Abstract
V(D)J recombination is regulated through changes in chromatin structure that allow recombinase proteins access to recombination signal sequences and through changes in three-dimensional chromatin organization that bring pairs of distant recombination signal sequences into proximity. The Tcra/Tcrd locus is complex and undergoes distinct recombination programs in double negative and double positive thymocytes that lead to the assembly of Tcrd and Tcra genes, respectively. Our studies provide insights into how locus chromatin structure is regulated and how changes in locus chromatin structure can target and then retarget the recombinase to create developmental progressions of recombination events. Our studies also reveal distinct locus conformations in double negative and double positive thymocytes and suggest how these conformations may support the distinct recombination programs in the two compartments.
Keywords: T cell receptor, chromatin, V(D)J recombination, T lymphocyte
Introduction
Antigen-specific immunity depends on the display of diverse and clonally distributed antigen receptors on the surfaces of T and B lymphocytes. These receptors, T cell receptors (TCRs) and surface immunoglobulins (Ig), are acquired as T and B lymphocytes develop in the thymus and bone marrow, respectively. Antigen receptor diversity depends, in large measure, on V(D)J recombination, the somatic DNA recombination process that is used to assemble mature TCR and Ig genes (1, 2). V(D)J recombination selects individual variable (V), diversity (D) and joining (J) gene segments from large arrays of these gene segments, and joins them to create the variable region exon of the mature, rearranged gene.
V(D)J recombination is directed by the lymphoid-specific recombinase activing gene (RAG)1 and RAG2 protein complex (referred to as RAG) (1–3). RAG can recognize recombination signal sequences (RSSs) that flank each TCR or Ig V, D, or J gene segment, and with two RSSs in a synaptic complex, can generate double-strand breaks at the borders of the RSSs and the coding segments. DNA repair proteins then join the four ends to create novel coding joints (D-to-J, V-to-D or V-to-J, which remain chromosomal) and signal joints (which are often extrachromosomal). V(D)J recombination creates diverse antigen receptor repertoires through the combinatorial nature of the recombination process and nucleotide loss and addition at the site of the coding joint. T lymphocyte development generates T cells that bear either an αβ or a γδ TCR (4). Notably, the four TCR genes that encode these receptors are organized into three genetic loci: Tcrb, encoding TCRβ chain; Tcrg, encoding the TCRγ chain; and Tcra/Tcrd, encoding both TCRα and TCRδ chains. During T lymphocyte development, Tcrg, Tcrd and Tcrb all undergo recombination in CD4−CD8− double negative (DN) thymocytes, when RAG is first expressed. Successful recombination of Tcrg and Tcrd promotes assembly of a γδ TCR and commitment to the γδ T cell lineage. Successful Tcrb recombination, on the other hand, promotes assembly of a pre-TCR and commitment to the αβ lineage. Such committed cells downregulate recombinase expression, enter the cell cycle and differentiate to the CD4+CD8+ double positive (DP) stage. Newly generated DP thymocytes then re-express RAG, initiate Tcra recombination, and ultimately assemble a cell surface αβ TCR. DP thymocyte fate then depends on TCR specificity: those with useful TCRs are saved (positive selection) whereas those with autoreactive TCRs are eliminated (negative selection). Therefore, TCR gene assembly not only creates a T cell repertoire, but also instructs multiple cell fate decisions during T cell development.
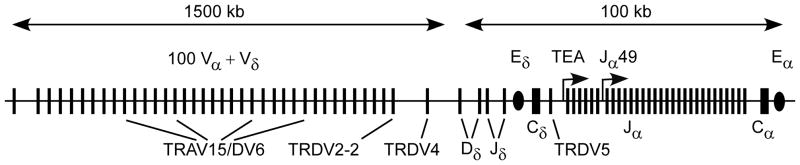
In both the B and T cell lineages, V(D)J recombination events are thought to be developmentally programmed through changes in chromatin structure and chromatin organization that provide RAG proteins access to pairs of RSSs (5–7). Such developmental programming takes multiple forms, but nowhere is the task more complex than at the Tcra/Tcrd locus (4, 8). This locus contains more than 160 gene segments that undergo recombination at two different stages of T cell development to form two different TCR proteins (Fig. 1). The major portion of the locus (about 1.5 Mb) is devoted to an array of about 100 V gene segments. Downstream of this, a 0.1 Mb region includes two Dδ and Jδ gene segments, followed by Cδ, and an array of 61 Jα gene segments, followed by Cα. Tcrd gene assembly in DN thymocytes occurs in two steps, Vδ-to-Dδ and Dδ-to-Jδ, whereas Tcra gene assembly in DP thymocytes occurs in a single step, Vα-to-Jα. Both TCRs draw from the same array of V gene segments, but in fact only a small number of V gene segments typically rearrange to Dδ in DN thymocytes, and are therefore considered Vδ gene segments, whereas the vast majority of V gene segments (including some Vδ) can rearrange to Jα in DP thymocytes, and are therefore considered Vα gene segments.
Figure 1. Organization of the Tcra/Tcrd locus.
Gene segments are depicted by filled rectangles, enhancers are depicted by filled ovals, and prominent promoters in the Jα array are depicted by bent arrows. The commonly used Vδ gene segments (TRAV15/DV6 family, TRDV2-2, TRDV4, TRDV5) are identified. Note that TRDV4 rearranges preferentially in fetal thymocytes.
The dynamics of Tcra/Tcrd locus recombination events are fascinating: Tcrd alleles typically undergo complete (Vδ-to-Dδ-to-Jδ) or partial (Vδ-to-Dδ or Dδ-to-Jδ) recombination in DN thymocytes; in those thymocytes that commit to the αβ lineage, Tcrd rearrangements can then be excised by Vα-to-Jα recombination (4, 8). Strikingly, there can be multiple cycles of Vα-to-Jα recombination. Early DP thymocytes initially join an available 3’ Vα gene segment to a Jα segments at the 5’ end of the Jα array, and these initial, or primary, Vα-to-Jα recombination events can then be replaced by secondary events that join progressively more 5’ Vα gene segments to progressively more 3’ Jα gene segments. The recombination process is ultimately terminated by downregulation of recombinase expression in positively selected DP thymocytes (or by cell death in those DP thymocytes that are not selected). Multiple cycles of Vα-to-Jα recombination increase the efficiency of thymocyte development by providing individual DP thymocytes with several opportunities to be positively selected.
The unique behavior of the Tcra/Tcrd locus raises many questions about mechanisms of developmental control. What targets initial recombination events in DN thymocytes to Dδ and Jδ gene segments? What features of Vδ gene segments distinguish them from Vα gene segments and promote their rearrangement in DN thymocytes? What retargets recombination events to the Jα array in DP thymocytes, and what directs the 5’-to-3’ progression of Jα usage? What, if anything, promotes a reciprocal progression across the Vα array? These questions have been the subject of intensive investigation in our laboratory and in other laboratories for many years; we will discuss each of them in turn. Central to the discussion are two concepts: accessibility, which has guided the field of V(D)J recombination for many years, and locus conformation, which has been recognized more recently.
The “accessibility hypothesis” was developed from the observation that V(D)J recombination events at the Igh locus correlated developmentally with the activation of transcription of the unrearranged gene segments (germline transcription) (9). It was proposed that chromatin structure forms an intrinsic barrier that prevents RAG proteins from accessing RSSs, and that an accessible state (characterized by active transcription and recombination) could be reached through developmentally regulated changes to chromatin structure. In the ensuing years, it has been shown that TCR and Ig locus transcriptional enhancers and promoters are essential for and impart developmental control to V(D)J recombination in chromatin (10–17), and that they do so by recruiting chromatin remodeling enzymes that covalently modify histones and that can move or remove nucleosomes (18–21). Recent studies have also revealed that a passive view of “accessibility” is likely an oversimplification since RAG2 has been found to interact directly with a modified form of histone H3 (H3K4me3) and appears to be recruited to H3K4me3 modified nucleosomes genome-wide (22–24). Remarkably, RAG1 appears to distribute independent of RAG2, and to localize selectively to regions of antigen receptor loci with accessible RSSs, presumably through direct RAG1-RSS interactions (23).
Because V(D)J recombination represents a transaction between two RSSs that may be widely separated in antigen receptor loci, the three dimensional organization or conformation of antigen receptor loci is also thought to be a critical regulator of the recombination process (25). Using pairs of DNA probes in three dimensional fluorescence in situ hybridization (3D-FISH) experiments, it has been found that Ig and TCR loci adopt relatively extended configurations in non-lymphoid cells and undergo developmental stage-specific contraction events in lymphoid cells to set the stage for V(D)J recombination (26–28). Thus, the Igh locus contracts in pro-B cells to promote VH-to-DJH recombination, and does so in a fashion that brings all VH gene segments into roughly equal proximity to DJH gene segments (29). It then decontracts in pre-B cells, presumably to limit further VH-to-DJH recombination and promote allelic exclusion (27).
Accessibility control of Tcra/Tcrd gene recombination
Efficient Tcrd gene recombination in DN thymocytes depends on the Tcrd enhancer (Eδ). Elimination of Eδ by gene targeting inhibits, but does not completely block, both Vδ-toDδ and Dδ-to-Jδ recombination events (11). This likely reflects reduced accessibility of Dδ-Jδ chromatin: germline Tcrd transcripts, initiating predominantly at Dδ2 and Jδ1 (30), are reduced, but not eliminated, by Eδ-deletion, and histone modifications such as H3 and H4 acetylation and H3K4 di- and tri-methylation are diminished across the Dδ and Jδ segments as well (BH and MSK, unpublished). Thus, Eδ appears to collaborate with local promoters to target the Dδ and Jδ segments for recombination. However, we have found no appreciable effect of Eδ deletion on chromatin structure at the common Vδ gene segments (BH and dMSK, unpublished), arguing that Vδ gene segments are made accessible in an Eδ-independent fashion. In previous experiments we found that the commonly used Vδ gene segments (in particular TRDV5, TRDV2-2 and members of the TRAV15/TRDV6 family) are actively transcribed and display histone modifications characteristic of accessible chromatin in DN thymocytes (31). Thus we assume that these V gene segments are defined as Vδ on the basis of Eδ-independent promoter activity and chromatin remodeling in DN thymocytes. It appears unlikely that physical location in the Tcra/Tcrd locus defines them as Vδ, since some (TRDV5, TRDV2-2) are Dδ-proximal, whereas others (TRAV15/TRDV6 family) are hundreds of kb away. It also appears unlikely that unique properties of their RSSs define them as Vδ, since the Dδ 5’RSS rearranges effectively with both Vα and Vδ RSSs (32).
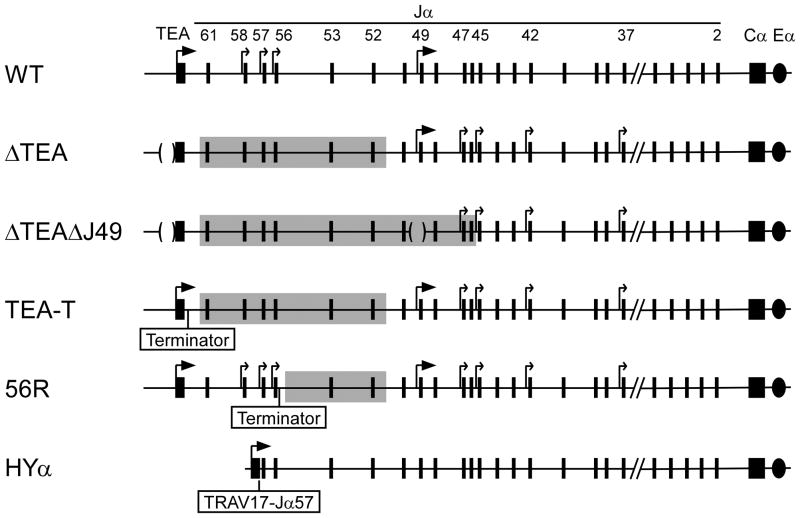
The transition to the DP stage is marked by a downregulation of Eδ activity, an upregulation of Eα activity, and a retargeting of recombination events from Tcrd gene segments to Tcra gene segments(33). Gene targeting experiments revealed Eα to be required for essentially all Vα-to-Jα recombination events (10). However, as noted previously, the earliest Vα-to-Jα recombination events (so-called primary rearrangements) are targeted preferentially to the most 5’ Jα gene segments (34, 35). This 5’ targeting depends on two Eα-dependent promoters, T early-α (TEA) and Jα49 (15, 36). Primary rearrangements are directed almost exclusively to two clusters of Jα segments, one immediately downstream of TEA, and one in proximity of Jα49. Elimination of these promoters by gene-targeting suppressed recombination events involving these Jα segments, and caused residual primary recombination events to be scattered across the central portion of the Jα array (Fig. 2) (36). This retargeting was associated with upregulated transcription from a set of otherwise cryptic germline promoters associated with central Jα gene segments.
Figure 2. Regulation of Jα chromatin accessibility.
Wild-type and genetically manipulated versions of the Jα array are depicted. ΔTEA and ΔTEAΔJ49 carry deletions of the TEA and J49 promoters. TEA-T and 56R carry insertions of a transcription terminator downstream of TEA or Jα56, respectively. HYα carries an insertion of a TRAV17-Jα57 rearrangement. Large arrows identify major promoters (TEA, J49). Small arrows identify minor or cryptic promoter activity. Shaded portions of the ΔTEA,ΔTEAΔJ49, TEA-T and 56R alleles identify areas of diminished chromatin modifications and disrupted Vα-to-Jα recombination.
These studies established germline Jα promoters as local regulators of recombination, but did not establish a mechanism. Several years ago, we asked whether transcription across 5’ Jα RSSs was critical for their recombination by introducing a transcription terminator into the Jα array (37, 38). Termination of transcription downstream of Jα56 impaired rearrangement of Jα53 and Jα52 (37), whereas termination of transcription downstream of TEA impaired rearrangement of the entire Jα61-Jα52 interval (Fig. 2) (38). Importantly, blockade of TEA transcription mimicked exactly the recombination defect in TEA promoter-deleted mice, indicating that the TEA promoter targets 5’ Jα recombination events by driving transcription across this region. Notably, like TEA promoter deletion, termination of TEA transcription caused the derepression of cryptic promoters associated with central Jα gene segments, indicating that these promoters are normally suppressed by transcriptional interference. Thus, TEA transcription focuses primary Vα to Jα recombination through a combination of positive effects across 5’ Jα gene segments and negative effects across central Jα gene segments.
We have also addressed the effects of TEA transcription on 5’ Jα chromatin. Wild-type alleles are characterized by very high levels of several histone modifications, including H3 acetylation, H3K4 di- and tri-methylation, and H3K36 tri-methylation in the region from Jα61 to Jα56. All of these modifications are potently suppressed by transcriptional blockade (37, 38). Transcriptional elongation also influences nucleosome organization. We recently identified three well positioned nucleosomes spanning the Jα61 region on inaccessible Eα-deficient alleles and demonstrated nucleosome loss and repositioning on accessible wild-type alleles (19). Transcriptional blockade reversed several of these changes, indication that in addition to covalently modifying nucleosomes, transcriptional elongation can move and remove nucleosomes. We suspect that accessible alleles are actually mosaics with nucleosomes missing at some sites and covalently modified at others, in this manner simultaneously promoting RAG1 interaction with RSS DNA and RAG2 interaction with a nearby H3K4 tri-methylated nucleosome.
These studies provide a reasonably satisfying view of how Jα segments are targeted for primary recombination events. To understand the targeting of secondary recombination, we analyze an allele engineered to mimic a primary rearrangement: Tcrd gene segments, the TEA promoter and 5’ Jα gene segments were eliminated, and a Vα-Jα57 rearrangement replaced the germline Jα57 gene segment (39). We found that this configuration targeted histone modifications preferentially to the Jα segments immediately downstream of the introduced Vα-Jα57, and that Vα-to-Jα recombination events that replaced the Vα-Jα57 rearrangement in early DP thymocytes were targeted to this region as well (40). As such, we think that the promoter of the rearranged Vα effectively replaces the TEA promoter and locally targets recombination to downstream Jα gene segments through similar mechanisms. Moreover, since at each round of recombination the promoter of the rearranged Vα would be repositioned further 3’ along the Jα array, the net effect would be a stepwise progression of chromatin remodeling and recombinase targeting events. Of note, all of our inferences about recombinase targeting on the different Tcra alleles discussed above have recently been confirmed in studies analyzing RAG1 binding by chromatin immunoprecipitation (Y.Ji, B.Hao, E.M.Oltz, M.S.Krangel and D.G.Schatz, unpublished observations).
Can regulated changes in accessibility account for preferential use of 3’Vα gene segments in primary Vα to Jα recombination and a progression from 3’ to 5’ across the Vα array? We suspect that accessibility is indeed part of the story, since the 3’ one-third of the Vα array displays dramatic, Eα-dependent increases in histone modifications in DP thymocytes (31). The ability of Eα to activate these V segment promoters over a distance of 500 kb may provide these proximal V gene segments with an advantage over central and distal V gene segments as competitors for RAG proteins. However, we suspect that the real key to this behavior is a unique conformation of the Tcra/Tcrd locus that is adopted in DP thymocytes.
Conformational control of Tcra/Tcrd gene recombination
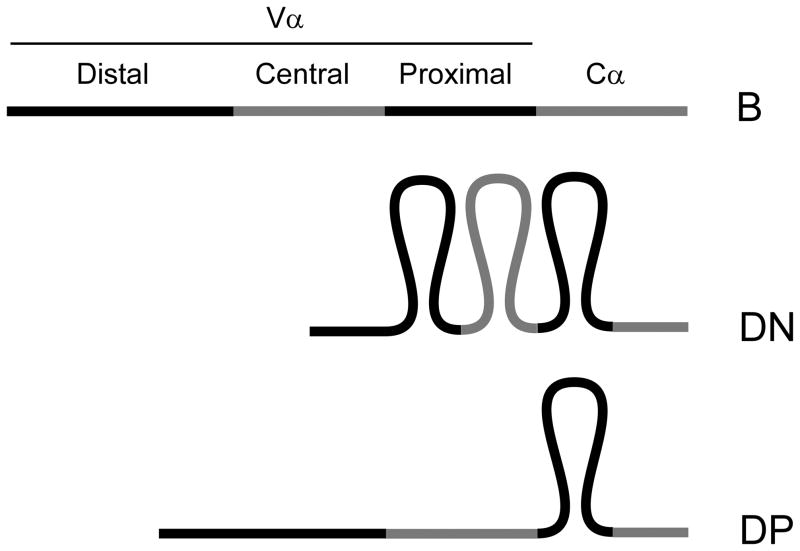
Recent 3D-FISH analysis using 12 genomic markers spanning the 3Mb Igh locus provided a detailed characterization of the conformational change that occurs between pre-pro B and pro B cells (29). In pre-pro B cells, the locus is relatively extended, with most VH gene segments relatively distant from DH and JH gene segments; in contrast, the locus is much more compact in pro-B cells, with both distal and proximal VH gene segments moved into close proximity of DH and JH gene segments. This conformation presumably provides all VH gene segments a similar opportunity for VH-to-DHJH rearrangement, which is likely of particular importance since an individual allele can undergo only a single round of VH-to-DHJH rearrangement. We reasoned that the Tcra/Tcrd locus might display more complex conformational properties since the pattern of recombination events switches between the DN and DP stages of thymocyte development. Like the Igh locus in pro-B cells, the Tcra/Tcrd locus undergoes a single round of Vδ-to-Dδ-to-Jδ rearrangement, and this rearrangement must access both proximal and more distant Vδ gene segments. However, as noted above, Vα-to-Jα recombination in DP thymocytes can occur multiple times on an allele and tends to be targeted to the more 3’ of the available Vα gene segments at each round of recombination. We recently addressed this issue by comparing Tcra/Tcrd locus conformation in B cells, DN thymocytes and DP thymocytes using four DNA probes in 3D-FISH experiments (41). We found that, as compared to splenic B cells, the locus was contracted in both DN and DP thymocytes. Remarkably, however, we detected two distinct modes of contraction in the two thymocyte compartments: in DN thymocytes the locus is contracted along its entire length, whereas in DP thymocytes, the locus displays a 3’ contracted and 5’ decontracted conformation (Fig. 3). Hence, the 3’ end of the locus (including proximal but not central and distal V gene segments) is contracted in DN thymocytes and remains contracted upon transition to the DP stage, whereas the 5’ end of the locus (including distal and central V gene segments) is contracted in DN thymocytes and then decontracts upon transition to the DP stage (41). We interpret this 5’ decontraction to be a mechanism that would force initial Vα-to-Jα recombination events to use the relatively proximal Vα gene segments and that would similarly bias Vα usage during subsequent rounds of recombination. This idea is supported by proximal VH- biased rearrangements documented in mice carrying a decontracted Igh locus: Igh transgenic (39), Pax5−/− (42), Ikaros−/− (43) and YY1−/− mice (44). However, the Tcra/Tcrd locus would be the only example in which a decontracted state would promote a bias to V(D)J recombination events that is of physiological significance.
Figure 3. Tcra/Tcrd locus conformational states.
The diagram depicts the relatively extended configuration of the locus in B cells, the fully contracted configuration in DN thymocytes, and the unique 3’ contracted and 5’ decontracted configuration in DP thymocytes. The V array is segregated into distal (5’), central, and proximal (3’) regions.
The molecular mechanisms of antigen receptor locus contraction and decontraction are not well understood. Although deficiencies in transcription factors Pax5, Ikaros and YY1 disrupt the contracted Igh locus configuration, it is unclear how they do so. Since enhancers are thought to activate promoters by looping interactions, and since Eα in particular is known to activate promoters at a distance of 500 kb, we imagined that Eδ and Eα might function to establish locus conformational states. However, examination of the conformation of Eα- and Eδ-deficient alleles in DN and DP thymocytes provided no evidence that locus contraction depended on these elements (41). On the contrary, we think it likely that the ability of Eα to activate promoters over the proximal 1/3 of the locus in DP thymocytes may be a consequence of the contracted configuration of this portion of the locus.
The regulation of antigen receptor locus conformation thus remains a major unanswered question. However, there are several molecules previously implicated as mediators of chromatin architecture, including SATB1, CTCF, and CTCF-associated cohesin, that are potential candidates (45–47). As a transcription factor and a chromatin organizer, SATB1 is thought to bridge specific AT-rich DNA sequences to the nuclear matrix and to function as an anchor for tethering chromatin into loops. It is directly implicated in the formation of a complex looped structure at the Th2 cytokine locus (48). CTCF and cohesin have been shown to colocalize at many sites genome-wide (49–51), and have been directly implicated as mediators of long-distance interactions at the Ifng (52), H19/Igf2 (53), and beta-globin loci (54). Moreover, colocalized CTCF and cohesin binding sites are abundant at both the Igh and Igk loci in developing B cells (55). However, binding profiles across the Tcra/Tcrd locus in DN and DP thymocytes have not yet been reported, and any roles in locus conformation remain purely speculative.
Conclusions
The Tcra/Tcrd locus provides an intriguing system in which to explore the rules governing gene segment usage during V(D)J recombination. Our studies reveal how regulated changes in local chromatin accessibility are established and how developmentally regulated changes in accessibility are likely critical to establish the dynamics of V(D)J recombination events at the Tcra/Tcrd locus. Our studies also suggest how changes in locus conformation may impart a critical second level of control. Finally, although we have discussed accessibility and conformation as two independent parameters that direct V(D)J recombination events at the locus, we suspect that this view is substantially oversimplified, since looping of DNA over long distances has the potential to inhibit (eg., enhancer-blocking) or potentiate enhancer-promoter interactions. These considerations suggest that it will be essential to establish an integrated view of conformational and accessibility effects to bring our understanding of Tcra/Tcrd locus regulation to the next level.
Acknowledgments
Work in the authors’ laboratory was supported by NIH grant R37 GM41052.
References
- 1.Schatz DG, Spanopoulou E. Biochemistry of V(D)J recombination. Curr Top Microbiol Immunol. 2005;290:49–85. doi: 10.1007/3-540-26363-2_4. [DOI] [PubMed] [Google Scholar]
- 2.Bassing CH, Swat W, Alt FW. The mechanism and regulation of chromosomal V(D)J recombination. Cell. 2002;109 (Suppl):S45–55. doi: 10.1016/s0092-8674(02)00675-x. [DOI] [PubMed] [Google Scholar]
- 3.Matthews AG, Oettinger MA. RAG: a recombinase diversified. Nat Immunol. 2009;10:817–21. doi: 10.1038/ni.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krangel MS. Mechanics of T cell receptor gene rearrangement. Curr Opin Immunol. 2009;21:133–9. doi: 10.1016/j.coi.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krangel MS. T cell development: better living through chromatin. Nat Immunol. 2007;8:687–94. doi: 10.1038/ni1484. [DOI] [PubMed] [Google Scholar]
- 6.Krangel MS. Gene segment selection in V(D)J recombination: accessibility and beyond. Nat Immunol. 2003;4:624–30. doi: 10.1038/ni0703-624. [DOI] [PubMed] [Google Scholar]
- 7.Cobb RM, Oestreich KJ, Osipovich OA, Oltz EM. Accessibility control of V(D)J recombination. Adv Immunol. 2006;91:45–109. doi: 10.1016/S0065-2776(06)91002-5. [DOI] [PubMed] [Google Scholar]
- 8.Krangel MS, Carabana J, Abbarategui I, Schlimgen R, Hawwari A. Enforcing order within a complex locus: current perspectives on the control of V(D)J recombination at the murine T-cell receptor alpha/delta locus. Immunol Rev. 2004;200:224–32. doi: 10.1111/j.0105-2896.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 9.Yancopoulos GD, Alt FW. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985;40:271–81. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- 10.Sleckman BP, Bardon CG, Ferrini R, Davidson L, Alt FW. Function of the TCR α enhancer in αβ and γδ T cells. Immunity. 1997;7:505–15. doi: 10.1016/s1074-7613(00)80372-6. [DOI] [PubMed] [Google Scholar]
- 11.Monroe RJ, Sleckman BP, Monroe BC, Khor B, Claypool S, Ferrini R, Davidson L, Alt FW. Developmental regulation of TCR δ locus accessibility and expression by the TCR δ enhancer. Immunity. 1999;10:503–13. doi: 10.1016/s1074-7613(00)80050-3. [DOI] [PubMed] [Google Scholar]
- 12.Ferrier P, Krippl B, Blackwell TK, Furley AJ, Suh H, Winoto A, Cook WD, Hood L, Costantini F, Alt FW. Separate elements control DJ and VDJ rearrangement in a transgenic recombination substrate. EMBO J. 1990;9:117–25. doi: 10.1002/j.1460-2075.1990.tb08087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauzurica P, Krangel MS. Temporal and lineage-specific control of T cell receptor α/δ gene rearrangement by T cell receptor α and δ enhancers. J Exp Med. 1994;179:1913–21. doi: 10.1084/jem.179.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lauzurica P, Krangel MS. Enhancer-dependent and -independent steps in the rearrangement of a human T cell receptor δ transgene. J Exp Med. 1994;179:43–55. doi: 10.1084/jem.179.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villey I, Caillol D, Selz F, Ferrier P, de Villartay JP. Defect in rearrangement of the most 5' TCR-J α following targeted deletion of T early α (TEA): implications for TCR α locus accessibility. Immunity. 1996;5:331–42. doi: 10.1016/s1074-7613(00)80259-9. [DOI] [PubMed] [Google Scholar]
- 16.Whitehurst CE, Chattopadhyay S, Chen J. Control of V(D)J recombinational accessibility of the Dβ1 gene segment at the TCR β locus by a germline promoter. Immunity. 1999;10:313–22. doi: 10.1016/s1074-7613(00)80031-x. [DOI] [PubMed] [Google Scholar]
- 17.Bories JC, Demengeot J, Davidson L, Alt FW. Gene-targeted deletion and replacement mutations of the T-cell receptor β-chain enhancer: the role of enhancer elements in controlling V(D)J recombination accessibility. Proc Natl Acad Sci U S A. 1996;93:7871–6. doi: 10.1073/pnas.93.15.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osipovich O, Cobb RM, Oestreich KJ, Pierce S, Ferrier P, Oltz EM. Essential function for SWI-SNF chromatin-remodeling complexes in the promoter-directed assembly of Tcrb genes. Nat Immunol. 2007;8:809–16. doi: 10.1038/ni1481. [DOI] [PubMed] [Google Scholar]
- 19.Kondilis-Mangum HD, Cobb RM, Osipovich O, Srivatsan S, Oltz EM, Krangel MS. Transcription-dependent mobilization of nucleosomes at accessible TCR gene segments in vivo. J Immunol. 2010;184:6970–7. doi: 10.4049/jimmunol.0903923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathieu N, Hempel WM, Spicuglia S, Verthuy C, Ferrier P. Chromatin remodeling by the T cell receptor (TCR)-β gene enhancer during early T cell development: Implications for the control of TCR-β locus recombination. J Exp Med. 2000;192:625–36. doi: 10.1084/jem.192.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMurry MT, Krangel MS. A role for histone acetylation in the developmental regulation of VDJ recombination. Science. 2000;287:495–8. doi: 10.1126/science.287.5452.495. [DOI] [PubMed] [Google Scholar]
- 22.Matthews AG, Kuo AJ, Ramón-Maiques S, Han S, Champagne KS, Ivanov D, Gallardo M, Carney D, Cheung P, Ciccone DN, Walter KL, Utz PJ, Shi Y, Kutateladze TG, Yang W, Gozani O, Oettinger MA. RAG2 PHD finger couples histone H3 lysine 4 trimethylation with V(D)J recombination. Nature. 2007;450:1106–10. doi: 10.1038/nature06431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji Y, Resch W, Corbett E, Yamane A, Casellas R, Schatz DG. The in vivo pattern of binding of RAG1 and RAG2 to antigen receptor loci. Cell. 2010;141:419–31. doi: 10.1016/j.cell.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Subrahmanyam R, Chakraborty T, Sen R, Desiderio S. A plant homeodomain in RAG-2 that binds Hypermethylated lysine 4 of histone H3 is necessary for efficient antigen-receptor-gene rearrangement. Immunity. 2007;27:561–71. doi: 10.1016/j.immuni.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jhunjhunwala S, van Zelm MC, Peak MM, Murre C. Chromatin architecture and the generation of antigen receptor diversity. Cell. 2009;138:435–48. doi: 10.1016/j.cell.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosak ST, Skok JA, Medina KL, Riblet R, Le Beau MM, Fisher AG, Singh H. Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science. 2002;296:158–62. doi: 10.1126/science.1068768. [DOI] [PubMed] [Google Scholar]
- 27.Roldán E, Fuxa M, Chong W, Martinez D, Novatchkova M, Busslinger M, Skok JA. Locus 'decontraction' and centromeric recruitment contribute to allelic exclusion of the immunoglobulin heavy-chain gene. Nat Immunol. 2005;6:31–41. doi: 10.1038/ni1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skok JA, Gisler R, Novatchkova M, Farmer D, de Laat W, Busslinger M. Reversible contraction by looping of the Tcra and Tcrb loci in rearranging thymocytes. Nat Immunol. 2007;8:378–87. doi: 10.1038/ni1448. [DOI] [PubMed] [Google Scholar]
- 29.Jhunjhunwala S, van Zelm MC, Peak MM, Cutchin S, Riblet R, van Dongen JJ, Grosveld FG, Knoch TA, Murre C. The 3D structure of the immunoglobulin heavy-chain locus: implications for long-range genomic interactions. Cell. 2008;133:265–79. doi: 10.1016/j.cell.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carabana J, Ortigoza E, Krangel MS. Regulation of the murine Dδ2 promoter by upstream stimulatory factor 1, Runx1, and c-Myb. J Immunol. 2005;174:4144–52. doi: 10.4049/jimmunol.174.7.4144. [DOI] [PubMed] [Google Scholar]
- 31.Hawwari A, Krangel MS. Regulation of TCR δ and α repertoires by local and long-distance control of variable gene segment chromatin structure. J Exp Med. 2005;202:467–72. doi: 10.1084/jem.20050680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee YN, Alt FW, Reyes J, Gleason M, Zarrin AA, Jung D. Differential utilization of T cell receptor TCRα/TCRδ locus variable region gene segments is mediated by accessibility. Proc Natl Acad Sci U S A. 2009;106:17487–92. doi: 10.1073/pnas.0909723106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernandez-Munain C, Sleckman BP, Krangel MS. A developmental switch from TCR δ enhancer to TCR α enhancer function during thymocyte maturation. Immunity. 1999;10:723–33. doi: 10.1016/s1074-7613(00)80071-0. [DOI] [PubMed] [Google Scholar]
- 34.Thompson SD, Pelkonen J, Hurwitz JL. First T cell receptor α gene rearrangements during T cell ontogeny skew to the 5' region of the Jα locus. J Immunol. 1990;145:2347–52. [PubMed] [Google Scholar]
- 35.Guo J, Hawwari A, Li H, Sun Z, Mahanta SK, Littman DR, Krangel MS, He YW. Regulation of the TCRα repertoire by the survival window of CD4+CD8+ thymocytes. Nat Immunol. 2002;3:469–76. doi: 10.1038/ni791. [DOI] [PubMed] [Google Scholar]
- 36.Hawwari A, Bock C, Krangel MS. Regulation of T cell receptor α gene assembly by a complex hierarchy of germline Jα promoters. Nat Immunol. 2005;6:481–9. doi: 10.1038/ni1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abarrategui I, Krangel MS. Regulation of T cell receptor-α gene recombination by transcription. Nat Immunol. 2006;7:1109–15. doi: 10.1038/ni1379. [DOI] [PubMed] [Google Scholar]
- 38.Abarrategui I, Krangel MS. Noncoding transcription controls downstream promoters to regulate T-cell receptor α recombination. EMBO J. 2007;26:4380–90. doi: 10.1038/sj.emboj.7601866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buch T, Rieux-Laucat F, Forster I, Rajewsky K. Failure of HY-specific thymocytes to escape negative selection by receptor editing. Immunity. 2002;16:707–18. doi: 10.1016/s1074-7613(02)00312-6. [DOI] [PubMed] [Google Scholar]
- 40.Hawwari A, Krangel MS. Role for rearranged variable gene segments in directing secondary T cell receptor α recombination. Proc Natl Acad Sci U S A. 2007;104:903–7. doi: 10.1073/pnas.0608248104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shih HY, Krangel MS. Distinct contracted conformations of the Tcra/Tcrd locus during Tcra and Tcrd recombination. J Exp Med. 2010;207:1835–41. doi: 10.1084/jem.20100772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuxa M, Skok J, Souabni A, Salvagiotto G, Roldan E, Busslinger M. Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene. Genes Dev. 2004;18:411–22. doi: 10.1101/gad.291504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reynaud D, Demarco IA, Reddy KL, Schjerven H, Bertolino E, Chen Z, Smale ST, Winandy S, Singh H. Regulation of B cell fate commitment and immunoglobulin heavy-chain gene rearrangements by Ikaros. Nat Immunol. 2008;9:927–36. doi: 10.1038/ni.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu H, Schmidt-Supprian M, Shi Y, Hobeika E, Barteneva N, Jumaa H, Pelanda R, Reth M, Skok J, Rajewsky K, Shi Y. Yin Yang 1 is a critical regulator of B-cell development. Genes Dev. 2007;21:1179–89. doi: 10.1101/gad.1529307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galande S, Purbey PK, Notani D, Kumar PP. The third dimension of gene regulation: organization of dynamic chromatin loopscape by SATB1. Curr Opin Genet Dev. 2007;17:408–14. doi: 10.1016/j.gde.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Phillips JE, Corces VG. CTCF: master weaver of the genome. Cell. 2009;137:1194–211. doi: 10.1016/j.cell.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bose T, Gerton JL. Cohesinopathies, gene expression, and chromatin organization. J Cell Biol. 2010;189:201–10. doi: 10.1083/jcb.200912129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai S, Lee CC, Kohwi-Shigematsu T. SATB1 packages densely looped, transcriptionally active chromatin for coordinated expression of cytokine genes. Nat Genet. 2006;38:1278–88. doi: 10.1038/ng1913. [DOI] [PubMed] [Google Scholar]
- 49.Parelho V, Hadjur S, Spivakov M, Leleu M, Sauer S, Gregson HC, Jarmuz A, Canzonetta C, Webster Z, Nesterova T, Cobb BS, Yokomori K, Dillon N, Aragon L, Fisher AG, Merkenschlager M. Cohesins functionally associate with CTCF on mammalian chromosome arms. Cell. 2008;132:422–33. doi: 10.1016/j.cell.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Rubio ED, Reiss DJ, Welcsh PL, Disteche CM, Filippova GN, Baliga NS, Aebersold R, Ranish JA, Krumm A. CTCF physically links cohesin to chromatin. Proc Natl Acad Sci U S A. 2008;105:8309–14. doi: 10.1073/pnas.0801273105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wendt KS, Yoshida K, Itoh T, Bando M, Koch B, Schirghuber E, Tsutsumi S, Nagae G, Ishihara K, Mishiro T, Yahata K, Imamoto F, Aburatani H, Nakao M, Imamoto N, Maeshima K, Shirahige K, Peters JM. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature. 2008;451:796–801. doi: 10.1038/nature06634. [DOI] [PubMed] [Google Scholar]
- 52.Hadjur S, Williams LM, Ryan NK, Cobb BS, Sexton T, Fraser P, Fisher AG, Merkenschlager M. Cohesins form chromosomal cis-interactions at the developmentally regulated IFNG locus. Nature. 2009;460:410–3. doi: 10.1038/nature08079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han L, Lee DH, Szabo PE. CTCF is the master organizer of domain-wide allele-specific chromatin at the H19/Igf2 imprinted region. Mol Cell Biol. 2008;28:1124–35. doi: 10.1128/MCB.01361-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Splinter E, Heath H, Kooren J, Palstra RJ, Klous P, Grosveld F, Galjart N, de Laat W. CTCF mediates long-range chromatin looping and local histone modification in the β-globin locus. Genes Dev. 2006;20:2349–54. doi: 10.1101/gad.399506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Degner SC, Wong TP, Jankevicius G, Feeney AJ. Cutting edge: developmental stage-specific recruitment of cohesin to CTCF sites throughout immunoglobulin loci during B lymphocyte development. J Immunol. 2009;182:44–8. doi: 10.4049/jimmunol.182.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]