Abstract
Delivery of bone marrow cells (BMCs) to the heart has substantially improved cardiac function in most rodent models of myocardial infarction (MI), but clinical trials of BMC therapy have led to only modest improvements. Rodent models typically involve intra-myocardial injection of BMCs from distinct donor individuals that are healthy, unlike autologous BMCs used for clinical trials that are from post-MI individuals. Using BMCs from post-MI donor mice, we discovered that recent MI impaired BMC therapeutic efficacy. MI led to myocardial inflammation and an increased inflammatory state in the bone marrow, changing the BMC composition and reducing their efficacy. Injection of a general anti-inflammatory drug or a specific interleukin-1 inhibitor to post-MI donor mice prevented this impairment. Our findings offer an explanation of why human trials have not matched the success of rodent experiments, and suggest potential strategies to improve the success of clinical autologous BMC therapy.
INTRODUCTION
Cell therapy for cardiac remodeling after myocardial infarction (MI) has been the focus of intense research and debate in the last several years (1,2). Delivery of bone marrow-derived cells (BMCs) to the infarcted myocardium has been reported to be therapeutic in the improvement of post-MI cardiac function in most animal experiments, improving pump capacity and myocardial wall thickness, and limiting infarct size, although the mechanism of the therapeutic effect has been controversial. BMCs may be therapeutic by playing a stem cell role and differentiating (or fusing) to regenerate cardiomyocytes, playing a paracrine role by delivering cytoprotective compounds or growth factors to the injured myocardium, or both. Importantly, we and others have shown that delivery of products of cell secretion can be therapeutic in a cell-free setting, and even injection of cell-free extract from BMC lysates can be beneficial to the same degree as intact cells for most functional parameters studied (3-5).
The results of human clinical trials have been less compelling than the preclinical rodent experiments (6-9). A modest improvement in cardiac function post-MI has been reported in some (6,7) but not all double-blind placebo-controlled clinical trials (8). Clearly, there is a need for a greater understanding of the mechanisms behind the therapeutic effects observed in rodents, and of what can thwart them in a clinical setting. Notably, harvest of bone marrow from mice requires euthanasia of the donor mouse, so rodent BMC therapy experiments cannot be autologous and typically involve healthy donor mice. However, clinical patients undergoing autologous BMC therapy have had a recent MI. Therefore, the cells being used in rodent experiments may be a poor approximation of actual BMCs used for clinical autologous cell therapy, regardless of the state of the recipient heart.
Supporting this hypothesis, various functional properties of certain populations of cells isolated from bone marrow or blood of patients with chronic ischemic heart disease are impaired (10,11). However, the effect of acute MI on the therapeutic properties of the bone marrow is relatively unexplored, other than efforts concentrating on molecular changes that prevent mobilization of cells like putative progenitor cells into the blood (12).
Acute cardiomyocyte death immediately initiates a robust host reaction involving regional myocardial inflammation and a systemic inflammatory response (13). This includes stimulation of the bone marrow, leading to an increased hematopoietic and putative progenitor cell migration from the bone marrow to the circulation (14-17) and resulting in cellular and molecular alterations within the bone marrow compartment (12). We therefore hypothesized that acute MI influences the therapeutic efficacy of the donor BMCs, irrespective of the state of the recipient heart. Using a rodent model in which the BMC donors and recipients are separate individuals, which is not practical in autologous human cell transplants, we show here that the therapeutic potential of BMCs is impaired in donors with MI, and that acute MI leads to an increased inflammatory state in the bone marrow that is responsible for the impairment of BMC-based therapy, in association with interleukin-1 (IL-1) mediated inflammatory response after MI.
RESULTS
Impairment of BMC therapeutic efficacy by donor MI
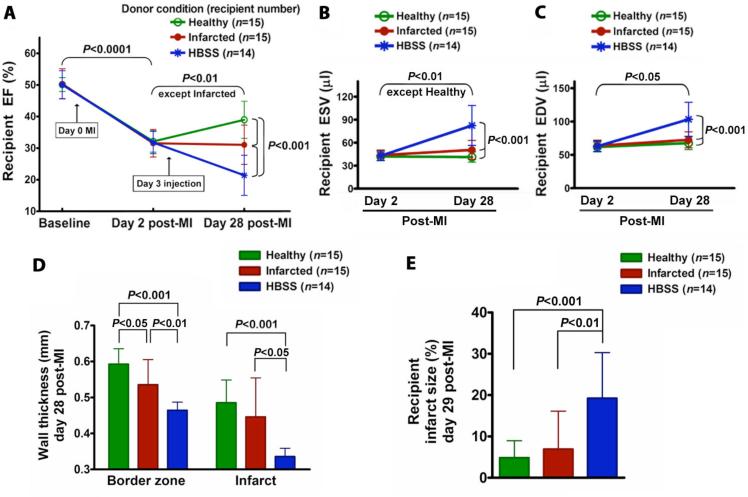
To determine if donor MI influences the therapeutic efficacy of BMCs for treatment of MI, we implanted BMCs into groups of infarcted recipient mice, keeping the recipient conditions constant but varying the donor conditions with respect to MI (no MI vs. 3 days post-MI). The recipients were all injected on day 3 post-MI (a stage of physiological response to MI comparable to that used in the clinic) using ultrasound-guided intra-myocardial injection (18). The recipient echocardiographic parameters at baseline and day 2 post-MI were similar between experimental groups. Differences in recipient myocardial function after implantation of BMCs from healthy and infarcted donors are shown in Fig. 1. In all groups, the recipient left ventricular ejection fraction (EF) declined uniformly from a baseline of 50.2±3.9% to 31.8±3.7% on day 2 post-MI before cell injection. Injection of Hank's buffered saline solution (HBSS) vehicle on day 3 was associated with continued deterioration of EF by day 28 post-MI. In contrast, injection of healthy donor BMCs significantly improved the 28 day post-MI EF (Fig. 1A), as we have reported previously (4). However, the 28 day post-MI EF in the infarcted donor group was not improved and significantly worse than the healthy donor group, despite good viability (98% assessed by trypan blue staining) in all BMCs assessed before implantation.
Fig. 1.
Effect of donor MI on BMC therapeutic efficacy. Donor BMCs were harvested from healthy and 3 day post-MI mice. Recipient and donor mice randomly underwent a parasternotomy MI by the left anterior descending artery ligation ~3 mm below the tip of the left atrium. All data are mean ± SD. (A) Recipient EF. Healthy donor BMCs significantly improved recipient 28 day post-MI EF. However, 28 day post-MI EF in the infarcted donor group was only preserved and significantly different from the healthy donor group. Recipient 28 day post-MI EF in the negative control HBSS group was significantly reduced and significantly different from the healthy and infarcted donor groups. Data are summarized numerically in Table S1. (B) Recipient ESV. (C) Recipient EDV. (D) Recipient anterior wall thickness at border zone and infarct area. (E) Recipient infarct size.
By day 28 post-MI, healthy donor BMCs, but not infarcted donor BMCs, prevented an increase in the recipient end-systolic volume (ESV), although the ESV in both healthy and infarcted donor groups was significantly different from the deterioration seen in the group from HBSS (Fig. 1B). None of the donor BMC conditions prevented an increase in the recipient end-diastolic volume (EDV), although the 28 day post-MI EDV of groups receiving BMCs from healthy and infarcted donors was significantly smaller than in those receiving HBSS (Fig. 1C). In the healthy and infarcted donor groups, recipient anterior wall thickness at the infarct and border zone was significantly thicker and infarct size was significantly smaller than those in the HBSS group, and the border zone wall thickness showed a significant difference between healthy and infarcted donor groups (Fig. 1, D and E).
These results demonstrate that donor MI reduces BMC therapeutic efficacy, leaving them less capable of preventing the decline in recipient cardiac function post-MI.
Morphology and proliferation of implanted BMCs
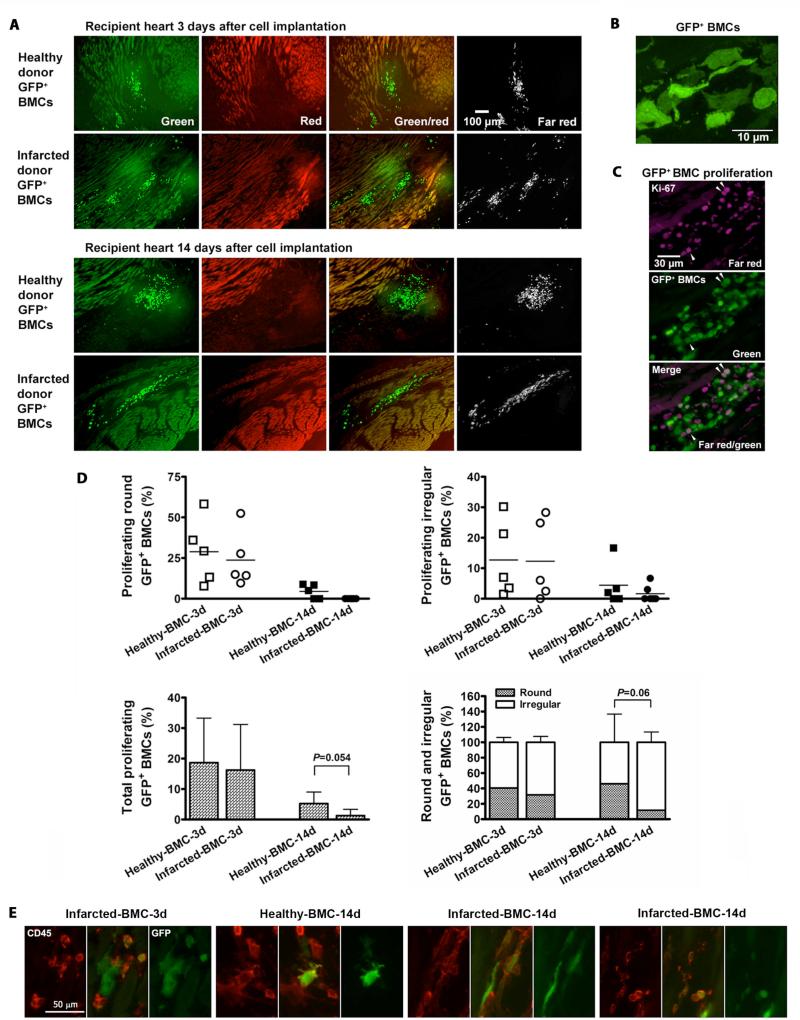
To track the implanted BMCs, we used healthy and infarcted donor mice that were transgenic for enhanced green fluorescent protein (GFP) for one experiment. Many round and irregular GFP+ BMCs from healthy and infarcted donors were found around the infarct border zone of recipient hearts on days 3 and 14 after cell injection (Fig. 2, A and B). On day 3 post-injection, there was no significant difference in proliferation between the healthy and infarcted donor BMCs; while on day 14 post-injection, the healthy donor BMCs showed a trend toward increased proliferation versus infarcted donor BMCs, in which proliferation was barely detectable; and there was also a trend on day 14 toward a greater percentage of irregular BMCs from infarcted donors versus healthy donors (Fig. 2, C and D, and fig. S1), although statistical significances were not reached. Round BMCs tended to be CD45+, while irregular BMCs were either CD45+ or CD45- with no apparent pattern based on donor condition (Fig. 2E). No evidence of differentiation into cardiomyocytes was observed, consistent with our previous findings (4).
Fig. 2.
Implanted donor BMCs in recipient infarcted hearts. BMCs harvested from GFP+ transgenic mice under healthy and 3 day post-MI conditions were injected into myocardium of 3 day post-MI wild-type mice. (A) By detection of endogenous GFP fluorescence and immunofluorescence staining for GFP using a far-red secondary antibody and no red fluorophores, GFP+ cells were identified as those visible only by green and far red fluorescence but not red fluorescence. (B) Confocal imaging reveals two categories of GFP+ BMC morphology, round and irregular. (C) Ki-67 immunofluorescence (magenta) revealed proliferation in a subset of round and irregular GFP+ BMCs. Arrows show proliferating GFP+ BMCs. (D) Percentage distribution of proliferating and total (including non-proliferating) round and irregular GFP+ BMCs of healthy and infarcted donors on days 3 and 14 post-injection. (E) Examples of CD45+ and CD45- implanted BMCs, taken from (left to right) MI d3, healthy d14, and two examples from MI d14. For these images only, brightness was optimized to demonstrate presence or absence of stain, rather than keeping it constant for all images. Green=GFP; red=CD45.
Greater impairment of BMCs by severe donor MI
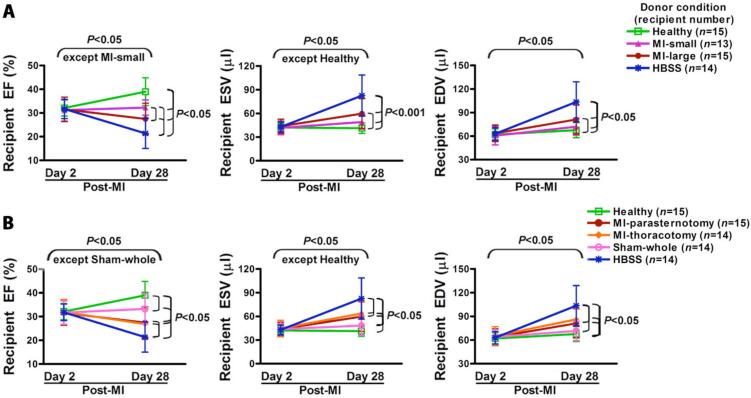
If donor MI was the cause of reduction in BMC therapeutic efficacy, we reasoned that donor infarcts of greater severity would lead to greater impairment. To test this, donor mice randomly underwent the MI surgery but with ligation at different positions of the left anterior descending (LAD) artery to induce different infarct sizes (described in Supplementary Methods). Donor BMCs were harvested from these mice on day 3 post-donor-MI, and injected into recipient hearts on day 3 post-recipient-MI. BMCs from donors with small infarcts were only partially impaired while BMCs from donors with large infarcts were more impaired (Fig. 3A).
Fig. 3.
Influence of donor MI severity on BMC therapeutic impairment. All data are mean ± SD. (A) Injection of BMCs from small-MI donors led to preservation of recipient EF on day 28 post-MI; whereas injection of BMCs from large-MI donors resulted in a significant decrease in the 28 day post-MI EF and there was a significant difference between small and large MI donor groups (P<0.05). Moreover, the injection of BMCs from healthy donors led to recipient 28 day post-MI EF that was significantly higher than that of the small and large MI donor BMC groups and the HBSS group. The recipient 28 day post-MI EF in the small and large MI donor BMC groups was significantly higher than that of the HBSS group (P<0.05). (B) Thoracotomy MI led to the same reduction in BMC therapeutic efficacy as the original parasternotomy MI and the sham surgery slightly reduced the BMC therapeutic efficacy. The data are summarized numerically in Table S2.
We next tested whether impairment of BMCs was specifically due to severe donor MI and/or simply reflected the consequences of surgical manipulation in this model. Donor mice underwent either thoracotomy (no bone injury) MI, parasternotomy MI, or sham surgery that damaged skin, bone, and myocardium but did not occlude coronary blood flow (described in Supplementary Methods). Donor BMCs were harvested from these mice on day 3 post-donor-MI or post-sham surgery, and injected into recipient hearts on day 3 post-recipient-MI. As shown in Fig. 3B, both MI procedures led to similar reductions in BMC therapeutic efficacy, which was only slightly reduced by sham surgery.
Evidence of systemic inflammatory state and pro-inflammatory changes in bone marrow composition after MI
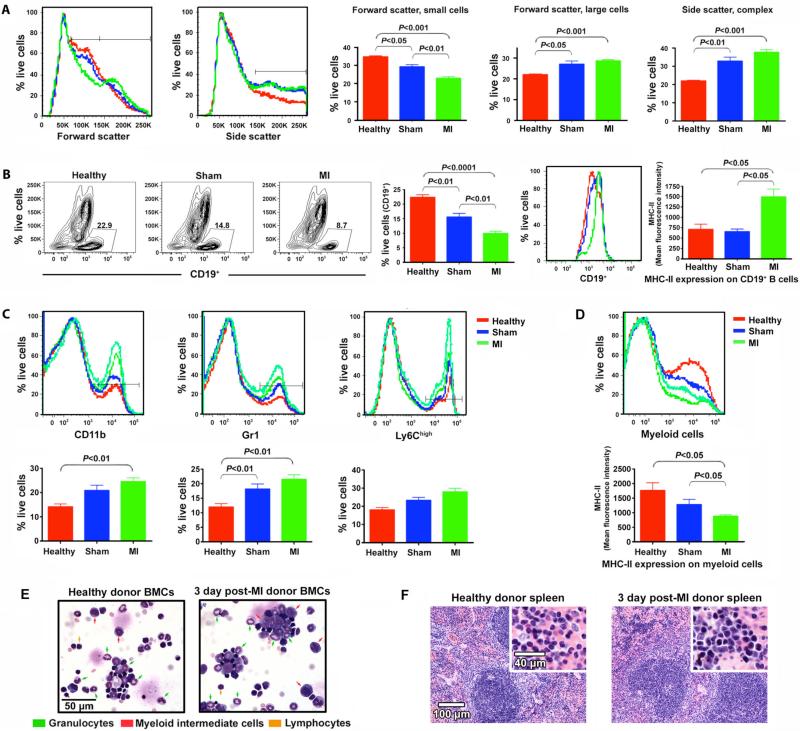
We hypothesized that the decreased efficacy of harvested BMCs might be a result of MI-induced systemic inflammatory changes that influence the composition of the bone marrow. To confirm that BMC therapeutic efficacy was impaired during a period of active inflammation, both in the bone marrow and systemically, we first asked whether donor BMC composition is altered at 3 days post-MI in a manner consistent with an acute inflammatory response. BMCs harvested at this time were quantitatively analyzed by flow cytometry. Comparison of forward and side characteristics, markers of cell size and granularity respectively, revealed a decrease in small cells and an increase in larger cells post-MI BMCs (Fig. 4A). Both sham and post-MI BMCs had increased side scatter properties relative to BMCs from healthy mice. Consistent with loss of a population of small cells, there was a significant decrease in CD19+ B cells in post-MI mice relative to controls. Interestingly, major histocompatibility complex class II (MHC-II) expression was upregulated on these B cells (Fig. 4B). The increase in larger cells with increased side scatter properties suggests an impact on the myeloid compartment in the bone marrow. We thus compared the frequency of myeloid subsets as defined by the cell surface expression of the myeloid markers CD11b, Gr1, and/or Ly6Chigh. The frequency of myeloid cells was increased in both sham and post-MI mice relative to healthy controls (Fig. 4C). However, as reported in human MI patients (19) and consistent with MI-induced systemic inflammation, MHC-II expression was downregulated on myeloid cells in post-MI but not sham treated bone marrow (Fig. 4D).
Fig. 4.
MI-induced systemic inflammatory changes and alteration in bone marrow composition. (A) BMCs from healthy, sham, or post-MI donors (3 days post-MI/sham) were analyzed by flow cytometry. Data is representative of two independent experiments, each involving 3-4 mice/condition. For each parameter, histograms contain a representative curve from individual healthy (red), sham (blue), or post-MI (green) donors in the live gate. Each graph represents the results of 6-7 mice/condition. Error bars = SEM. (B) Contour plot depicts frequency of CD19+ B cells for individual mice. The right-most histogram depicts MHC-II expression on CD19+ cells from the contour plot. Graphs are as in A. (C) Representative histograms of CD11b, Gr1 and Ly6Chigh of BMCs in the live gate. Tracings from two post-MI mice are shown due to increased heterogeneity in this group. Graphs are as in A. (D) MHC-II expression on myeloid cells. (E) Cytospin preparations of BMCs of healthy and 3 day post-MI donor mice. Results are representative of three samples/group. (F) Representative spleen histology. Increased erythropoiesis is evident as dense collections of darkly staining nuclei in red pulp at upper left in post-MI image and higher-power view shows increased erythroid cells and scattered granulocytes post-MI.
The donor MI-induced inflammatory state was also reflected by an increase in immature intermediate myeloid cells in bone marrow cytospins (Fig. 4E), which showed a trend toward more of several classes of inflammatory cells (including mature granulocytes and myeloid cells at an intermediate stage of maturation) in the BMCs from post-MI donors, although with the small number of donors analyzed statistical significance was not reached. To confirm that day 3 post-MI was a period of active systemic inflammation, we harvested spleens from healthy and infarcted mice to seek differences in inflammatory state. Upon blinded examination of tissue sections by a trained pathologist (S.K.), the spleens of the MI donors were observed to have increased intrasplenic hematopoiesis, especially erythropoiesis (Fig. 4F), with modest germinal center reactive changes in the white pulp, relative to the healthy donor spleens; suggestive of increased systemic inflammatory response in the MI donors. While we do not propose that these changes specifically are responsible for the impairment of donor BMC therapeutic efficacy in recipients, these changes, which coincide with the point at which donor BMCs were therapeutically impaired, are consistent with a systemic inflammatory response in progress.
Timing correlations between BMC impairment and inflammatory response post-donor-MI
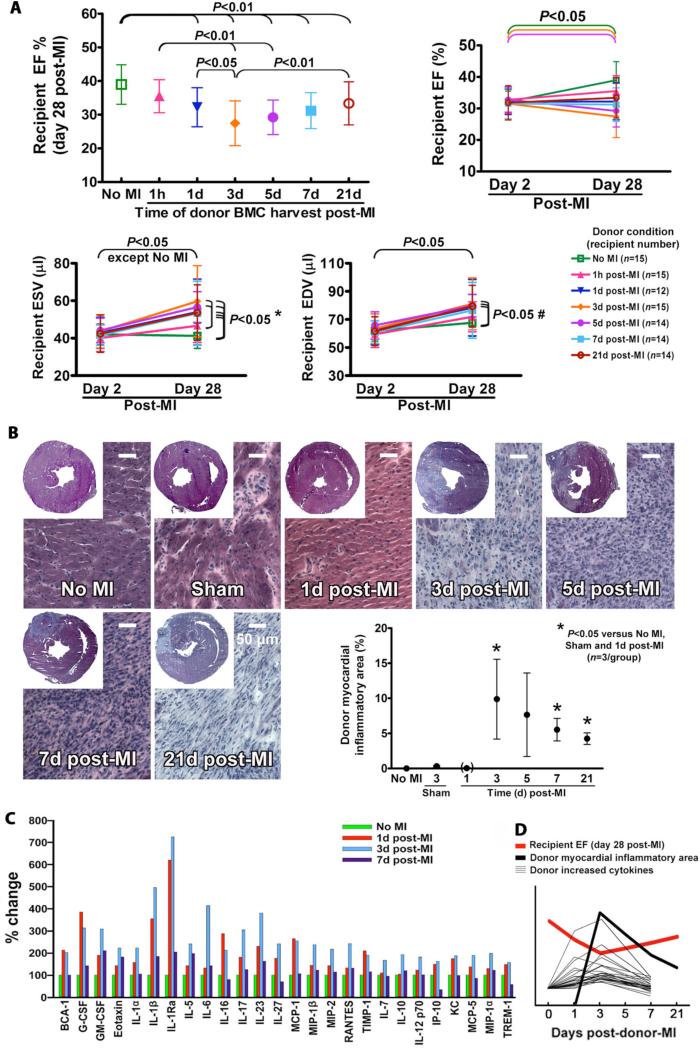
If acute inflammation caused by MI is responsible for the observed reduction in BMC therapeutic efficacy, then the therapeutic impairment of BMCs should develop and then resolve with increasing time of BMC harvest post-donor-MI as the inflammatory response develops and resolves. BMCs were harvested from donor mice undergoing the parasternotomy large MI at varying times for 21 days post-donor-MI, along with a no-MI donor positive control. Recipients always received BMC injections on day 3 post-recipient-MI, and EF was always measured on day 28 post-recipient-MI (Fig. 5A). The therapeutic efficacy of BMCs harvested 1 hour post-donor-MI were similar to those from non-MI donors but only preserved recipient 28 day post-MI function relative to day 2; BMCs harvested 1 day post-donor-MI were significantly impaired. BMCs harvested at 3 and 5 days post-donor-MI were the most impaired, leading to a significant decline in recipient 28 day post-MI function. BMCs from 7 day post-MI donors were still impaired, but less so. BMCs from 21 day post-MI donors showed even less impairment and a significant increase in efficacy versus 3 day post-MI donors. BMCs harvested from 1 hour to 21 day post-MI donors did not prevent an increase in the ESV and EDV (Fig. 5A and Table S2). These results show that donor BMCs harvested at varying times post-MI are increasingly impaired over the first 3 days but are less impaired when harvested after 7 days, consistent with the time course of a post-MI inflammatory response as reported in the literature (20,21).
Fig. 5.
Correlations between BMC impairment and inflammatory response post-donor-MI. All data are mean ± SD. (A) Time courses of BMC impairment post-donor-MI. Therapeutic changes in BMCs were increasingly impaired over the first 3 days post-donor-MI but were less impaired after 7 days. * P<0.05 (no MI donors versus 1d, 3d, 5d, 7d and 21d post-MI donors; 1h post-MI donors versus 3d and 5d post-MI donors); # P<0.05 (no MI donors versus 3d, 5d and 21d post-MI donors). (B) Development of localized myocardial inflammation post-donor-MI detected by hematoxylin and eosin staining. Note that representative sections shown are from border zone rather than infarct scar. (C) Increased changes in pro-inflammatory cytokines and other proteins at varying times post-donor-MI in serum. Abbreviations of cytokines are shown in fig. S2. (D) A summarized illustration originated from (A), (B) and (C) shows a consistent correlation between time courses post-donor-MI of BMC therapeutic impairment and subsequent restoration (red line, EF), appearance of donor localized myocardial inflammation (black line, percent volume of LV), and elevation and subsequent reduction in serum levels of increased pro-inflammatory cytokines and other proteins (thinner black lines). Because these are relative, units are not shown on the Y-axis.
To confirm that the time course of donor BMC impairment post-MI is consistent with the development of donor myocardial inflammation specifically in our mice, the extent of local inflammation was determined over time by estimating the percent volume of the left ventricle composed of inflammatory infiltrate in post-MI and sham surgery hearts. A concentrated inflammatory cell infiltrate was evident by hematoxylin and eosin staining in the infarct beginning on day 3 post-MI, which gradually lessened over subsequent days (Fig. 5B). There was no measurable concentrated area of inflammation on day 1, so the assay assigned a value of 0 at this time point, but there was an increase in cellular infiltration between cardiomyocytes, indicating that inflammatory response had indeed started by day 1. Sham-operated hearts showed only a few extremely small regions of inflammation corresponding to the suture being passed through myocardium without ligation. This histological study indicates that post-MI myocardial inflammation significantly increases over the first 3 days, consistent with the time course of BMC impairment.
We anticipated that the progressive increase in localized myocardial inflammation would reflect changes over time in the systemic inflammatory response, providing the link between tissue injury in the donor heart and changes in the donor bone marrow. To test this, we measured serum levels of a panel of cytokines at varying times post-donor-MI. Serum at 1 and 3 days post-MI but not 7 days showed an increase in several pro-inflammatory cytokines, chemokines, and related proteins (Fig. 5C and fig. S2). Notably, there was an increase in three members of IL-1 family at 1 to 3 days post-MI relative to no-MI controls. IL-1β and IL-1 receptor antagonist (IL-1Ra) were increased by 354% to 725% at 1 to 3 days post-MI; but IL-1α was transiently increased by 223% only on day 3 post-MI. A number of others such as granulocyte colony stimulating factor (G-CSF), IL-6, and monocyte chemoattractant protein (MCP)-1 were also transiently increased on day 1 and/or day 3; most of which were back to near baseline levels by day 7. Several cytokines showed no notable changes, and several others decreased over time post-MI (Fig. S2). These observed changes in cytokine levels further confirm that donor BMC impairment post-MI peaks and resolves over the natural time course of the inflammatory response, consistent with the hypothesis that donor localized myocardial inflammation leads to pro-inflammatory changes in the bone marrow that impair therapeutic efficacy of BMCs harvested during this time (Fig. 5D).
IL-1-mediated inflammatory response as a mechanism for donor MI induction of BMC impairment
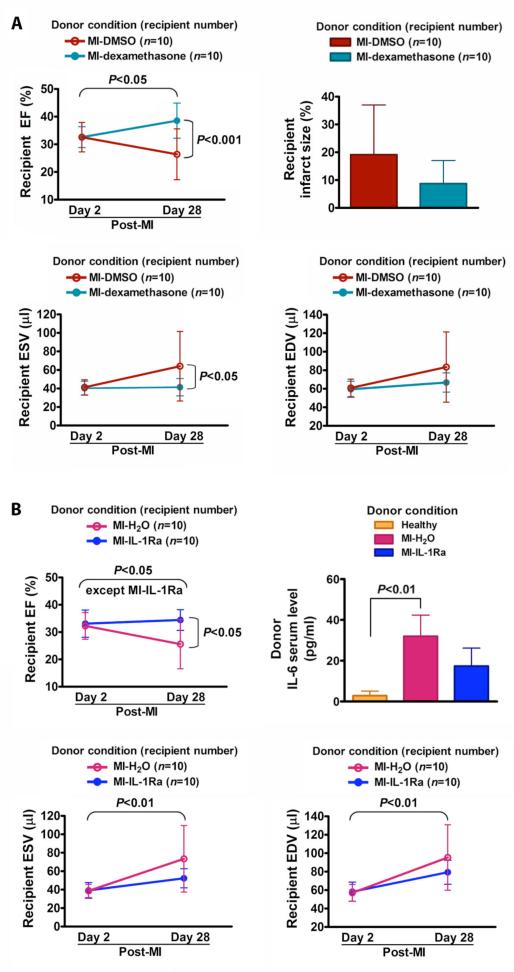
We endeavored to establish a mechanistic link of pro-inflammatory cytokine signaling between the post-MI inflammation and BMC impairment by injection of anti-inflammatory drug or inhibitor into the infarcted donor mice. We used a broad-spectrum anti-inflammatory drug, dexamethasone; and an IL-1 inhibitor, IL-1Ra (a receptor antagonist), to see if the donor inflammatory response mediated by the activated inflammatory cytokines (such as IL-1) is associated with the donor BMC impairment. As expected, BMCs from vehicle (DMSO)-injected post-MI donors did not prevent a significant decline in recipient 28 day post-MI EF (26.4±9.2% versus 32.6±5.3% for day 2, P<0.05), similar to non-injected post-MI donor BMCs. However, BMCs from dexamethasone-injected post-MI donors improved recipient 28 day post-MI EF (38.5±6.3% versus 26.4±9.2% for DMSO-injected post-MI donors, P<0.001), comparable to the therapeutic effect of implanting no-MI donor BMCs, and showed a trend toward reduced recipient infarct size although significance was not achieved (Fig. 6A). This demonstrates that post-MI donor inflammation plays a role in the therapeutic impairment. In testing effects of the IL-1 inhibitor, BMCs from vehicle (H2O)-injected post-MI donors also did not prevent a significant decline in recipient 28 day post-MI EF (25.5±8.9% versus 32.2±4.8% for day 2, P<0.05). Notably, BMCs from IL-1Ra-injected post-MI donors improved recipient 28 day post-MI EF (34.3±3.8% versus 25.5±8.9% for H2O-injected post-MI donors, P<0.05; Fig. 6B). As a confirmation of bioactivity, serum levels of IL-6 were reduced in IL-1Ra-injected post-MI donors, although statistical significance was not reached due to the small group size. These findings suggest that MI-induced BMC impairment is caused by a process inhibitable by dexamethasone (22) or IL-1Ra, such as IL-1-mediated inflammation.
Fig. 6.
Prevention of BMC impairment by injection of anti-inflammatory drug or inhibitor to MI donor mice. All data are mean ± SD. (A) Preventive effect of dexamethasone administrated to post-MI donor mice on BMC impairment. BMCs were harvested from 3 day post-donor-MI mice that were administered dexamethasone or DMSO (vehicle) immediately after the left anterior descending artery ligation, and 24 and 48 hours post-MI. (B) Preventive effect of IL-1Ra injected into donor mice pre- and post-MI on BMC impairment. BMCs were harvested from 3 day post-donor-MI mice that received IL-1Ra or H2O (vehicle) daily for 5 days (2 days pre-MI, 1 hour pre-MI, and 24 and 48 hours post-MI).
DISCUSSION
Our results indicate that donor MI-induced inflammatory response leads to the therapeutic impairment of BMCs, making them less able to prevent a decline in cardiac function when implanted into post-MI recipient hearts. We and others have demonstrated that BMCs from healthy donor animals exert a therapeutic effect (3,4,9,23,24). However, in human autologous BMC therapy clinical trials (6,7), in which the donor BMCs and recipient heart belong to the same individual, the effect of MI on the (donor) BMCs cannot be distinguished from the effect of MI on the recipient heart. Notably, the experiments described here with inbred mice used different animals as donors and recipients, which enabled us to vary the disease state of the donors while keeping the recipient conditions constant throughout. As a result, any differences between groups were entirely due to differences in the donor BMCs, a situation that cannot be assessed in patients receiving autologous cells because they are their own donors. Our results reveal that one reason that clinical autologous cell trials have shown less obvious therapeutic effects than most rodent experiments may be that the BMCs used in the human acute MI trials are impaired by the post-MI condition of the patients.
Literature suggests that chronic disease conditions can cause reductions in functional properties of cells from the bone marrow and peripheral blood (10,25,26). However, the acute nature of the BMC response to donor MI is unlikely to be explained thoroughly by these functional declines. Because MI is a highly inflammatory event that occurs rapidly and causes inflammatory changes in the bone marrow (20,21,27), we hypothesized that these inflammatory changes underlie the reduction in BMC therapeutic efficacy. It is well-known that acute MI activates innate immune mechanisms immediately initiating the host inflammatory response that increases the number or activation state of inflammatory cells in the circulation and the infarcted heart itself (13), as well as distant sites such as the spleen (28). Moreover, donor bone marrow on day 3 post-MI showed several characteristics consistent with an inflammatory reaction. Notably, there is a consistent correlation between the time courses post-MI of impairment and subsequent restoration of BMC therapeutic efficacy, appearance of inflammatory infiltrate in the heart, and elevation and subsequent reduction in levels of many pro-inflammatory cytokines, which supports a link between the inflammatory response and the therapeutic changes in the BMCs (Fig. 5D).
Moreover, the therapeutic impairment induced by donor MI is prevented by injection of either a broad-spectrum anti-inflammatory drug or an IL-1 inhibitor into the infarcted donor mice, which suggests the existence of an inflammatory cytokine signaling link between the infarcted heart and the bone marrow. Following MI, dying myocytes trigger an exuberant systemic and loco-regional inflammatory response through activating the nuclear factor-κB system, which induces activation and upregulation of chemokines, as well as release of many pro-inflammatory cytokines such as IL-1β, IL-6, and tumor necrosis factor-α. IL-1 signaling is known to play a significant role in initiating and regulating the inflammatory response post-MI (13). Therefore, based on our finding that administration of exogenous IL-1 receptor antagonist (IL-1Ra) to the infarcted donor mice prevents the therapeutic impairment of BMCs and reduces the IL-6 serum level of the infarcted donors, we conclude that MI-induced IL-1-mediated inflammatory response leads to pro-inflammatory changes in donor bone marrow composition and reduced BMC therapeutic efficacy. A caveat to this experiment is that measurement of cytokines in serum rather than plasma can be confounded by cytokines released during the clotting process, so absolute cytokine levels as measured may be inaccurate. However, the relative change in levels over time normalized to the healthy baseline value clearly shows the expected increase and decrease of members of the IL-1/6 pathway in agreement with several published reports (29-31). Because the more general anti-inflammatory drug dexamethasone is more effective than IL-1Ra in preventing the BMC impairment, other pathways may also play a role. It should be emphasized that we do not propose anti-inflammatory drug treatment as a clinical solution due to known dangers of anti-inflammatory drugs in post-MI patients (32), but these experiments clearly illuminate a mechanistic link between inflammation and the reduced BMC efficacy.
This might result in an increased number or activation state of inflammatory cells among the BMCs that get implanted into the infarcted recipient heart. In support of this reasoning, the day 3 post-MI BMCs include more Ly6Chigh inflammatory myeloid cells. Ly6Chigh monocytes are reported to be the pro-inflammatory arm of the monocyte response to MI (33), and might impair healing when implanted into the infarcted myocardium. The decrease in MHC-II expression was also intriguing. This has been reported in post-MI human patients (19) and would be consistent with the increase in the immunosuppressive cytokine IL-10 observed at day 3 in post-MI mice. Alternatively, local signals from the MI that trigger the egress of potentially beneficial cells from the bone marrow could also reduce therapeutic efficacy of the remaining BMCs. Accordingly, while most cytokines peaked on day 3 post-MI, a small number of cytokines induced by MI such as G-CSF and MCP-1 showed an increase beginning on day 1. G-CSF and MCP-1 have the potential to enhance cell migration and have been reported to mediate mobilization of bone marrow stem cells in experimental studies (34-36) and clinical trials (37). Hence, it is possible that the BMCs on day 3 post-MI are less therapeutic due to mobilization of stem cells and other cells away from the bone marrow. A third possibility is that, because we transfer a constant total number of cells, the local increase in inflammatory cells may dilute, out-compete, or even suppress the beneficial cells. While it is currently unclear which of these explanations, which are not mutually exclusive, predominates in the decreased efficacy of post-MI BMCs, it is clear that the composition of implanted BMCs is altered by the MI-induced inflammatory response. Future studies will focus on elucidating the exact changes that occur within this cellular compartment and how each of those changes influence the therapeutic effects of the implanted BMCs.
In summary, we have shown that acute MI results in specific changes in the bone marrow, such that implantation of BMCs from these mice into infarcted hearts of other mice is less able to prevent the decline in cardiac function. The impairment in therapeutic efficacy caused by donor MI correlates well with the development and resolution of the acute inflammatory response, and is prevented by injection of IL-1Ra to the donor MI mice. Based on these results, we propose that the donor MI increases the inflammatory state of the BMCs, which alters their composition and/or activation state to leave them less therapeutic. Of clinical relevance, it has been reported in human trials that autologous BMC therapy is more effective if performed several days after acute MI (6,7), but our findings suggest that the optimal time for post-MI harvest of autologous BMCs may be different than the optimal time for the administration of such BMCs to the heart. Optimal therapy may require either that one consideration be prioritized over the other, or that steps are taken to prevent these changes in the bone marrow.
MATERIALS AND METHODS
All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco.
Donor and recipient mice
Male C57BL/6J mice at age 10 weeks (Jackson Laboratory, Bar Harbor, ME) were randomly assigned to BMC donor and recipient groups. Male C57BL/6-Tg (CAG-EGFP) 1Osb/J age 10 weeks mice (Jackson Laboratory) were used for GFP+ BMC tracking.
Myocardial infarction
MI was surgically induced as described previously (18). Briefly, mice were anesthetized with 2% isoflurane and received analgesics (buprenorphine 0.1 mg/kg, subcutaneous injection) at time of surgery. The heart was exposed via a parasternotomy and the LAD was permanently ligated ~3 mm below the tip of the left atrium. Recipient mice underwent the same parasternotomy MI and were under consistent conditions from experiment to experiment. The mortalities during and after MI surgery were 2% and 1%, respectively.
BMC harvest and injection
The protocol for BMC harvest and injection can be found in the Supplementary Methods. Each donor mouse provided BMCs for 5 recipient mice in order to have multiple donors per group and to aid in blinding. Recipient mice were always injected at 3 days post-MI. Injection of HBSS served as a negative control. The allocation of BMC treatment for MI recipients was random, and investigators were blinded to the identity of BMCs during harvest and injection. The quality of all injections was assessed according to ultrasound images as they occurred, and 98% of intramyocardial injections were judged to be optimal.
Echocardiography
Echocardiography of recipients anesthetized with 1.25% isoflurane was performed at baseline, 2 days post-MI (one day before BMC injection) and 28 days post-MI using a Vevo660 micro-ultrasound system (VisualSonics Inc., Toronto). Echocardiograms were obtained in 2-D mode at parasternal long-axis view to measure the left ventricular ESV and EDV. EF was calculated with the formula: EF (%) = [(EDV – ESV)/ EDV] × 100. Wall thickness was measured at the apical-segment (infarct) and mid-segment (infarct border zone) of the anterior wall and at the basal segment of the anterior and posterior walls, respectively. The investigator who performed echocardiograms and measured echocardiographic parameters was blinded to the identity of BMC treatment among groups. Echocardiographic parameters were re-measured by one additional blinded researcher and found to be consistent.
Immunofluorescent histology
The protocols for detection of implanted GFP+ BMCs, GFP+ BMC proliferation and detection of CD45+/GFP+ BMCs in recipient hearts can be found in the Supplementary Methods.
Flow cytometry
The fluorescent antibodies for cell-surface markers used for flow cytometry analysis can be found in the Supplementary Methods.
Quantitation of tissue inflammation
For quantitative analysis of post-MI myocardial inflammatory state, mouse hearts were arrested in diastole with saturated KCl injected into the left ventricular chamber and removed. Hearts were embedded in O.C.T. compound, frozen in a bath of 2-methylbutane with dry ice and stored at -80°C. Hearts were sliced transversely at 10 μm thickness with an interval of 500 μm between each section. Seven sections evenly distributed from apex to base of ventricle were stained with hematoxylin and eosin for inflammatory area analysis. Inflammatory area (%) = [area of cellular accumulation/(whole myocardial area – cavities)] ×100. Sections were read blinded and scored for the extent of inflammation.
Quantitation of myocardial infarct size
Mouse hearts were arrested in diastole, embedded in O.C.T. compound and frozen. Hearts were sliced transversely at 10 μm thickness with an interval of 300 μm between each section. Seven sections from apex to base of ventricle were stained with Masson trichrome for infarct size measurement by a midline arc length method (38). Sections were read blinded and scored for the extent of fibrosis.
Anti-inflammatory drug and inhibitor
Dexamethasone (Sigma-Aldrich) was dissolved in dimethyl sulfoxide (DMSO) at 50 mg/ml. All dilutions were made in sterile phosphate-buffered saline, and all injections (dexamethasone 2 mg/kg (22), 0.2 ml/mouse) were given intraperitoneally. The final concentration of DMSO was 0.5%.
Recombinant mouse IL-1Ra (Prospec-Tany Technogene Ltd., Israel) was dissolved in sterile H2O at 250 μg/ml. All injections (IL-1Ra 50 μg/0.2 ml/mouse) were given intraperitoneally.
Statistics
Variables in each group are presented as means ± SD. Differences are determined by two-way repeated measures ANOVA with subsequent Bonferroni's post hoc test to compare means between multiple (>2) groups on days 2 and 28 post-MI, by one-way ANOVA with Bonferroni's post hoc test to compare means between multiple groups and by two-tailed paired t test to compare means between day 2 and day 28 post-MI in each group. A value of P<0.05 is considered statistically significant. Each cardiac function experiment is a single experiment carried out with group sizes greater than needed for power of 0.8; flow cytometry data are representative of 2 independent experiments.
Supplementary Material
Acknowledgments
We thank Randall Lee for helpful discussion and advice, Sebastian Peck at the University of California, San Francisco Biological Imaging Development Center for help with confocal imaging, and Jianqin Ye, Brian Cook, and Shirley Mihardja for technical and physical assistance.
Funding: This work was supported by NIH grants R01 HL086917, R03 EB005802, and R21 HL097129, and University of California Discovery Grant bio04-10481.
Footnotes
Acute myocardial infarction-induced inflammatory response in bone marrow cell donors leads to the therapeutic impairment of the cells, making them less able to prevent a decline in cardiac function when implanted into infarcted recipient hearts.
Author contributions: X.W. helped conceive of and performed most of the experiments and data analysis, and J.T. performed the preliminary study, with technical help from V.C.L., D.J.H., D.L.T., P.Y.M., Y.Z., B.T.C., S.A.S., K.P., R.S., M.J.B. and R.E.S. M.L.S. conceived of the project and supervised most of the experiments. M.H., S.K., Y.Y. and L.C. provided collaborative experimental support and ideas for the project. X.W. and M.L.S. wrote the manuscript.
Competing interests: The authors declare that they have no competing interests.
REFERENCES
- 1.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin-Puig S, Wang Z, Chien KR. Lives of a heart cell: tracing the origins of cardiac progenitors. Cell Stem Cell. 2008;2:320–331. doi: 10.1016/j.stem.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 4.Yeghiazarians Y, Zhang Y, Prasad M, Shih H, Saini SA, Takagawa J, Sievers RE, Wong ML, Kapasi NK, Mirsky R, Koskenvuo J, Minasi P, Ye J, Viswanathan MN, Angeli FS, Boyle AJ, Springer ML, Grossman W. Injection of bone marrow cell extract into infarcted hearts results in functional improvement comparable to intact cell therapy. Mol Ther. 2009;17:1250–1256. doi: 10.1038/mt.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Sievers RE, Prasad M, Mirsky R, Shih H, Wong ML, Angeli FS, Ye J, Takagawa J, Koskenvuo JW, Springer ML, Grossman W, Boyle AJ, Yeghiazarians Y. Timing of bone marrow cell therapy is more important than repeated injections after myocardial infarction. Cardiovasc Pathol. 2010 doi: 10.1016/j.carpath.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 7.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 8.Rosenzweig A. Cardiac cell therapy--mixed results from mixed cells. N Engl J Med. 2006;355:1274–1277. doi: 10.1056/NEJMe068172. [DOI] [PubMed] [Google Scholar]
- 9.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 10.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 11.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 12.Thum T, Fraccarollo D, Galuppo P, Tsikas D, Frantz S, Ertl G, Bauersachs J. Bone marrow molecular alterations after myocardial infarction: Impact on endothelial progenitor cells. Cardiovasc Res. 2006;70:50–60. doi: 10.1016/j.cardiores.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leone AM, Rutella S, Bonanno G, Abbate A, Rebuzzi AG, Giovannini S, Lombardi M, Galiuto L, Liuzzo G, Andreotti F, Lanza GA, Contemi AM, Leone G, Crea F. Mobilization of bone marrow-derived stem cells after myocardial infarction and left ventricular function. Eur Heart J. 2005;26:1196–1204. doi: 10.1093/eurheartj/ehi164. [DOI] [PubMed] [Google Scholar]
- 15.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 17.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 18.Springer ML, Sievers RE, Viswanathan MN, Yee MS, Foster E, Grossman W, Yeghiazarians Y. Closed-chest cell injections into mouse myocardium guided by high-resolution echocardiography. Am J Physiol Heart Circ Physiol. 2005;289:H1307–1314. doi: 10.1152/ajpheart.00164.2005. [DOI] [PubMed] [Google Scholar]
- 19.Haeusler KG, Schmidt WU, Foehring F, Meisel C, Guenther C, Brunecker P, Kunze C, Helms T, Dirnagl U, Volk HD, Villringer A. Immune responses after acute ischemic stroke or myocardial infarction. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.10.053. [DOI] [PubMed] [Google Scholar]
- 20.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164:665–677. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fishbein MC, Maclean D, Maroko PR. Histopathologic Evolution of Myocardial-Infarction. Chest. 1978;73:843–849. doi: 10.1378/chest.73.6.843. [DOI] [PubMed] [Google Scholar]
- 22.Krakauer T, Buckley M. Dexamethasone attenuates staphylococcal enterotoxin B-induced hypothermic response and protects mice from superantigen-induced toxic shock. Antimicrob Agents Chemother. 2006;50:391–395. doi: 10.1128/AAC.50.1.391-395.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 24.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 25.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 26.Kissel CK, Lehmann R, Assmus B, Aicher A, Honold J, Fischer-Rasokat U, Heeschen C, Spyridopoulos I, Dimmeler S, Zeiher AM. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J Am Coll Cardiol. 2007;49:2341–2349. doi: 10.1016/j.jacc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 27.Li M, Georgakopoulos D, Lu G, Hester L, Kass DA, Hasday J, Wang Y. p38 MAP kinase mediates inflammatory cytokine induction in cardiomyocytes and extracellular matrix remodeling in heart. Circulation. 2005;111:2494–2502. doi: 10.1161/01.CIR.0000165117.71483.0C. [DOI] [PubMed] [Google Scholar]
- 28.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guillen I, Blanes M, Gomez-Lechon MJ, Castell JV. Cytokine signaling during myocardial infarction: sequential appearance of IL-1 beta and IL-6. Am J Physiol. 1995;269:R229–235. doi: 10.1152/ajpregu.1995.269.2.R229. [DOI] [PubMed] [Google Scholar]
- 30.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PWF, D'Agostino RB. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction - The Framingham Heart Study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 31.Puhakka M, Magga J, Hietakorpi S, Penttila I, Uusimaa P, Risteli J, Peuhkurinen K. Interleukin-6 and tumor necrosis factor alpha in relation to myocardial infarct size and collagen formation. J Card Fail. 2003;9:325–332. doi: 10.1054/jcaf.2003.38. [DOI] [PubMed] [Google Scholar]
- 32.Bulkley BH, Roberts WC. Steroid-Therapy during Acute Myocardial-Infarction - Cause of Delayed Healing and of Ventricular Aneurysm. Am J Med. 1974;56:244–250. doi: 10.1016/0002-9343(74)90603-2. [DOI] [PubMed] [Google Scholar]
- 33.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 35.Birdsall HH, Green DM, Trial J, Youker KA, Burns AR, MacKay CR, LaRosa GJ, Hawkins HK, Smith CW, Michael LH, Entman ML, Rossen RD. Complement C5a, TGF-beta 1, and MCP-1, in sequence, induce migration of monocytes into ischemic canine myocardium within the first one to five hours after reperfusion. Circulation. 1997;95:684–692. doi: 10.1161/01.cir.95.3.684. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Li Y, Chen X, Chen J, Gautam SC, Xu Y, Chopp M. MCP-1, MIP-1, IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture. Hematology. 2002;7:113–117. doi: 10.1080/10245330290028588. [DOI] [PubMed] [Google Scholar]
- 37.Engelmann MG, Theiss HD, Hennig-Theiss C, Huber A, Wintersperger BJ, Werle-Ruedinger AE, Schoenberg SO, Steinbeck G, Franz WM. Autologous bone marrow stem cell mobilization induced by granulocyte colony-stimulating factor after subacute ST-segment elevation myocardial infarction undergoing late revascularization: final results from the G-CSF-STEMI (Granulocyte Colony-Stimulating Factor ST-Segment Elevation Myocardial Infarction) trial. J Am Coll Cardiol. 2006;48:1712–1721. doi: 10.1016/j.jacc.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 38.Takagawa J, Zhang Y, Wong ML, Sievers RE, Kapasi NK, Wang Y, Yeghiazarians Y, Lee RJ, Grossman W, Springer ML. Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length-based approaches. J Appl Physiol. 2007;102:2104–2111. doi: 10.1152/japplphysiol.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.