Abstract
A limited number of studies have investigated language in Huntington's disease (HD). These have generally reported abnormalities in rule-governed (grammatical) aspects of language, in both syntax and morphology. Several studies of verbal inflectional morphology in English and French have reported evidence of over-active rule processing, such as over-suffixation errors (e.g., walkeded) and over-regularizations (e.g., digged). Here we extend the investigation to noun inflection in Hungarian, a Finno-Ugric agglutinative language with complex morphology, and to genetically proven pre-symptomatic Huntington's disease (pre-HD). Although individuals with pre-HD have no clinical, motor or cognitive symptoms, the underlying pathology may already have begun, and thus sensitive behavioral measures might reveal already-present impairments. Indeed, in a Hungarian morphology production task, pre-HD patients made both over-suffixation and over-regularization errors. The findings suggest the generality of over-active rule processing in both HD and pre-HD, across languages from different families with different morphological systems, and for both verbal and noun inflection. Because the neuropathology in pre-HD appears to be largely restricted to the caudate nucleus and related structures, the findings further implicate these structures in language, and in rule-processing in particular. Finally, the need for effective treatments in HD, which will likely depend in part on the ability to sensitively measure early changes in the disease, suggests the possibility that inflectional morphology, and perhaps other language measures, may provide useful diagnostic, tracking, and therapeutic tools for assessing and treating early degeneration in pre-HD and HD.
Keywords: pre-symptomatic Huntington's disease, basal ganglia, caudate nucleus, language production, regular and irregular morphology, Hungarian
Introduction
This study examines particular aspects of language processing in individuals with genetically proven pre-symptomatic Huntington's Disease (pre-HD) – specifically regular and irregular noun morphology in an agglutinative language, Hungarian. Although pre-HD individuals exhibit apparently intact motor, cognitive, and linguistic functioning, the underlying pathology may already be present (Stout, et al., 2011). Thus, carefully designed and sensitive language measures might reveal already-present impairments in pre-HD individuals. Such tests could have potential diagnostic value for predicting the onset of the symptoms, possibly allowing for appropriate timing of treatment. Moreover, since in the very early stages of HD, including in pre-HD, the neuropathology seems to be relatively restricted to the caudate nucleus and related structures (Harris, et al., 1999; Henley, et al., 2009; Kipps, et al., 2005; Kloppel, et al., 2008), the investigation of language in pre-HD may provide a useful avenue for examining the dependence of language on these structures. Because the present study focuses on Hungarian, an agglutinative language with complex morphology that has not been examined before in either HD or pre-HD, the results should be useful for testing the cross-linguistic validity of previous research on HD that has investigated similar aspects of language in both English (Longworth, Keenan, Barker, Marslen-Wilson, & Tyler, 2005; Ullman, et al., 1997) and French (Teichmann, Dupoux, Kouider, & Bachoud-Levi, 2006; Teichmann, et al., 2005). Finally, although previous studies of pre-HD have examined language-related tasks such as verbal fluency (Larsson, Almkvist, Luszcz, & Wahlin, 2008; Lawrence, et al., 1998) or artificial grammar (De Diego-Balaguer, et al., 2008), to our knowledge this is the first study that specifically examines morphology, or indeed any other particular component of the language system, in pre-HD.
Huntington's Disease (HD)
HD is a rare autosomal dominantly inherited progressive neurodegenerative disorder (Huntington Study Group, 1996). Abnormalities seems to be largely restricted to the caudate nucleus and related structures at early stages of the disease (Henley, et al., 2009; Kipps, et al., 2005; Kloppel, et al., 2008). The degeneration leads to involuntary choreiform (hyperkinetic) movements, and eventually to personality changes and dementia (Reiner, et al., 1988). The timing of symptom onset is variable, though it usually occurs around ages 35 to 45. The disease inevitably leads to death within 10 to 15 years. The mutation in HD involves the expansion of a trinucleotide (Cytosine-Adenine-Guanine) repeat number in the Huntingtin gene. In healthy individuals, the CAG repeat number ranges from 9 to 35, while in patients with HD the range is 39–121 (Kremer, et al., 1994; Rubinsztein, et al., 1996; The Huntington's Disease Collaborative Research Group, 1993). Individuals who demonstrate no apparent motor, cognitive and linguistic impairments but have positive genetic test result for the expanded triplicate repeat are called pre-symptomatic HD, or pre-HD (Nopoulos, et al., 2010). There is a need for effective treatments in HD. Such treatments will likely depend in part on the ability to sensitively measure early changes in the disease (e.g., to know when to begin administering medications). Although neuroimaging techniques can apparently pick up some neuropathology in pre-HD (Henley, et al., 2009; Kipps, et al., 2005; Kloppel, et al., 2008), it is not clear whether such techniques are more sensitive than behavioral measures. Moreover, evidence suggests that markers of disease progression, particularly at pre-clinical stages of the disease, are lacking (Witjes-Ane, et al., 2007). Given that previous evidence suggests that aspects of language, including morphology, may depend in part on the caudate nucleus (Beretta, et al., 2003; Teichmann, et al., 2006; Ullman, et al., 1997; Ullman & Pierpont, 2005), measures of morphological aspects of language might indeed be useful for revealing early stages of the disease, even in pre-HD.
Huntington's Disease and Language
Considerable evidence suggests that certain aspects of language are abnormal in HD. In particular, syntax seems to be compromised in the disorder. Studies of sentence processing in HD suggest impairments of syntax that are tied to caudate abnormalities (Teichmann, Dupoux, Cesaro, & Bachoud-Levi, 2008; Teichmann, et al., 2005). Similarly, studies of spontaneous speech in HD have found apparent syntactic deficits, characterized by the production of sentences with simplified grammatical structure (Illes, 1989; Murray & Lenz, 2001; Podoll, Caspary, Lange, & Noth, 1988). In contrast, the status of lexical/semantics in the disorder seems to be less clear. On the one hand, in one study HD subjects did not show word-finding difficulties or other semantic deficits in their spontaneous speech (Podoll, et al., 1988). However, another study reported that HD patients exhibited impaired performance at tasks involving certain aspects of lexical/semantics, in particular the Boston Naming Test and category fluency (Chenery, Copland, & Murdoch, 2002). Yet other studies have found impaired letter fluency in HD (Langbehn & Paulsen, 2007; Stout, et al., 2011). Similarly, research has suggested that both letter and category fluency appear to be impaired in pre-HD individuals (Larsson, et al., 2008).
Thus, the picture from these studies appears to be somewhat mixed. Syntax appears to be impaired, while the status of lexical/semantics remains less clear. However, one of the problems drawing such conclusions is that it is difficult to separately probe grammatical (rule-governed) aspects of language, such as syntax, and lexical/semantic aspects, while holding other factors constant (e.g., motivation, attention, phonological complexity, etc.).
To address this quite general problem, a large body of research has emerged that examines the distinction between regular and irregular inflectional morphology, such as in English past tenses. Irregularly inflected forms are at least partially idiosyncratic, in that they cannot be fully predicted by their stems (e.g., sing-sang, bring-brought, fling-flung), and thus must depend on some sort of stored (lexicalized) representation. In contrast, regulars are fully rule-governed (stem + -ed affix), and thus do not need to be stored, but rather can depend instead on rule-based (grammatical) composition. Since regulars and irregulars can be matched on many factors (e.g., frequency, phonological complexity), and can be tested in the same task in the same contexts, one can examine the distinction between irregular and regular morphology in order to probe lexical and grammatical aspects of language while holding other factors constant.
Whereas numerous studies have investigated regular and irregular morphology in various languages in other disorders (Ullman, 2004, 2008), only a handful of studies have examined this contrast in HD, and none in pre-HD. To our knowledge, these studies have thus far examined only verb-related morphology, specifically only in English past tense (Longworth, et al., 2005; Ullman, et al., 1997) and French present and future tenses (Teichmann, et al., 2006; Teichmann, et al., 2005). As we shall see, overall the preponderance of findings seems to suggest abnormal performance on rule-based processing but relative normal performance on lexical-based processing, as compared to healthy control subjects.
Ullman et al. (1997) tested 17 native-English speaking HD patients and 8 neurologically intact control subjects. Participants were asked to produce the past-tenses of English regular, irregular and novel (made-up) verbs. Error rates constituted the dependent variable. As compared to the control subjects, HD patients produced significantly more over-regularization errors (e.g., digged) and regular and novel verbs with over-affixations, as reflected in multiple affixes or syllabic affixes (e.g., walkeded, walk-id). Moreover, the number of both over-regularization and over-affixation errors correlated with the patients' level of hyperkinesia. In contrast, over-regularizations did not correlate with the patients' performance at lexical retrieval, as measured by the Boston Naming Test, suggesting that even though HD can lead to lexical retrieval problems (see above), these did not explain the over-regularization errors. Ullman et al. (1997) concluded that overall, the evidence suggests that the basal ganglia abnormalities in HD leading to hyperkinesias also lead to over-active rule use. Longworth et al. (2005) also investigated regular and irregular past-tense inflection in (mild and moderate) HD. Like Ullman et al. (1997), the patients made errors on both regular and irregular verbs, with the most common error being over-regularizations. They also examined priming, and found normal priming facilitation for regulars (e.g., walked primes walk) as well as irregulars (e.g., taught primes teach), though only the mild HD patients were examined, since the moderate HD patients were unable to perform the task.
Teichman and colleagues (2005) tested French verbal inflectional morphology in a production task in 30 native-French speaking HD patients (both early and later stages) and healthy control subjects. Participants were presented with regular and irregular French verbs and novel verbs that were either regular or “subregular” (i.e., they were irregular in not following the default, main rules). Compared to controls, morphology was relatively spared in HD, expect for subregular novel verbs, on which HD patients demonstrated impaired performance. Specifically, rather than apply the subregular (irregular) pattern, they often over-regularized by applying the main rule, or produced double suffixations (the excessive application of regular `–era' suffix, such as `garoust-er-era'). Agreeing with Ullman et al. (1997), they interpreted the pattern as a consequence of over-active rule use in HD. Along somewhat similar lines, Teichmann et al. (2006) examined regular and irregular inflectional morphology in HD with acceptability ratings and lexical decision, and found abnormalities in rule processing but not in lexical processing.
Thus, studies of inflectional morphology in HD have largely reported problems with rule processing, in particular over-active rules. However, it is not clear whether such problems might be found even in pre-HD, in which the neuropathology is more restricted to the caudate nucleus and related structures, or in other languages with more complex morphology such as Hungarian.
Hungarian noun morphology
In Hungarian, which is an agglutinative language with rich inflectional morphology, inflected forms consist of a stem followed by one or more distinct inflectional suffixes (Abondolo, 1988). In the present study, we focus on the singular and plural forms and nominative and accusative cases of nouns. The singular form involves zero morphology (no affixes), while the plural marker is the suffix –Vk (where -V marks a linking vowel whose phonology agrees with the stem, following the rules of vowel harmony). Nominative case is associated with the subject (the agent role) and also involves zero morphology. Accusative case is associated with the direct object (the patient/theme role) and involves the suffix -Vt. Thus, the singular nominative form of a noun involves the stem only (e.g., `kert'; garden), the plural nominative form consists of the stem plus the plural suffix (e.g., `kertek', `kert+ek'; garden+PL), the singular accusative form involves the stem plus the accusative suffix (e.,g., `kertet', `kert+et'; garden+ACC), and the plural accusative form consists of the stem plus both the plural suffix and the accusative suffix (e.g., `kerteket', `kert+ek+et'; garden+PL+ACC).
Hungarian nouns have different stem classes that can be grouped into regular and irregular nouns on the basis of their morpho-phonological behavior. Although both regular and irregular nouns take the suffixes described above, the two types of nouns differ in their stems. In regular nouns, the stem either does not change, or changes according to productive morpho-phonological rules such as vowel lengthening (Table 1). The morpho-phonological rules observed in regular nouns are productive, applying regularly to any noun form (including neologisms), provided that the general phonological contexts required for their application are met (Rounds, 2001). In contrast, Hungarian irregular nouns exhibit at least partially idiosyncratic morpho-phonological changes to their stems. For example, to create the plural form lovak `horses', the uninflected stem ló `horse' must undergo modification to lov prior to the attachment of the suffix –Vk. Table 1 illustrates the regulars and the irregulars in the four forms under investigation. For further details on Hungarian morphology, see (Rounds, 2001).
Table 1.
Examples of singular and plural forms of regular and irregular nouns (singular – SG; plural –PL; accusative – ACC).
| Type | SG → PL Nominative (stem + /Vk/) | SG Accusative (stem + /Vt/) | PL Accusative (stem + /Vk/ + /Vt/) | |
|---|---|---|---|---|
| Regular nouns | No change | kert → kert-ek1 | kert-et | kert-ek-et |
| `garden' - garden-PL | garden-ACC | garden-PL-ACC | ||
| Vowel lengthening | róka → rók-ák | rók-át | rók-ák-at | |
| `fox' - fox-PL | fox-ACC | fox-PL-ACC | ||
| Irregular nouns | Epenthetic | kéreg→ kérg-ek | kérg-et | kérg-ek-et |
| `bark' - bark-PL | bark-ACC | bark-PL-ACC | ||
| Shortening | madár → madar-ak | madar-at | madar-ak-at | |
| `bird' - bird-PL | bird-ACC | bird-PL-ACC | ||
| 'v'-inserting | ló → lov-ak | lov-at | lov-ak-at | |
| `horse' - horse-PL | horse-ACC | horse-PL-ACC | ||
The vowel 'e' is a linking vowel. Depending on the word stem, sometimes the stem may need a linking vowel in order to facilitate the pronunciation of the suffixed noun.
The present study
The present study fills several empirical gaps. First, to our knowledge, it is the first study to examine inflectional morphology in pre-symptomatic HD. The presence of any impairments in pre-HD would implicate the caudate nucleus and related structures in this language domain. Additionally, it might lead to useful diagnostic tests for identifying the onset of symptoms, as well as for following the progression of the disorder. Second, by investigating Hungarian, the study extends the investigation of inflectional morphology cross-linguistically to an agglutinative language that has greater morphological complexity than previously studied languages in HD, and that comes from a different language family (Finno-Ugric rather than Indo-European). Finally, whereas previous studies of inflectional morphology in HD have been restricted to verbal morphology, here we extend the investigation by examining noun morphology, to test the generalizeability of previously reported patterns. Although this was the first study of pre-HD, as well as the first of Hungarian morphology in (pre-)HD, we broadly expected errors that might reflect over-active rule use, such as some sort of over-affixation errors and over-regularizations.
Results
Seven genetically proven pre-HD participants and seven age- and education-matched healthy control subjects took part in the study (see Subjects in Methods). The relatively small number of pre-HD participants was due to the relative paucity of these subjects in Hungary. All subjects completed a Hungarian noun morphology production task (see Task in Methods). The production of the three types of morphologically complex forms were elicited from pictures: the accusative singular form of the noun, the nominative plural form, and the accusative plural form. Nominative singular forms of all nouns were also elicited, as a control condition to ensure that subjects were familiar with each word; all subjects achieved 100% accuracy on this condition. The dependent variables of the morphologically complex forms were accuracy and error rates for various types of errors (for details see Methods).
Statistical analyses were performed, first of all, with Mann-Whitney tests to compare performance (accuracy and number of errors of different error types) between the pre-HD and control groups, and second, with Wilcoxon tests to compare performance on regular vs. irregular nouns within each subject group. The interaction between the pre-HD and control groups and irregular and regular forms was analyzed by Bradley's collapsed and reduced technique (Sawilowsky, 1990). These non-parametric tests were employed because the dependent variables did not follow a normal distribution.
As shown in Table 2, the pre-HD group had lower accuracy than the control group over both noun types (93.8% vs. 100%, p = 0.001), as well as at both regulars (p = 0.02) and irregulars (p = 0.001). All pre-HD subjects made errors on irregulars, and four of the seven made errors on regulars. In contrast, the control group made no errors at all on either noun type; that is, they performed at ceiling. In addition, the pre-HD subjects were less accurate on irregular (89%) than regular (98%) nouns (Wilcoxon paired sample test: Z = −2.37, p = 0.02; see Figure 1). Analyzing the interaction between groups (pre-HD vs. control) and regularity (regular vs. irregular), Bradley's collapsed and reduced technique revealed that the difference between the performance on regular vs. irregular forms was significantly greater in the pre-HD group than in the controls (U(14) = −3.35, p<0.001).
Table 2.
Production of morphologically complex nouns (plural, accusative, plural + accusative) in pre-HD and control subjects.
| Pre-HD group (N=7) | Control group (N=7) | Group difference (Mann-Whitney test) | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (%) | Mean | Mean (%) | |||
| Irregular (39) | Accuracy (ló: lóvat, lovak, lovakat) | 34.71 (1.98) | 89.01 | 39 | 100 | U(14)=49, Z=−3.35, p=0.001** |
| Error Types | ||||||
| Substitution (*lovak for lóvat) | 0.43 (0.79) | 1.10 | 0 | 0 | U(14) = 17.5, Z = −1.47, p = 0.14 | |
| Under-suffixation (*lovak for lovakat) | 0.43 (0.54) | 1.10 | 0 | 0 | U(14) = 14.00, Z = −1.88, p = 0.06 | |
| Over-suffixation (*lovakat for lovak) | 1.29 (1.60) | 3.30 | 0 | 0 | U(14) = 10.50, Z = −2.24, p = 0.02* | |
| Over-regularization (*lót for lovat) | 2.14 (1.46) | 5.49 | 0 | 0 | U(14) = 0, Z = −3.36, p = 0.001** | |
| Regular (42) | Accuracy (kert: kertet, kertek, kerteket) | 41.29 (0.76) | 98.30 | 42 | 100 | U(14) = 38.50, Z = −2.26, p = 0.02* |
| Error Types | ||||||
| Substitution (*kertek for kertet) | 0.14 (0.38) | 0.34 | 0 | 0 | U(14) = 21.0, Z = −1.0, p = 0.32 | |
| Under-suffixation (*kertek for kerteket) | 0.14 (0.38) | 0.34 | 0 | 0 | U(14) = 21.0, Z = −1.0, p=0.32 | |
| Over-suffixation (*kerteket for kertek) | 0.43 (0.54) | 1.02 | 0 | 0 | U(14) = 14.00, Z = −1.88, p = 0.06 | |
| Total (81) | Accuracy | 76.00 (2.24) | 93.83 | 81 | 100 | U(14) = 49, Z = −3.36, p = 0.001** |
| Error Types | ||||||
| Substitution | 0.57 (0.79) | 0.71 | 0 | 0 | U(14) = 14.0, Z = −1.87, p = 0.06 | |
| Under-suffixation | 0.57 (0.79) | 0.71 | 0 | 0 | U(14) = 14.0, Z = −1.870, p = 0.06 | |
| Over-suffixation | 1.71 (1.80) | 2.12 | 0 | 0 | U(14) = 10.5, Z = −2.25, p = 0.02* | |
p < 0.05
p < 0.01
Figure 1.
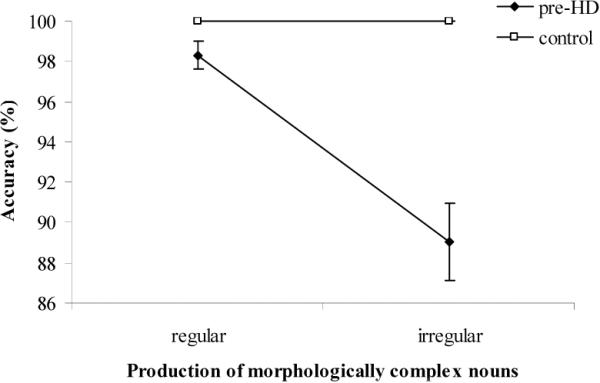
Accuracy (percentages) on regular and irregular morphologically complex noun forms by subject group. The control group performed at ceiling both on regular and irregular nouns, whereas the pre-HD group was less accurate on irregulars than regulars. Error bars represent Standard Error of Mean.
Further analyses were conducted separately for each of the four error types (substitution, under-suffixation over-suffixation, and over-regularization; see Table 2). For irregular forms, the error rate differences between the pre-HD and control groups did not reach significance either for substitution errors (p = 0.14) or under-suffixation errors (p = 0.06). In contrast, the pre-HD subjects produced significantly more over-suffixation errors (p = 0.02) and especially over-regularization errors (p = 0.001) than the controls. For regular forms, on which the pre-HD patients made very few errors, there were, not surprisingly, no significant differences between the pre-HD and control groups on any of the error types, although even in this condition the group difference in over-suffixation rates showed a trend (p = 0.06). Over both regular and irregular nouns, only over-suffixation errors showed a significant difference between the two groups (p = 0.02).
Discussion
This is the first study examining inflectional morphology in pre-HD, or Hungarian morphology in either HD or pre-HD. In the absence of any apparent motor, cognitive or other deficits, the pre-HD patients showed impairments at producing inflected forms for both regular and irregular nouns. Their most common and reliable errors were over-suffixation and over-regularization errors. Irregular nouns were produced less accurately than regular nouns.
The results seem to support the view that Huntington's disease is associated with over-active rule use (Ullman, et al., 1997), and that this pattern can be observed even in pre-HD. Both the over-suffixation and over-regularization errors are consistent with over-active affixation. Note that whereas in English, over-suffixation was characterized as multiple or syllabified affixation (see above), in Hungarian it was instead reflected by the attachment of the accusative suffix to correctly pluralized forms (e.g. *`lovakat'; horse+PL+ACC instead of `lovak'; horse+Pl, or `lovat'; horse+PL). The reasons for this difference are not clear, though it might be due to the fact that in Hungarian noun morphology more than one affix is available (in this case, Vk, Vt), and moreover these can be combined to create a well-formed word. Further investigations are needed to examine this issue.
The greater error rate observed on irregular than regular nouns is also unclear. However, it might be at least partly explained by additional processing required for irregulars as compared to regulars: whereas for both types of nouns the suffix is applied, for irregulars the stem is also transformed. Whether this transformation relies on lexical retrieval (of a memorized irregular stem) and/or rule processing in Hungarian remains unclear. Thus, whereas the data presented here strengthen previous findings of over-active rule use in (pre-)HD, they remain agnostic about lexical deficits. Therefore the present findings do not specifically demonstrate a dissociation between grammatical and lexical processes (Ullman et al., 1997; Ullman, 2004), though they are consistent with it. In contrast, certain aspects of the data appear to be inconsistent with the theoretical position that morphological errors on regulars are due not to morphological rule impairments but to phonological deficits, which are claimed to result in the phonological simplification of regulars in patients who have trouble with these forms (e.g., resulting in the utterance of walk instead of walked) (Bird, Lambon Ralph, Seidenberg, McClelland & Patterson, 2003). The present study seems to be problematic for this view, at least for (pre-)HD, since the over-suffixation errors made by the patients were more rather than less phonologically complex than the correct forms (e.g., kerteket for kertek).
Overall, the findings go some way towards addressing the empirical gaps that the study set out to address. The results demonstrate that inflectional morphology can indeed be impaired in pre-HD, moreover in similar ways as in HD. The observed pattern of errors supports the view that pre-HD, as well as HD, is associated with over-active rule use (Ullman, et al., 1997). The fact that these results were observed in pre-HD further implicates the basal ganglia in rule processing, and in particular the caudate nucleus and related structures. However, it remains unclear whether the basal ganglia themselves or the frontal regions they are heavily connected to are implicated in over-active rule use in pre-HD and HD: it may be disinhibited frontal cortex rather than the basal ganglia per se that are at least partly responsible for these errors (Ullman, 2006). The findings also suggest the cross-linguistic generalizability of the pattern of over-active rule use, from verbs to nouns, and even to Hungarian, an agglutinative language that is very different from English and French, and comes from a different language family. Finally, the results of this study suggest the possibility that inflectional morphology, and perhaps other language measures, may provide useful diagnostic tools for predicting the onset of symptoms in HD, possibly facilitating the appropriate timing of treatment.
Methods
Subjects
Fourteen adult participants took part in this study. All were right-handed native speakers of Hungarian. The pre-HD group consisted of 7 participants (2 females) with a mean age of 31.86 (SD = 11) and a mean education of 15.14 years (SD = 1.77). All pre-HD participants had a positive family history for HD and had genetic testing for the expanded triplet repeat in the “huntingtin” gene at IT-15. Six of the subjects had more than 39 CAG repeats (range from 42 to 50) and one subject was borderline with 37 CAG repeats. Based on standardized neurological examinations (including Unified Huntington's Disease Rating Scales, Huntington Study Group, 1996) administered independently by two neurologists specializing in HD at the University of Szeged Hospital, it was determined that the 7 subjects were free of clinical neurological and motor symptoms (UHDRS scores of zero for all pre-HD individuals). No subject showed evidence of dementia on the Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975), with all subjects (range 27–30) showing normal performance. The neurologically intact control group consisted of 7 subjects (2 females). This control group head a mean age of 32.29 (SD = 11.1) and a mean education of 15.14 years (SD = 1.57). All of them had negative family history for HD and other neurodegenerative diseases. The study was conducted in accordance with the Declaration of Helsinki. All procedures were carried out with the adequate understanding and written consent of the subjects and with the approval of University of Szeged.
Task
All subjects participated in an experiment involving a noun morphology production task. This is a picture-naming subtest of a longer protocol developed previously for Hungarian language-impaired children by Pléh, Palotás and Lorik (2002), and used previously in several studies (e.g., Nemeth, et al., 2008; Pleh, Lukacs, & Racsmany, 2003). The test items consisted of 42 regular nouns and 39 irregular nouns. The noun stems ranged between 2 and 3 syllables. Regular and irregular nouns were matched on word frequency (t(79) = −0.927, p = 0.357), word length in syllable (U(81) = 664.5, p = 0.115), and phonological complexity measured via the obstruction of consonants (i.e., two or more adjacent consonants) (χ2(1, N = 81) = 0.065, p = 0.798). The 81 test items were presented in the same pseudorandomized order to all subjects. For each item, subjects saw four pictures, each of which was designed to elicit one of four forms: the nominative singular form of the noun, the accusative singular form, the nominative plural form, and the accusative plural form, presented one at a time in that order, via computer. For example, to elicit the nominative singular of `fish', participants were presented with a picture showing a single fish; for the nominative plural, a picture depicting multiple fish; for the accusative singular, a picture of a fisherman catching a fish; and for the accusative plural, a picture of a fisherman catching many fish. Participants were prompted with questions such as “What are these?” or “What is the fisherman catching?”. Before beginning the task, participants were given instructions as well as three practice items.
Responses were recorded and coded by the experimenters. A response was coded as correct if it exactly matched the target form; otherwise, it was considered an error. Errors for regular nouns were classified into the following types: substitution, under-suffixation, and over-suffixation. Substitution errors entailed the production of the accusative suffix instead of the plural suffix, or vice versa. For example, for the noun kert `garden', the production of *kertet `garden-Acc' instead of kertek `garden-Pl'. Under-suffixation errors entailed the production of either just the plural suffix or just the accusative suffix in contexts where both the accusative and plural are required (e.g., *kertek `garden-Pl' or *kertet `garden-Acc' for kerteket `garden-Pl-Acc'). Over-suffixation errors entailed the production of both the accusative and plural suffixes in contexts where only the plural or only the accusative is required (e.g., *kerteket `garden-Pl-Acc' for kertek `garden-Pl' or kertet `garden-Acc'). Note that forms with more than one instance of the same affix, such as kertekek or kertetet, would also have been counted as over-suffixation errors, although no such forms were produced. Errors for irregular nouns consisted of the same types as for regular nouns except that they additionally included over-regularization errors, where an irregular noun such as ló `horse' was regularized, as in *lót instead of lovat `horse-Acc' or *lók instead of lovak `horse-Pl Therefore, over-regularization errors are only found on irregular nouns, when the plural affix is attached to the unchanged stem.
Highlights.
➢ Our study examined the language production in pre-symptomatic Huntington's Disease (pre-HD). > Impaired performance found on the production of morphologically complex nouns. > Language measures may provide diagnostic tools for assessing pre-HD.
Acknowledgements
Support for this project was provided by Bolyai Scholarship and the Hungarian National Research Fund (OTKA) K 82068 to DN, and NIH R01 MH58189 and NIH R01 HD049347 to MTU.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflict of interest and have no financial disclosure.
References
- Abondolo DM. Hungarian Inflectional Morphology. Akadémiai Kiadó; Budapest: 1988. [Google Scholar]
- Beretta A, Campbell C, Carr TH, Huang J, Schmitt LM, Christianson K, Cao Y. An ER-fMRI investigation of morphological inflection in German reveals that the brain makes a distinction between regular and irregular forms. Brain and Language. 2003;85(1):67–92. doi: 10.1016/s0093-934x(02)00560-6. [DOI] [PubMed] [Google Scholar]
- Bird H, Lambon Ralph MA, Seidenberg MS, McClelland JL, Patterson K. Deficits in phonology and past-tense morphology: What's the connection? Journal of Memory and Language. 2003;48(3):502–526. [Google Scholar]
- Chenery HJ, Copland DA, Murdoch BE. Complex language functions and subcortical mechanisms: evidence from Huntington's disease and patients with non-thalamic subcortical lesions. International Journal of Language and Communicantion Disorders. 2002;37(4):459–474. doi: 10.1080/1368282021000007730. [DOI] [PubMed] [Google Scholar]
- De Diego-Balaguer R, Couette M, Dolbeau G, Durr A, Youssov K, Bachoud-Levi AC. Striatal degeneration impairs language learning: evidence from Huntington's disease. Brain. 2008;131:2870–2881. doi: 10.1093/brain/awn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state: A practical ” method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Harris GJ, Codori AM, Lewis RF, Schmidt E, Bedi A, Brandt J. Reduced basal ganglia blood flow and volume in pre-symptomatic, gene-tested persons at-risk for Huntington's disease. Brain. 1999;122:1667–1678. doi: 10.1093/brain/122.9.1667. [DOI] [PubMed] [Google Scholar]
- Henley S, Wild EJ, Hobbs NZ, Scahill RI, Ridgway GR, MacManus D, Tabrizi SJ. Relationship between CAG repeat length and brain volume in premanifest and early Huntington's disease. Journal of Neurology. 2009;256(2):203–212. doi: 10.1007/s00415-009-0052-x. [DOI] [PubMed] [Google Scholar]
- Huntington Study Group The Unified Huntington's Disease Rating Scale: Reliability and Consistency. Movement Disorders. 1996;11(2):136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- Illes J. Neurolinguistic features of spontaneous language production dissociate three forms of neurogenerative disease: Alzheimer's, Huntington's, and Parkinson's. Brain and Language. 1989;37:628–642. doi: 10.1016/0093-934x(89)90116-8. [DOI] [PubMed] [Google Scholar]
- Kipps CM, Duggins AJ, Mahant N, Gomes L, Ashburner J, McCusker EA. Progression of structural neuropathology in preclinical Huntington's disease: a tensor based morphometry study. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76(5):650–655. doi: 10.1136/jnnp.2004.047993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppel S, Draganski B, Golding CV, Chu C, Nagy Z, Cook PA, Frackowiak RS. White matter connections reflect changes in voluntary-guided saccades in pre-symptomatic Huntington's disease. Brain. 2008;131:196–204. doi: 10.1093/brain/awm275. [DOI] [PubMed] [Google Scholar]
- Kremer B, Goldberg P, Andrew SE, Theilmann J, Telenius H, Zeisler J, Hayden MR. A worldwide study of the Huntington's disease mutation. The sensitivity and specificity of measuring CAG repeats. The New England Journal of Medicine. 1994;330(20):1401–1406. doi: 10.1056/NEJM199405193302001. [DOI] [PubMed] [Google Scholar]
- Langbehn DR, Paulsen JS. Predictors of diagnosis in Huntington disease. Neurology. 2007;68(20):1710–1717. doi: 10.1212/01.wnl.0000261918.90053.96. [DOI] [PubMed] [Google Scholar]
- Larsson MU, Almkvist O, Luszcz MA, Wahlin TB. Phonemic fluency deficits in asymptomatic gene carriers for Huntington's disease. Neuropsychology. 2008;22(5):596–605. doi: 10.1037/0894-4105.22.5.596. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Hodges JR, Rosser AE, Kershaw A, ffrench-Constant C, Rubinsztein DC, Robbins TV, Sahakian BJ. Evidence for specific cognitive deficits in preclinical Huntington's disease. Brain. 1998;121:1329–1341. doi: 10.1093/brain/121.7.1329. [DOI] [PubMed] [Google Scholar]
- Longworth CE, Keenan SE, Barker RA, Marslen-Wilson WD, Tyler LK. The basal ganglia and rule-governed language use: evidence from vascular and degenerative conditions. Brain. 2005;128(3):584–596. doi: 10.1093/brain/awh387. [DOI] [PubMed] [Google Scholar]
- Murray LL, Lenz LP. Productive syntax abilities in Huntington's and Parkinson's diseases. Brain and Cognition. 2001;46(1–2):213–219. doi: 10.1016/s0278-2626(01)80069-5. [DOI] [PubMed] [Google Scholar]
- Nemeth D, Dye C, Gardian G, Janacsek K, Sefcsik T, Klivenyi P, Ullman MT. Functional morphology in pre-symptomatic Huntington's Disease. In: Grosvald M, Soares D, editors. Western Conference on Linguistics. Vol. 19. University of California; Davis: 2008. pp. 241–251. [Google Scholar]
- Nopoulos PC, Aylward EH, Ross CA, Mills JA, Langbehn DR, Johnson HJ, Paulsen JS. Smaller intracranial volume in prodromal Huntington's disease: evidence for abnormal neurodevelopment. Brain. 2010;134:137–142. doi: 10.1093/brain/awq280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleh C, Lukacs A, Racsmany M. Morphological patterns in Hungarian children with Williams syndrome and the rule debates. Brain and Language. 2003;86(3):377–383. doi: 10.1016/s0093-934x(02)00537-0. [DOI] [PubMed] [Google Scholar]
- Pléh C, Palotás G, Lorik J. A new screening method for Hungarian language impaired children. Akadémiai (in Hungarian); Budapest: 2002. [Google Scholar]
- Podoll K, Caspary P, Lange HW, Noth J. Language functions in Huntington's disease. Brain. 1988;111:1475–1503. doi: 10.1093/brain/111.6.1475. [DOI] [PubMed] [Google Scholar]
- Reiner A, Albin R, Anderson K, D'amato C, Penney J, Young A. Differential loss of striatal projection neurons in Huntington's Disease. Proceedings of the National Academy of Science USA. 1988;85:5733–5777. doi: 10.1073/pnas.85.15.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounds C. Hungarian: An essential grammar. Routledge; New York: 2001. [Google Scholar]
- Rubinsztein DC, Leggo J, Coles R, Almqvist E, Biancalana V, Cassiman JJ, Hayden MR. Phenotypic characterization of individuals with 30–40 CAG repeats in the Huntington disease (HD) gene reveals HD cases with 36 repeats and apparently normal elderly individuals with 36–39 repeats. American Journal of Human Genetics. 1996;59(1):16–22. [PMC free article] [PubMed] [Google Scholar]
- Sawilowsky SS. Nonparametric tests of interaction in experimental design. Review of Educational Research. 1990;60(1):91. [Google Scholar]
- Stout JC, Paulsen JS, Queller S, Solomon AC, Whitlock KB, Campbell JC, Aylward EH. Neurocognitive signs in prodromal Huntington disease. Neuropsychology. 2011;25(1):1–14. doi: 10.1037/a0020937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann M, Dupoux E, Cesaro P, Bachoud-Levi AC. The role of the striatum in sentence processing: evidence from a priming study in early stages of Huntington's disease. Neuropsychologia. 2008;46(1):174–185. doi: 10.1016/j.neuropsychologia.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Teichmann M, Dupoux E, Kouider S, Bachoud-Levi AC. The role of the striatum in processing language rules: Evidence from word perception in Huntington's Disease. Journal of Cognitive Neuroscience. 2006;18(9):1555–1569. doi: 10.1162/jocn.2006.18.9.1555. [DOI] [PubMed] [Google Scholar]
- Teichmann M, Dupoux E, Kouider S, Brugieres P, Boisse M-F, Baudic S, Bachoud-Levi AC. The role of the striatum in rule application: the model of Huntington's Disease at early stage. Brain. 2005;128(5):1155–1167. doi: 10.1093/brain/awh472. [DOI] [PubMed] [Google Scholar]
- The Huntington's Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72(6):971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Ullman MT. Contributions of memory circuits to language: The declarative/procedural model. Cognition. 2004;92(1–2):231–270. doi: 10.1016/j.cognition.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Ullman MT. Is Broca's area part of a basal ganglia thalamocortical circuit? Cortex. 2006;42(4):480–485. doi: 10.1016/s0010-9452(08)70382-4. [DOI] [PubMed] [Google Scholar]
- Ullman MT. The role of memory systems in disorders of language. In: Stemmer B, Whitaker HA, editors. Handbook of the Neuroscience of Language. Elsevier Ltd; Oxford, UK: 2008. pp. 189–198. [Google Scholar]
- Ullman MT, Corkin S, Coppola M, Hickok G, Growdon JH, Koroshetz W, Pinker S. A neural dissociation within language: Evidence that the mental dictionary is part of declarative memory, and that grammatical rules are processed by the procedural system. Journal of Cognitive Neuroscience. 1997;9(2):266–276. doi: 10.1162/jocn.1997.9.2.266. [DOI] [PubMed] [Google Scholar]
- Ullman MT, Pierpont EI. Specific language impairment is not specific to language: the procedural deficit hypothesis. Cortex. 2005;41(3):399–433. doi: 10.1016/s0010-9452(08)70276-4. [DOI] [PubMed] [Google Scholar]
- Witjes-Ane MN, Mertens B, van Vugt JP, Bachoud-Levi AC, van Ommen GJ, Roos RA. Longitudinal evaluation of “presymptomatic” carriers of Huntington's disease. J Neuropsychiatry Clin Neurosci. 2007;19(3):310–317. doi: 10.1176/jnp.2007.19.3.310. [DOI] [PubMed] [Google Scholar]


