Abstract
Background
The clinical manifestations of food allergy include diarrhea and systemic anaphylaxis (shock), which can occur together or by themselves in different individuals. Although ingested food antigens need to be absorbed to induce shock, it is not known whether they need to be absorbed to induce diarrhea.
Objective
Identify mechanisms that determine whether food allergy induces diarrhea versus shock and determine whether diarrhea requires absorption of ingested antigens. Methods: These issues were studied in mice in active, passive and hybrid immunization models. The active model was used to determine the allergic diarrhea susceptibility of J chain- and pIgR-deficient mice, which are unable to secrete IgA. The hybrid model was used to determine whether intravenously administered antigen-specific IgG antibody, which is not secreted into the gut, can protect against allergic diarrhea as well as shock.
Results
Shock, but not diarrhea was induced in naïve mice by intravenous IgE anti-TNP antibody, followed by oral TNP-bovine serum albumin, whereas both were induced in mice presensitized with intraperitoneal ovalbumin/alum plus oral ovalbumin. More TNP-bovine serum albumin was required to induce shock than diarrhea in presensitized mice and intravenous IgG anti-TNP antibody, which is not secreted into the gut, protected these mice against both diarrhea and shock. Consistent with this, OVA-immunized J chain- and pIgR-deficient mice, which have high serum IgA but little intestinal IgA, resisted diarrhea induction.
Conclusion
Intestinal immunity and oral Ag dose determine whether diarrhea and/or systemic anaphylaxis are induced and ingested Ag must be absorbed to induce either response.
Keywords: IgA, IgE, IgG, J chain, polymeric Ig receptor
Introduction
“Food allergy” is a general term that includes several different food-induced clinical syndromes. These range from mild abdominal discomfort to severe swelling of the mouth, tongue and pharynx (angioedema), vomiting and diarrhea, hives, asthma and shock (systemic anaphylaxis) 1–4. These syndromes can occur independently or in combination and are not hierarchical – self-limited symptoms, such as diarrhea or vomiting, most frequently occur in the absence of life-threatening shock, while shock can occur in the absence of gastrointestinal symptoms 1–4. Although mild food allergy can be induced by both antibody-dependent and independent mechanisms, gastrointestinal symptoms, hives, and shock that develop rapidly after food ingestion are IgE- and mast cell-dependent immediate hypersensitivity reactions 1–5. Even though the milder forms of IgE-mediated food allergy far outnumber the life-threatening forms, food ingestion is the most common cause of systemic anaphylaxis, with ~50,000 emergency room visits and ~150 deaths annually in the U.S. 2, 6, 7. Additionally, food allergy, in common with most allergic disorders, appears to be dramatically increasing in frequency in developed countries and has no satisfactory treatment 6–9.
Several rodent models have been developed to study food allergy 5, 10, 11. All involve the initial administration of an Ag in the context of inflammation, followed by Ag ingestion. In one such model, in which mice are primed by i.p. inoculation with ovalbumin (OVA) plus alum, then inoculated several times with OVA by oral gavage (o.g.) 5, 12, mice can develop shock (detected as hypothermia), which usually becomes evident within 5 min and nadirs at 15–25 min; and watery diarrhea, which is apparent by 30–60 min. Although both of these responses depend on IgE-, FcεRIα and mast cells, they differ in the mast cell-secreted mediators that are most important (histamine for shock; platelet activating factor (PAF) and possibly serotonin for diarrhea) 5, 13. Furthermore, like human food allergy, the mouse models can be manipulated so that diarrhea occurs without shock and vice versa.
This suggested that further examination of these models might shed light on the factors that alternatively promote shock vs. diarrhea. We have taken this approach with studies that evaluated two possibilities. Because mice in our model must be inoculated o.g. with OVA several times before they develop allergic diarrhea and this clinical feature is associated with large increases in intestinal mast cell number 5, 12, we hypothesized that local allergic inflammation may be needed to induce allergic diarrhea. We were also influenced by the much greater effectiveness of serum IgA and IgG Ab than gut secretory IgA at blocking IgE-dependent shock that is induced by Ag ingestion 12. Because serum Ig could only inhibit an Ag-mediated process if that Ag must be absorbed systemically, and a requirement for systemic Ag absorption had not been demonstrated for the induction of allergic diarrhea, we hypothesized that Ag still present in the gut lumen might induce allergic diarrhea, but not shock, by activating mucosal mast cells that are located immediately adjacent to the lumen 14. By supporting the first hypothesis, but disproving the second, the studies reported here suggest that the purposeful induction of a strong IgG Ab response to a food allergen should be useful for treating intestinal as well as systemic features of food allergy.
Methods
Mice
BALB/c background wild-type (WT) and transgenic mice in which an IL-9 transgene is regulated by the intestinal fatty acid binding protein promoter (iIL-9 tgn) 15, BALB/c background polymeric immunoglobulin receptor (pIgR)–deficient mice (Jackson Lab, Bar Harbor, Me), 16, BALB/c background J-chain–deficient mice (a gift from Dennis Metzger, Albany Medical College), 17 and BALB/c background FcγRIIb–deficient mice 18 were all bred in house. All experimental procedures were performed with approval from the Institutional Animal Care and Use Committees of the Cincinnati Children’s Hospital Research Foundation, which follows the ‘‘Guide for the Care and Use of Laboratory Animals’’ prepared by the Institute of Laboratory Animal Resources, National Research Council, and published by the National Academy Press.
Reagents
OVA, BSA and Pristane were purchased from Sigma (St Louis, Mo). OVA and BSA were labeled with trinitrophenyl (TNP) 19.
Hybridomas
Hybridomas that secrete mouse IgE anti-TNP (IGEL 2a) and mouse IgG1 anti-TNP (1B7.11) were purchased from the American Type Culture Collection (Rockville, MD) and grown in pristane-primed athymic nude mice. A hybridoma that secretes mouse IgG1 anti-fluorescein isothiocyanate (CG7) was produced in house. IgG1 mAbs were purified by ammonium sulfate precipitation, followed by diethylaminoethyl-cellulose (DE-52) cation exchange chromatography, while IgE anti-TNP ascites was used without purification.
Fecal pellet IgA extraction
IgA was extracted from fecal pellets as described 12.
Passive anaphylaxis
Mice (5–6/group (gp) unless otherwise noted) were injected intravenously (i.v.) with 10 μg IgE anti-TNP mAb followed 24 hr later by oral gavage (o.g.) with TNP-OVA or TNP-bovine serum albumin (BSA) plus Evans blue in 300 μl 0.15M NaCl. Systemic anaphylaxis severity as reflected by hypothermia was determined by rectal thermometry 13. All studies were repeated at least twice.
Active anaphylaxis
Mice (5–6/gp) were injected intraperitoneally (i.p.) with 50 μg OVA adsorbed to 1 mg alum. Starting 14 d later, these mice were inoculated by o.g. 3 times/week (wk) with 50 mg OVA plus Evans blue in 300 μl 0.15M NaCl. Mice were observed for up to 60 min after OVA inoculation for development of diarrhea (intestinal anaphylaxis) and hypothermia.
Mixed (hybrid) anaphylaxis
Mice (5–6/gp) were inoculated i.p. with OVA/alum. Fourteen days later, they were inoculated o.g. every 2–3 d with OVA until they developed both hypothermia and diarrhea. Mice were then injected i.v. with 10 μg of IgE anti-TNP mAb or saline. The next day, mice were injected i.v. with 2 mg of IgG1 anti-TNP mAb or IgG1 isotype control mAb and challenged 1 hour (hr) later o.g. with 5–50 mg of TNP-BSA plus Evans blue and observed for the next hr for development of hypothermia and diarrhea.
Evaluation of diarrhea
Mice were evaluated for by a blinded observer for the development of diarrhea, defined as the development of watery green stools by 60 min after o.g. inoculation with antigen plus Evans blue. Attempts to quantitate the severity of diarrhea by measuring stool volume of the time between o.g. inoculation and development of diarrheal stool were not productive.
ELISAs
IgA and IgE concentrations were determined by sandwich ELISAs in which microtiter plate wells were coated with anti-mouse IgA mAb (BD Bioscience, Franklin Lakes, NJ) or rat IgGa anti-mouse IgE mAb (EM-95) followed by sample and purified IgA or IgE standard (BD Biosciences). After incubation for 60 minutes (min), bound IgA or IgE were detected with biotin-anti-mouse IgAmAb (BD Bioscience), or biotin-rat IgG2a anti-mouse IgE mAb (RIE4), respectively, followed sequentially by horseradish peroxidase-streptavidin and SuperSignal ELISA substrate (Pierce Biotechnology).
Statistics
The significance of differences in temperature and Ig concentrations between groups of mice was compared with the Mann-Whitney t test. Differences in the occurrence of diarrhea between groups were compared with the Pearson chi-square test (GraphPad Prism 5.0; GraphPad software, La Jolla, Calif). A p value <0.05 was considered significant. Means ± SEMs are shown in all figures. All studies were performed at least twice with consistent results.
Results
Passive immunization with IgE Ab is sufficient for Ag ingestion to induce shock, but not diarrhea in immunologically naïve mice
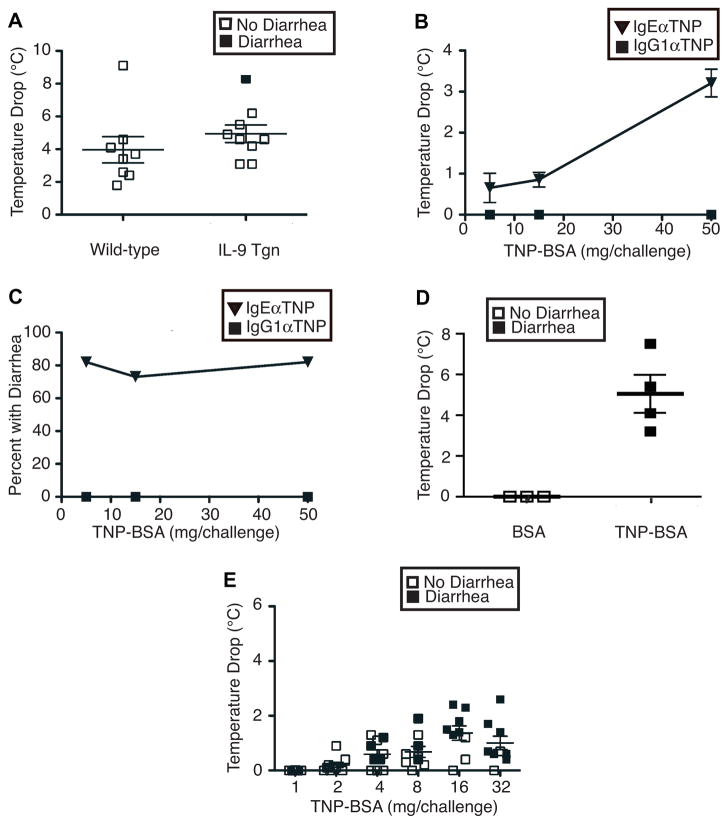
Initial experiments determined whether increased levels of Ag-specific IgE Ab are sufficient for ingestion of the targeted Ag to induce systemic shock and/or allergic diarrhea. Intravenous injection of WT mice with IgE anti-TNP mAb failed, by itself, to induce shock (hypothermia) or diarrhea (data not shown; however, o.g. of these IgE-primed mice the next day with 50 mg of TNP-BSA, induced shock (hypothermia), although it failed to induce diarrhea (Fig. 1A). To determine if the presence of small intestinal mastocytosis and increased intestinal permeability would be sufficient for IgE priming and Ag challenge to induce diarrhea as well as shock, we subjected iIL-9 Tgn mice to the same treatment that had been given to WT mice. Despite the constitutive small intestinal mastocytosis and increased intestinal permeability in iIL-9 Tgn mice15, only 1 of 9 developed diarrhea (Fig. 1A). Thus, IgE antibody is sufficient to prime immunologically intact, naïve mice for development of systemic anaphylaxis in response to Ag ingestion, while induction of allergic diarrhea requires additional stimuli that go beyond increased small intestinal mastocytosis and permeability.
Figure 1. IgE-mediated shock and diarrheal responses to ingested antigens.
A. Naïve BALB/c WT and iIL-9 Tgn mice were injected i.v. with 10 μg of IgE anti-TNP mAb, then challenged o.g. 24 hr later with 50 mg of TNP-BSA. Rectal temperatures and development of diarrhea were assessed during the next 60 min. B and C. BALB/c mice (5–6/group) were inoculated i.p. with OVA/alum, than o.g. every 2–3 d with OVA until they developed both hypothermia and diarrhea. Mice were then injected i.v. with either 10 μg of IgE anti-TNP mAb or 2 mg of IgG anti-TNP mAb, challenged the next d o.g. with 5, 15 or 50 mg of TNP-BSA and followed for the next hr for development of hypothermia and diarrhea. D. BALB/c mice sensitized to OVA as in B were challenged o.g. with 50 mg of BSA or TNP-BSA and followed for the next hr for development of hypothermia and diarrhea. E. BALB/c mice sensitized to OVA as in B were injected with 10 μg of IgE anti-TNP mAb, challenged o.g. the next d with the doses of TNP-BSA shown and followed for the next hr for development of hypothermia and diarrhea.
Active intestinal immunization allows passive immunization with IgE mAb, but not IgG1 mAb, to prime for intestinal anaphylaxis
We hypothesized that pre-existing intestinal allergic inflammation could allow IgE mAb to induce allergic diarrhea in response to ingested Ag. To test this, mice were first immunized i.p. with OVA/alum, then challenged o.g. every 2–3 d with OVA until challenge induced both diarrhea and shock. These mice were then injected i.v. with IgE anti-TNP mAb and challenged o.g. the next day with 5–50 mg of TNP-bovine serum albumin (TNP-BSA). TNP-BSA-challenged mice developed both shock (hypothermia, Fig. 1B) and diarrhea (Fig. 1C), while mice challenged with BSA that was not haptenated developed neither shock nor diarrhea (Fig. 1D). Most mice that developed shock (a temperature drop > 1°C) also developed diarrhea, while many mice that had diarrhea failed to develop shock (Fig. 1E). Thus, once allergic inflammation has been induced in the gut, less Ag is required to induce diarrhea than to induce shock (Fig. 1B-E). In contrast to priming with IgE anti-TNP mAb, priming OVA-immune mice with IgG1 anti-TNP mAb failed to allow the development of either shock (Fig. 1B) or allergic diarrhea (Fig. 1C) in response to TNP-BSA ingestion.
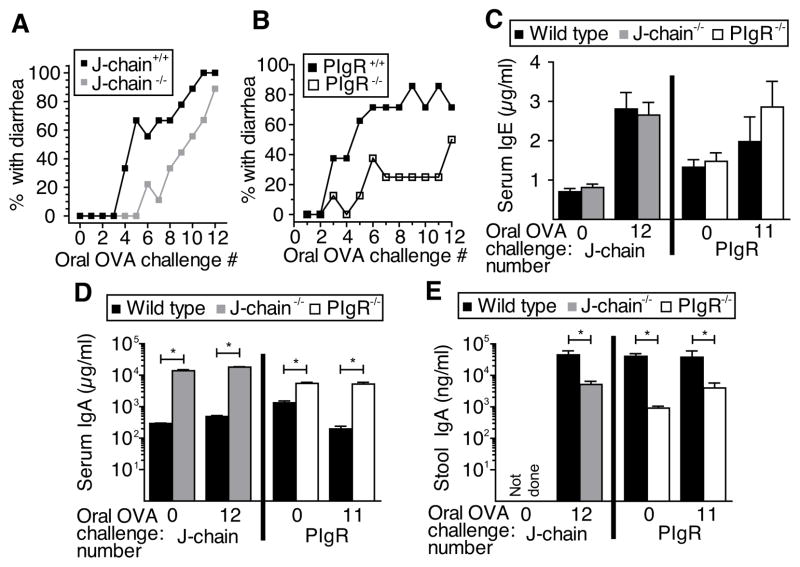
Serum IgA rather than secreted IgA delays development of intestinal anaphylaxis
We have previously demonstrated that Ag-specific IgA Ab is much more effective at blocking the IgE-mediated systemic anaphylactic (shock) response to ingested Ab if it is present in serum than in gut secretions 12. This indicated that ingested Ag must be absorbed systemically to induce systemic shock (we define systemic absorption as transit of Ag in the intestinal lumen through the intestinal villus epithelium; by this definition, Ag in the lamina propria of the intestine has been systemically absorbed). The same requirement was not necessarily true for the induction of IgE-mediated allergic diarrhea by ingested Ag, inasmuch as allergic diarrhea is a local, rather than a systemic process that requires pre-existing local allergic inflammation and requires less ingested Ag than induction of shock. In addition, the presence of some intestinal mast cells immediately adjacent to the intestinal lumen 14 suggested that allergic diarrhea might be induced directly by Ag in the gut without a requirement for its systemic absorption. To evaluate this possibility, we first compared the development of allergic diarrhea in actively immunized wild-type vs. J chain-deficient mice (Fig. 2A) and wild-type vs. pIgR- deficient mice (Fig. 2B), with the foreknowledge that defects in Ig secretion allow normal serum levels of IgE (Fig. 2C) in the J chain- and pIgR-deficient mice, while they cause increased levels of serum IgA (Fig. 2D) and decreased levels of intestinal IgA (Fig 2E) in these mice. In contrast to our expectations, allergic diarrhea was slower to develop in the J chain-deficient (Fig. 2A) and pIgR-deficient mice (Fig. 2B) than in wild-type mice. Because Ag in the gut lumen can only be neutralized by luminal Ig while serum Ig can neutralize Ag that has been systemically absorbed, the slower development of allergic diarrhea in mice that have high serum IgA but low luminal IgA suggests that ingested Ag needs to be absorbed systemically to induce allergic diarrhea.
Figure 2. Serum, not secreted IgA delays development of intestinal anaphylaxis.
In separate experiments, J-chain- or pIgR-sufficient or deficient mice (10/gp) were inoculated i.p. with OVA/alum, then challenged multiple times with OVA o.g. until they developed allergic diarrhea. The percentages of WT and either (A) J-chain deficient or (B) pIgR-deficient mice that developed diarrhea within 60 min after each oral OVA challenge are shown. Blood was drawn and fresh feces collected prior to the initial o.g. OVA challenge and after the 11th or 12th challenge. Total serum IgE (C), total serum IgA (D), and total stool IgA levels (D) were determined by ELISA. *p<0.05.
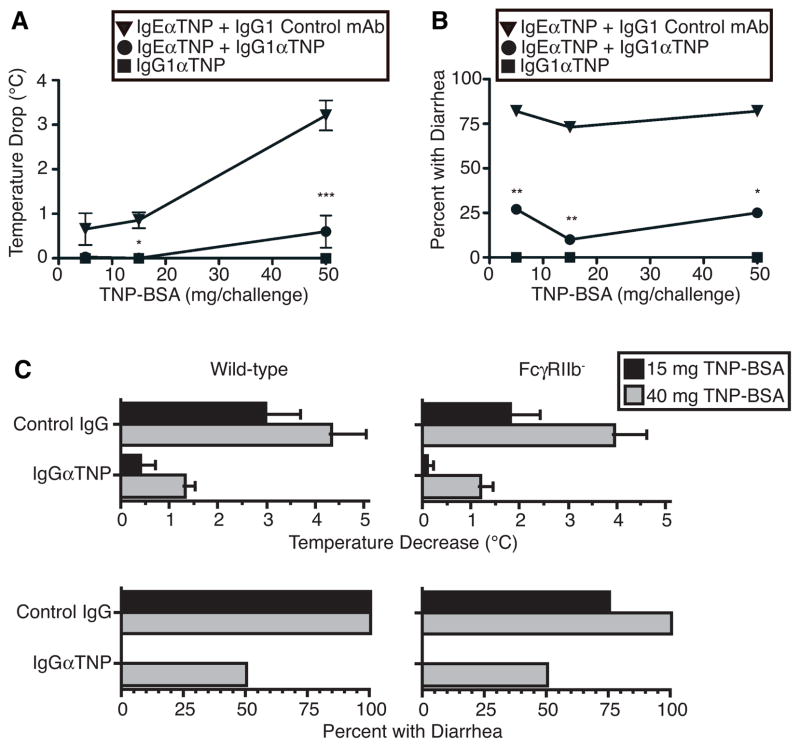
Ag-specific IgG1 suppresses IgE-mediated shock and diarrheal responses to oral Ag challenge through an FcγRIIb-independent mechanism
To confirm this interpretation, we used our hybrid active/passive immunization model to determine whether Ag-specific IgG mAb, which is not secreted into the gut 20, can suppress IgE-mediated allergic diarrheal as well as shock responses to ingested Ag. Indeed, IgG1 anti-TNP blocked both shock and diarrhea in OVA-immune mice that were primed with IgE anti-TNP mAb and challenged orally with 5 – 50 mg of TNP-BSA (Fig. 3A and B). IgG1 might inhibit IgE mediated shock and allergic diarrhea by intercepting Ag before it could be bound by mast cell-associated IgE and/or by forming a IgG1/Ag complex that inhibits FcεRI-mediated mast cell degranulation by crosslinking FcεRI with the inhibitory receptor, FcγRIIb. To determine whether IgG inhibition of IgE-mediated anaphylactic shock and allergic diarrhea involves an interaction between IgG-containing immune complexes and FcγRIIb, we compared the ability of IgG1 anti-TNP to inhibit these IgE-mediated responses in wild-type vs. FcγRIIb-deficient mice on the same BALB/c background. Results show that IgG-mediated inhibition of IgE-mediated allergic diarrhea is at least as effective in FcγRIIb-deficient mice as in wild-type mice (Fig. 3C). Thus, IgG-mediated inhibition of IgE-mediated allergic diarrhea most likely results from interception of absorbed Ag by IgG Ab before it can bind to mast cell-associated IgE mAb, rather than from inhibition of signaling by IgG immune complex ligation of FcγRIIb.
Figure 3. Ag-specific IgG1 suppresses IgE-mediated shock and diarrheal responses to oral Ag challenge through an FcγRIIb-independent mechanism.
BALB/c mice (5–6/gp) that had been sensitized to OVA as in Fig. 1B were injected i.v. with 10 μg of IgE anti- TNP mAb. 24 hr later, these mice were injected i.v. with 2 mg of IgG1 anti-TNP mAb or control IgG1 mAb and challenged o.g. 30 min later with 5, 15, or 50 mg of TNP-BSA. Rectal temperature (A) and diarrhea (B) were assessed for 1 hr. In a separate experiment, BALB/c background WT and Fc RIIb-deficient mice (5–6/group) were sensitized with OVA as in Fig. 1B and injected i.v. with 10 μg of IgE anti-TNP mAb. Twenty-four hr later, these mice were inoculated i.v. with 2 mg of IgG1 anti-TNP mAb or control IgG1 mAb. Mice were challenged o.g. 1 hr later with 15 or 40 mg of TNP-BSA. Rectal temperature (C) and diarrhea (D) were assessed for the following hr.
Discussion
The studies described here: 1) explain how allergic diarrhea and systemic anaphylaxis can each occur by itself in food allergic patients; and 2) demonstrate that even allergic diarrhea requires systemic absorption of ingested allergen. Our observation that passive immunization with IgE anti-TNP, followed by ingestion of TNP-BSA, causes shock but not diarrhea indicates that basal mast cell numbers and quantities of Ag absorption are sufficient to induce shock, but not diarrhea. This occurs even when iIL-9 Tgn mice, which have increased permeability and mast cell number in the small intestine, are passively immunized and challenged. Because most fluid resorption occurs in the large intestine, it seems likely that allergic diarrhea requires increased permeability (and perhaps, mastocytosis) of this organ in addition to the small intestine. This is consistent with the requirement for active enteral immunization, which induces mastocytosis and increased permeability in both the small and large intestine 15, 20, 21, to allow mice to develop allergic diarrhea in response to passive IgE sensitization and oral Ag challenge.
Once actively immunized mice become able to develop allergic diarrhea, however, we find that diarrhea can be triggered by less Ag than is required to trigger shock. Because shock is caused by vascular leak (which decreases intravascular volume) 22, this most likely reflects a requirement for Ag activation of vascular mast cells, rather than gut mast cells to induce systemic anaphylaxis. If so, the induction of diarrhea without shock could be explained by the higher concentration of absorbed Ag in intestinal tissue than in the vasculature following ingestion of a given Ag dose. In contrast, a requirement for induction of gut mastocytosis to induce diarrhea while basal numbers of vascular mast cells are sufficient to induce systemic anaphylaxis could explain why some individuals experience systemic disease without intestinal symptoms. We cannot, however, rule out other possible mechanisms that could differentially induce allergic diarrhea vs. shock. One possibility is production of different sets of vasoactive mediators (e.g.; predominant production of PAF and serotonin, which are associated with allergic diarrhea5 vs. predominant production of histamine, which is associated with mast cell-dependent shock13) by different mast cell populations. However, although different mast cell populations product distinct proteases23, we are not aware of evidence that different mast cell populations in the mouse secrete distinct subsets or different quantities of vasoactive mediators. A second possibility is that intestinal inflammation induced by repeated ovalbumin inoculation may permit the selective development of allergic diarrhea by increasing the sensitivity of gut epithelial, smooth muscle and/or vascular endothelial cells to mast cell-produced mediators. In this regard, we have demonstrated that IL-4 and IL-13 have this effect, even at relatively low concentrations22. Increased concentrations of IL-4 or IL-13 produced by T cells, basophils and/or nuocytes in the lamina propria might promote diarrhea without systemic shock by predominantly acting locally to increase the sensitivity of intestinal cells, but not systemic vascular endothelial cells to mast cell-produced mediators.
In contrast, we find that the induction of both localized and systemic anaphylaxis requires the absorption of ingested Ag, as shown by: 1) the delayed development of allergic diarrhea in J chain-deficient and pIgR-deficient mice, both of which have increased plasma IgA but very little secreted IgA; and 2) the ability of plasma IgG Ab to block both. Although crosslinking FcεRI with FcγRIIb inhibits IgE-dependent mast cell activation, IgG strongly inhibited both allergic diarrhea and shock in both FcγRIIb-sufficient and deficient mice, suggesting that IgG inhibits IgE-mediated anaphylaxis primarily by intercepting Ag before it can crosslink FcεRI-associated IgE.
Our results have the important practical consequence that induction of high levels of allergen-specific IgG, which can be induced by systemic immunization, should block both allergic diarrhea and systemic anaphylaxis. This is consistent with clinical observations that systemic desensitization therapy that increases IgG Ab levels without decreasing IgE Ab levels can reduce food allergy symptoms 24 and provides a mechanistic explanation of why specific Ag desensitization should be an efficacious and, provided that it can be done safely, useful therapy for food allergy.
Key Messages.
Intestinal immunity and oral antigen dose determine whether food allergy elicits local symptoms, such as diarrhea and/or systemic anaphylaxis.
Ingested antigen must be absorbed to induce either response.
Desensitization procedures that induce systemic IgG secretion should suppress both local and systemic food allergy.
Acknowledgments
Funding: This work was supported by a Department of Veterans Affairs Merit Award (FDF); NIH R01 AI073553 (SH), and an NIH T32 Training Grant (ZYK).
Abbreviations
- Ab
antibody
- BSA
bovine serum albumin
- gp
group
- d
day
- hr
hour
- iIL-9 Tg
transgenic mice in with an IL-9 transgene is regulated by the intestinal fatty acid binding protein promoter
- i.p
intraperitoneal
- i.v.
intravenous
- mAb
monoclonal antibody
- min
minute
- o.g
oral gavage
- OVA
chicken ovalbumin
- PAF
platelet activating factor
- pIgR
polymeric Ig receptor
- wk
week
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang J, Sampson HA. Food allergy. J Clin Invest. 2011;121:827–35. doi: 10.1172/JCI45434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125:S116–25. doi: 10.1016/j.jaci.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Berin MC, Mayer L. Immunophysiology of experimental food allergy. Mucosal Immunol. 2009;2:24–32. doi: 10.1038/mi.2008.72. [DOI] [PubMed] [Google Scholar]
- 4.Sampson HA. 9. Food allergy. J Allergy Clin Immunol. 2003;111:S540–7. doi: 10.1067/mai.2003.134. [DOI] [PubMed] [Google Scholar]
- 5.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666–77. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta R, Sheikh A, Strachan DP, Anderson HR. Time trends in allergic disorders in the UK. Thorax. 2007;62:91–6. doi: 10.1136/thx.2004.038844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2010;127:594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 8.Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;124:1549–55. doi: 10.1542/peds.2009-1210. [DOI] [PubMed] [Google Scholar]
- 9.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638–46. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh KY, Tsai CC, Wu CH, Lin RH. Epicutaneous exposure to protein antigen and food allergy. Clin Exp Allergy. 2003;33:1067–75. doi: 10.1046/j.1365-2222.2003.01724.x. [DOI] [PubMed] [Google Scholar]
- 11.Li XM, Schofield BH, Huang CK, Kleiner GI, Sampson HA. A murine model of IgE-mediated cow's milk hypersensitivity. J Allergy Clin Immunol. 1999;103:206–14. doi: 10.1016/s0091-6749(99)70492-6. [DOI] [PubMed] [Google Scholar]
- 12.Strait RT, Mahler A, Hogan S, Khodoun M, Shibuya A, Finkelman FD. Ingested allergens must be absorbed systemically to induce systemic anaphylaxis. J Allergy Clin Immunol. 2011;127:982–9.e1. doi: 10.1016/j.jaci.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strait RT, Morris SC, Yang M, Qu XW, Finkelman FD. Pathways of anaphylaxis in the mouse. J Allergy Clin Immunol. 2002;109:658–68. doi: 10.1067/mai.2002.123302. [DOI] [PubMed] [Google Scholar]
- 14.Alizadeh H, Urban JF, Jr, Katona IM, Finkelman FD. Cells containing IgE in the intestinal mucosa of mice infected with the nematode parasite Trichinella spiralis are predominantly of a mast cell lineage. J Immunol. 1986;137:2555–60. [PubMed] [Google Scholar]
- 15.Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansen FE, Pekna M, Norderhaug IN, Haneberg B, Hietala MA, Krajci P, et al. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J Exp Med. 1999;190:915–22. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendrickson BA, Conner DA, Ladd DJ, Kendall D, Casanova JE, Corthesy B, et al. Altered hepatic transport of immunoglobulin A in mice lacking the J chain. J Exp Med. 1995;182:1905–11. doi: 10.1084/jem.182.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in FcγRII-deficient mice. Nature. 1996;379:346–9. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 19.Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE- mediated anaphylaxis in vivo through both antigen interception and FcγRIIb cross-linking. J Clin Invest. 2006;116:833–41. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandtzaeg P, Bjerke K, Kett K, Kvale D, Rognum TO, Scott H, et al. Production and secretion of immunoglobulins in the gastrointestinal tract. Ann Allergy. 1987;59:21–39. [PubMed] [Google Scholar]
- 21.Forbes D, Patrick M, Perdue M, Buret A, Gall DG. Intestinal anaphylaxis: in vivo and in vitro studies of the rat proximal colon. Am J Physiol. 1988;255:G201–5. doi: 10.1152/ajpgi.1988.255.2.G201. [DOI] [PubMed] [Google Scholar]
- 22.Strait RT, Morris SC, Smiley K, Urban JF, Jr, Finkelman FD. IL-4 exacerbates anaphylaxis. J Immunol. 2003;170:3835–42. doi: 10.4049/jimmunol.170.7.3835. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds DS, Stevens RL, Lane WS, Carr MH, Austen KF, Serafin WE. Different mouse mast cell populations express various combinations of at least six distinct mast cell serine proteases. Proc Natl Acad Sci U S A. 1990;87:3230–4. doi: 10.1073/pnas.87.8.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nowak-Wegrzyn A, Sampson HA. Future therapies for food allergies. J Allergy Clin Immunol. 2011;127:558–73. doi: 10.1016/j.jaci.2010.12.1098. quiz 74–5. [DOI] [PMC free article] [PubMed] [Google Scholar]