Conference Summary
Manganese (Mn) is a well established neurotoxin associated with specific damage to the basal ganglia in humans. The phenotype associated with Mn neurotoxicity was first described in two workers with occupational exposure to Mn oxide.(Couper, 1837) Although the description did not use modern clinical terminology, a parkinsonian illness characterized by slowness of movement (bradykinesia), masked facies, and gait impairment (postural instability) appears to have predominated. Nearly 100 years later an outbreak of an atypical parkinsonian illness in a Chilean Mn mine provided a phenotypic description of a fulminant neurologic disorder with parkinsonism, dystonia, and neuropsychiatric symptoms.(Rodier J, 1955) Exposures associated with this syndrome were massive and an order of magnitude greater than modern exposures.(Rodier J, 1955; Hobson et al., 2011) The clinical syndrome associated with Mn neurotoxicity has been called manganism.
Modern exposures to Mn occur primarily through occupations in the steel industry and welding. These exposures are often chronic and varied, occurring over decades in the healthy workforce. Although the severe neurologic disorder described by Rodier and Couper are no longer seen, several reports have suggested a possible increased risk of neurotoxicity in these workers.(Racette et al., 2005b; Bowler et al., 2007; Harris et al., 2011) Based upon limited prior imaging and pathologic investigations into the pathophysiology of neurotoxicity in Mn exposed workers,(Huang et al., 2003) many investigators have concluded that the syndrome spares the dopamine system distinguishing manganism from Parkinson disease (PD), the most common cause of parkinsonism in the general population, and a disease with characteristic degenerative changes in the dopaminergic system.(Jankovic, 2005)
The purpose of this symposium was to highlight recent advances in the understanding of the pathophysiology of Mn associated neurotoxicity from C. elegans to humans. Dr. Aschner’s presentation discussed mechanisms of dopaminergic neuronal toxicity in C. elegans and demonstrates a compelling potential role of Mn in dopaminergic degeneration. Dr. Guilarte’s experimental, non-human primate model of Mn neurotoxicity suggests that Mn decreases dopamine release in the brain without loss of neuronal integrity markers, including dopamine. Dr. Racette’s presentation demonstrates a unique pattern of dopaminergic dysfunction in active welders with chronic exposure to Mn containing welding fumes. Finally, Dr. Dydak presented novel magnetic resonance (MR) spectroscopy data in Mn exposed smelter workers and demonstrated abnormalities in the thalamus and frontal cortex for those workers. This symposium provided some converging evidence of the potential neurotoxic impact of Mn on the dopaminergic system and challenged existing paradigms on the pathophysiology of Mn in the central nervous system.
Keywords: Manganese, parkinsonism, PET, MRI
C. elegans and the role of dopamine in manganese-induced neurodegeneration
Michael Aschner, PhD
Departments of Pediatrics, Pharmacology and The Kennedy Center for Research on Human Development, Vanderbilt University Medical Center, Nashville, TN, USA
Manganese homeostasis is crucial given the delicate relationship between its essentiality and toxicity. Control over Mn concentrations in all tissues, including the central nervous system (CNS) is maintained by several mechanisms including the divalent metal transporter (DMT1).(Andrews, 1999) The latter belongs to the family of natural resistance-associated macrophage protein (NRAMP).(Garrick et al., 2003; Gruenheid et al., 1995; Gunshin et al., 1997) In the basal ganglia, an area known to accumulate the highest Mn concentrations, DMT1 levels are also the highest in the CNS.(Huang and Lin J. L., 2004) The existence of multiple DMT proteins which are differentially regulated at the transcriptional and post-translational levels and within specific tissues (intestine, liver, kidney, brain) and under various conditions [Mn or iron (Fe) levels, infection], has been a challenge in elucidating their specific functions. To better characterize DMT1 function, studies in our lab have utilized a C. elegans model. C. elegans offers many distinct advantages. Its small size (~1.5mm adult), short lifespan (~3 weeks) and rapid lifecycle (~ 3 days)(Leung et al., 2008; Sulston et al., 1983) permit rapid analysis. A C. elegans hermaphrodite produces ~300 progeny thus providing ample number of animals for analysis. C. elegans with <1000 cells is transparent allowing for direct visualization of cells. Furthermore, RNA interference (RNAi) and chromosomal deletion can be achieved with relative ease and provide critical information on mutant strains and sensitivity to environmental insults.(Jiang and Zheng W., 2005; Lund et al., 2002; Reichert and Menzel R., 2005) Strains can also be frozen and thawed, and proteins can be labeled with green fluorescence (GFP) and easily visualized.
In recent studies in our lab we identified and cloned three functional C. elegans DMT1 orthologues SMF-1, SMF-2 and SMF-3.(Au et al., 2009) These proteins were shown to play distinct roles in Mn transport and support an evolutionary conserved function for DMT1 isoforms in the regulation of Mn uptake. Specifically, our results have documented that SMF-3 is the major Mn transporter in the worm. smf-3(ok1035) was shown to be the mutant most resistant to acute Mn exposure and the only mutant that displayed a significant decrease in Mn content upon Mn treatment suggesting that SMF-3 depletion may not be compensated for by SMF-1 or SMF-2. smf-3 was also shown to be down-regulated both transcriptionally and post-translationally upon exposure to Mn thus limiting the toxic accumulation of Mn in the worm. In contrast, SMF-2 was shown to partially protect against Mn exposure, most likely by allowing: Mn excretion, its sequestration, or the modulation of Mn uptake via the other DMT1-like isoforms, or a combination of the above. We posited that SMF-2 most likely functions as a sensor of environmental Mn levels, and a downstream signaling pathway would either impact Mn uptake via SMF-3 and SMF-1 or Mn excretion via as of yet uncharacterized transporters. Finally, SMF-1’s contribution to Mn uptake was minor compared to SMF-3 and we posited that it is a counterpart of NRAMP1 in the worm, and is predominantly required for Fe uptake.
These studies were conducted as a first step in establishing that both Mn uptake and toxicity mechanisms involving DMTs are conserved from nematodes to man. Given the evolutionary conserved function for DMT1 isoforms in C. elegans, we then used this platform as a tool to dissect out the role of various neurotransmitters in mediating Mn neurotoxicity. As such, additional studies were conducted in which it was demonstrated that dysfunction in the dopamine re-uptake transporter, DAT-1, sensitizes the worm to Mn neurotoxicity.(Benedetto et al., 2010) Analogous to observations in mammalian studies, Mn toxicity in the worm was also associated with elevated formation of reactive oxygen species (ROS) and reduced longevity. Consistent with the oxidative stress associated with Mn exposure, SKN-1 (the worm’s NRF-2 homolog) overexpression protected from Mn-induced toxicity. Furthermore, increased extracellular, but not intracellular dopamine (DA) concentrations due to direct DA exposure or genetic manipulation potentiated the Mn-induced lethality, oxidative-stress, and the worms’ reduction in lifespan.(Benedetto et al., 2010) Finally, we have also shown that the dual-oxidase, BLI-3, is involved in the DA-dependent Mn-induced toxicity and that bli-3 loss-of-function attenuates the DA-dependency of Mn toxicity.
Taken together these studies show that in Mn-treated C. elegans, physiological aspects of parkinsonism are recapitulated. Specifically, we corroborate the importance of NRAMP/DMT orthologues in the toxicity process,(Salazar et al., 2008; Song et al., 2007; Zhang et al., 2009) the specificity and dose-dependency of the DAergic neurodegeneration,(Barzilai and Melamed E., 2003) the involvement of the dopamine transporter (DAT),(Afonso-Oramas et al., 2009) the synergy between DA and Mn,(Prabhakaran et al., 2008) as well as the role of Mn-induced oxidative stress in the neurodegenerative process,(Milatovic et al., 2007; Milatovic et al., 2009) Our finding on the role of extracellular DA in mediating Mn neurotoxicity also have important implications for contemporary treatment modalities of Parkinson disease (PD) since different strategies are necessary for controlling extracellular vs. intracellular DA levels. The most commonly used treatment for PD, L-DOPA, could accelerate or exacerbate DAergic neurodegeneration over a protracted time period. However, only one clinical trial has investigated the impact of L-DOPA on disease progression and no worsening in PD was found.(Fahn, 1999) If the dual-oxidases also oxidize DA in vertebrates, the ideal pharmacologic treatment for PD would maintain high affinity for DA receptors and DAT, but serve as poor substrates for the dual-oxidases. (Benedetto et al., 2010) Alternatively, one could limit the extent of the DAergic neurodegeneration by directly inhibiting the dual-oxidases. Promoting Nrf2 activity could also offer a beneficial strategy in limiting Mn-induced toxicity. Importantly, the series of studies described herein establishes the critical benefit in conducting biochemical and genetic investigations in the C. elegans model. Clearly, studies on environment-gene interactions in age-related DAergic neurodegenerative disorders are highly amenable in the nematode, providing crucial information on mechanisms associated with Mn-induced neurotoxicity.
Dysregulation of in vivo dopamine release in the striatum of manganese-exposed non-human primates measured by PET
Tomas Guilarte, PhD.
Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University, New York, NY, USA
Accumulation of Mn in the CNS can result in a parkinsonian syndrome with clinical features that may overlap with idiopathic PD.(Perl and Olanow C. W., 2007; Guilarte, 2010) The neuropathology in PD is well characterized and comprises the progressive loss of dopamine-containing neurons in the substantia nigra pars compacta. The loss of dopamine neurons leads to a dramatic decrease in dopamine concentrations and the structural loss of dopamine terminal markers in the caudate and putamen, the brain regions that are primarily affected and account for the movement abnormalities in PD. Unlike PD, the underlying neurobiology of Mn-induced parkinsonism has been a subject of great debate in the scientific literature.(Guilarte, 2010) Historically, there has been a paucity of data and the available evidence has been contradictory on the mechanism(s) by which Mn induces movement abnormalities. Accordingly, our laboratory undertook a multidisciplinary study using non-human primates to address the early neurological effects of exposure to moderate levels of Mn on behavior and motor function. These longitudinal studies comprise assessment of cognitive and motor function in conjunction with neuroimaging studies that interrogate structural and functional aspects of the dopaminergic system using Positron Emission Tomography (PET). Finally, verification of in vivo PET findings was performed using a variety of neuropathological endpoints.
The results of these studies indicate that moderate levels of Mn exposure produce subtle changes in fine motor control and small decrements in motor function.(Guilarte et al., 2006b; Guilarte et al., 2008a; Schneider et al., 2006) These motor function changes were associated with a marked decrease of in vivo dopamine release in the absence of a change in dopamine transporter levels in the caudate/putamen(Guilarte et al., 2006a; Guilarte et al., 2008a) measured by PET in the same animals. We also found a small but significant decrease in D2-dopamine receptor levels in the caudate and putamen measured by PET. The neuropathological studies confirmed that Mn exposure did not alter levels of tyrosine hydroxylase or other structural markers of dopamine terminals in the caudate and putamen, or dopamine concentrations.(Guilarte et al., 2006b; Guilarte et al., 2008a) Thus, the findings from our studies clearly indicate that the movement abnormalities in non-human primates associated with exposure to moderate levels of Mn were not the result of dopamine neuron loss; but in fact, they are associated with the inability of dopaminergic neurons to release dopamine. One important aspect of our comprehensive non-human primate studies is that there are a number of cognitive tasks that are assessed along with motor function tests. We have found that in the same Mn-exposed animals that express subtle deficits in motor function, they also express working memory deficits.(Schneider et al., 2009) Since dopamine plays an important role in working memory in the frontal cortex,(Aalto et al., 2005; Landau et al., 2009) we are currently investigating whether the same level of Mn exposure that result in impairments of in vivo dopamine release in the caudate/putamen, also affects dopamine release in the frontal cortex. We have previously shown that Mn exposure in these non-human primates results in significant neurodegenerative changes in the frontal cortex,(Guilarte et al., 2008b) a brain region that has not been previously associated with Mn neurotoxicity. The in vivo dopamine release studies in the frontal cortex using PET are being performed in parallel with assessment of working memory in the same animals. The results from these studies should provide valuable information that may help explain the role of the dopamine system in various aspects of Mn-induced neurological dysfunction.
Molecular Imaging of Mn Exposed Humans
Brad A. Racette, M.D.
Department of Neurology, Washington University School of Medicine, St. Louis, MO, USA
Several single-photon emission computed tomography (SPECT) or PET-based approaches use different radiotracers to assess various aspects of presynaptic dopaminergic nigrostriatal neurons.(Brooks et al., 2003) PET has the advantage of substantially greater resolution. We have recently used FDOPA PET to investigate the pathophysiology of Mn associated neurotoxicity in welders. FDOPA PET primarily reflects neuronal decarboxylase activity that converts FDOPA into the charged molecule [18F]dopamine that is subsequently trapped in the brain.(Martin and Perlmutter Joel S., 1994) Multiple studies in humans and nonhuman primates suggest that FDOPA uptake reflects nigrostriatal function. FDOPA uptake is decreased in PD patients compared to normal subjects (Martin et al., 1989; Heiss and Hilker R., 2004) and striatal uptake modestly correlates with parkinsonian signs.(Ishikawa et al., 1996) Several studies have attempted to correlate striatal uptake of FDOPA with postmortem measures of striatal dopamine or numbers of substantia nigra pars compacta (SNpc) cell bodies of nigrostriatal neurons but FDOPA probably best correlates with dopamine content.(Snow et al., 1993; Pate et al., 1993; Yee et al., 2001) FDOPA PET has greater sensitivity to detect dysfunction of the nigrostriatal system than clinical examination and identifies dopaminergic dysfunction in pre-symptomatic relatives several years prior to symptoms in genetic studies for PD(Brooks, 1991; Doudet et al., 1998; Shinotoh et al., 1996) and other genetic parkinsonisms.(Kishore et al., 1996)
FDOPA PET studies in patients with Mn toxicity provide conflicting results. Wolters et al. examined four workers with parkinsonism due to ventilatory malfunction in a ferromanganese smelter.(Wolters et al., 1989) All four subjects had normal FDOPA PET and T1 MRI scans despite blood Mn levels 7-700 times the normal value. Repeat FDOPA PET and MRI scans in four of these patients, four years later were reportedly normal.(Shinotoh et al., 1997) Kim et al performed FDOPA PET on a welder with Mn exposure and clinically typical idiopathic PD and found reduced FDOPA uptake in the left putamen.(Kim et al., 1999) These same authors later described two additional cases of patients with parkinsonism and Mn exposure with dopaminergic imaging using [123I]-(1r)-2b-carboxymethoxy-3b-(4-iodophenyl)tropane ([123I]-β-CIT) SPECT.(Kim et al., 2002) One subject was a welder and the other worked in a foundry. Both had reduced striatal uptake of [123I]-β-CIT suggesting dysfunction of the nigrostriatal pathway. Unfortunately, coincident PD cannot be excluded in those subjects with dopaminergic dysfunction, especially in older subjects.
To investigate the integrity of the dopamine system in Mn exposed humans, we imaged 20 asymptomatic welders exposed to Mn containing fumes, 20 subjects with PD, and 20 non-exposed reference subjects using FDOPA PET.(Criswell et al., 2011) The mean Unified Parkinson Disease Rating Scale motor subsection part 3 (UPDRS3) was 8.3 (±3.82) as compared to 1.08 (±1.32) (ANOVA; p<0.001) demonstrating mild parkinsonian signs. Despite having mild parkinsonian signs, these subjects had no neurologic symptoms or neurologic diagnoses. For each subject we identified basal ganglia volumes of interest and generated the specific uptake of FDOPA, Ki, for each region. Repeated measures general linear model analysis demonstrated a significant interaction between diagnostic group and region F(4, 112) = 15.36, p < 0.001. Caudate Ki’s were significantly lower in asymptomatic welders (0.0098 ± 0.0013 min−1) compared to non-exposed reference subjects (0.0111 ± 0.0012 min−1, p = 0.002). The regional pattern of uptake in welders was most affected in the caudate > anterior putamen > posterior putamen. PD patients demonstrated the typical regional pattern of uptake with most severe reductions in the posterior putamen > anterior putamen > caudate. This study demonstrates dysfunction in the nigrostriatal dopamine system in active, Mn exposed welders without neurologic symptoms. We did not find a dose-response relationship between FDOPA Ki and cumulative welding exposure. However, the upregulation of dopa decarboxylase in dopamine deficiency states may obscure a dose-response relationship. The caudate Ki reduction in welders may represent an early (asymptomatic) marker of Mn neurotoxicity and may be distinct from the pattern of dysfunction found in symptomatic PD. Our data cannot exclude a specific regulatory effect of welding fume and Mn on dopa decarboxylase. Use of a radioligand that binds to VMAT2 may provide a better dose-response relationship since VMAT2 is not upregulated in dopamine deficiency states like the dopamine transporter and dopa decarboxylase.
We have also reported FDOPA PET in two subjects with Mn exposure due to end stage liver disease (ESLD). Patients with ESLD accumulate Mn in the brain due to impaired hepatic excretion.(Burkhard et al., 2003) This provides a potentially informative model to study the neurotoxic effects of chronic Mn exposure since these patients presumably have consistent elevations in blood Mn. The first subject presented with a severe parkinsonian illness with symmetric signs and severe gait impairment and had response to levodopa with improvement in her UPDRS3 from 45 to 33.5.(Racette et al., 2005a) MRI demonstrated increased T1 signal in the globus pallidum and FDOPA PET revealed marked reduction in FDOPA uptake with a caudate: posterior putamen ratio of 1.43. More recently, we described another ESLD subject who had mild parkinsonism with a UDPRS3 of 12 who also had an elevated pallidal index, indicating Mn deposition in the brain. This subject had reduced FDOPA uptake in the caudate (24.7% reduction), anterior putamen (28.0% reduction), and posterior putamen (29.3% reduction).(Criswell SC et al., 2011) The caudate: posterior putamen ratio was 0.99.
These human studies suggest that early Mn neurotoxicity results in dopaminergic dysfunction that primarily affects the caudate and may be associated with cognitive and behavioral symptoms.(Bowler et al., 2007) However, as the syndrome progresses to a more severe motor phenotype, the neurotoxicity affects the posterior putamen. This latter pattern is similar to that seen in PD patients and provides a possible pathophysiologic link between Mn neurotoxicity and the neurodegeneration seen in PD.
Altered Brain GABA and NAA Concentrations in Occupational Manganese Exposure measured by in vivo MRS
Ulrike Dydak, PhD.
School of Health Sciences, Purdue University, West Lafayette, IN, USA
While Mn neurotoxicity is clearly linked to a dysfunctional dopaminergic system,(Burton and Guilarte T. R., 2009; Criswell et al., 2011) the exact roles of the neurotransmitters glutamate and γ-aminobutyric acid (GABA) are less well understood. GABA is an inhibitory neurotransmitter, involved in projecting neuronal signals in the basal ganglia and thalamic regions and coordinating the body’s fine movement. Thus, GABA plays an important role in many movement disorders.(Galvan and Wichmann T., 2007) Several animal studies have suggested increased striatal GABA levels following Mn exposure.(Anderson et al., 2008; Bonilla, 1978; Gianutsos and Murray M. T., 1982; Gwiazda et al., 2002) Due to novel developments in the non-invasive imaging technique of magnetic resonance spectroscopy (MRS) over the past decade, it has become feasible to measure in vivo brain GABA levels in addition to many other brain metabolites in subjects exposed to Mn. Furthermore, Mn deposition in their brains may be visualized by T1-weighted MRI.
A recent study on 10 Mn-exposed smelters and 10 matched controls was conducted using the MEGA-PRESS MRS sequence(Mescher et al., 1998) to determine GABA concentrations(Edden and Barker P. B., 2007) in a mainly thalamic brain region and a standard short echo time MRS sequence to measure six more brain chemicals in several brain regions, including the frontal cortex, globus pallidus, thalamus and putamen.(Dydak et al., 2011) A 3D high-resolution T1-weighted MR imaging sequence was used to investigate differences in Mn-deposition in the brain as determined by the Pallidal Index (PI), a ratio of the T1-weighted signal intensities referenced to a white matter region in the brain with no to little Mn deposition.
While a clear group difference was found between exposed and non-exposed subjects using the PI (p = 0.007), only seven out of the ten exposed subjects did show a hyper-intense signal in the T1-weighted images, revealing Mn deposition. Thus, no clear cutoff value for the PI could be determined to discriminate between the two groups. The question as to why a small percentage of exposed workers did not show brain Mn deposition could not be explained by the duration of exposure, different work practices, age, blood Mn levels or any other factors studied in this group. It is possible that the size of samples in this study may not be sufficient to reveal the difference; however, it is also possible that a protective mechanism may exist among these subjects.
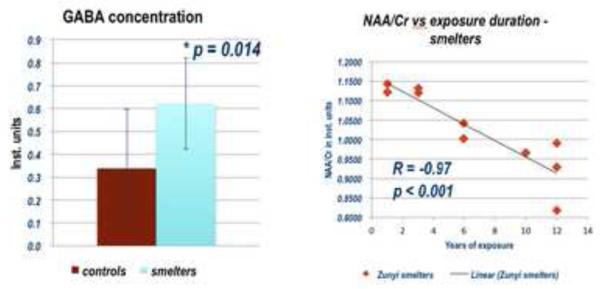
MRS revealed two major findings: First, the concentration of N-acetylaspartate (NAA), a marker of neuronal integrity, was found to be decreased in the frontal cortex of Mn-exposed workers (p = 0.042). Further, this decrease showed a significant correlation with duration of exposure (R = −0.93, p < 0.001). Second, the neurotransmitter GABA was increased by 80% on average in the thalamus and adjacent brain regions in the Mn-exposed group. (Figure 1) The findings of increased GABA levels corroborate the findings in rodent studies mentioned above for the first time in humans. Since GABA levels did not correlate with the MRI signal hyperintensity indicative of high Mn deposition in the brain, and NAA was found to be decreased particularly in the frontal cortex, where less Mn is deposited than in the basal ganglia, Mn neurotoxicity may rather be defined by the intrinsic vulnerability of neuronal systems to injury caused by Mn than the amount of accumulated Mn, a notion already previously suggested.(Burton and Guilarte T. R., 2009) Finally, with the GABA findings mirroring findings in movement disorders, and decreased NAA levels reflecting increased neuronal dysfunction, the MRS findings of this study may lead to new approaches identifying early presymptomatic effects of Mn neurotoxicity. Confirmation of these results in larger study cohorts is in progress.
Figure 1.
GABA Concentrations in the Thalamus in Smelter Workers and Control Subjects
Future Directions
This symposium highlighted paradigm shifting research implicating Mn as a potential dopamine neuron toxin. The mechanisms of this toxicity remain unclear. Dr. Aschner provides compelling evidence of the mechanisms underlying Mn associated dopaminergic toxicity. Dr. Guilarte’s work suggests that dopamine release is impaired but without neuronal degeneration. The human studies presented by Drs. Criswell and Racette demonstrate dopaminergic dysfunction in Mn exposed welders but the molecular imaging modalities do not necessarily indicate neurodegeneration. The human studies conducted by Drs. Dydak and Zheng broaden the spectrum of neurotransmitters impaired by Mn exposure and offers a potentially reliable new marker of Mn-associated early stage neuronal injury. Ultimately, human imaging-pathologic correlation will provide the unprecedented advantage to address lingering questions raised by the work presented at this symposium. The research progress presented by the speakers in this symposium also suggests that these imaging techniques may provide in vivo evidence to verify, compare, or discover protective or symptomatic treatments.(Jiang et al., 2006; Zheng et al., 2009)
Acknowledgements
This work was supported by funds from the National Institutes of Health: ES10563 (MA), ES010975 (TG), ES013743 (BAR), K24ES017765 (BAR), Clinical Science Translational Award NCRR UL1 RR024992 (SRC), ES-017498 (UD), ES-008146 (WZ); Michael J. Fox Foundation (BAR); the American Parkinson Disease Association (APDA) Advanced Research Center at Washington University, the Greater St. Louis Chapter of the APDA (BAR,SRC).; U.S. Department of Defense Contract # USAMRMC W81XWH-05-1-0239 (WZ, UD, MA); Chinese Science Technology Ministry Grant #2006BAI06B02 (UD,WZ) and Guangxi Science and Technology Commission Grant #0991129 (UD, WZ)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aalto S, Bruck A, Laine M, Nagren K, Rinne JO. Frontal and temporal dopamine release during working memory and attention tasks in healthy humans: a positron emission tomography study using the high-affinity dopamine D2 receptor ligand [11C]FLB 457. J Neurosci. 2005;25:2471–2477. doi: 10.1523/JNEUROSCI.2097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonso-Oramas D, Cruz-Muros I, Alvarez d l R, Abreu P, Giraldez T, Castro-Hernandez J, Salas-Hernandez J, Lanciego JL, Rodriguez M, Gonzalez-Hernandez T. Dopamine transporter glycosylation correlates with the vulnerability of midbrain dopaminergic cells in Parkinson’s disease. Neurobiol. Dis. 2009;36:494–508. doi: 10.1016/j.nbd.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Anderson JG, Fordahl SC, Cooney PT, Weaver TL, Colyer CL, Erikson KM. Manganese exposure alters extracellular GABA, GABA receptor and transporter protein and mRNA levels in the developing rat brain. Neurotoxicology. 2008;29:1044–1053. doi: 10.1016/j.neuro.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews NC. The iron transporter DMT1. Int. J Biochem. Cell Biol. 1999;31:991–994. doi: 10.1016/s1357-2725(99)00065-5. [DOI] [PubMed] [Google Scholar]
- Au C, Benedetto A, Anderson J, Labrousse A, Erikson K, Ewbank JJ, Aschner M. SMF-1, SMF-2 and SMF-3 DMT1 orthologues regulate and are regulated differentially by manganese levels in C. elegans. PLoS. One. 2009;4:e7792. doi: 10.1371/journal.pone.0007792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai A, Melamed E. Molecular mechanisms of selective dopaminergic neuronal death in Parkinson’s disease. Trends Mol. Med. 2003;9:126–132. doi: 10.1016/s1471-4914(03)00020-0. [DOI] [PubMed] [Google Scholar]
- Benedetto A, Au C, Avila DS, Milatovic D, Aschner M. Extracellular dopamine potentiates mn-induced oxidative stress, lifespan reduction, and dopaminergic neurodegeneration in a BLI-3-dependent manner in Caenorhabditis elegans. PLoS. Genet. 2010;6 doi: 10.1371/journal.pgen.1001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla E. Increased GABA content in caudate nucleus of rats after chronic manganese chloride administration. J Neurochem. 1978;31:551–552. doi: 10.1111/j.1471-4159.1978.tb02672.x. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Roels HA, Nakagawa S, Drezgic M, Diamond E, Park R, Koller W, Bowler RP, Mergler D, Bouchard M, Smith D, Gwiazda R, Doty RL. Dose-effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup. Environ. Med. 2007;64:167–177. doi: 10.1136/oem.2006.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DJ. Detection of preclinical Parkinson’s disease with PET. Geriatrics. 1991;46:25–30. [PubMed] [Google Scholar]
- Brooks DJ, Frey KA, Marek KL, Oakes D, Paty D, Prentice R, Shults CW, Stoessl AJ. Assessment of neuroimaging techniques as biomarkers of the progression of Parkinson’s disease. Exp. Neurol. 2003;184(Suppl 1):S68–S79. doi: 10.1016/j.expneurol.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Burkhard PR, Delavelle J, Du PR, Spahr L. Chronic parkinsonism associated with cirrhosis: a distinct subset of acquired hepatocerebral degeneration. Arch. Neurol. 2003;60:521–528. doi: 10.1001/archneur.60.4.521. [DOI] [PubMed] [Google Scholar]
- Burton NC, Guilarte TR. Manganese neurotoxicity: lessons learned from longitudinal studies in nonhuman primates. Environ. Health Perspect. 2009;117:325–332. doi: 10.1289/ehp.0800035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couper J. On the effects of black oxide of manganese when inhaled into the lungs. British Annals of Medical Pharmacology. 1:41–42. [Google Scholar]
- Criswell SC, Perlmutter JS, Videen TO, Crippin JS, Moerlein SM, Flores HP, Birke AM, Racette BA. Reduced Uptake of [18F]FDOPA PET in End Stage Liver Disease with Elevated Manganese Levels. In Press. [DOI] [PMC free article] [PubMed]
- Criswell SR, Perlmutter JS, Videen TO, Moerlein SM, Flores HP, Birke AM, Racette BA. Reduced uptake of [18F]FDOPA PET in asymptomatic welders with occupational manganese exposure. Neurology. 2011;76:1296–1301. doi: 10.1212/WNL.0b013e3182152830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudet DJ, Chan GL, Holden JE, McGeer EG, Aigner TA, Wyatt RJ, Ruth TJ. 6-[18F]Fluoro-L-DOPA PET studies of the turnover of dopamine in MPTP-induced parkinsonism in monkeys. Synapse. 1998;29:225–232. doi: 10.1002/(SICI)1098-2396(199807)29:3<225::AID-SYN4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Dydak U, Jiang YM, Long LL, Zhu H, Chen J, Li WM, Edden RA, Hu S, Fu X, Long Z, Mo XA, Meier D, Harezlak J, Aschner M, Murdoch JB, Zheng W. In vivo measurement of brain GABA concentrations by magnetic resonance spectroscopy in smelters occupationally exposed to manganese. Environ. Health Perspect. 2011;119:219–224. doi: 10.1289/ehp.1002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RA, Barker PB. Spatial effects in the detection of gamma-aminobutyric acid: improved sensitivity at high fields using inner volume saturation. Magn Reson. Med. 2007;58:1276–1282. doi: 10.1002/mrm.21383. [DOI] [PubMed] [Google Scholar]
- Fahn S. Parkinson disease, the effect of levodopa, and the ELLDOPA trial. Earlier vs Later L-DOPA. Arch. Neurol. 1999;56:529–535. doi: 10.1001/archneur.56.5.529. [DOI] [PubMed] [Google Scholar]
- Galvan A, Wichmann T. GABAergic circuits in the basal ganglia and movement disorders. Prog. Brain Res. 2007;160:287–312. doi: 10.1016/S0079-6123(06)60017-4. [DOI] [PubMed] [Google Scholar]
- Garrick MD, Dolan KG, Horbinski C, Ghio AJ, Higgins D, Porubcin M, Moore EG, Hainsworth LN, Umbreit JN, Conrad ME, Feng L, Lis A, Roth JA, Singleton S, Garrick LM. DMT1: a mammalian transporter for multiple metals. Biometals. 2003;16:41–54. doi: 10.1023/a:1020702213099. [DOI] [PubMed] [Google Scholar]
- Gianutsos G, Murray MT. Alterations in brain dopamine and GABA following inorganic or organic manganese administration. Neurotoxicology. 1982;3:75–81. [PubMed] [Google Scholar]
- Gruenheid S, Cellier M, Vidal S, Gros P. Identification and characterization of a second mouse Nramp gene. Genomics. 1995;25:514–525. doi: 10.1016/0888-7543(95)80053-o. [DOI] [PubMed] [Google Scholar]
- Guilarte TR. Manganese and Parkinson’s disease: a critical review and new findings. Environ. Health Perspect. 2010;118:1071–1080. doi: 10.1289/ehp.0901748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Burton NC, McGlothan JL, Verina T, Zhou Y, Alexander M, Pham L, Griswold M, Wong DF, Syversen T, Schneider JS. Impairment of nigrostriatal dopamine neurotransmission by manganese is mediated by pre-synaptic mechanism(s): implications to manganese-induced parkinsonism. J. Neurochem. 2008a;107:1236–1247. doi: 10.1111/j.1471-4159.2008.05695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Burton NC, Verina T, Prabhu VV, Becker KG, Syversen T, Schneider JS. Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. J. Neurochem. 2008b;105:1948–1959. doi: 10.1111/j.1471-4159.2008.05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK, McGlothan JL, Verina T, Wong DF, Zhou Y, Alexander M, Rohde CA, Syversen T, Decamp E, Koser AJ, Fritz S, Gonczi H, Anderson DW, Schneider JS. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp. Neurol. 2006a;202:381–390. doi: 10.1016/j.expneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, McGlothan JL, Degaonkar M, Chen MK, Barker PB, Syversen T, Schneider JS. Evidence for cortical dysfunction and widespread manganese accumulation in the nonhuman primate brain following chronic manganese exposure: a 1H-MRS and MRI study. Toxicol. Sci. 2006b;94:351–358. doi: 10.1093/toxsci/kfl106. [DOI] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Gwiazda RH, Lee D, Sheridan J, Smith DR. Low cumulative manganese exposure affects striatal GABA but not dopamine. Neurotoxicology. 2002;23:69–76. doi: 10.1016/s0161-813x(02)00002-5. [DOI] [PubMed] [Google Scholar]
- Harris RC, Lundin JI, Criswell SR, Hobson A, Swisher LM, Evanoff BA, Checkoway H, Racette BA. Effects of parkinsonism on health status in welding exposed workers. Parkinsonism Relat Disord. 2011 doi: 10.1016/j.parkreldis.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss WD, Hilker R. The sensitivity of 18-fluorodopa positron emission tomography and magnetic resonance imaging in Parkinson’s disease. Eur. J. Neurol. 2004;11:5–12. doi: 10.1046/j.1351-5101.2003.00709.x. [DOI] [PubMed] [Google Scholar]
- Hobson A, Seixas N, Sterling D, Racette BA. Estimation of particulate mass and manganese exposure levels among welders. Ann Occup. Hyg. 2011;55:113–125. doi: 10.1093/annhyg/meq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Weng YH, lu CS, Chu NS, Yen TC. Dopamine transporter binding in chronic manganese intoxication. J Neurol. 2003;250:1335–1339. doi: 10.1007/s00415-003-0214-1. [DOI] [PubMed] [Google Scholar]
- Huang WH, Lin JL. Acute renal failure following ingestion of manganese-containing fertilizer. J Toxicol. Clin. Toxicol. 2004;42:305–307. doi: 10.1081/clt-120037433. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Dhawan V, Chaly T, Margouleff C, Robeson W, Dahl JR, Mandel F, Spetsieris P, Eidelberg D. Clinical significance of striatal DOPA decarboxylase activity in Parkinson’s disease. J. Nucl. Med. 1996;37:216–222. [PubMed] [Google Scholar]
- Jankovic J. Searching for a relationship between manganese and welding and Parkinson’s disease. Neurology. 2005;64:2021–2028. doi: 10.1212/01.WNL.0000166916.40902.63. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zheng W. Cardiovascular toxicities upon manganese exposure. Cardiovasc. Toxicol. 2005;5:345–354. doi: 10.1385/ct:5:4:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YM, Mo XA, Du FQ, Fu X, Zhu XY, Gao HY, Xie JL, Liao FL, Pira E, Zheng W. Effective treatment of manganese-induced occupational Parkinsonism with p-aminosalicylic acid: a case of 17-year follow-up study. J. Occup. Environ. Med. 2006;48:644–649. doi: 10.1097/01.jom.0000204114.01893.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Kim JM, Kim JW, Yoo CI, Lee CR, Lee JH, Kim HK, Yang SO, Chung HK, Lee DS, Jeon B. Dopamine transporter density is decreased in parkinsonian patients with a history of manganese exposure: What does it mean? Mov Disord. 2002;17:568–575. doi: 10.1002/mds.10089. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim JW, Ito K, Lim HS, Cheong HK, Kim JY, Shin YC, Kim KS, Moon Y. Idiopathic parkinsonism with superimposed manganese exposure: utility of positron emission tomography. Neurotoxicology. 1999;20:249–252. [PubMed] [Google Scholar]
- Kishore A, Wszolek ZK, Snow BJ, Fuente-Fernandez R, Arwert F, Wijker M, Schulzer M, Calne DB, Vingerhoets FJ. Presynaptic nigrostriatal function in genetically tested asymptomatic relatives from the pallido-ponto-nigral degeneration family. Neurology. 1996;47:1588–1590. doi: 10.1212/wnl.47.6.1588. [DOI] [PubMed] [Google Scholar]
- Landau SM, Lal R, O’Neil JP, Baker S, Jagust WJ. Striatal dopamine and working memory. Cereb. Cortex. 2009;19:445–454. doi: 10.1093/cercor/bhn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung MC, Williams PL, Benedetto A, Au C, Helmcke KJ, Aschner M, Meyer JN. Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol. Sci. 2008;106:5–28. doi: 10.1093/toxsci/kfn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J, Tedesco P, Duke K, Wang J, Kim SK, Johnson TE. Transcriptional profile of aging in C. elegans. Curr. Biol. 2002;12:1566–1573. doi: 10.1016/s0960-9822(02)01146-6. [DOI] [PubMed] [Google Scholar]
- Martin WR, Palmer MR, Patlak CS, Calne DB. Nigrostriatal function in humans studies with positron emission tomography. Ann. Neurol. 1989;26:535–542. doi: 10.1002/ana.410260407. [DOI] [PubMed] [Google Scholar]
- Martin WRW, Perlmutter JS. Assessment of fetal tissue transplantation in Parkinson’s disease: does PET play a role? Neurology. 1994;44:1777–1780. doi: 10.1212/wnl.44.10.1777. [DOI] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR. Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Yin Z, Gupta RC, Sidoryk M, Albrecht J, Aschner JL, Aschner M. Manganese induces oxidative impairment in cultured rat astrocytes. Toxicol. Sci. 2007;98:198–205. doi: 10.1093/toxsci/kfm095. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Zaja-Milatovic S, Gupta RC, Yu Y, Aschner M. Oxidative damage and neurodegeneration in manganese-induced neurotoxicity. Toxicol. Appl. Pharmacol. 2009;240:219–225. doi: 10.1016/j.taap.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate BD, Kawamata T, Yamada T, McGeer EG, Hewitt KA, Snow BJ, Ruth TJ, Calne DB. Correlation of striatal fluorodopa uptake in the MPTP monkey with dopaminergic indices. Ann. Neurol. 1993;34:331–338. doi: 10.1002/ana.410340306. [DOI] [PubMed] [Google Scholar]
- Perl DP, Olanow CW. The neuropathology of manganese-induced Parkinsonism. J Neuropathol. Exp. Neurol. 2007;66:675–682. doi: 10.1097/nen.0b013e31812503cf. [DOI] [PubMed] [Google Scholar]
- Prabhakaran K, Ghosh D, Chapman GD, Gunasekar PG. Molecular mechanism of manganese exposure-induced dopaminergic toxicity. Brain Res. Bull. 2008;76:361–367. doi: 10.1016/j.brainresbull.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Racette BA, Antenor JA, McGee-Minnich L, Moerlein SM, Videen TO, Kotagal V, Perlmutter JS. [18F]FDOPA PET and clinical features in parkinsonism due to manganism. Mov Disord. 2005a;20:492–496. doi: 10.1002/mds.20381. [DOI] [PubMed] [Google Scholar]
- Racette BA, Tabbal SD, Jennings D, Good L, Perlmutter JS, Evanoff B. Prevalence of parkinsonism and relationship to exposure in a large sample of Alabama welders. Neurology. 2005b;64:230–235. doi: 10.1212/01.WNL.0000149511.19487.44. [DOI] [PubMed] [Google Scholar]
- Reichert K, Menzel R. Expression profiling of five different xenobiotics using a Caenorhabditis elegans whole genome microarray. Chemosphere. 2005;61:229–237. doi: 10.1016/j.chemosphere.2005.01.077. [DOI] [PubMed] [Google Scholar]
- Rodier J. Manganese poisoning in Moroccan miners. Brit J Industr Med. 12:21–35. doi: 10.1136/oem.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar J, Mena N, Hunot S, Prigent A, Alvarez-Fischer D, Arredondo M, Duyckaerts C, Sazdovitch V, Zhao L, Garrick LM, Nunez MT, Garrick MD, Raisman-Vozari R, Hirsch EC. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson’s disease. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18578–18583. doi: 10.1073/pnas.0804373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Clark K, Bouquio C, Syversen T, Guilarte TR. Effects of chronic manganese exposure on working memory in non-human primates. Brain Res. 2009;1258:86–95. doi: 10.1016/j.brainres.2008.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Koser AJ, Fritz S, Gonczi H, Syversen T, Guilarte TR. Effects of chronic manganese exposure on cognitive and motor functioning in non-human primates. Brain Res. 2006;1118:222–231. doi: 10.1016/j.brainres.2006.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinotoh H, Snow BJ, Chu NS, Huang CC, Lu CS, Lee C, Takahashi H, Calne DB. Presynaptic and postsynaptic striatal dopaminergic function in patients with manganese intoxication: a positron emission tomography study. Neurology. 1997;48:1053–1056. doi: 10.1212/wnl.48.4.1053. [DOI] [PubMed] [Google Scholar]
- Shinotoh H, Vingerhoets FJ, Schulzer M, Snow BJ. The presymptomatic period in a patient with idiopathic parkinsonism. Parkinsonism. Relat Disord. 1996;2:127–130. doi: 10.1016/1353-8020(96)00010-7. [DOI] [PubMed] [Google Scholar]
- Snow BJ, Tooyama I, McGeer EG, Yamada T, Calne DB, Takahashi H, Kimura H. Human positron emission tomographic [18F]fluorodopa studies correlate with dopamine cell counts and levels. Ann. Neurol. 1993;34:324–330. doi: 10.1002/ana.410340304. [DOI] [PubMed] [Google Scholar]
- Song N, Jiang H, Wang J, Xie JX. Divalent metal transporter 1 up-regulation is involved in the 6-hydroxydopamine-induced ferrous iron influx. J Neurosci. Res. 2007;85:3118–3126. doi: 10.1002/jnr.21430. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Wolters EC, Huang CC, Clark C, Peppard RF, Okada J, Chu NS, Adam MJ, Ruth TJ, Li D, Calne DB. Positron emission tomography in manganese intoxication. Ann. Neurol. 1989;26:647–651. doi: 10.1002/ana.410260510. [DOI] [PubMed] [Google Scholar]
- Yee RE, Irwin I, Milonas C, Stout DB, Huang SC, Shoghi-Jadid K, Satyamurthy N, DeLanney LE, Togasaki DM, Farahani KF, Delfani K, Janson AM, Phelps ME, Langston JW, Barrio JR. Novel observations with FDOPA-PET imaging after early nigrostriatal damage. Mov. Disord. 2001;16:838–848. doi: 10.1002/mds.1168. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang J, Song N, Xie J, Jiang H. Up-regulation of divalent metal transporter 1 is involved in 1-methyl-4-phenylpyridinium (MPP(+))-induced apoptosis in MES23.5 cells. Neurobiol. Aging. 2009;30:1466–1476. doi: 10.1016/j.neurobiolaging.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Zheng W, Jiang YM, Zhang Y, Jiang W, Wang X, Cowan DM. Chelation therapy of manganese intoxication with para-aminosalicylic acid (PAS) in Sprague-Dawley rats. Neurotoxicology. 2009;30:240–248. doi: 10.1016/j.neuro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]