Abstract
Background
Information comparing subjective and objective measurements of adherence to study medications and the effects of adherence on treatment-related differences in asthma clinical trials are limited.
Objective
We sought to compare subjective and objective measurements of children’s adherence to inhaled corticosteroids or placebo and to determine whether adherence to study medications modified treatment-related differences in outcomes.
Methods
In an ancillary study conducted in 3 of 8 Childhood Asthma Management Program Clinical Centers, adherence was assessed by using self-reported and objective data in 5- to 12-year-old children with mild or moderate asthma who were randomly assigned to 200 μg of inhaled budesonide twice per day (n = 84) or placebo (n = 56) for 4 years. The κ statistic was used to evaluate agreement between self-reported adherence (daily diary cards) and objectively measured adherence (number of doses left in study inhalers). Multivariable analyses were used to determine whether adherence to study treatment modified treatment-related differences in outcomes.
Results
Adherence of less than 80% was seen in 75% of 140 children when adherence was measured objectively but only in 6% of children when measured by means of self-report. There was poor agreement between objective and subjective measurements of adherence of at least 80% (κ = 0.00; 95% CI, −0.05 to 0.04); self-reported adherence over the 4-year period generally overestimated objectively measured adherence (93.6% vs 60.8%, P < .0001). There was little evidence to indicate that adherence modified treatment-related differences in outcomes.
Conclusion
Researchers should use objective rather than self-reported adherence data to identify clinical trial participants with low levels of adherence to study treatment.
Keywords: Asthma, adherence, compliance, children, lung growth, inhaled corticosteroids, budesonide, clinical trial
Asthma is a common respiratory disorder characterized by chronic airway inflammation, impaired pulmonary function, and episodic respiratory symptoms.1,2 Results of randomized clinical trials in children indicate that anti-inflammatory therapy with daily inhaled corticosteroids (ICSs) improves symptom control and reduces the risk of asthma exacerbations.3–5 Although it is known that patients’ adherence to therapy is often incomplete and decreases over time,6–8 the effects of partial adherence to ICS therapy on efficacy and safety end points in clinical trials are unclear.3,5,9
To address these gaps in knowledge, we designed and conducted an ancillary adherence study within the Childhood Asthma Management Program (CAMP), a large, multicenter, placebo-controlled, randomized clinical trial designed to evaluate the long-term effects of budesonide, an ICS, in children with mild or moderate asthma.10 The objectives of this ancillary study were (1) to characterize children’s adherence to study medications and compare self-reported and objective measures of medication use and (2) to determine whether adherence to study medications modified the observed effect of daily budesonide therapy (compared with placebo) on clinical and pulmonary function, as well as height and bone mineral density outcomes.
METHODS
Study participants
A detailed description of the CAMP study design has been reported elsewhere.10 Between December 1993 and September 1995, children from 5 through 12 years of age at 8 clinical centers were screened for enrollment in the CAMP trial. Participants had to have mild or moderate asthma (presence of symptoms at least twice weekly, use of an inhaled bronchodilator at least twice weekly, or use of daily medication for asthma) and airway hyperresponsiveness to inhaled methacholine (a 20% decrease in FEV1 with a methacholine dose of ≤12.5 mg/mL). Children with other clinically significant pulmonary disease and selected nonpulmonary conditions were excluded.
Children were randomly assigned to budesonide, nedocromil, or matching placebo in CAMP. Assignments were made by using permuted-blocks randomization with stratification according to clinic. The ancillary adherence study was designed to be conducted within the main CAMP study at 3 of the 8 clinical centers (Baltimore, Denver, and San Diego). Enrollment into the ancillary adherence study at these 3 clinical centers began after the start of enrollment in the main CAMP study, and therefore not all children enrolled in the main CAMP study were also enrolled in the ancillary adherence study. The ancillary adherence study included objective monitoring of children’s adherence to study treatments. Because the current report is focused on adherence to ICS treatment, analyses were restricted to children who were randomized to budesonide or budesonide placebo and were enrolled in the ancillary study. Informed consent was obtained from the children’s parents or guardians. The study, including adherence measurements, was approved by the local institutional review board at each center.
Study treatments and visits
Participants were randomly assigned to receive 200 μg of budesonide twice daily delivered by means of two 100-μg actuations of an inhaler or a matching placebo inhaler (Turbuhaler; AstraZeneca, London, United Kingdom). Albuterol administered through a metered-dose inhaler was used as needed for symptom relief and to prevent exercise-induced bronchospasm. A written action plan was used to guide rescue treatment, which included a short course of oral prednisone for asthma exacerbations. The treatment protocol included a pre-established algorithm to escalate asthma therapy in children with inadequate asthma control, beginning with the addition of 168 μg of inhaled beclomethasone dipropionate twice daily to the study medications. If asthma control remained unsatisfactory, replacement or addition of other long-term control medications was allowed. It was permissible to use a pre-established algorithm to taper the study medication to a dose of zero by using stepwise reductions from 100% to 50% to 0% to account for remission. Algorithms also guided the resumption of the full dose of the study medication. After several baseline study visits, there were follow-up visits at 2 and 4 months after randomization and every 4 months afterward over a period of 4.3 years (average) until March through June 1999 (end of the treatment period) in the main study. The follow-up period for this ancillary study was 4 years.
Adherence measures
Self-reported use of study medication was recorded daily on a study diary card by children or their parents/guardians. The diary cards were continued through the entire period of the clinical trial. Diary cards were collected at study visits or returned by mail to the clinical centers and reviewed for completeness. If a participant failed to complete or return diary cards for a period of 1 or more months in the interval between study visits, then self-reported adherence data at the follow-up visit were considered missing. Objective medication adherence was based on the recording of the number of doses remaining in each Turbuhaler device by a research assistant. If a participant failed to return 1 or more Turbuhaler devices at a study visit (ie, sufficient study medication for ≥1 month), then objective adherence data at that visit were considered missing. Participants were informed that diaries and medication use would be reviewed but were not told specifically that the number of puffs of medications dispensed would be counted. Also, counting of doses dispensed from Turbuhaler devices was not done in the presence of children or families.
Outcome measures
Clinical
Urgent care visits to a physician’s office because of asthma, number of courses of prednisone, need for treatment escalation (percentage of days taking beclomethasone or additional asthma medications), and change in daily diary measures of symptom control (symptom frequency, number of asthma episode-free days per month, uses of albuterol for symptoms and to prevent exercise-induced bronchospasm per week, and number of nighttime awakenings per month) during the 4-year treatment period were examined. Because we were interested in the change in health status with long-term therapy, we calculated the difference between the measurement obtained at the last visit while taking study medications from the measurement obtained at the baseline visit for clinical outcomes, pulmonary function (except airway hyper-responsiveness [see below]), and height and bone density.
Pulmonary function
Changes in the prebronchodilator and postbronchodilator spirometric values (FEV1 [in liters], FEV1 percent predicted, forced vital capacity [FVC; in liters], and FEV1/FVC ratio [as a percentage]) and airway hyperresponsiveness during the 4-year treatment period were detected.10 Airway responsiveness to methacholine was measured as the PC20 value. To estimate the change in airway hyperresponsiveness with treatment, we used the ratio of the PC20 value at the last visit while taking study medications and the PC20 value at the baseline visit.
Height and bone density
Change in height (in centimeters and percentages) and bone mineral density during the 4-year treatment period and projected final height were measured.10
Statistical analysis
Descriptive statistics are presented as means (SDs), medians (ranges), or percentages, as appropriate. Comparisons of patients’ characteristics between groups used t tests, Wilcoxon rank sum tests, or χ2 tests. Adherence was defined as the percentage of prescribed dose used (ie, [No. of doses used/Prescribed no. of doses] × 100%) and accounted for dose reductions in study medications that occurred between study visits. Adherence data (self-reported and objectively measured) at each follow-up visit were truncated at 100% to prevent overuse or “dumping” (deliberate emptying of inhaler before study visits to conceal nonadherence) during some follow-up periods from masking underuse during others.11 Adherence data collected at each visit were used to compute the yearly mean adherence during the 4-year treatment period. The mean of the yearly values was used to calculate the overall mean adherence over the 4-year study (adherence4yrs). The Wilcoxon signed-rank test was used to compare participants’ self-reported and objectively measured adherence4yrs values. The κ statistic for agreement (and 95% CI) was used to compare the self-reported adherence (based on daily diary cards) with the objectively measured adherence (based on the number of doses remaining in study inhalers).12 The likelihood of missing self-report data at visits in which objective adherence data were missing was calculated by using a logistic regression model. Changes in self-reported and objectively measured yearly mean adherence during the study period were evaluated by using linear regression models.
As in the main CAMP study, multivariable regression models were developed to estimate the effect of budesonide on outcomes. Regression models accounted for the possible correlation of repeated measurements within subjects.13,14 In each model the predictor variables were the treatment group (budesonide vs placebo [reference]), baseline value for the outcome measure, age, race or ethnic group, sex, clinical center, duration of asthma, severity of asthma, skin test reactivity, and any factor associated with treatment assignment (budesonide vs placebo) at the baseline visit with a P value of less than .20. Then to determine whether the mean adherence to study medications over the 4-year treatment period (adherence4yrs) modified the effects of budesonide on outcomes, adherence4yrs was categorized as low (<50% adherence4yrs [approximately 25th percentile among study participants]), medium (50% to 79% adherence4yrs [approximately 25th to 75th percentile]), or high (≥80% adherence4yrs [approximately 75th percentile]). Then a treatment-adherence4yrs interaction term (ordinal variable representing low, medium, and high adherence4yrs groups) was included in the multivariable regression model for each outcome. If the treatment group–adherence4yrs interaction term was significant (P < .05) for an outcome, 3 multivariable regression models were fitted (one for each adherence4yrs stratum) for that outcome, after excluding the adherence4yrs value and treatment-adherence4yrs interaction term. A 2-sided P value of less than .05 was used to detect a statistically significant difference for all analyses. Computations were performed with STATA version 8.2 software (StataCorp, College Station, Tex).
RESULTS
Baseline characteristics
Of the 198 children who were assigned to budesonide or budesonide placebo and enrolled in the main CAMP study at these 3 clinical centers, 148 (74.7%) were enrolled in this ancillary adherence study. Eight (5.4% of 148) children did not provide any objective adherence data during the follow-up period, although they did complete the main CAMP study visits. These 8 children were similar in age, race/ethnicity, and sex to the 140 children in whom we collected objective adherence data, but the former group were more likely to have been classified as having moderate asthma (88% vs 46%, P = .03) and had a lower prebronchodilator FEV1 percent predicted (78% vs 93%, P < .01). Because this report is focused on adherence to ICS treatment, analyses were subsequently restricted to the 140 children in whom objective adherence data were collected (Table I), representing 71% of participants assigned to budesonide or budesonide placebo in the 3 clinical centers and 27% of 519 participants assigned to budesonide or budesonide placebo in all 8 main CAMP trial clinical centers. Participants in the ancillary study were more likely to be non-Hispanic (89% vs 78%) and had a slightly lower median asthma symptom scores (0.4 vs 0.5) compared with children assigned to budesonide or budesonide placebo at the other 5 CAMP clinical centers; the 2 groups were otherwise similar.
TABLE I.
Baseline characteristics
| Characteristic | Current study (ancillary adherence study) | Other CAMP participants (n = 379) | P value† | |||
|---|---|---|---|---|---|---|
| Budesonide (n = 84) | Placebo (n = 56) | P value* | Combined group (n = 140) | |||
| Demographics | ||||||
| Age (y) | 8.9 ± 2.0 | 8.9 ± 2.2 | .86 | 8.9 ± 2.0 | 9.1 ± 2.1 | .36 |
| Race/ethnic group, no. (%) | .75 | .049 | ||||
| Non-Hispanic white | 63 (75.0) | 39 (69.6) | 102 (72.9) | 249 (65.7) | ||
| Non-Hispanic black | 11 (13.1) | 11 (19.6) | 22 (15.7) | 48 (12.7) | ||
| Hispanic | 6 (7.1) | 3 (5.4) | 9 (6.4) | 37 (9.8) | ||
| Other | 4 (4.8) | 3 (5.4) | 7 (5.0) | 45 (11.9) | ||
| Sex, no. (%) | .73 | .32 | ||||
| Female | 38 (45.2) | 27 (48.2) | 65 (46.4) | 157 (41.4) | ||
| Male | 46 (54.8) | 29 (51.8) | 75 (53.6) | 222 (58.6) | ||
| Clinical measures based on patients’ report | ||||||
| Age at onset of asthma (y) | 3.1 ± 2.2 | 2.6 ± 2.4 | .28 | 2.9 ± 2.3 | 3.1 ± 2.4 | .32 |
| Years since asthma diagnosis | 5.3 ± 2.6 | 5.0 ± 2.9 | .43 | 5.1 ± 2.8 | 5.1 ± 2.6 | .96 |
| Severity of asthma, no. (%) | .73 | .11 | ||||
| Mild | 46 (54.8) | 29 (51.8) | 75 (53.6) | 173 (45.7) | ||
| Moderate | 38 (45.2) | 27 (48.2) | 65 (46.4) | 206 (54.4) | ||
| Ever hospitalized for asthma, no. (%) | 21 (25) | 16 (29) | .70 | 37 (26) | 128 (34) | .14 |
| Days of oral steroids in past 6 mo, median (range) | 0 (0–21) | 0 (0–18) | .52 | 0 (0–21) | 0 (0–42) | .95 |
| Diary card measures | ||||||
| Symptom score, median (range) | 0.4 (0–1) | 0.4 (0–1) | .79 | 0.4 (0–1) | 0.5 (0–1) | .01 |
| Asthma episode-free days (no./mo), median (range) | 8.3 (0–21.7) | 7.6 (0–30.4) | .86 | 7.7 (0–30.4) | 9.8 (0–29.3) | .50 |
| Use of albuterol for symptoms (puffs/wk), median (range) | 7.9 (0–42.5) | 8 (0–42) | .94 | 8 (0–42.5) | 7.5 (0–51.5) | .79 |
| Nighttime awakenings (no./mo), median (range) | 0 (0–4.3) | 0 (0–5.4) | .56 | 0 (0–5.4) | 0 (0–7.6) | .30 |
| Pulmonary function | ||||||
| Spirometric values before bronchodilator | ||||||
| FEV1, % predicted | 93.4 ± 14.0 | 92.4 ± 16.0 | .69 | 93.0 ± 14.8 | 93.8 ± 14.3 | .60 |
| Spirometric values after bronchodilator | ||||||
| FEV1 (% predicted) | 102.3 ± 13.8 | 102.2 ± 12.8 | .97 | 102.3 ± 13.4 | 103.5 ± 12.7 | .33 |
| FEV1 (L) | 1.8 ± 0.5 | 1.7 ± 0.5 | .33 | 1.8 ± 0.5 | 1.8 ± 0.5 | .18 |
| FVC after bronchodilator (L) | 2.1 ± 0.7 | 2.0 ± 0.6 | .34 | 2.1 ± 0.6 | 2.2 ± 0.6 | .18 |
| FEV1/FVC ratio (%) | 85.3 ± 6.2 | 85.3 ± 6.6 | .96 | 85.3 ± 6.3 | 85.3 ± 6.7 | .98 |
| Airway responsiveness to methacholine (PC20; mg/mL), geometric mean (95% CI) | 1.0 (0.8–1.3) | 1.0 (0.7–1.4) | >.99 | 1.0 (0.8–1.2) | 1.1 (1.0–1.3) | .43 |
| Height and bone density | ||||||
| Height (cm) | 134.0 ± 14.2 | 132.0 ± 13.4 | .41 | 133.2 ± 13.9 | 134.3 ± 13.0 | .43 |
| Height (percentile) | 58.7 ± 27.0 | 47.4 ± 30.6 | .03 | 54.3 ± 28.9 | 56.2 ± 28.5 | .50 |
| Bone mineral density (g/cm2) | 0.66 ± 0.12 | 0.67 ± 0.11 | .77 | 0.66 ± 0.12 | 0.65 ± 0.10 | .07 |
Numbers represent means ± SDs, unless otherwise noted.
P value for comparison of the budesonide and budesonide placebo groups in the current study.
P value for comparison of the combined group in the current study versus other CAMP participants randomized to budesonide or budesonide placebo in all 8 clinical sites.
The budesonide and placebo groups in the ancillary study were similar across multiple asthma and atopic characteristics at the baseline visit. However, despite similar heights when measured in centimeters (134 vs 132 cm, P = .41), the budesonide group had a significantly higher baseline height percentile compared with the placebo group (59th vs 47th percentile, P = .03). In the main CAMP trial the baseline height percentiles in children assigned to budesonide (57th percentile) or budesonide placebo (54th percentile) were not significantly different (P = .29).
Effects of budesonide on outcomes within the ancillary study
For more information on the effects of budesonide on outcomes within the ancillary study, see Table II. Significant improvements were noted across several outcome measures in the budesonide group compared with the placebo group: the percentage of children with urgent care visits for asthma, the percentage of days of beclomethasone or other asthma medications, symptom scores, asthma episode-free days, FEV1/FVC ratio, and airway responsiveness to methacholine. Other than FEV1/FVC ratio and airway responsiveness, there were no significant differences in the other measures of pulmonary function between the budesonide and placebo groups, including the change in postbronchodilator FEV1 percent predicted (2.46% vs −1.88%, respectively; P =.11).
TABLE II.
Outcomes in the budesonide and placebo groups within the ancillary study
| Budesonide (n = 84) | Placebo (n = 56) | P value* | |
|---|---|---|---|
| Clinical outcomes | |||
| Urgent care visits caused by asthma, no. (%) | 21 (25) | 25 (45) | .02 |
| No. of courses of prednisone (no./100 person years) | 109 (87) | 145 (110) | .07 |
| Percentage of days on beclomethasone or other asthma medications | 5.7 (18.2) | 18.4 (33.3) | <.01 |
| Change in daily diary card measures | |||
| Symptom score | −0.38 (0.34) | −0.13 (0.39) | <.001 |
| Asthma episode-free days (no./mo) | 14.1 (10.7) | 9.8 (12.9) | .048 |
| Use of albuterol for symptoms (puffs/wk) | −7.7 (9.1) | −4.1 (11.1) | .055 |
| Nighttime awakenings (no./mo) | −0.59 (1.22) | −0.60 (2.54) | .98 |
| Pulmonary function | |||
| Changes in spirometric values before bronchodilator | |||
| FEV1 (L) | 0.88 (0.40) | 0.78 (0.46) | .19 |
| FEV1 (% predicted) | 2.45 (13.0) | −1.88 (17.56) | .11 |
| FVC (L) | 1.17 (0.49) | 1.12 (0.47) | .58 |
| FEV1/FVC ratio (%) | −1.11 (6.51) | −3.81 (9.09) | .048 |
| Changes in spirometric values after bronchodilator | |||
| FEV1 (L) | 0.93 (0.42) | 0.89 (0.40) | .61 |
| FEV1 (% predicted) | 1.28 (12.08) | −0.96 (14.53) | .34 |
| FVC (L) | 1.16 (0.50) | 1.12 (0.43) | .59 |
| FEV1/FVC ratio (%) | −1.74 (5.32) | −2.15 (7.01) | .70 |
| Airway responsiveness to methacholine, ratio of follow-up to baseline values | 2.43 (3.86) | 1.11 (3.13) | <.001 |
| Height and bone density | |||
| Change in height (cm) | 20.8 (5.5) | 21.4 (5.3) | .57 |
| Change in height percentile | −6.1 (16.3) | 0.9 (15.7) | .02 |
| Projected final height (cm) | 172.1 (10.2) | 170.4 (10.7) | .36 |
| Change in bone mineral density (g/cm2) | 0.182 (0.102) | 0.176 (0.110) | .73 |
Values represent means (SDs), unless otherwise stated.
P values based on multivariable regression model for differences between groups (see the Methods section).
There was also a significant reduction in height percentile in the budesonide group compared with that seen in the placebo group; however, there were no significant differences in the change in height (in centimeters), projected final height, or change in bone mineral density between treatment groups.
Adherence to study treatment within the ancillary study
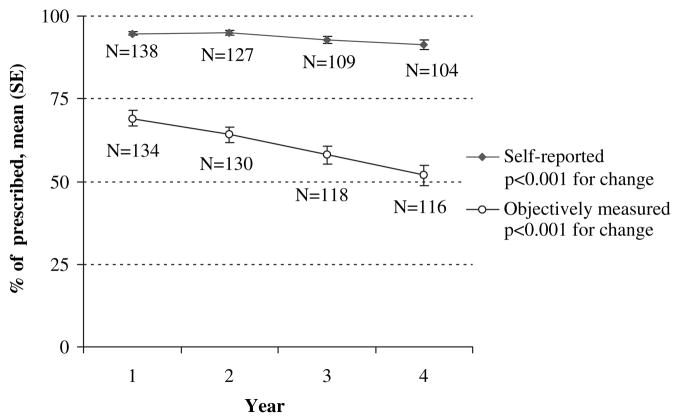
A significant decrease occurred in the yearly mean adherence based on objective data (from 69% in year 1 to 52% in year 4, P < .001, Fig 1). A significant, although smaller, decrease also occurred in mean adherence based on self-report (from 95% in year 1 to 91% in year 4, P < .001).
FIG 1.
Objective and self-reported adherence by study year within the ancillary study. Self-reported (solid diamonds) and objective (open circles) adherence at years 1, 2, 3, and 4 of the study are shown. The number of children in whom adherence data were available each year is shown adjacent to each data point.
Objectively measured adherence4yrs values were significantly lower than self-reported adherence4yrs values (60.8% vs 93.6%, P < .0001). Whereas only 8 (5.8%) participants had self-reported adherence4yrs values of less than 80%, 75% of participants had objectively measured adherence4yrs values of less than 80%, and 27% of participants had objectively measured adherence4yrs values of less than 50%. The κ statistic for agreement between objectively and subjectively measured adherence of at least 80% was poor (κ = 0.00; 95% CI, −0.05 to 0.04).
The proportion of children with objectively measured adherence4yrs values of less than 80% was similar in the budesonide and placebo treatment groups (P =.56). The overall mean objectively measured adherence4yrs value (budesonide vs placebo, 60.1% vs 62.8%; P =.53) and the decrease in yearly mean objective adherence (P =.55) were also similar in the 2 treatment groups.
Objective adherence data were missing at more study visits than self-reported data (number of visits with missing objective vs self-reported data, mean [SE]: 4 [0.2] vs 3 [0.3]; P =.0004). Missing objective adherence data at 4 or more visits was associated with lower adherence at follow-up visits in which these data were available (adherence4yrs if missing data at ≥4 vs <4 visits: 53.6% vs 68.3%; P =.0002). Children with missing objective adherence data at a study visit were significantly more likely to also have missing self-reported adherence data at that same visit (odds ratio, 3.1; 95% CI, 2.2–4.3).
Adherence to study treatment and outcomes within the ancillary study
For more information on adherence to study treatment and outcomes within the ancillary study, see Table III. In multivariable analyses, adherence did not modify treatment-related differences (budesonide vs placebo) in most outcomes. However, there was a significant adherence–treatment group interaction for the 4-year change in FEV1 percent predicted (postbronchodilator and prebronchodilator). To examine this further, we conducted analyses within strata defined by adherence4yrs values (low [n = 34 children], medium [n = 61 children], and high [n = 34 children]). In the high-adherence stratum the children treated with budesonide had a significantly greater change in the mean postbronchodilator FEV1 percent predicted compared with that seen in children treated with placebo (change in budesonide minus change in placebo treatment groups, 12.8%; 95% CI, 2.9% to 22.8%; P = .01). There were no significant differences in the change in postbronchodilator FEV1 percent predicted between treatment groups in the low-adherence stratum (budesonide minus placebo, 4.8%; 95% CI, −5.1% to 14.6%; P = .33) or in the medium-adherence stratum (budesonide minus placebo, −2.3%; 95% CI, −8.0% to 3.4%; P = .43).
TABLE III.
Effect of adherence to study treatment on differences in outcomes between the budesonide and placebo treatment groups within the ancillary study
| Treatment group–adherence4yrs interaction, P value* | |
|---|---|
| Clinical outcomes | |
| Urgent care visits caused by asthma (yes vs no); odds ratio | .58 |
| No. of courses of prednisone (no./100 person years) | .56 |
| Percentage of days on beclomethasone or other asthma medications | .71 |
| Change in daily diary card measures | |
| Symptom score | .84 |
| Asthma episode-free days (no./mo) | .61 |
| Use of albuterol for symptoms (puffs/wk) | .30 |
| Nighttime awakenings (no./mo) | .58 |
| Pulmonary function | |
| Changes in spirometric values before bronchodilator | |
| FEV1 (L) | .24 |
| FEV1 (% predicted) | .02 |
| FVC (L) | .32 |
| FEV1/FVC ratio (%) | .30 |
| Changes in spirometric values after bronchodilator | |
| FEV1 (L) | .38 |
| FEV1 (% predicted) | .03 |
| FVC (L) | .61 |
| FEV1/FVC ratio (%) | .26 |
| Airway responsiveness to methacholine, ratio of follow-up to baseline values | .25 |
| Height and bone density | |
| Change in height (cm) | .41 |
| Change in height percentile | .31 |
| Projected final height (cm) | .20 |
| Change in bone mineral density (g/cm2) | .46 |
Values represent P values for interaction between treatment group and adherence4yrs values in multivariable regression models (see the Methods section).
In the high-adherence stratum, budesonide also significantly improved the prebronchodilator FEV1 percent predicted (difference, 16.4%; 95% CI, 4.4% to 28.5%; P =.01) but not in the other adherence strata (difference in the low-adherence stratum, 7.4%; 95% CI, −4.2% to 19.1%; P = .20; difference in the medium-adherence stratum, 2.6%; 95% CI, −3.6% to 8.8%; P =.41).
DISCUSSION
Two primary findings emerged from this ancillary study: (1) three quarters of children had mean objectively measured adherence of less than 80%, and (2) self-report data failed to detect most children with objectively measured adherence of less than 80%. In addition, in the subset of children who were at least 80% adherent to study medications, the prebronchodilator and postbronchodilator FEV1 percent predicted was significantly greater in children assigned to budesonide compared with that seen in those assigned to placebo. However, this finding must be interpreted in light of the absence of an adherence effect on other outcomes seen in the budesonide group in the main CAMP trial (eg, airway hyperresponsiveness).
The dramatic difference between self-reported and objectively measured mean adherence and the poor agreement between these 2 measures in this study are consistent with results of previous investigations in children and adults, including those that used electronic medication monitors.15–17 In this report we found that self-reported adherence over the 4-year study period generally overestimated objectively measured adherence by about 30%. Findings from this study also indicate that relying on self-reported medication use to assess time trends in adherence is likely to be misleading. Although self-reported data suggested adherence to study medications was greater than 90% throughout the 4-year treatment period, objective data were far less encouraging, indicating that participants’ use of study medications had decreased to about 50% by the end of the 4 years. A similar decrease in objectively measured adherence was demonstrated in a 27-month clinical trial of budesonide therapy for children with asthma,7 underscoring the limited effectiveness of efforts incorporated into clinical trials (including CAMP) to promote and sustain participants’ adherence, including extensive asthma education, asthma diary monitoring, and regular nurse coordinator follow-up. The multiple supports to promote adherence in the trial are typically not available in clinical practice, suggesting that adherence to therapy is likely to be even lower in nonresearch settings.
In the main CAMP study5 the change in postbronchodilator FEV1 percent predicted was the primary outcome; secondary out-comes included airway hyperresponsiveness, other measures of lung function (eg, FEV1/FVC ratio), asthma symptom control (eg, need for albuterol), and adverse events (eg, bone density and children’s height). As in the main CAMP study, budesonide improved multiple outcomes during the 4-year treatment period in the subset of children enrolled in this ancillary adherence study, including symptom control (eg, urgent care visits and symptom scores) and airway responsiveness. Also, as in the main CAMP study, treatment with budesonide did not significantly improve postbronchodilator FEV1 percent predicted during the 4-year treatment period in the subset of children enrolled in this ancillary adherence study. In the main CAMP study report,5 children assigned to budesonide appeared to have higher postbronchodilator FEV1 percent predicted during the first year of study treatment, but differences between treatment groups disappeared by the end of the study. Results of this ancillary study suggest that the decrease in adherence during the 4-year treatment period (resulting in lower overall mean levels of adherence) might have attenuated the effect of budesonide on the primary outcome because the benefit of budesonide therapy (vs placebo) on postbronchodilator FEV1 percent predicted was only observed in the stratum of children with high levels of adherence to study medications. However, although a similar adherence effect was observed for the change in prebronchodilator FEV1 percent predicted, the level of adherence to study treatment did not significantly modify the effect of budesonide on multiple other outcomes, which suggests that the adherence-related findings might be due to chance (type I error). Alternatively, the lack of an “adherence effect” on other outcomes might suggest that relatively modest levels of ICS use (ie, different thresholds of adherence) could be effective for other outcomes.18–22 Also, this ancillary study, which only included about one quarter (27%) of the 519 participants assigned to budesonide or budesonide placebo in the main CAMP trial, might have been underpowered to assess the extent to which adherence modifies the effects of budesonide therapy on outcomes other than prebronchodilator and postbronchodilator FEV1 percent predicted.
The strengths of this study include the prospective collection of objective adherence data within a large, multicenter clinical trial comparing the long-term effects of ICSs and placebo on multiple clinical outcomes in children with asthma.
This study also has several limitations. Medication measurement, such as a count of the number of doses remaining between follow-up visits, does not provide information on day-to-day patterns of adherence, unlike electronic measures of adherence.23 Also, although measurements of doses left are more accurate than self-reported adherence, comparisons with electronic data indicate that adherence among study participants might have nevertheless been significantly overestimated.11 Medication measurements, for example, cannot identify deliberate emptying of an inhaler immediately preceding a study visit (“dumping”), a deceptive practice that has been noted in other clinical trials.24 Misclassification of adherence might have contributed to an underestimation of the role of adherence in mediating the observed treatment effects. Also, objective adherence data were not available at every study visit. However, concerns about measurement bias resulting from missing data are not limited to analyses based on objective adherence data alone. Self-reported adherence data were also incomplete, were more likely to be missing when objective data were missing, and overestimated objectively measured adherence. The study did not collect data on adherence to supplemental courses of ICSs and systemic corticosteroids that were prescribed during the course of the study. Thus it is possible that differences in the use of supplemental corticosteroids across treatment groups might have been a source of confounding in the analyses, resulting in minimizing or exaggerating the observed relationship between adherence to study medications and outcomes. Also, it is possible that those with higher levels of adherence to budesonide therapy might have had other beneficial self-care habits, such as avoidance of environmental irritants (eg, cigarette smoke) and allergens; these self-selection factors might have contributed to selection bias in the analyses of outcomes by adherence groups.25 However, adherence did not vary by treatment group (inhaled budesonide and placebo groups), and therefore selection bias is not likely to be a concern.
In conclusion, use of self-report data (ie, diary cards) to identify pediatric participants with asthma who are nonadherent to therapy in research studies is unjustified because unrecognized non-adherence to therapy in research studies can lead to an underestimate of the pharmacologic effects of study medications, especially for outcomes more sensitive to dose response.
Acknowledgments
The Childhood Asthma Management Program is supported by the National Heart, Lung, and Blood Institute (NHLBI; contracts NO1-HR-16044, 16045, 16046, 16047, 16048, 16049, 16050, 16051, and 16052). The adherence ancillary study was supported by the NHLBI (HL048999).
Ancillary Study Credit Roster—Johns Hopkins University, Baltimore, Md: C. S. Rand, R. A. Wise, N. F. Adkinson, Jr, N. Bollers, P. Eggleston, K. Huss, K. Hyatt, B. Lertiz, M. Pessaro, S. Philips, L. Plotnick, M. Pulsifer, B. Wheeler, and K. Weeks; National Jewish Medical and Research Center, Denver, Colo: B. Bender, S. Szefler, K. Brelsford, J. Bridges, J. Ciacco, R. Covar, M. Eltz, M. Flynn, M. Gleason, J. Hassell, M. Hefner, C. Hendrickson, D. Hettleman, C. G. Irvin, J. Jacobs, T. Junk-Blanchard, A. Kamada, A. Liu, H. S. Nelson, S. Nimmagadda, K. Sandoval, J. Sheridan, J. Spahn, D. Sundstrom, T. Washington, M. P. White, and E. Willcutt; Kaiser Permanente Medical Center and University of California–San Diego, San Diego, Calif: R. Zeiger, J. G. Easton, N. Friedman, L. L. Galbreath, E. Hanson, K. Harden, A. Horner, A. Jalowayski, E. M. Jenson, K. King, A. Lincoln, B. Lopez, M. Magiari, M. H. Mellon, A. Moscona, K. Mostafa, C. A. Nelle, S. Pizzo, E. Rodriguez, K. Sandoval, M. Schatz, and N. W. Wilson.
Abbreviations used
- adherence4yrs
Overall mean adherence over the 4-year study
- CAMP
Childhood Asthma Management Program
- FVC
Forced vital capacity
- ICS
Inhaled corticosteroid
Footnotes
Disclosure of potential conflict of interest: J. A. Krishnan receives research support from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI). S. J. Szefler has consultant arrangements with GlaxoSmithKline, Genentech, Merck, Boehringer-Ingelheim, Novartis, and Schering-Plough and receives research support from the NIH/NHLBI’s Childhood Asthma Management Program, the NHLBI’s Childhood Asthma Research and Education, the NIH/NHLBI’s Asthma Clinical Research Network, the NIH/National Institute of Allergy and Infectious Diseases’s Inner City Asthma Consortium, GlaxoSmithKline, National Jewish Health/NHLBI Asthma Net, and the National Institute of Environmental Health Sciences/US Environmental Protection Agency’s Children’s Environmental Health Center grant. R. S. Zeiger has consultant arrangements with AstraZeneca, Aerocrine, Genentech, Merck and Co, Schering-Plough, MedImmune, Sunovion, and Centocor and receives research support from Aerocrine, Genentech, Merck and Co, and GlaxoSmithKline. R. A. Wise has consultant arrangements with AstraZeneca. C. S. Rand is an advisor for the Merck Foundation/MCAN and has consultant arrangements with TEVA. The rest of the authors declare that they have no relevant conflicts of interest.
Clinical implications: Objective measures of adherence are needed to assess adherence to ICS therapy.
References
- 1.Martinez FD. Present and future treatment of asthma in infants and young children. J Allergy Clin Immunol. 1999;104:169–74. doi: 10.1016/s0091-6749(99)70058-8. [DOI] [PubMed] [Google Scholar]
- 2.Dixon AE, Irvin CG. Early intervention of therapy in asthma. Curr Opin Pulm Med. 2005;11:51–5. doi: 10.1097/01.mcp.0000148663.53798.72. [DOI] [PubMed] [Google Scholar]
- 3.Verberne AA, Frost C, Roorda RJ, van der Laag H, Kerrebijn KF. One year treatment with salmeterol compared with beclomethasone in children with asthma. The Dutch Pediatric Asthma Study Group. Am J Respir Crit Care Med. 1997;156:685–7. doi: 10.1164/ajrccm.156.3.9611067. [DOI] [PubMed] [Google Scholar]
- 4.Simons FE. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. Canadian Beclomethasone Diproprionate-Salmeterol Xinafoate Study Group. N Engl J Med. 1997;337:1659–65. doi: 10.1056/NEJM199712043372304. [DOI] [PubMed] [Google Scholar]
- 5.The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054–63. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 6.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–97. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 7.Jonasson G, Carlsen KH, Mowinckel P. Asthma drug adherence in a long term clinical trial. Arch Dis Child. 2000;83:330–3. doi: 10.1136/adc.83.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmons MS, Nides MA, Rand CS, Wise RA, Tashkin DP. Trends in compliance with bronchodilator inhaler use between follow-up visits in a clinical trial. Chest. 1996;109:963–8. doi: 10.1378/chest.109.4.963. [DOI] [PubMed] [Google Scholar]
- 9.Pauwels Ra, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. Early intervention with budesonide in mild persistent asthma: a randomized, double-blind trial. Lancet. 2003;361:1071–6. doi: 10.1016/S0140-6736(03)12891-7. [DOI] [PubMed] [Google Scholar]
- 10.Childhood Asthma Management Program Research Group. The Childhood Asthma Management Program (CAMP): design, rationale, and methods. Controlled Clin Trials. 1999;20:91–120. [PubMed] [Google Scholar]
- 11.Rand CS, Wise RA, Nides M, Simmons MS, Bleecker ER, Kusek JW, et al. Metered-dose inhaler adherence in a clinical trial. Am Rev Respir Dis. 1992;146:1559–64. doi: 10.1164/ajrccm/146.6.1559. [DOI] [PubMed] [Google Scholar]
- 12.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 13.Huber PJ. The behavior of maximum likelihood estimates under non-standard conditions. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability; Berkeley (CA): University of California Press; 1980. pp. 221–33. [Google Scholar]
- 14.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 15.Coutts JA, Gibson NA, Paton JY. Measuring compliance with inhaled medication in asthma. Arch Dis Child. 1992;67:332–3. doi: 10.1136/adc.67.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milgrom H, Bender B, Ackerson L, Bowry P, Smith B, Rand C. Noncompliance and treatment failure in children with asthma. J Allergy Clin Immunol. 1996;98:1051–7. doi: 10.1016/s0091-6749(96)80190-4. [DOI] [PubMed] [Google Scholar]
- 17.Krishnan JA, Riekert KA, McCoy JV, Stewart DY, Schmidt S, Chanmugam A, et al. Corticosteroid use after hospital discharge among high-risk adults with asthma. Am J Respir Crit Care Med. 2004;170:1281–5. doi: 10.1164/rccm.200403-409OC. [DOI] [PubMed] [Google Scholar]
- 18.Donahue JH, Weiss ST, Livingston JM, Goetsch MA, Greineder DK, Platt R. Inhaled steroids and the risk of hospitalization for asthma. JAMA. 1997;277:887–91. [PubMed] [Google Scholar]
- 19.Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332–6. doi: 10.1056/NEJM200008033430504. [DOI] [PubMed] [Google Scholar]
- 20.Boushey HA, Sorkness CA, King TS, Sullivan SD, Fahy JV, Lazarus SC, et al. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med. 2005;352:1519–28. doi: 10.1056/NEJMoa042552. [DOI] [PubMed] [Google Scholar]
- 21.Rand CS, Bildberback A, Schiller K, Edelman JM, Hustad CM, Zeiger RS, et al. Adherence with montelukast or fluticasone in a long-term clinical trial: results from the Mild Asthma Montelukast Versus Inhaled Corticosteroid Trial. J Allergy Clin Immunol. 2007;119:916–23. doi: 10.1016/j.jaci.2006.12.664. [DOI] [PubMed] [Google Scholar]
- 22.Jones CA, Clement LT, Morphew T, Kwong KY, Hanley-Lopez J, Lifson F, et al. Achieving and maintaining asthma control in an urban pediatric disease management program: the Breathmobile Program. J Allergy Clin Immunol. 2007;119:1445–53. doi: 10.1016/j.jaci.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 23.Rand CS, Wise RA. Measuring adherence to asthma medication regimens. Am J Respir Crit Care Med. 1994;149(suppl):S69–76. doi: 10.1164/ajrccm/149.2_Pt_2.S69. [DOI] [PubMed] [Google Scholar]
- 24.Simmons MS, Nides MA, Rand CS, Wise RA, Tashkin DP. Unpredictability of deception in compliance with physician-prescribed bronchodilator inhaler use in clinical trial. Chest. 2000;118:290–5. doi: 10.1378/chest.118.2.290. [DOI] [PubMed] [Google Scholar]
- 25.The Coronary Drug Project Research Group. Influence of adherence to treatment and response of cholesterol on mortality in the coronary drug project. N Engl J Med. 1980;303:1038–41. doi: 10.1056/NEJM198010303031804. [DOI] [PubMed] [Google Scholar]