Abstract
Objectives
Oral squamous cell carcinoma (OSCC) is the most commonly diagnosed oral malignancy in humans and cats and frequently invades bone. The objective of this study was to determine if feline OSCC serves as a relevant model of human OSCC in terms of osteolytic behavior and expression of bone resorption agonists.
Materials and Methods
Novel feline OSCC cell lines (SCCF2 and SCCF3) were derived from spontaneous carcinomas. Gene expression and osteolytic behavior were compared to an established feline OSCC cell line (SCCF1) and three human OSCC cell lines (UMSCC-12, A253 and SCC25). Interaction of OSCC with bone and murine pre-osteoblasts (MC3T3) was investigated using in vitro co-culture techniques. In vivo bioluminescent imaging, faxitron radiography and microscopy were used to measure xenograft growth and bone invasion in nude mice.
Results
Human and feline OSCC expressing the highest levels of parathyroid hormone-related protein (PTHrP) were associated with in vitro and in vivo bone resorption and osteoclastogenesis. MC3T3 cells had increased receptor activator of nuclear factor κB ligand (RANKL) expression and reduced osteoprotegerin (OPG) expression in conditioned medium from bone-invasive SCCF2 cells compared to minimally bone invasive SCCF3 cells, which was partially reversed with a neutralizing anti-PTHrP antibody. Human and feline OSCC cells cultured in bone-conditioned medium had increased PTHrP secretion and proliferation.
Conclusion
Feline OSCC-induced bone resorption was associated with tumor cell secretion of PTHrP and with increased RANKL : OPG expression ratio in mouse preosteoblasts. Bone-CM increased OSCC proliferation and secretion of PTHrP. The preclinical models of feline OSCC recapitulated the bone-invasive phenotype characteristic of spontaneous OSCC and will be useful to future preclinical and mechanistic studies of bone invasive behavior.
Introduction
Cancer of the oral cavity was diagnosed in an estimated 263,900 patients, globally, in 2008.1 In 2010, the American Cancer Society estimated that 25,800 people in the U.S. would be diagnosed with oral and pharyngeal cancer and 5,830 people would die.2 Approximately 90% of oral and oropharyngeal tumors are squamous cell carcinoma (OSCC).3–5 There has been minimal improvement in the 5-year disease-specific survival for OSCC, which is currently 61% for all stages combined.6 Development of successful therapies depends on the utility of OSCC animal models that faithfully recapitulate complex tumor–host interactions including angiogenesis, invasion and metastasis.7
OSCC frequently invades bone and is associated with osteoclastic bone resorption.8,9 Bone-invasion contributes to the clinical morbidity of OSCC patients and is associated with poorer prognosis.10–15 Despite the frequency and clinical impact of bone invasion in OSCC, the mechanisms responsible for osteoclastic bone resorption and bone invasion remain poorly understood.
Multiple animal models are available for the study of OSCC; however, many are designed to study the early stages of carcinogenesis and involve exposing tissues of the oral cavity of hamsters, mice and rats to carcinogenic agents such as dimethylbenzanthracine (DMBA) and 4-nitroquinolone oxide (4NQO), or involve injection of primary or established OSCC cell lines subcutaneously in syngeneic or immunocompromised rodent models resulting in noninvasive tumor growth.7,16 There are few preclinical in vivo models that recapitulate the bone-invasive behavior of OSCC in order to evaluate therapeutic agents. The objective of this study was to develop a relevant in vitro and in vivo model of OSCC-associated bone resorption utilizing cell lines derived from primary OSCC tumors from humans and domestic cats. As in humans, OSCC is the most commonly diagnosed tumor of the oral cavity in cat17,18 and has a highly invasive, osteolytic phenotype19,20 with similarities in clinical progression and pathology compared to human OSCC.21 Characterization of feline OSCC cell lines not only provides additional tools for studying mechanisms and treatment of bone resorption in OSCC, but will support the utility of cats with OSCC as a spontaneous preclinical model of the human disease. Cell lines derived from primary OSCC tumors in cats in addition to human OSCC cell lines were evaluated to determine if the bone-invasive phenotype and expression of parathyroid hormone related-protein (PTHrP, a stimulator of osteoclastic bone resorption) receptor activator of nuclear factor κB ligand (RANKL, an activator of osteoclastogenesis) and osteoprotegerin (OPG, the soluble receptor of RANKL and inhibitor of osteoclastogenesis) was similar between the two species, and to better characterize bone-invasive feline OSCC as a model of human OSCC.
Materials and Methods
Established and novel cell lines
A253 (human salivary SCC), SCC25 (human lingual SCC), NHDF (human dermal fibroblast) and MC3T3 (murine preosteoblast) cell lines were purchased from ATCC (Manassas, VA). UMSCC12 cells (human laryngeal SCC) were provided by Dr. Thomas Carey at the University of Michigan. SCCF1 cells (feline laryngeal SCC) were previously derived and characterized.22 SCCF2 cells were derived from a bone-invasive gingival SCC of a 7-year-old cat and SCCF3 cells were derived from a lingual SCC of a 12-year-old cat using published methods.22 Tumor-associated fibroblasts (TAF) from a feline gingival OSCC unrelated to SCCF2 and SCCF3 were isolated using similar methods. Methods used to derive, maintain and characterize cell lines (cytokeratin immunohistochemistry, proliferation rate, karyotype analysis, electron microscopy) and to stably transfect cells with luciferase are described in the supplemental information.
In vitro bone resorption and osteoclastogenesis
Four (4) mm diameter disks of calvarial bone were harvested from 5 to 8-day-old mouse pups and co-cultured with NHDF or OSCC cells. Formalin-fixed bone disks were stained for tartrate-resistant acid phosphatase (TRAP) activity using a commercially available kit (Sigma-Aldrich, St. Louis, MO) as previously described.23 Disk area was calculated (Image-Pro plus histomorphometry software, Bathesda, MD) and compared between each cell line. Bone marrow mononuclear cells (BMMC) were flushed from femurs, tibias and humeri of 4-to-6-week-old male mice and cultured in αMEM growth medium supplemented with 30 ng/ml recombinant murine RANKL (Peprotech Inc., Rocky Hills, NJ) and 5 ng/ml recombinant murine MCSF (R&D Systems, Minneapolis, MN) for 72 hours. Medium was replaced with 1:1 mixture of αMEM growth medium and OSCC-conditioned medium (CM) or unconditioned medium (DMEM growth medium)for 6 days. Adherent cells were formalin-fixed and TRAP-stained as previously described.24 . Fibroblasts were used in co-culture experiments to produce medium that was cell culture-conditioned but did not contain OSCC-derived factors (negative controls).
Orthotopic nude mouse model of bone invasive OSCC
Animal procedures were approved by the Institutional Lab Animal Care and Use Committee at The Ohio State University. Luciferase-expressing OSCC cell lines (UMSCC12Luc, SCCF1Luc, SCCF2Luc and SCCF3Luc) were injected into 2 groups of five, 6-week-old male nu/nu mice (NCI, Frederick, MD), with or without the addition of Matrigel (BD Matrigel™ , high concentration, phenol red free, Franklin Lakes, NJ). OSCC cells were injected through the gingival mucosa, lateral to the maxillary incisors, at a density of 5×105 to 1×106 cells suspended in 0.1ml of vehicle. Mice were sacrificed 90 days post-injection or earlier when xenografts reached 1cm in diameter. Bioluminescent imaging was performed every 1 to 2 weeks using the IVIS 100 system as previously described.21 Results were analyzed using LivingImage® software, version 2.2 (Caliper Life Sciences). Ex vivo imaging of submandibular and cervical lymph nodes, lungs, liver, spleen and kidneys was performed on all mice immediately following BLI and euthanasia. Bone loss was qualitatively evaluated using a Faxitron cabinet X-ray system (Hewlett-Packard, McMinnville, OR). Skulls were formalin-fixed, decalcified in 10% EDTA pH 7.4, paraffin embedded and stained with hematoxylin and eosin (HE) for microscopic determination of bone invasion. Enzymatic histochemistry for TRAP-positive osteoclasts was performed on tissues from representative mice as previously described.25
Expression of bone resorption genes in OSCC and MC3T3 cells
OSCC cells were evaluated using real-time reverse transcriptase polymerase chain reaction (RT-PCR) for expression of PTHrP, colony stimulating factor-1 (CSF-1), RANKL and OPG using methods previously described.24 Primer sequences are provided as supplemental information. Gene expression was normalized to beta-2 microglobulin.
MC3T3 cells were cultured in 50% serum-free α-MEM medium supplemented with 0.1% BSA and 50% serum-free OSCC-CM or unconditioned medium for 3 hours, at which time RANKL and OPG expression was measured by real-time RT-PCR. All medium was pre-incubated with previously validated chicken anti human PTHrP neutralizing IgY or preimmune IgY at 100 μg/ml for 1 hour at 37°C. 26,27 Primer sequences were previously published24 and are included in supplemental materials.
Effect of bone-derived factors on OSCC proliferation and expression of PTHrP
NHDF or OSCC cells were grown in 96-well plates in 50% growth medium / 50% CM (murine bone-CM or fibroblast-CM). A commercial MTT assay (Promega, Madison, WI) was performed according to the manufacturer’s instructions after 96 hours of culture.
OSCC cells were cultured in serum-free bone-CM, MC3T3-CM, or 12 ng/ml recombinant human TGF-β1 (R&D). OSCC cells were cultured in 50% unconditioned serum-free medium and 50% conditioned serum-free medium for 3 hours (mRNA expression) or for 24 hours (PTHrP secretion). PTHrP mRNA expression was measured using real-time RT-PCR and the concentration of PTHrP secreted into the culture medium was determined with a PTHrP immunoradiometric assay (Diagnostic Systems Laboratories Inc., Webster, TX, USA) according to the manufacturer’s instructions.
Statistical analysis
Results are displayed with means and standard error as indicated. Data were analyzed using Student’s t-test or ANOVA and Bonferroni’s post hoc test. Normalized gene expression data (ΔCT) were analyzed using Student’s t-test or ANOVA and Bonferroni’s post hoc test, and displayed as relative expression compared to the cell line with the lowest expression or control samples as indicated. Data with P values less than 0.05 were considered statistically significant. All statistical comparisons were performed with STATA Intercooled 10 software (Cary, NC).
Results
Cell line derivation and morphologic characterization
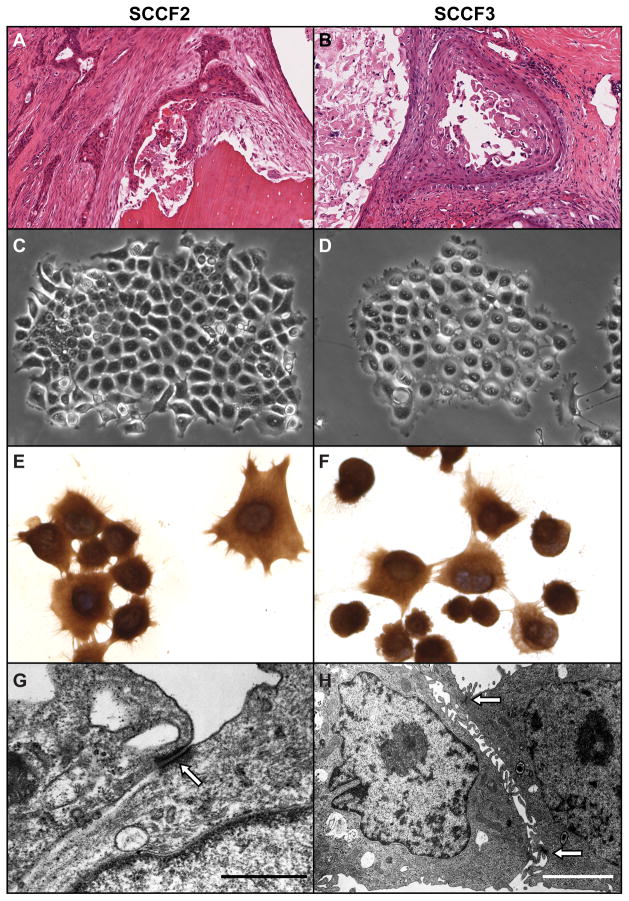
The SCCF2 primary tumor was a well differentiated maxillary OSCC with evidence of bone resorption (figure 1A). The SCCF3 primary tumor was a well differentiated SCC which had infiltrated and expanded the entire thickness of the tongue (figure 1B). Both tumors had morphologic characteristics typical of OSCC which included islands and cords of neoplastic epithelium with squamous differentiation and formation of keratin pearls. SCCF2 and SCCF3 cells grew in vitro as colonies of adherent round to polygonal cells that maintained close cell-to-cell contact (figures 1C and D) and were pancytokeratin-positive (figures 1E and F). Transmission electron microscopy revealed that SCCF2 and SCCF3 cells formed desmosomes (figures 1G and H) and tonofilaments typical of epithelial cells. The average doubling time of SCCF2 cells (27 ± 3 hours) was slightly shorter than SCCF3 cells (30 hours ± 3 hours) (data not shown).
Figure 1. SCCF2 and SCCF2 morphology.
SCCF2 and SCCF3 cell lines were derived from spontaneous feline OSCC cancers. A. SCCF2 originated from a bone invasive maxillary OSCC . OSCC cells formed thin cords occasionally surrounding foci of keratin and sloughed tumor cells, within dense fibrous stroma and adjacent to a fragment of partially resorbed, necrotic, lamellar bone (Hematoxylin and eosin (HE)). B. SCCF3 was derived from an invasive lingual OSCC. The tumor was composed of large cystic spaces lined by neoplastic squamous epithelium and contained large quantities of keratin and necrotic tumor cells. SCCF2 (C) and SCCF3 (D) cells both grew in vitro as colonies of adherent cells with typical round to polygonal cell morphology. SCCF2 (E) and SCCF3 (F) cells grown on glass slides were cytokeratin positive (Pancytokeratin immunocytochemistry, DAB and hematoxylin counterstain). SCCF2 (G) and SCCF3 (H) cells were grown on glass and were examined using transmission electron microscopy. Both cell types demonstrated desmosomes characteristic (open arrow) of epithelial cells (Scale bars: G, 0.7 μM; H, 2.5 μM).
SCCF2 and SCCF3 cells were aneuploid with multiple marker chromosomes (figure S1). SCCF2 cells were 4n with chromosomal additions to B1 (short arm), D4 and F1. No normal D4 chromosomes were observed and no Y chromosomes were observed. Approximately one half of the SCCF3 cells were diploid and the remaining cells were tetraploid; however, all had the same chromosomal abnormalities which included additions to B1 (long arm), C2, F2 and deletion of part of D1. No normal E3 chromosomes were observed in SCCF3 cells.
Human UMSCC12 and Feline SCCF2 cells induced bone resorption and osteoclastogenesis
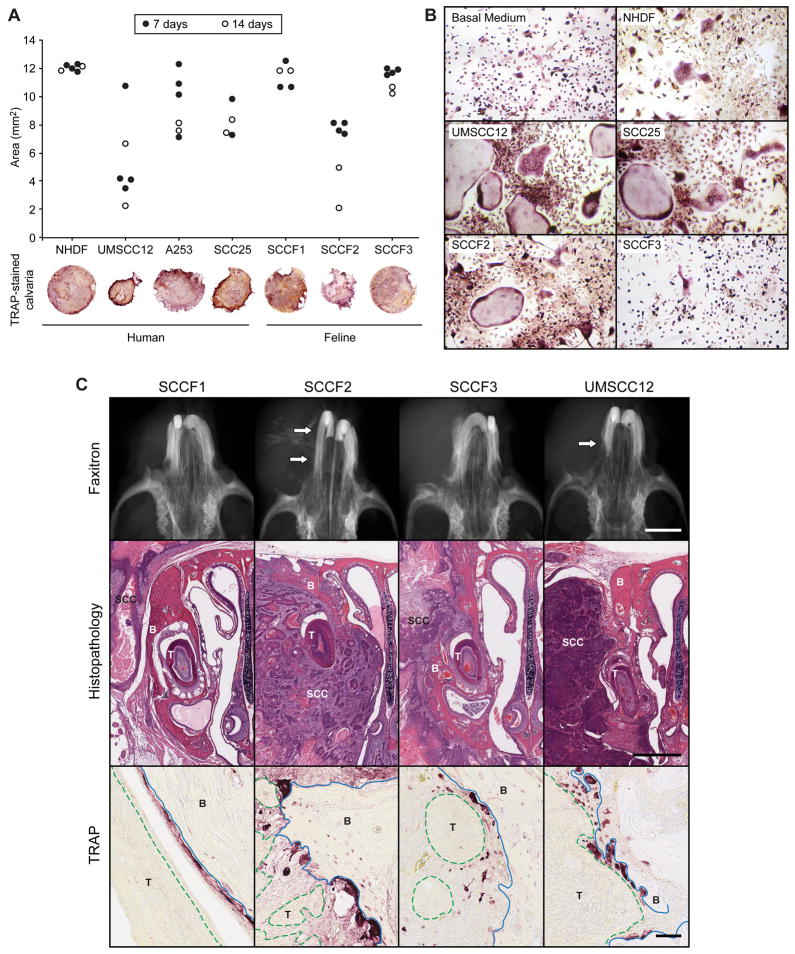
In order to model in vitro OSCC-associated bone resorption, feline and human OSCC cells were co-cultured with murine calvarial bone. UMSCC12 and SCCF2 cells were associated with pronounced bone loss (figure 2A). UMSCC12, SCC25 and SCCF2 CM stimulated the formation of large multinucleated TRAP-positive osteoclasts in murine BMMC cultures (figure 2B). In contrast, few osteoclasts were formed in the presence of SCCF3 and NHDF medium. Similarly few osteoclasts formed in TAF-CM (data not shown).
Figure 2. Feline and human OSCC cells induced in vitro and in vivo osteoclastic bone resorption.
A. Murine calvarial bone discs (4 mm diameter) were co-cultured with OSCC cells for 7 (black circles) or 14 (white circles) days. Calvaria were fixed, stained for TRAP activity, photographed and evaluated for changes in bone area using histomorphometry. SCCF2 (feline) and UMSCC12 (human) cells stimulated the most bone resorption. B. Murine BMSCs were cultured in CM from human and feline OSCC cells. UMSCC12, SCC25 and SCCF2 cells stimulated the formation of numerous, large osteoclasts. C. In vivo, minimal to no evidence of bone resorption was radiographically evident in SCCF1 and SCCF3-tumor-bearing mice. Moderate to marked reactive bone resorption (white arrows) was observed in the SCCF2Luc and UMSCC12Luc-bearing mice. SCCF1Luc xenografts were rarely associated with bone resorption despite close proximity of tumor to bone. SCCF2Luc xenografts were characterized by marked bone loss (xenograft indicated by ‘SCC’, tooth by ‘T’ and bone by ‘B’) and invasion with the formation of numerous large, TRAP-positive osteoclasts. Osteoclasts are red-stained cells on the surface of bone (blue solid line) adjacent to OSCC xenografts (green dashed line, labeled ‘T’). Less bone invasion was observed in the SCCF3Luc xenografts; however, osteoclastic bone resorption was occasionally observed. UMSCC12Luc frequently invaded bone and stimulated osteoclastic bone resorption. Scale bars: Faxitron: 1 mm; Histopathology: 1 mm; TRAP: 50 μm.
SCCF1Luc, SCCF2Luc, SCCF3Luc and UMSCC12Luc differed in xenograft growth, bone-invasive behavior and metastasis in vivo (table 1). BLI was used to evaluate xenograft growth with and without Matrigel, and to detect regional and distant metastasis ex vivo (figures S2 and table 1). In the absence of Matrigel, SCCF3Luc was the only cell line to yield progressive xenograft growth in all mice and was associated with the most rapid tumor progression (19 to 28 days). Without Matrigel, xenografts developed from SCCF2Luc cells in 4 of 5 mice, from UMSCC12Luc cells in 3 of 5 mice, and from SCCF1Luc cells in 2 of 5 mice. UMSCC12Luc xenografts demonstrated the slowest rate of tumor progression (83–90 days). Interestingly, Matrigel increased the incidence of xenograft formation to 5 of 5 mice for UMSCC12Luc and SCCF1Luc cells, but reduced the incidence of sustained xenograft growth in SCCF2Luc-injected mice.
Table 1. Behavior of OSCC xenografts in nude mice.
Mice were injected with the indicated cell lines, with and without matrigel, and were observed for tumor growth using in vivo bioluminescent imaging. Mice were removed from the study when xenografts reached 1cm diameter, or at 90 days following injection if xenografts failed to reach 1 cm. ‘Days’ indicates the average number of days to removal with the range in parantheses. Osteolysis and invasion was characterized subjectively as none, minimal, mild, moderate and marked. Metastasis were detected in cervical lymph nodes and lungs using ex vivo bioluminescent imaging, and the metastatic events that were confirmed using histologic examination are indicated.
| Cell Line | Vehicle | N | Progressive Growth | Days (range) | Maxillary osteolysis and Invasion | Metastasis BLI / Histo |
|---|---|---|---|---|---|---|
| UMSCC12Luc | PBS | 5 | 3 | 86 (83–90) | Moderate to marked | 2 / 2 Lymph node |
| PBS + Matrigel | 5 | 5 | 74 (49–90) | Moderate to marked | 1 / 0 Lymph node | |
| SCCF1Luc | PBS | 5 | 2 | 70 (49–90) | None | |
| PBS + Matrigel | 5 | 5 | 50 (35–64) | Minimal to none | 1 / 0 Lymph node | |
| SCCF2Luc | PBS | 5 | 4 | 49 (46–57) | Marked | 2 / 2 Lungs |
| PBS + Matrigel | 5 | 1 | 47 | Marked | ||
| SCCF3Luc | PBS | 5 | 5 | 25 (19–28) | Mild | |
| PBS + Matrigel | 5 | 5 | 21 (14–26) | Mild to moderate |
The greatest degree of osteoclastic bone resorption and maxillary invasion was observed in SCCF2Luc and UMSCC12Luc-bearing mice (table 1 and figure 2C. Mild to moderate bone resorption was observed in SCCF3Luc-bearing mice, and minimal to no bone resorption was observed in SCCF1Luc-bearing mice. Bone invasion was associated with numerous TRAP-positive osteoclasts in resorption pits on the maxillary surfaces adjacent to the xenograft (figure 2C).
Osteolytic OSCC expressed the most PTHrP and stimulated MC3T3 expression of RANKL
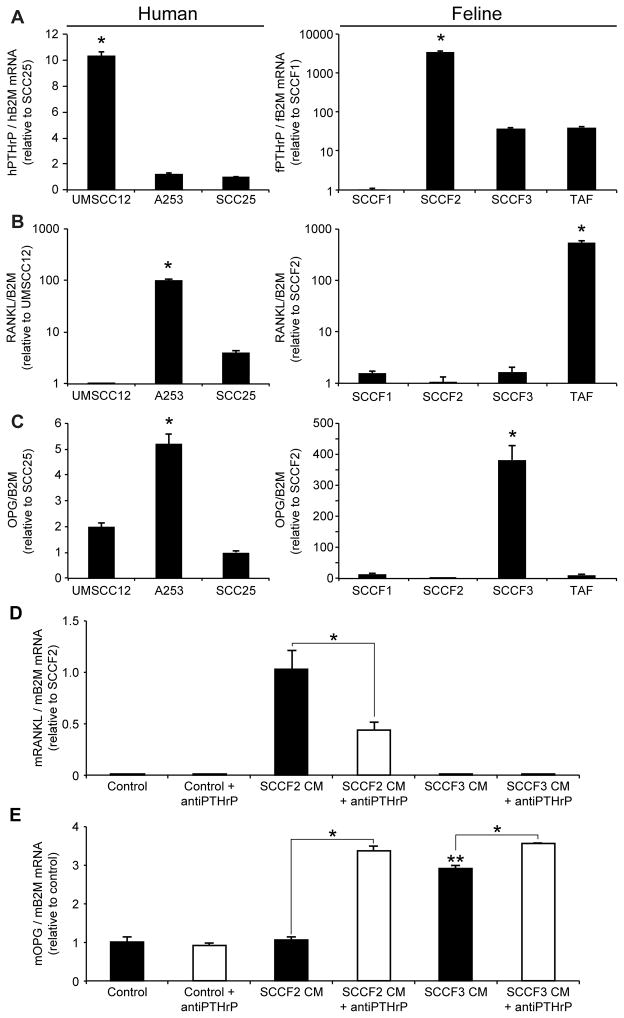
UMSCC12 and SCCF2 cells expressed significantly greater PTHrP mRNA (5-fold and 50-fold) compared to A253 and SCC25 or SCCF3 and TAF cells respectively (figure 3A). The PTHrP mRNA levels corresponded to secreted PTHrP (figure 4C) and the ability to stimulate osteoclastic bone resorption. RANKL expression was relatively low in UMSCC12 and SCCF2 cells (figure 3B) compared to A253 cells and TAF cells, suggesting that osteoclast activation does not rely solely on OSCC-expression of RANKL. UMSCC12 expressed the highest levels of CSF-1 mRNA (a cytokine capable of stimulating osteoclast formation) compared to either A253 or SCC25 which may have contributed to osteoclastic bone resorption. In contrast, bone-invasive SCCF2 cells expressed the lowest levels of CSF-1 mRNA compared to the other feline OSCC cell lines (figure S3). TAF cells expressed the highest levels of CSF-1 and RANKL mRNA compared to the feline OSCC cell lines, suggesting that the tumor stroma may have a significant role in stimulating osteoclast formation in bone invasive OSCC. Interestingly, cells associated with less bone resorption (A253 and SCCF3), expressed the highest levels of OPG (figure 3C).
Figure 3. Bone-invasive OSCC cells expressed more PTHrP and less OPG compared to minimally bone-invasive OSCC cells, and stimulated RANKL expression in murine preosteoblasts.
A. UMSCC12 cells expressed the most PTHrP mRNA compared to A253 and SCC25 cells, and SCCF2 cells expressed the most PTHrP compared to SCCF1 and SCCF3 cells. B. A253 and TAF cells expressed the most RANKL. C. A253 and SCCF3 cells (both were associated with low degrees of bone resorption) expressed the greatest amount of OPG mRNA (A,B, and C: *P<0.05, ANOVA and Bonferroni post hoc test). D. RANKL expression was not detected in untreated or SCCF3-CM-treated MC3T3 cells, but was induced by SCCF2-CM. Anti-PTHrP neutralizing antibody reduced the effect of SCCF2-CM on RANKL expression (*P<0.05, Student’s T-Test). E. SCCF3-CM stimulated OPG expression in MC3T3 cells compared to untreated and SCCF2-CM-treated MC3T3 cells (**P<0.05, ANOVA and Bonferroni post hoc test). SCCF2-CM had no effect of OPG expression compared to untreated cells. Addition of anti-PTHrP antibody to SCCF2-CM increased MC3T3-expression of OPG expression. Anti-PTHrP antibody also increased OPG expression in SCCF3-CM, but to a smaller degree compared to SCCF2-CM (*P<0.05, Student’s T-Test).
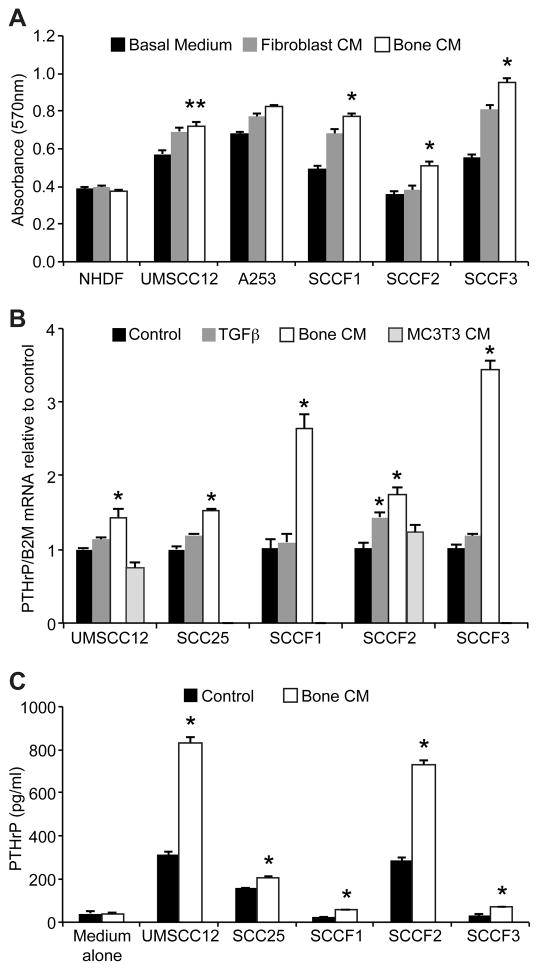
Figure 4. Bone-CM stimulated proliferation of OSCC cells and increased OSCC-expression of PTHrP.
A. An MTT assay was used to demonstrate that bone-CM stimulated OSCC cell proliferation (*P<0.05, compared to unconditioned medium or fibroblast-CM, **P<0.05 compared to unconditioned medium, ANOVA and Bonferroni’s post-hoc-test). Bone or fibroblast-CM did not stimulate proliferation of human dermal fibroblasts (NHDF). B. Bone-CM stimulated OSCC-expression of PTHrP mRNA compared to unconditioned medium (*P<0.05, ANOVA and Bonferroni’s post-hoc-test). There was no effect of MC3T3-CM on PTHrP expression on OSCC (B and data not shown). TGF-β1 (12 ng/ml) stimulated PTHrP expression in SCCF2 cells (*P<0.05, ANOVA and Bonferroni’s post-hoc-test). C. Bone-CM stimulated PTHrP secretion from OSCC cells into culture medium compared to control medium (*P<0.05, 2-tailed t-test).
RANKL expression was induced in MC3T3 cells by SCCF2-CM (high PTHrP expression) but not by SCCF3-CM (low PTHrP expression, figure 3D), which corresponded to the high degree of bone resorption and osteoclastogenesis associated with the SCCF2 cell line. Preincubation of medium with anti-human PTHrP neutralizing IgY significantly reduced the RANKL expression response to SCCF2-CM (figure 3D). SCCF3-CM stimulated the expression of OPG in MC3T3 cells (figure 3E). In contrast, SCCF2-CM did not stimulate MC3T3 expression of OPG. Interestingly, addition of anti-PTHrP neutralizing antibody to SCCF2-CM resulted in an increase in MC3T3 expression of OPG, suggesting that the level of OPG expression in MC3T3 cells treated with SCCF2-CM was the result of balanced inhibitory and stimulatory signals on MC3T3-expression of OPG. Neutralization of SCCF2-derived PTHrP alleviated inhibition of OPG expression, resulting in levels similar to that observed in SCCF3-CM. Addition of PTHrP neutralizing antibody to SCCF3-CM had a smaller stimulatory affect on MC3T3 expression of OPG, which may be explained by reduced PTHrP expression observed in the SCCF3 cell line. Taken together, exposure of MC3T3 cells to SCCF2-CM increased RANKL expression relative to OPG expression (a pro-osteoclastogenesis expression profile), which was partially reversed by the addition of a PTHrP neutralizing antibody.
Effect of bone-CM on OSCC proliferation and expression of PTHrP
OSCC cells, but not NHDF fibroblasts, proliferated more rapidly cultured in bone-CM compared to unconditioned medium (UMSCC12, figure 4A) or fibroblast-CM (A253, SCCF1, SCCF2 and SCCF3). Bone-CM stimulated OSCC-expression of PTHrP mRNA (figure 4B). TGF-β1 stimulated SCCF2-expression of PTHrP mRNA; however, TGF-β1 did not significantly stimulate PTHrP expression in the other OSCC cell lines. There was no effect of MC3T3-CM on OSCC-expression of PTHrP (figure 4B). Bone-CM stimulated PTHrP secretion into culture medium in all OSCC cell lines evaluated (figure 4C).
Discussion
We have developed a unique model of OSCC-associated osteoclastic bone resorption utilizing cell lines derived from human and feline cancers. There are few orthotopic models of head and neck cancer that demonstrate the bone-invasive behavior typical of the natural disease. Previously, in vivo studies of bone invasive OSCC included two osteolytic OSCC cell lines; BHY, derived from a human gingival OSCC,28 and SCCVII, derived from a C(3)H/HeN murine mouth floor OSCC.29 Both BHY and SCCVII cells are typically injected percutaneously into the masseter muscle to achieve mandibular invasion. Other cell lines reported to have bone invasive activity in vivo include UMSCC1 injected percutaneously ventral to the mandible,30 UMSCC11A, UMSCC11B, BICR31 and BICR56 injected intraorally into the floor of the mouth,31,32 and HSC3 injected percutaneously adjacent to the parietal bone.33 Although murine models have proven to be indispensable to studies investigating the mechanisms and treatment of OSCC, a model of spontaneous, naturally occurring OSCC would be of great benefit to researchers and patients. Our characterization of feline OSCC supports the utility of naturally occurring OSCC in cats as a spontaneous model of human OSCC. The prognosis of OSCC in cats is poor and there are few treatment options, making this population ideal for preclinical evaluations of novel treatment strategies.
Human OSCC karyotypes are frequently complex with numerous numeric and structural abnormalities.34,35 Both SCCF2 and SCCF3 cells had complex karyotypes (3 or more numeric and/or structural changes) with numerous marker chromosomes. Early alterations in the progression of human oral cancer include loss of DNA at chromosome 9p21 (p16), alteration of 17p13 (P53) and gain at 11q13 (cyclin D).36 Interestingly, no normal D4 chromosomes were observed in the SCCF2 cells (homologue of human chromosome 9 and site of p16).37,38 Neither cell line had abnormalities of E1 (homologue of human chromosome 17 and locus of TP53)37,38 or gains in D1 (homologue of human chromosome 11q and locus for cyclin D).37,38 Further study of the integrity and function of p16, p53 and cyclin D in the feline cell lines is warranted. Matrigel improved xenograft growth in SCCF1Luc and UMSCC12Luc cells but did not add to the high rate of SCCF3Luc engraftment and tumor growth. Matrigel appeared to improve tumor growth in the early stages of xenograft formation in SCCF2Luc-bearing mice, but surprisingly, the tumors regressed in all but 1 mouse. The bone invasive phenotype on UMSCC12 and SCCF2 cells in vivo was similar to the ability of these cells to induce bone resorption in vitro. Conversely, the low degree of in vivo bone resorption observed in SCCF1 and SCCF3 xenografts was consistent with the minimal in vitro bone resorption associated with these cell lines.
The human and feline OSCC cell lines evaluated in this study all expressed PTHrP, at varying levels, in agreement with previous reports showing that PTHrP is commonly expressed in human OSCC tissue33,39–42 and cell lines,42 and in feline OSCC tissue20 and SCCF1 cells.22 OSCC-expression of PTHrP influences in vitro tumor cell proliferation, migration and invasiveness43 and participates in osteoclastogenesis by increasing osteoblast expression of RANKL.33,42,44 In addition to PTHrP, OSCC-derived factors that have been associated with osteoclast formation and bone invasion include interleukin-6 (IL-6), IL-8, IL-11 and tumor necrosis factor α (TNFα).33,45,46 Although we have previously shown that TNFα expression was absent or minimally expressed in bone invasive feline OSCC tumors (in stark contrast to expression of PTHrP),20 it is possible that OSCC-derived factors other than PTHrP contribute to the bone invasive phenotype. Of interest, OSCC expression of RANKL was not associated with osteoclast formation or bone invasion in this study. It has previously been suggested that the ability of OSCC cells to stimulate stromal expression of RANKL or to inhibit stromal expression of OPG is more important than OSCC tumor cell expression of RANKL. For example, the osteolytic behavior of BHY cells has been attributed to their inhibitory effect on stromal expression of OPG, rather than tumor cell expression of RANKL. 47
We demonstrated that bone-invasive SCCF2 cells stimulated RANKL and suppressed OPG expression in MC3T3 cells compared to the less invasive SCCF3 cells, which was partially reversed by the addition of a PTHrP neutralizing antibody. Similarly, BHY cells have been shown to express PTHrP and to stimulate RANKL expression in MC3T3 cells,42,44 and a PTHrP neutralizing antibody partially inhibited the BHY-stimulation of RANKL expression in ST-2 cells (rat osteoblasts) and osteoclast formation in vitro.33 In a recent report, knockdown of PTHrP in murine SCCVII cells inhibited osteoclastic bone resorption of the mandible in mice.29
OSCC cell lines proliferated more rapidly and had increased expression and secretion of PTHrP when cultured in bone-CM. The factors responsible for these effects are unknown; however, bone is a reservoir of numerous growth factors with the ability to promote tumor progression and include TGF-β1, fibroblast growth factor (FGF), insulin-like growth factors (IGFs) I and II, platelet derived growth factor (PDGF), and bone morphogenic proteins (BMP).48 In fact, TGF-β1 is known to stimulate PTHrP expression in a variety of tumor cells and is suspected to function in a vicious cycle of tumor growth and bone invasion in skeletal metastasis of human breast cancer.49 We have observed that bone-CM contains latent TGF-β1 using a commercially available TGF-β1 ELISA (data not shown), and TGF-β1 stimulated PTHrP expression in SCCF2 cells. Our findings are in agreement with those of Takayama et al., who demonstrated that PTHrP expression in SCCVII cells was stimulated by TGF-β1.29 In all cell lines evaluated, the stimulatory effect of TGF-β1 on PTHrP expression was less than the stimulation by bone-CM, suggesting that TGF-β1 in bone-CM is not solely responsible for the increased expression of PTHrP. Calcium released from resorbing bone has been proposed to play a role in stimulating tumor-expression of PTHrP in bone-metastatic breast cancer,50 and resorbing bone has been shown to release calcium into culture medium.24 It is possible that bone-derived calcium in the CM contributed to the stimulation of PTHrP in the OSCC cell lines.
We have developed a novel in vitro and in vivo model of OSCC-associated osteoclastic bone resorption using feline OSCC cells. We observed that the ability of feline OSCC cells to stimulate osteoclastic bone resorption corresponded to PTHrP expression, and that conditioned medium from bone invasive feline OSCC was associated with increased RANKL expression and reduced OPG expression in murine preosteoblasts in a manner that was partially PTHrP-dependant. Bone-CM and TGF-β1 stimulated OSCC-expression of PTHrP, supporting the hypothesis that OSCC invasion into bone was facilitated by a vicious cycle of tumor-derived PTHrP and bone-derived factors. These preclinical models of OSCC recapitulate the bone invasive phenotype characteristic of the disease in both humans and cats, and will be useful to future studies of bone invasive OSCC.
Supplementary Material
Acknowledgments
The authors thank Alan Flechtner and Anne Saulsbery for tissue processing and preparation of slides; Shelly Haramia for slide scanning; Tim Vojt for assistance with figures; and Jadwiga Labanowska at the Molecular Cytogenetics Shared Resource at Ohio State University for karyotyping. Funding This work was supported by the Morris Animal Foundation [grant number D08FE-023, PI: Rosol]; and the National Cancer Institute [award number F32CA130458, PI: Martin].
Footnotes
Conflict of Interest
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011 doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2009. Atlanta: American Cancer Society; 2009. [Google Scholar]
- 3.Funk GF, Karnell LH, Robinson RA, Zhen WK, Trask DK, Hoffman HT. Presentation, treatment, and outcome of oral cavity cancer: a National Cancer Data Base report. Head Neck. 2002;24(2):165–180. doi: 10.1002/hed.10004. [DOI] [PubMed] [Google Scholar]
- 4.Silverman S Jr, editor. Oral Cancer. 4. Hamilton, Ontario, Canada: BC Decker Inc; 1998. [Google Scholar]
- 5.Silverman S., Jr Demographics and occurrence of oral and pharyngeal cancers. The outcomes, the trends, the challenge. J Am Dent Assoc. 2001;132 (Suppl):7S–11S. doi: 10.14219/jada.archive.2001.0382. [DOI] [PubMed] [Google Scholar]
- 6.Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2006. 2009 http://seer.cancer.gov.proxy.lib.ohio-state.edu/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site. Updated 2009.
- 7.Smith LP, Thomas GR. Animal models for the study of squamous cell carcinoma of the upper aerodigestive tract: a historical perspective with review of their utility and limitations. Part A. Chemically-induced de novo cancer, syngeneic animal models of HNSCC, animal models of transplanted xenogeneic human tumors. Int J Cancer. 2006;118(9):2111–2122. doi: 10.1002/ijc.21694. [DOI] [PubMed] [Google Scholar]
- 8.Kimura Y, Sumi M, Sumi T, Ariji Y, Ariji E, Nakamura T. Deep Extension from Carcinoma Arising from the Gingiva: CT and MR Imaging Features. AJNR Am J Neuroradiol. 2002;23(3):468–472. [PMC free article] [PubMed] [Google Scholar]
- 9.Ito M, Izumi N, Cheng J, et al. Jaw bone remodeling at the invasion front of gingival squamous cell carcinomas. J Oral Pathol Med. 2003;32(1):10–17. doi: 10.1034/j.1600-0714.2003.00139.x. [DOI] [PubMed] [Google Scholar]
- 10.Patel RS, Dirven R, Clark JR, Swinson BD, Gao K, O'Brien CJ. The prognostic impact of extent of bone invasion and extent of bone resection in oral carcinoma. Laryngoscope. 2008;118(5):780–785. doi: 10.1097/MLG.0b013e31816422bb. [DOI] [PubMed] [Google Scholar]
- 11.Munoz Guerra MF, Naval Gias L, Campo FR, Perez JS. Marginal and segmental mandibulectomy in patients with oral cancer: a statistical analysis of 106 cases. J Oral Maxillofac Surg. 2003;61(11):1289–1296. doi: 10.1016/s0278-2391(03)00730-4. [DOI] [PubMed] [Google Scholar]
- 12.Shaw RJ, Brown JS, Woolgar JA, Lowe D, Rogers SN, Vaughan ED. The influence of the pattern of mandibular invasion on recurrence and survival in oral squamous cell carcinoma. Head Neck. 2004;26(10):861–869. doi: 10.1002/hed.20036. [DOI] [PubMed] [Google Scholar]
- 13.Ogura I, Kurabayashi T, Amagasa T, Okada N, Sasaki T. Mandibular bone invasion by gingival carcinoma on dental CT images as an indicator of cervical lymph node metastasis. Dentomaxillofac Radiol. 2002;31(6):339–343. doi: 10.1038/sj.dmfr.4600726. [DOI] [PubMed] [Google Scholar]
- 14.Ogura I, Kurabayashi T, Sasaki T, Amagasa T, Okada N, Kaneda T. Maxillary bone invasion by gingival carcinoma as an indicator of cervical metastasis. Dentomaxillofac Radiol. 2003;32(5):291–294. doi: 10.1259/dmfr/25125369. [DOI] [PubMed] [Google Scholar]
- 15.Wong RJ, Keel SB, Glynn RJ, Varvares MA. Histological pattern of mandibular invasion by oral squamous cell carcinoma. Laryngoscope. 2000;110(1):65–72. doi: 10.1097/00005537-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Lu SL, Herrington H, Wang XJ. Mouse models for human head and neck squamous cell carcinomas. Head Neck. 2006;28(10):945–954. doi: 10.1002/hed.20397. [DOI] [PubMed] [Google Scholar]
- 17.Dorn CR, Priester WA. Epidemiologic analysis of oral and pharyngeal cancer in dogs, cats, horses, and cattle. J Am Vet Med Assoc. 1976;169(11):1202–1206. [PubMed] [Google Scholar]
- 18.Stebbins KE, Morse CC, Goldschmidt MH. Feline oral neoplasia: a ten-year survey. Vet Pathol. 1989;26(2):121–128. doi: 10.1177/030098588902600204. [DOI] [PubMed] [Google Scholar]
- 19.Morris J, Dobson J. Head and Neck. In: Morris J, Dobson J, editors. Small Animal Oncology. Alder Press Ltd; Oxford: Blackwell Science Ltd; 2001. pp. 94–124. [Google Scholar]
- 20.Martin CK, Tannehill-Gregg SH, Wolfe TD, Rosol TJ. Bone-invasive oral squamous cell carcinoma in cats: pathology and expression of parathyroid hormone-related protein. Vet Pathol. 2011;48(1):302–312. doi: 10.1177/0300985810384414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tannehill-Gregg SH, Levine AL, Rosol TJ. Feline head and neck squamous cell carcinoma: a natural model for the human disease and development of a mouse model. Veterinary and Comparative Oncology. 2006;4(2):84–97. doi: 10.1111/j.1476-5810.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 22.Tannehill-Gregg S, Kergosien E, Rosol TJ. Feline head and neck squamous cell carcinoma cell line: characterization, production of parathyroid hormone-related protein, and regulation by transforming growth factor-betaIn vitro cellular & developmental biology. Animal. 2001;37(10):676–683. doi: 10.1290/1071-2690(2001)037<0676:FHANSC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Meghji S, Morrison MS, Henderson B, Arnett TR. pH dependence of bone resorption: mouse calvarial osteoclasts are activated by acidosis. Am J Physiol Endocrinol Metab. 2001;280(1):E112–9. doi: 10.1152/ajpendo.2001.280.1.E112. [DOI] [PubMed] [Google Scholar]
- 24.Shu ST, Martin CK, Thudi NK, Dirksen WP, Rosol TJ. Osteolytic bone resorption in adult T-cell leukemia/lymphoma. Leuk Lymphoma. 2010;51(4):702–714. doi: 10.3109/10428191003646697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thudi NK, Martin CK, Nadella MV, et al. Zoledronic acid decreased osteolysis but not bone metastasis in a nude mouse model of canine prostate cancer with mixed bone lesions. Prostate. 2008;68(10):1116–1125. doi: 10.1002/pros.20776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosol TJ, Steinmeyer CL, McCauley LK, et al. Studies on chicken polyclonal anti-peptide antibodies specific for parathyroid hormone-related protein (1–36) Vet Immunol Immunopathol. 1993;35(3–4):321–337. doi: 10.1016/0165-2427(93)90042-3. [DOI] [PubMed] [Google Scholar]
- 27.Dougherty KM, Blomme EA, Koh AJ, et al. Parathyroid hormone-related protein as a growth regulator of prostate carcinoma. Cancer Res. 1999;59(23):6015–6022. [PubMed] [Google Scholar]
- 28.Erdem NF, Carlson ER, Gerard DA. Characterization of gene expression profiles of 3 different human oral squamous cell carcinoma cell lines with different invasion and metastatic capacities. J Oral Maxillofac Surg. 2008;66(5):918–927. doi: 10.1016/j.joms.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 29.Takayama Y, Mori T, Nomura T, Shibahara T, Sakamoto M. Parathyroid-related protein plays a critical role in bone invasion by oral squamous cell carcinoma. Int J Oncol. 2010;36(6):1387–1394. doi: 10.3892/ijo_00000623. [DOI] [PubMed] [Google Scholar]
- 30.Simon C, Nemechek AJ, Boyd D, et al. An orthotopic floor-of-mouth cancer model allows quantification of tumor invasion. Laryngoscope. 1998;108(11 Pt 1):1686–1691. doi: 10.1097/00005537-199811000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Henson B, Li F, Coatney DD, et al. An orthotopic floor-of-mouth model for locoregional growth and spread of human squamous cell carcinoma. J Oral Pathol Med. 2007;36(6):363–370. doi: 10.1111/j.1600-0714.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- 32.Prime SS, Eveson JW, Stone AM, et al. Metastatic dissemination of human malignant oral keratinocyte cell lines following orthotopic transplantation reflects response to TGF-beta 1. J Pathol. 2004;203(4):927–932. doi: 10.1002/path.1603. [DOI] [PubMed] [Google Scholar]
- 33.Kayamori K, Sakamoto K, Nakashima T, et al. Roles of interleukin-6 and parathyroid hormone-related peptide in osteoclast formation associated with oral cancers: significance of interleukin-6 synthesized by stromal cells in response to cancer cells. Am J Pathol. 2010;176(2):968–980. doi: 10.2353/ajpath.2010.090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin C, Jin Y, Wennerberg J, Annertz K, Enoksson J, Mertens F. Cytogenetic abnormalities in 106 oral squamous cell carcinomas. Cancer Genet Cytogenet. 2006;164(1):44–53. doi: 10.1016/j.cancergencyto.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Martin CL, Reshmi SC, Ried T, et al. Chromosomal imbalances in oral squamous cell carcinoma: examination of 31 cell lines and review of the literature. Oral Oncol. 2008;44(4):369–382. doi: 10.1016/j.oraloncology.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lingen MW, Kumar V. Head and Neck. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. 7. Philadelphia, Pennsylvania: Elsevier Saunders; 2005. p. 773. [Google Scholar]
- 37.Murphy WJ, Davis B, David VA, et al. A 1.5-Mb-resolution radiation hybrid map of the cat genome and comparative analysis with the canine and human genomes. Genomics. 2007;89(2):189–196. doi: 10.1016/j.ygeno.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. [Accessed 08/22, 2010];Cat Genome Project: Genetic Maps of the Domestic Cat. http://home.ncifcrf.gov/ccr/lgd/comparative_genome/catgenome/genmaps/physicalmap.asp. Updated 1997.
- 39.Fujikawa M, Takata Y, Okamura K, et al. Hypercalcemia associated with parathyroid hormone-related protein at the terminal stage of uncomplicated squamous cell carcinoma in the head and neck region. Head Neck. 2002;24(1):56–62. doi: 10.1002/hed.10008. [DOI] [PubMed] [Google Scholar]
- 40.Kornberg LJ, Villaret D, Popp M, et al. Gene expression profiling in squamous cell carcinoma of the oral cavity shows abnormalities in several signaling pathways. Laryngoscope. 2005;115(4):690–698. doi: 10.1097/01.mlg.0000161333.67977.93. [DOI] [PubMed] [Google Scholar]
- 41.Tsuchimochi M, Kameta A, Sue M, Katagiri M. Immunohistochemical localization of parathyroid hormone-related protein (PTHrP) and serum PTHrP in normocalcemic patients with oral squamous cell carcinoma. Odontology. 2005;93(1):61–71. doi: 10.1007/s10266-005-0049-6. [DOI] [PubMed] [Google Scholar]
- 42.Deyama Y, Tei K, Yoshimura Y, et al. Oral squamous cell carcinomas stimulate osteoclast differentiation. Oncol Rep. 2008;20(3):663–668. [PubMed] [Google Scholar]
- 43.Yamada T, Tsuda M, Ohba Y, Kawaguchi H, Totsuka Y, Shindoh M. PTHrP promotes malignancy of human oral cancer cell downstream of the EGFR signaling. Biochem Biophys Res Commun. 2008;368(3):575–581. doi: 10.1016/j.bbrc.2008.01.121. [DOI] [PubMed] [Google Scholar]
- 44.Ishikuro M, Sakamoto K, Kayamori K, et al. Significance of the fibrous stroma in bone invasion by human gingival squamous cell carcinomas. Bone. 2008;43(3):621–627. doi: 10.1016/j.bone.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Shibahara T, Nomura T, Cui NH, Noma H. A study of osteoclast-related cytokines in mandibular invasion by squamous cell carcinoma. Int J Oral Maxillofac Surg. 2005;34(7):789–793. doi: 10.1016/j.ijom.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Hwang YS, Lee SK, Park KK, Chung WY. Secretion of IL-6 and IL-8 from lysophosphatidic acid-stimulated oral squamous cell carcinoma promotes osteoclastogenesis and bone resorption. Oral Oncol. 2011 doi: 10.1016/j.oraloncology.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 47.Jimi E, Furuta H, Matsuo K, Tominaga K, Takahashi T, Nakanishi O. The cellular and molecular mechanisms of bone invasion by oral squamous cell carcinoma. Oral Dis. 2011;17(5):462–468. doi: 10.1111/j.1601-0825.2010.01781.x. [DOI] [PubMed] [Google Scholar]
- 48.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350(16):1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 49.Chirgwin JM, Guise TA. Molecular mechanisms of tumor-bone interactions in osteolytic metastases. Crit Rev Eukaryot Gene Expr. 2000;10(2):159–178. [PubMed] [Google Scholar]
- 50.Yoneda T, Hiraga T. Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem Biophys Res Commun. 2005;328(3):679–687. doi: 10.1016/j.bbrc.2004.11.070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.