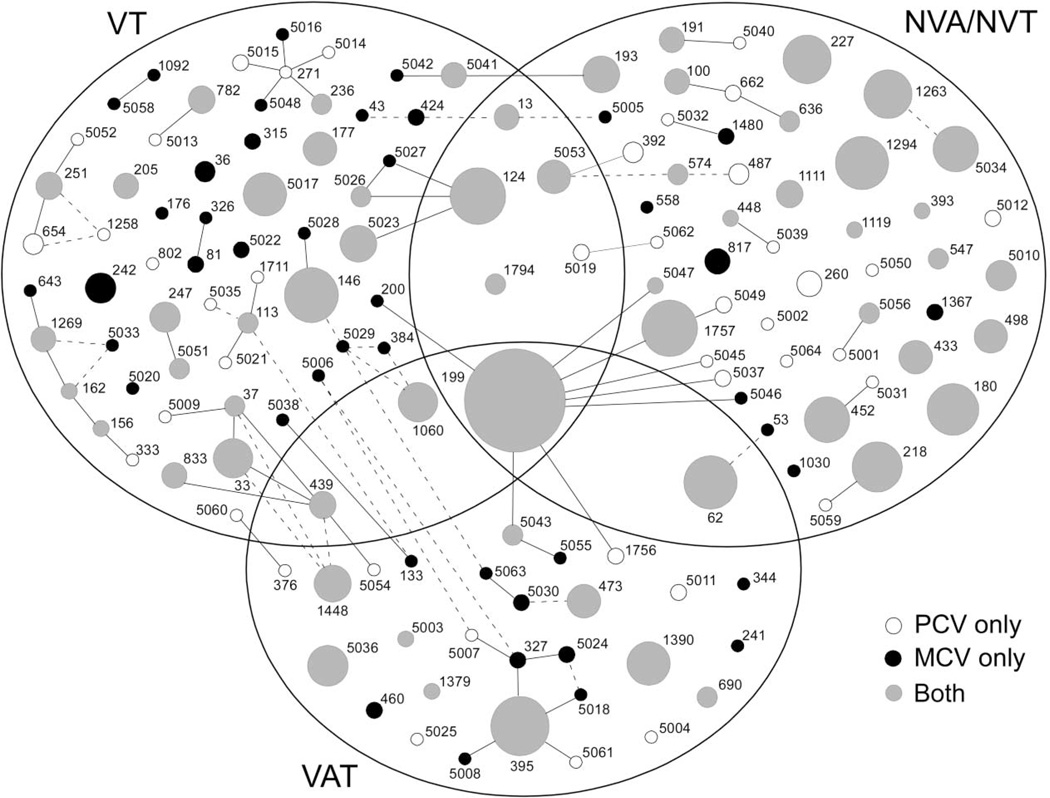
These isolates comprised 143 STs, whose distribution by community (MCV vs. PCV) and serotype (VT, NVA/NVT, and VAT) is shown in figure 1. The most frequent ST was ST199, which also had several single-locus variants in the collection; 59 STs were represented only once among the isolates (10% of isolates). For 59 other STs, isolates were recovered from both PCV and MCV communities; this accounted for 79% of all isolates and for 70% of all STs that were recovered more than once. In summary, a genetically diverse group of isolates was recovered from both MCV and PCV communities, and most STs obtained 2 or more times in our sample were recovered from both communities.
Figure 1.
Representation of the pneumococcal population structure in this study (all 590 isolates from carriage, otitis media, and invasive disease), modified from the output of eBURST (version 3). Each circle represents a single sequence type (ST), with the area proportional to the no. of isolates of that type. The Venn diagram indicates whether a given ST was found in vaccine serotype (VT), vaccine-associated serotype (VAT), or non–vaccine-associated nonvaccine serotype (NVA/NVT) isolates or in >1 of these types (these serotypes refer to the strains in our study, not to all known serotypes associated with the ST). White circles represent STs found only in pneumococcal conjugate vaccine (PCV) communities, black circles represent STs found only in meningococcal conjugate vaccine (MCV) communities, and gray circles represent STs found in both types of communities. Solid lines represent single-locus variants, and dashed lines represent double-locus variants not already connected through single-locus variants.
Mechanisms of serotype replacement: primarily outgrowth of STs also present in MCV communities
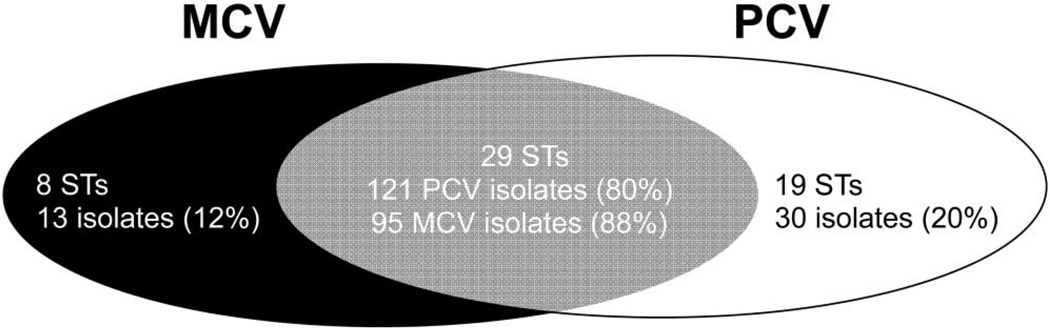
Most STs are associated with 1 or a few serotypes [17–19]. To determine whether serotype replacement was mainly the result of expansion of existing STs or the result of the appearance of novel STs, we classified each of the 259 NVA/NVT isolates by whether they belonged to STs that were present in MCV communities only, PCV communities only, or both (figure 2). Eighty percent of NVA/NVT isolates found in PCV communities belonged to STs that were also found in MCV communities. This indicates that, in the presence of the PCV, there is an expansion of STs that are also present in communities not exposed to PCV (and, by inference, were present in the PCV communities before vaccine exposure). Thirty (20%) of the NVA/NVT isolates from PCV communities did not match the clonal group of any isolates analyzed from the MCV communities; these 30 isolates comprised 19 different STs, the most frequent of which consisted of 4 isolates. By comparison, 95 (88%) of the NVA/NVT isolates in MCV communities belonged to STs also found in PCV communities. Thus, we conclude that most serotype replacement (NVA/NVTs isolated in PCV communities) resulted from expansion of STs also present in MCV communities.
Figure 2.
Analysis of serotype replacement. Serotype replacement (non–vaccine-associated nonvaccine serotype [NVA/NVT] isolates in pneumococcal conjugate vaccine [PCV] communities) was largely due to expansion of sequence types (STs) that already existed in control (meningococcal conjugate vaccine [MCV]) communities. The figure shows the distribution of STs among NVA/NVT isolates in PCV and MCV communities; 80% of NVA/NVT isolates in PCV communities and 88% of NVA/NVT isolates in MCV communities were of STs that were found in both communities.
Overall, there were 48 STs represented in NVA/NVT strains from PCV communities, more than the 37 seen in MCV communities. Because pneumococcal populations in each community were incompletely sampled (i.e., not all strains circulating in the community were included in the parent study, and not all strains in the parent study were subjected to MLST), the proportion of isolates unique to one community or another will tend to be overestimated (because some strains may have “matches” in the other community that are not seen in the study).
Mechanisms of serotype replacement: one isolate that may indicate PCV-induced capsular switching
We searched our MLST results for evidence of NVA/NVT isolates that were present in PCV communities but not MCV communities and that belonged to STs associated with VTs in either PCV or MCV communities. Such isolates would be expected if selection pressure from PCV led to the selection of previously VT strains that had acquired genetic material for biosynthesis of NVA/NVT capsules. Of the 590 pneumococcal isolates, only 1 was found with these characteristics, a serotype 35F strain of ST124. Seventeen (3%) other isolates in our collection also belonged to clonal group 124, and all of these isolates were serotype 14, the only serotype previously reported in the MLST database as being associated with clonal group 124.
Mechanisms of serotype replacement: no evidence of preferential outgrowth of particular STs
Having determined that most NVA/NVT pneumococci colonizing study participants in PCV communities were members of STs that were also found in MCV communities, we searched for evidence that particular STs may have preferentially expanded in the PCV communities— that is, that vaccination particularly favored specific STs over others. There was no evidence that specific STs contributed disproportionately more to serotype replacement than others. This is apparent from inspection of figure 1, in which most STs containing NVA/NVTs were relatively small and were included in both MCV and PCV communities. Quantitatively, the result of random-effects logistic regression testing for evidence that STs differed in their tendency to be present in PCV or MCV communities was nonsignificant (P = .22). Similar results were obtained when carriage, invasive disease, or OM isolates were considered separately (data not shown).
Impact of PCV7 on VT pneumococci: no evidence for clonal group differences in vaccine effectiveness
The effect of conjugate vaccination on NP carriage and invasive disease varies for different serotypes included in the vaccine: specifically, in the present population [6, 8] and others [1, 2, 20], the vaccine has shown lower efficacy or effectiveness against serotype 19F. To determine whether, beyond these serotype-specific effects, there are also ST-specific differences in vaccine effectiveness, we sought evidence that, among VT isolates, STs were differentially distributed between PCV and MCV populations.
Taking all VT isolates from NP carriage together, evidence of heterogeneity was found (P = .023 for a random effect of ST), suggesting that some STs were more completely inhibited in the PCV communities than others. However, this difference was attributable to the well-known lower effectiveness of PCV against serotype 19F. Considering the 19F population separately, no evidence of heterogeneity was found (P = 1.0 for serotype 19F and P = .39 for other VTs for a random effect of ST).
The same analysis was repeated for all VT isolates (from carriage and disease), and similar results were obtained, except that in this case even the combined 19F and other VT analysis, as well as the separate analyses, produced nonsignificant evidence of heterogeneity (P = .09, P = 1.0, and P = .39 for all VTs, 19F alone, and VTs except 19F, respectively). This finding can be seen semiquantitatively in figure 1: if particular STs were particularly susceptible to vaccine effects, one would expect to see many VT-associated STs found in MCV communities only; this was not the case, except for a few very rare STs (small black circles in figure 1).
VATs: possible differences in ST between PCV and MCV communities
Taking all VATs together, there was no evidence of a difference in response to the vaccine between STs (P = .44 for a random effect of ST). However, this finding may have reflected a lack of power. Only 2 STs (199, containing only 1 VAT, 19A; and 395, containing only type 6A) contained >10 VAT carriage isolates. For these 2 STs, a trend toward different representation in PCV versus MCV communities was observed, with 17 (53%) of 32 isolates of ST199/serotype 19A being found in PCV communities and 4 (25%) of 16 isolates of ST395/serotype 6A being found in PCV communities (odds ratio, 0.29; P = .075, Fisher’s exact test). Similar results were found for carriage, OM, and invasive disease isolates together.
DISCUSSION
Our results suggest that capsular serotype, rather than other aspects of a pneumococcal strain’s genetic makeup reflected by ST, was the primary determinant of vaccine effect in this cluster-randomized trial of PCV7. Invasive disease [8] and NP carriage [6, 10] of VT pneumococci were reduced in PCV communities compared with MCV communities; however, after accounting for the lesser effect of PCV on serotype 19F than other VTs, there was no evidence that the impact of the vaccine was variable for different STs. Likewise, NVA/NVT pneumococci were more commonly obtained from invasive disease (although not statistically significantly so) [8] and NP carriage [6, 10] in PCV than in MCV communities, but there was no evidence that particular STs among the NVA/NVTs expanded in the PCV communities more than others. Capsular switching may have occurred, and indeed 1 serotype 35F variant of ST124 (normally serotype 14) was detected in a PCV community. However, given that 151 NVA/NVT strains from PCV communities were analyzed, we can say that capsular switching made only a minor contribution to the process of serotype replacement during the present clinical trial.
For VATs, there was little evidence for differential representation of STs in PCV versus MCV communities. In this study as in others, serotype 19A tended to be more common in PCV communities (acting like an NVA/NVT, consistent with weak effectiveness of the vaccine against serogroup 19), whereas other VATs tended to be inhibited by cross-reactive immunity. Therefore, we suspect that, with a larger sample of VATs, we would have observed significant differences in ST representation, with serotype 19A–associated STs being overrepresented in PCV communities relative to STs associated with other VATs.
Our findings suggest that PCV7 acts as a “serotype filter”—that is, that the impact of PCV7 on the prevalence of a particular pneumococcal strain is predictable on the basis of the serotype of that strain, without reference to other aspects of the strain’s genetic background as determined by ST. VT strain transmission is inhibited in communities that receive PCV7, and inhibition is similar for all VT strains, with the exception that serotype 19F strains are less inhibited than other VT strains. NVA/NVT strains show an increased prevalence in carriage in PCV7 communities, but this increase is equivalent across all STs, a finding that is robust whether all isolates are considered together or whether carriage, OM, and invasive disease isolates are considered separately.
Mathematical models developed around the time of the introduction of PCV7 predicted that use of such a vaccine could lead to the expansion of existing NVA/NVT pneumococcal populations or to the appearance of novel serotypes or STs. The models suggested that if particular NVA/NVT strains were highly transmissible in the absence of competitors but were strongly inhibited by competition from VT pneumococci in the absence of vaccination, such strains might increase from very low frequencies in an unvaccinated population to much higher frequencies after vaccine introduction [11, 12]. This theoretical possibility did not occur after the introduction of PCV7 in the context of the present study; rather, many existing STs of NVA/ NVT strains increased in frequency. It was also suggested before the introduction of PCV that capsular switching may play an important role in the pneumococcal population’s response to conjugate vaccination [11, 12, 14]. If particular clonal characteristics other than capsular serotype (e.g., antimicrobial resistance or other determinants of fitness) accounted for the success of certain STs that expressed VT capsules before vaccination, then capsular switching might provide a ready means for such highly fit types to spread in the presence of vaccination. Novel capsule-switched strains were rare in the present study, consistent with earlier findings that capsular serotype seems to be a primary determinant of the abundance of particular strains of pneumococci [21].
Pneumococcal invasive disease has declined dramatically since the licensure of PCV7, despite an increase in disease from NVA/NVTs. A recent study examined clonal compositions of populations of invasive disease isolates from the United States, comparing isolates from 1999 (before PCV7 licensure) to those from 2001 and 2002 (after licensure) [17]. That study also found that the clonal makeup of each serotype was similar before and after vaccine introduction, although several novel STs were identified in the post licensure samples. Limited evidence of capsular switching was found in that study. Most isolates in the present study came from NP carriage in children in the target age group for the vaccine, although we also considered disease isolates from the same ages in the same communities. Each of our findings was reproduced whether we considered all isolates together or separated carriage isolates from disease isolates. Thus, our findings confirm for NP carriage (as well as disease) what was observed by Beall et al. [17] in invasive disease.
These findings are cause for cautious optimism about the future impact of PCV. If pneumococcal populations adapt to the selective pressure of conjugate vaccination mainly by increased frequency of STs associated with NVA/NVTs, then the appearance of new, highly transmissible, or highly virulent NVA/NVT STs may be less likely than if (contrary to observations to date) the use of PCV7 had provoked rapid evolution of pneumococci, with frequent capsular switching or rapid spread of previously unobserved STs. This optimistic interpretation should be tempered by the following caveats: (1) the trends observed in the first years of PCV7 use may or may not be maintained, because vaccine uptake increases worldwide over time and pneumococcal populations have the time to adapt to this growing selective pressure; (2) the rising prevalence of antimicrobial resistance in NVA/NVT pneumococci [22, 23] shows that pneumococcal populations are not evolutionarily static and will continue to adapt to vaccine pressure; and (3) the existence of globally successful, often multidrugresistant pneumococcal STs suggests that some factor other than serotype alone may contribute to the ability of pneumococci to spread efficiently [24], although recent theoretical work suggests that pneumococcal population structure may be largely explainable by neutral (nonselective) population processes [25].
Given these trends, continued surveillance of serotype, STs, and antimicrobial resistance patterns of pneumococci is warranted. Of particular concern is the monitoring of major increases in disease caused by pneumococci of previously minor serotypes and the accumulation of resistance to multiple antimicrobial classes in new serotypes.
Table 2.
Sequence types (STs) observed, by site of isolation, community of vaccine randomization, and serotype.
| Community of Vaccine Randomization |
NP Carriage | Otitis Media | Invasive Disease | |
|---|---|---|---|---|
| MCV | NVA/NVT | 62(6) 100(2) 180 193 199(3) 218 393 433(2) 448 452(4) 498(3) 547(2) 574 636(2) 817(4) 1263 1294(6) 1367(2) 1757(8) 1794 6 novel types (9 isolates) |
13 180(10) 191 227 558 1111(2) 1119 1294 1480 (2) 4 novel types (5 isolates) |
53 191(2) 199 218(8) 227(6) 433 1030 1263 1 novel type (1 isolate) |
| VT | 33(4) 36(3) 37(2) 43 113 124(7) 146(9) 156 177(2) 199 205(3) 242(5) 251 315(2) 326 |
33 81 146 176 236 242 247 251 424(2) 833 1060(2) 6 novel types (6 isolates) |
13(2) 33(2) 81 124(3) 146(5) 162 200 236 247(2) 251 643 782(2) 1060 1269 1711 |
|
| 384 439 782 833 1060(4) 1092 7 novel types (12 isolates) |
8 novel ST (9 isolates) |
|||
| VAT | 199 (15) 241 327(2) 395(12) 439(2) 460 473(3) 690 1379 1390(4) 1448(3) 5 novel ST (8 isolates) |
199(5) 395(3) 460 1390 4 novel ST(5 isolates) |
133 199(3) 344 1060 4 novel ST(4 isolates) |
|
| PCV | NVA/NVT | 62(8) 100 124 180 193(7) 199(4) 227(2) 260(2) 392(3) 433(4) 448 452(9) 487(3) 498(4) 547 574 (2) 636 662(2) 1111(2) 1263(9) 1294(9) 1757(11) 15 novel ST (28 isolates) |
62 180(2) 218 227 260(2) 1111 1119 1263(3) 1794 1 novel ST(1 isolate) |
62 100 180(2) 191 218(5) 227(4) 393 1294 5 novel ST (6 isolates) |
| VT | 33 124(3) 177(3) 236 247 251 654 802 833 1258 8 novel ST(13 isolates) |
177 199 205 251 271 333 654 1794 4 novel ST(5 isolates) |
13 33 37 113(2) 124(4) 146(3) 156 162 247(2) 654 782(2) 833 1269(3) 5 novel ST(6 isolates) |
|
| VAT | 62 199(17) 376 395(4) 439(2) |
199(2) 1390 |
199(4) 395(2) 1060 1390 1 novel ST (1 |
|
| 473(4) 690(2) 1379 1390(4) 1448(5) 1756(2) 8 novel ST (12 isolates) |
isolate) |
Acknowledgments
We gratefully acknowledge the field-workers, nurses, and staff of the Center for American Indian Health (Baltimore, Maryland) for their dedicated work on the American Indian PnCRM7 Efficacy Trial, from which the isolates analyzed here were collected. We also acknowledge the willing participation and commitment of the families of that trial. We appreciate the work of Melinda Bronsdon at the Centers for Disease Control and Prevention (CDC; Atlanta, Georgia) for isolation and serotyping of the nasopharyngeal strains and Alan Parkinson at the Arctic Investigations Program of the CDC (Anchorage, Alaska) for serotyping of the invasive disease and otitis media strains.
Financial support: National Institutes of Health (support to the present study via grant 1R01AI48935 to M.L.). The American Indian PnCRM7 Efficacy Trial and the nested nasopharyngeal study were supported by the National Institutes of Health, Wyeth Vaccines, the United States Agency for International Development, and the Centers for Disease Control and Prevention via support to M.S. and K.L.O.
Footnotes
Potential conflicts of interest: K.L.O. and M.S. have consulted for or have been a member of an advisory board for Wyeth Vaccines. All other authors report no potential conflicts.
Presented in part: 4th International Symposium on Pneumococci and Pneumococcal Diseases, Helsinki, 9–13 May 2004 (abstract PSV-31); 5th International Symposium on Pneumococci and Pneumococcal Diseases, Alice Springs, Australia, 2–6 April 2006.
References
- 1.Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19:187–195. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344:403–409. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 3.Cutts FT, Zaman SM, Enwere G, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365:1139–1146. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 4.Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 5.Dagan R, Lipsitch M. Ecological effects of vaccines and antibiotics. In: Tuomanen E, Mitchell T, Morrison DA, Spratt BG, editors. The pneumococcus. Washington, DC: ASM Press; 2004. pp. 283–313. [Google Scholar]
- 6.Millar EV, O’Brien KL, Watt JP, et al. Effect of community-wide conjugate pneumococcal vaccine use in infancy on nasopharyngeal carriage through 3 years of age: a cross-sectional study in a high-risk population. Clin Infect Dis. 2006;43:8–15. doi: 10.1086/504802. [DOI] [PubMed] [Google Scholar]
- 7.Reingold A. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease—United States, 1998–2003. MMWR. 2005;54:893–897. [PubMed] [Google Scholar]
- 8.O’Brien KL, Moulton LH, Reid R, et al. Efficacy and safety of a seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet. 2003;362:355–361. doi: 10.1016/S0140-6736(03)14022-6. [DOI] [PubMed] [Google Scholar]
- 9.Moulton LH, O’Brien KL, Kohberger R, et al. Design of a group-randomized Streptococcus pneumoniae vaccine trial. Control Clin Trials. 2001;22:438–452. doi: 10.1016/s0197-2456(01)00132-5. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien KL, Millar EV, Zell ER, et al. Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis. 2007;196:1211–20. doi: 10.1086/521833. (in this issue) [DOI] [PubMed] [Google Scholar]
- 11.Lipsitch M. Vaccination against colonizing bacteria with multiple serotypes. Proc Natl Acad Sci USA. 1997;94:6571–6576. doi: 10.1073/pnas.94.12.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipsitch M. Bacterial vaccines and serotype replacement: lessons from Haemophilus influenzae and prospects for Streptococcus pneumoniae. Emerg Infect Dis. 1999;5:336–345. doi: 10.3201/eid0503.990304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaffar S, Leach A, Hall AJ, et al. Preparation for a pneumococcal vaccine trial in The Gambia: individual or community randomisation? Vaccine. 1999;18:633–640. doi: 10.1016/s0264-410x(99)00277-7. [DOI] [PubMed] [Google Scholar]
- 14.Spratt BG, Greenwood BM. Prevention of pneumococcal disease by vaccination: does serotype replacement matter? Lancet. 2000;356:1210–1211. doi: 10.1016/S0140-6736(00)02779-3. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien KL, Bronsdon MA, Dagan R, et al. Evaluation of a medium (STGG) for transport and optimal recovery of Streptococcus pneumoniae from nasopharyngeal secretions collected during field studies. J Clin Microbiol. 2001;39:1021–1024. doi: 10.1128/JCM.39.3.1021-1024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bronsdon MA, O’Brien KL, Facklam RR, Whitney CG, Schwartz B, Carlone GM. Immunoblot method to detect Streptococcus pneumoniae and identify multiple serotypes from nasopharyngeal secretions. J Clin Microbiol. 2004;42:1596–1600. doi: 10.1128/JCM.42.4.1596-1600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beall B, McEllistrem MC, Gertz RE, Jr, et al. Pre- and postvaccination clonal compositions of invasive pneumococcal serotypes for isolates collected in the United States in 1999, 2001, and 2002. J Clin Microbiol. 2006;44:999–1017. doi: 10.1128/JCM.44.3.999-1017.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brueggemann AB, Griffiths DT, Meats E, Peto T, Crook DW, Spratt BG. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J Infect Dis. 2003;187:1424–1432. doi: 10.1086/374624. [DOI] [PubMed] [Google Scholar]
- 19.Brueggemann AB, Peto TE, Crook DW, Butler JC, Kristinsson KG, Spratt BG. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J Infect Dis. 2004;190:1203–1211. doi: 10.1086/423820. [DOI] [PubMed] [Google Scholar]
- 20.Dagan R, Givon-Lavi N, Zamir O, et al. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J Infect Dis. 2002;185:927–936. doi: 10.1086/339525. [DOI] [PubMed] [Google Scholar]
- 21.McCormick AW, Whitney CG, Farley MM, et al. Geographic diversity and temporal trends of antimicrobial resistance in Streptococcus pneumoniae in the United States. Nat Med. 2003;9:424–430. doi: 10.1038/nm839. [DOI] [PubMed] [Google Scholar]
- 22.Hanage WP, Huang SS, Lipsitch M, et al. Diversity and antibiotic resistance among nonvaccine serotypes of Streptococcus pneumoniae carriage isolates in the post-heptavalent conjugate vaccine era. J Infect Dis. 2007;195:347–352. doi: 10.1086/510249. [DOI] [PubMed] [Google Scholar]
- 23.Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354:1455–1463. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 24.McGee L, McDougal L, Zhou J, et al. Nomenclature of major antimicrobial-resistant clones of Streptococcus pneumoniae defined by the Pneumococcal Molecular Epidemiology Network. J Clin Microbiol. 2001;39:2565–2571. doi: 10.1128/JCM.39.7.2565-2571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fraser C, Hanage WP, Spratt BG. Neutral microepidemic evolution of bacterial pathogens. Proc Natl Acad Sci USA. 2005;102:1968–1973. doi: 10.1073/pnas.0406993102. [DOI] [PMC free article] [PubMed] [Google Scholar]