Abstract
Objective
To determine whether anatomical thigh muscle cross-sectional areas (MCSAs) and strength differ between osteoarthritis (OA) knees with frequent pain compared with contralateral knees without pain, and to examine the correlation between MCSAs and strength in painful versus painless knees.
Methods
48 subjects (31 women; 17 men; age 45–78 years) were drawn from 4796 Osteoarthritis Initiative (OAI) participants, in whom both knees displayed the same radiographic stage (KLG2 or 3), one with frequent pain (most days of the month within the past 12 months) and the contralateral one without pain. Axial MR images were used to determine MCSAs of extensors, flexors and adductors at 35% femoral length (distal to proximal) and in two adjacent 5 mm images. Maximal isometric extensor and flexor forces were used as provided from the OAI data base.
Results
Painful knees showed 5.2% lower extensor MCSAs (p=0.00003; paired t-test), and 7.8% lower maximal extensor muscle forces (p=0.003) than contra-lateral painless knees. There were no significant differences in flexor forces, or flexor and adductor MCSAs (p>0.39). Correlations between force and MCSAs were similar in painful and painless OA knees (0.44<r<0.66).
Conclusions
Knees with frequent pain demonstrate lower MCSAs and force of the quadriceps (but not of other thigh muscles) compared with contra-lateral knees without knee pain with the same radiographic stage. Frequent pain does not appear to affect the correlations between MCSAs and strength in OA knees. The findings suggest that quadriceps strengthening exercise may be useful in treating symptomatic knee OA.
Keywords: muscle, magnetic resonance imaging, strength, knee, osteoarthritis
INTRODUCTION
Osteoarthritis (OA) is recognized as a heterogeneous disease, associated with structural alterations of intra- and extra-articular tissues 1. A remarkable discordance between disease symptoms and radiographic changes has been reported, particularly at early disease stages 2,3. As shown by recent between-knee, within person comparisons, however, this discordance may be partly attributable to inter-person variation in pain perception 4. Further, radiography is limited to delineating pathological changes in the bones, whereas magnetic resonance (MR) imaging is capable of also visualizing other intra- and peri-articular structures, of which some (e.g. bone marrow lesions, synovitis) have been shown to display significant associations with joint pain 5–12.
Another potential extra-articular source for pain, and hence a potential explanation for the apparent discordance between radiographic disease and symptoms, is reduced muscle strength 13. Quadriceps weakness was shown to be a stronger determinant of functional disability and knee pain than radiographic disease stage 14,15, potentially due to failure of stabilizing the joint during physiological activity 16 and greater joint loading 17. It is currently unclear, however, whether quadriceps weakness results from disuse atrophy secondary to pain, or whether it precedes knee OA and represents an independent risk factor for the disease 18–21.
Significant reductions in anatomical muscle cross-sectional areas (MCSAs) of the quadriceps have been reported in (incident) knee OA 22–24 and may be responsible for loss of muscle strength. One recent longitudinal study using MRI reported the reduction in quadriceps MCSAs over two years in participants with established radiographic knee OA to be similar to the reduction observed in participants with risk factors, but without established knee OA 25. The extent of maximal voluntary muscle activation, however, also has been reported to be compromised in knee OA 15,26–29, and anxiety, lack of motivation, and other covariates may interfere with the ability to activate muscle fibers in patients with painful knee OA 16,30. Further, most studies have focused on the quadriceps, and the contribution of other thigh muscles to painful knee OA has not been comprehensively investigated.
The objective of the current study was to take a step in disentangling the relationship between knee pain, thigh muscle strength, muscle MCSAs, and radiographic knee OA. To eliminate between-person confounding from inter-subject differences in pain perception, thigh MCSAs and muscle strength were compared in participants with unilateral frequent knee pain (no pain in the contra-lateral knee) and an identical radiographic disease stage in both knees (between knee, within-person comparison). If the specific characteristic in question that differentiates both knees is rare (i.e. frequent pain versus no pain in contralateral knees with the same Kellgren Lawrence grade [KLG], this particular study design relies on large sample sizes for selecting the participants that display the specific between-knee differences of interest. For this reason, the above study design was applied to the Osteoarthritis Initiative (OAI) cohort that includes 4796 participants.
Using this design for selecting participants from the OAI, we addressed the following primary questions:
Do muscle strength and MCSAs differ between painful and (contra-lateral) painless OA knees, and do side differences vary between different thigh muscle groups (i.e. quadriceps, hamstrings and adductors)?
Does the specific muscle strength (strength / MCSAs) of the quadriceps and hamstrings differ between painful and (contra-lateral) painless OA knees?
Because a weaker correlation between muscle strength and MCSAs was observed in knees with unilateral end-stage knee osteoarthritis compared with contralateral knees without OA 31, and because frequent pain may potentially interfere with the ability to fully activate the available muscle fibers, we additionally investigated whether the correlation of muscle strength and MCSAs of the quadriceps and hamstrings differ between painful and (contra-lateral) painless OA knees. Further, sensitivity analyses were carried out to explore whether side differences and correlations differ between men and women, and whether they differ between cases with early (just osteophytes) and or advanced bilateral radiographic knee OA(osteophytes and joint space narrowing), whether they differ between participants with and without use of pain medication, and whether they depend on the duration of pain and on age. Lastly, it was explored whether averages of MCSA measurement from several MR images are more sensitive in detecting potential pain-related side-differences than analysis of a single MR image.
METHODS
Study design and sample selection
Data used in the preparation of this study were obtained from the Osteoarthritis Initiative (OAI) database, which is available for public access at http://www.oai.ucsf.edu/. Selection of OAI study subjects that matched the criteria of the current within-person, between-knee comparison design was performed using baseline and 12 months follow-up clinical and radiographic data (public-use data set 0.2.2 and 1.2.1). The study rationale and general inclusion criteria for the OAI (e.g. male or female sex, age 45–78, presence of symptoms and/or knee radiographic OA (rOA), or risk factors for developing knee OA) have been published 32,33 and are publicly available (http://oai.epi-ucsf.org/datarelease/). The participants were recruited at the University of Maryland School of Medicine (Baltimore), the Ohio State University (Columbus), the University of Pittsburgh, and the Memorial Hospital of Rhode Island (Pawtucket). Informed consent was obtained from all participants and the study was approved by the local ethics committees.
The radiographic grading used for participant selection relied on the fixed-flexion radiographs obtained at baseline. Calculated Kellgren Lawrence grades 34 (cKLG), were derived from OARSI atlas osteophyte and joint space narrowing (JSN) grades, which were assigned by centrally trained and certified readers at the clinical sites 35,36. Readers assessed each knee for presence/absence of definite marginal osteophytes (OARSI atlas grade 1–3 any medial and lateral, tibial and femoral osteophytes), and medial and lateral OAI JSN grades 1 (OARSI atlas grades 1–2) or 2 (OARSI atlas grade 3). Knees with a definite osteophyte and grade 0 OARSI-JSN were classified as cKLG2; based on previous recommendations the OAI graded knees with definite osteophytes and OARSI-JSN grade 1 and 2 as cKLG3 37.
Subjects used in the current analysis were selected as follows:
Presence of definite rOA and identical cKLG (i.e. either cKLG2 or cKLG3) in both knees at the baseline examination.
Frequent pain (Variable P01RKSX/P01LKSX; grade 2 = “pain, aching or stiffness in or around the knee” for at least one month during the past 12 months) in one knee and no pain (grade 0 = no pain in the past 12 months) in the other knee at the baseline examination. Knees with frequent pain will be termed “painful” and knees with no pain (according to variable P01RKSX/P01LKSX) will be termed “painless” knees throughout the study.
Maximal change of symptom status at 12 months follow-up in either knee to infrequent pain (grade 1 = pain in past 12 months, but not on most days of months), in order to avoid that subjects had more frequent pain in the formerly painless knee than in the former knee with frequent pain.
Of the 4796 OAI participants, 56 fulfilled the above criteria. In eight of these, no MR images of the thigh were available, so that a total of 48 participants were studied. Of the 48 participants, five did not have measurements of maximal isometric muscle forces.
MCSA analysis from MR image data
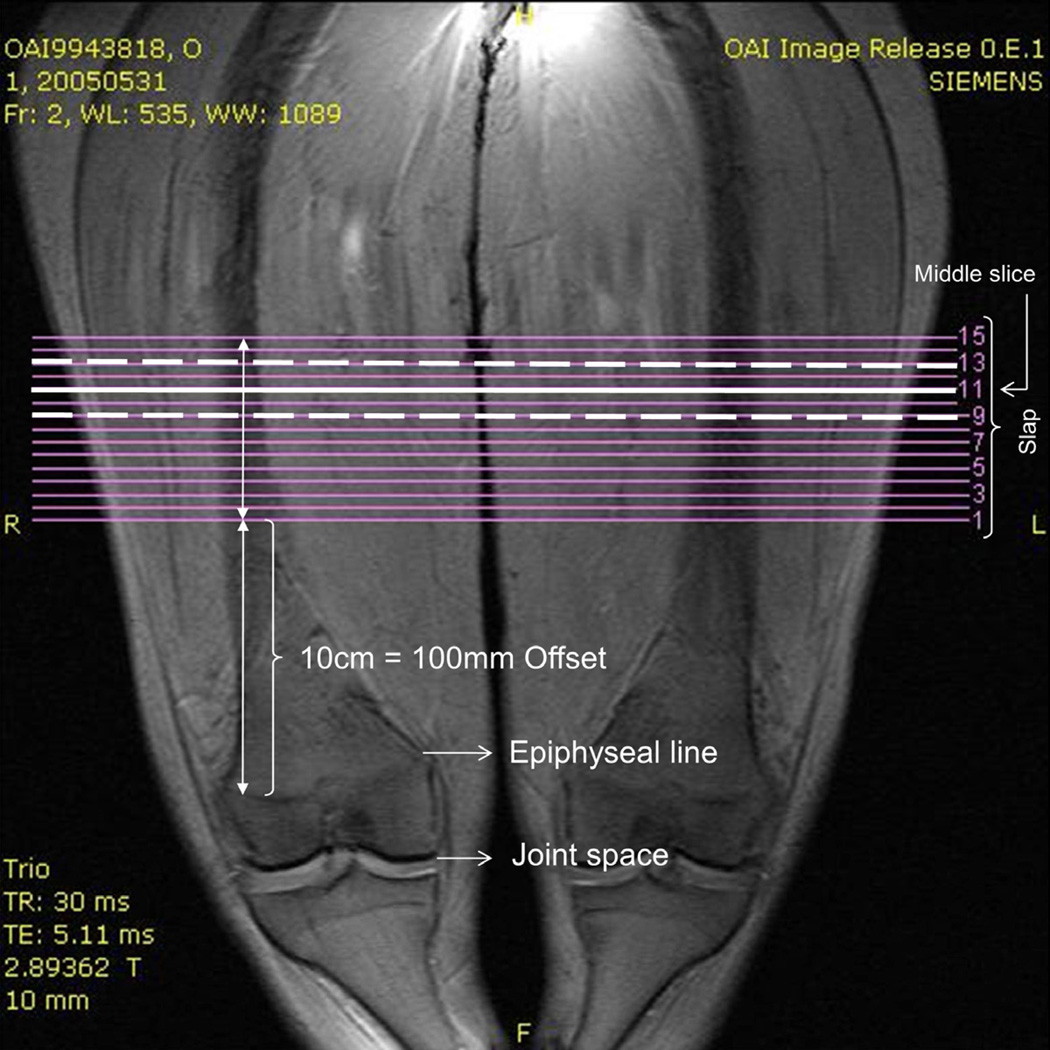
The analysis of thigh MCSAs relied on the public-use MR image data set 0.E.1 (baseline images). These were acquired using a 3 Tesla Magnetom Trio scanner (Siemens Healthcare Erlangen, Germany)33,38, with the participant positioned supine on the table. Coronal localizer images were used to delineate the distal femoral epiphyses (Fig. 1). Fifteen axial contiguous slices with 0.5cm slice thickness and an 0.977mm × 0.977mm in-plane resolution (field of view = 500mm, matrix = 512) of the thigh muscles were then acquired using a T1-weighted spin echo sequence (TR 500ms, TE 10ms; Fig. 2). Acquisition started 10cm proximal to the distal femoral epiphysis and extended 7.5cm proximally (Fig. 1). Details regarding the MRI techniques and protocols are available online (www.oai.ucsf.edu/datarelease/operationsmanuals.asp).
Figure 1.
Coronal localizer image: 15 axial images (0.5 cm) were acquired starting 100 mm proximal to the distal femoral epiphysis. Body height was used to determine an axial slice located at 35% femoral length. The slices located at 35% length and the slices proximal and distal to that slice were analysed.
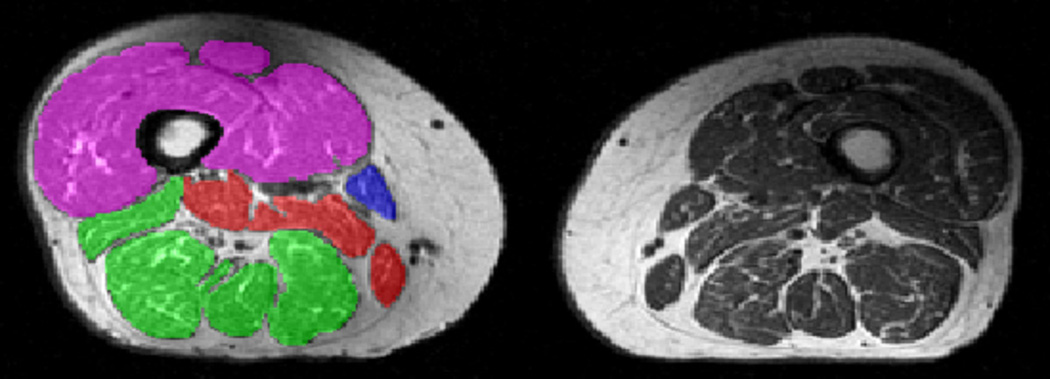
Figure 2.
Axial T1-weighted spin echo sequence delineating both thighs. Segmentation of the quadriceps (magenta), hamstrings (green), and adductors (red) are shown in the right thigh.
Note that due to the fixed distance (10cm) between the distal femoral epiphysis and the most distal MR image being acquired per OAI protocol, the position of the images relative to the femur and thigh musculature of the participants varied, depending on femoral length and body height. In order to adjust for this variability, three (of the 15 available) MR images, at intervals of 1cm, were selected based on body height. Because the thigh muscles (specifically the adductors) display larger MCSAs and greater correlations with total muscle volume proximally than distally 39, we selected the most proximal slice covered by the OAI muscle acquisitions in the largest person (1.88m) included in the current study. This position was estimated to be located at 35% of the femoral length (from distal to proximal), based on the relationship between body height, femoral length, and location of the distal femoral epiphysis previously determined in 48 OAI participants (Fig. 1) 40. Based on these relationships 40, different slice numbers within the acquisition were selected amongst the participants to ensure an anatomically consistent location.
Manual segmentation of the MCSAs of the quadriceps, the hamstrings, and the adductors (excluding the Sartorius) was performed (Fig. 2), as described previously 39,41 without the aid of (semi-)automated segmentation algorithms 42. Interstitial adipose tissue between the muscle groups, which has been previously shown to depend on age and BMI 43, was not included in the segmentations. Although the test-retest reproducibility was not assessed in this sample (because the OAI has not provided test-retest image data with repositioning), the test-retest precision for similar measurements (average of MCSAs in 3 slices spaced at 25%, 50% and 75% of the femur, with repositioning of the participant in the scanner) amounted to 1.7% for the quadriceps, 3.4% for the hamstrings, and 9.9% for the adductors 44.
Measurements of muscle strength and specific muscle strength
The maximal isometric forces of the quadriceps (variable V00_R/L_EmaxF) and of the hamstrings (variable V00_R/L_FmaxF), as measured at baseline, were taken from the OAI data base (http://www.oai.ucsf.edu/datarelease/forms.asp). These had been measured using the “Good Strength Chair” (Metitur Oy, Jycaskyla, Finland)45,46, for which satisfactory reliability (test-retest reproducibility) was reported previously 47. The participants had been positioned sitting, with the back erect and the legs hanging over the edge of the chair. A seatbelt had been used to stabilize the pelvis, the thigh and upper leg of the participant. After two warm-up trials with 50% effort, three measurements of the maximal isometric force (N) were taken of each knee at an angle of 60°, pushing the leg forward against the pad (extension) and pulling the leg back against the pad (flexion), respectively.
To determine the specific strength, the maximal isometric force measured in extension was divided by the MCSAs of the quadriceps, and maximal isometric force measured in flexion by the MCSAs of the hamstrings, in both knees of each participant.
Statistical analyses
The primary analyses focused on side-differences (pain versus no pain in knees with the same cKLG) in the MCSAs of the quadriceps, hamstrings, and adductors, and in side differences of the maximal isometric force in extension and flexion. To account for five parallel t-tests and to maintain a global error level of 5%, a p-value of <0.01 was considered to indicate statistical significance. P-values <0.05 (but not <0.01) in a single test were considered borderline significant. Sensitivity analyses comparing side differences in men versus women, cKLG2 versus cKLG3 knees, and participants with and without medication use were performed by comparing % differences in these strata. These exploratory analyses did not account for multiple testing. Linear regression analysis (Pearson correlation coefficients) was performed to explore the correlation between maximal isometric forces and MCSAs. Further, linear regression analysis was used to explore whether side-differences in isometric forces and MCSAs correlate with age or pain duration.
RESULTS
Demographics
Of the 48 participants in this sample, 17 were men and 31 women. The five participants who did not have maximal isometric force measurements were all women. The age of the participants ranged from 45 to 78 years (mean±SD = 63±9.3 years), the body height from 1.47 to 1.88m (mean±SD = 1.67m±0.10), the body weight from 52.3 to 121.8kg (mean±SD = 83.3±15.5kg), and the body mass index (BMI) from 21.2 to 44 (mean±SD = 29.9±4.8). Twenty-one participants displayed cKLG2 in both knees (6 men, 15 women), and 27 bilateral cKLG3 (11 men, 16 women).
In three participants, no information on limb dominance was available from the OAI data base (base on the question: “Which leg do you use to kick a ball?”), in 23 there was no side preference, in 21 the dominant knee was the frequently painful knee, and in only 1 the dominant knee was the painless knee. The painful knees displayed greater pain intensity (numerical rating scale = 3.7±2.6) than the contra-lateral painless knees (0.8±2.3), with 10 corresponding to the worst pain the participant could imagine. The pain subscale WOMAC score (Western Ontario and McMaster Universities, range 0–20, with 20 being the worst) was greater in the frequently painful (4.0±3.5) than in the painless knees (1.1±2.4). At 12 months follow-up, 9 participants still displayed frequently painful versus painless (contra-lateral) knees, 11 frequently painful versus infrequently painful (contra-lateral) knees, 13 infrequently painful vs. painless (contra-lateral) knees, and 15 bilateral, infrequently painful knees.
Primary analyses
Painful knees displayed significantly lower quadriceps MCSAs (p=0.00003) than painless contra-lateral knees, whereas the MCSAs of the hamstrings and adductors did not show significant differences. The percent differences were similar for all 48 participants (−5.2±7.7%) and for those 43 who also had muscle strength measurements (−4.6±7.6%; Table 1).
Table 1.
Anatomical muscle cross-sectional areas (MCSAs; three slices averaged), maximal isometric forces, and maximal isometric forces per unit MCSAs in painful versus painless knee (n=48 = all knees studied; n=43 = subsample studied that also had strength measurements)
| Painful knees | Painless knees | Diff. Painful vs. Painless | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean% | SD% | p value | |
| Anatomical muscle cross-sectional areas (MCSAs) in cm2 | |||||||
| Quadriceps (n=48) | 49.6 | 12.1 | 52.6 | 13.4 | −5.2 | 7.7 | 0.00003* |
| Hamstrings (n=48) | 31.8 | 7.9 | 31.8 | 7.4 | 0.0 | 9.6 | 0.98 |
| Adductors (n=48) | 14.1 | 5.5 | 14.4 | 5.7 | −0.5 | 19.8 | 0.40 |
| Quadriceps (n=43) | 50.4 | 12.2 | 53.2 | 13.6 | −4.6 | 7.6 | 0.00022* |
| Hamstrings (n=43) | 32.1 | 8.1 | 32.1 | 7.5 | 0.1 | 10.1 | 0.96 |
| Adductors (n=43) | 14.1 | 5.7 | 14.4 | 5.8 | −0.4 | 20.2 | 0.44 |
| Maximal isometric force in N | |||||||
| Extension (n=43) | 331.3 | 127.4 | 370.5 | 125.5 | −7.8 | 19.4 | 0.00288* |
| Flexion (n=43) | 141.1 | 58.5 | 146.9 | 65.3 | 4.6 | 47.5 | 0.68 |
| Specific maximal isometric force (per unit MCSA in N/cm2) | |||||||
| Extension (n=43) | 6.6 | 1.9 | 6.9 | 1.8 | −3.4 | 19.4 | 0.08 |
| Flexion (n=43) | 4.5 | 1.7 | 4.5 | 1.9 | 10.2 | 46.1 | 0.89 |
SD = standard deviation. The mean % and SD % of the difference (painful vs. painless knees) was determined across the individual pairwise differences between both knees of all participants. Negative differences refer to lower values in painful vs. painless (contralateral) knees.
The maximal isometric force measured in extension also was significantly lowered in painful vs. painless contra-lateral knees (−7.8%), but no significant difference was observed in maximal isometric forces measured in flexion (Table 1). The specific force (maximal isometric force per unit MCSA) in extension or flexion did not differ significantly between painful and painless knees (Table 1).
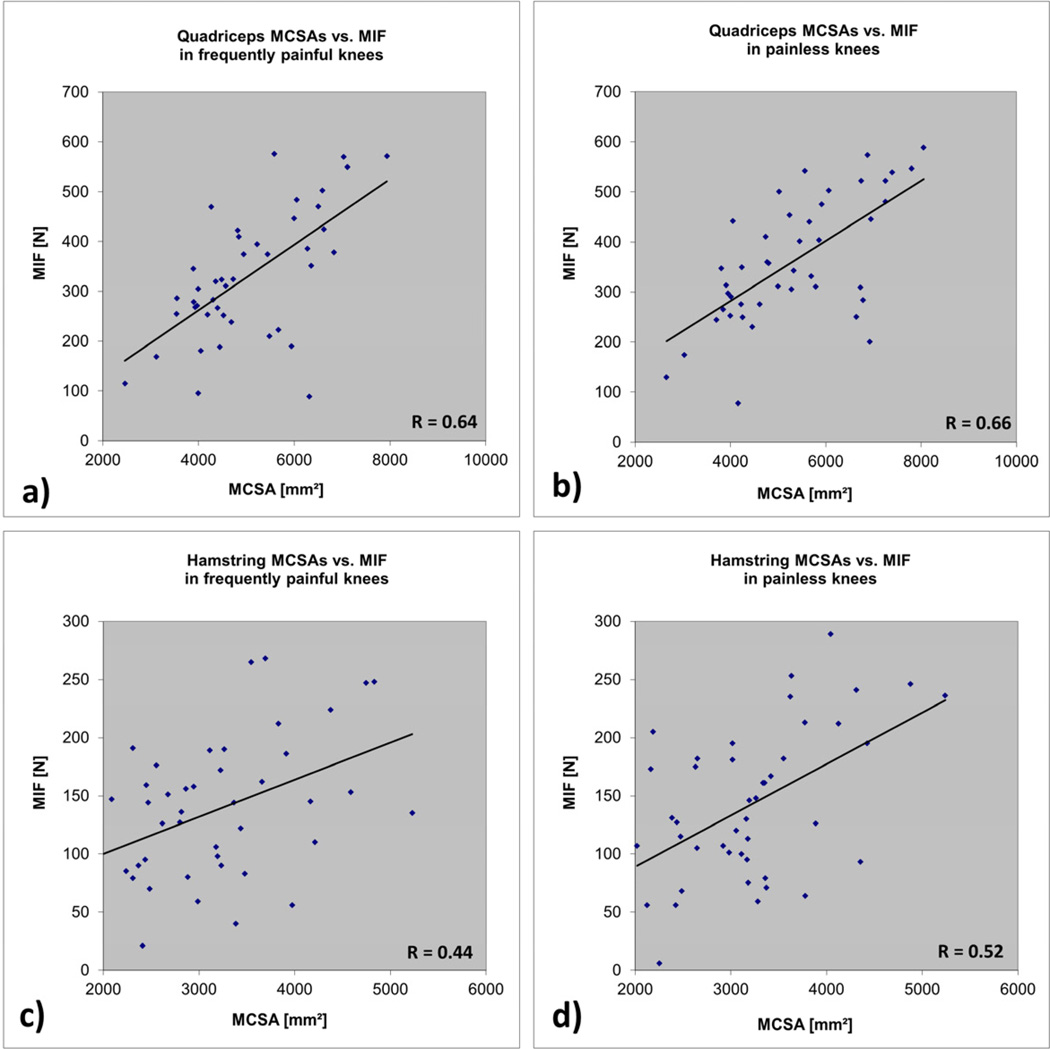
The correlation between maximal isometric force in extension and the quadriceps MCSA was r=0.64 in painful and r= 0.66 in painless knees (Fig. 3). The correlation between maximal isometric force in flexion and hamstring MCSA was r=0.44 in painful and r= 0.52 in painless knees (Fig. 3). The correlation between maximal isometric force measured in extension and that measured in flexion was r=0.69 in painful and r=0.79 in painless knees. All above correlations were statistically significant at p<0.01.
Figure 3.
| a) | Quadriceps |
| MCSAs vs. maximal isometric force in frequently painful knees | |
| b) | Quadriceps |
| MCSAs vs. maximal isometric force in painless knees | |
| c) | Hamstring |
| MCSAs vs. maximal isometric force in frequently painful knees | |
| d) | Hamstring |
| MCSAs vs. maximal isometric force in painless knees | |
Exploratory (sensitivity) analyses
The percent differences of the MCSAs between painful and painless knees were similar in men and in women, and similar in cKLG2 and cKLG3 strata (Table 2). The percent side-differences in quadriceps MCSAs also were similar for participants taking pain medication (−5.7±7.5%; n=31) versus those not taking pain medication (−4.3%±8.5%; n=17), and the same was observed for extension MIFs (−7.3%±16.2%; n=26 vs. −8.4±24.2%; n=17). Further, no significant correlation (Pearson correlation coefficients) was observed for between-knee differences vs. pain duration (p=0.10 to 0.98) or for between-knee differences vs. age (p=0.21 to 0.93). Further, side differences in quadriceps MCSAs were similar (−4.6%) when limiting the analysis to the 37 participants who had WOMAC score of zero in the knee defined as “painless” based on variable P01RKSX/P01LKSX.
Table 2.
Differences (%) between muscle cross-sectional areas (MCSAs), maximal isometric forces, and maximal isometric forces per unit MCSAs in painful versus painless knees in men and women, and in cKLG2 and cKLG3 strata
| All | Men | Women | cKLG2 | cKLG3 | |
|---|---|---|---|---|---|
| Anatomical muscle cross-sectional areas (MCSAs) | |||||
| Quadriceps § | −5.2±7.7% | −5.7±8.2% | −4.9±7.7% | −5.6±8.4% | −4.9±7.3% |
| Hamstrings_§ | 0.0±9.6% | 1.5±13.8% | −0.8±6.3% | 1.4±10.5% | −1.1±9.0% |
| Adductors § | −0.5±19.8% | −3.0±22.4% | 0.9±18.2% | −5.9±13.9% | 3.7±22.5% |
| Quadriceps § | −4.6±7.6% | −5.7±8.2% | −3.9±7.2% | −4.9±8.1% | −4.3±7.3% |
| Hamstrings_§ | 0.1±10.1% | 1.5±13.8% | −0.7±6.8% | 2.0±11.4% | −1.2±9.3% |
| Adductors § | −0.4±20.2% | −3.0±22.4% | 1.3±18.5% | −8.2±11.9% | 5.2±22.7% |
| Maximal isometrics forces | |||||
| Extension | −7.8±19.4% | −5.6±13.4% | −9.2±22.4% | −10.5±19.3% | −5.8±19.5% |
| Flexion | 9.6±47.5% | −1.0±27.1% | 16.5±55.8% | 10.6±64.4% | 8.9±33.0% |
| Maximal isometric force per unit MCSA | |||||
| Extension | −3.4±19.2% | 0.1±11.0% | −5.6±22.7% | −6.2±17.3% | −1.3±20.5% |
| Flexion | 10.2±46.1% | −0.8±29.5% | 17.4±53.0% | 8.4±59.3% | 11.5±35.8% |
Values for three slices averaged; KLG = Kellgren Lawrence Grade. The mean % differences (painful vs. painless knees) were determined across the individual pairwise differences between both knees in each stratum. Negative differences refer to lower values in painful vs. painless (contralateral) knees.
Analyses that were based on a single (transverse) MR image, rather than on an average of three slices displayed similar sensitivity to detecting side differences between painful and painless knees (Table 1 and 3). Further, the correlation between MCSAs and maximal isometric forces were very similar when data from one slice was used compared to using the average MCSAs from three contiguous slices (data not shown).
Table 3.
Anatomical muscle cross-sectional areas (MCSAs) in painful versus painless knees (analysis for single slice)
| Painful knees | Painless knees | Differences Painful vs. Painless | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean% | SD% | p-value | |
| Proximal slice | |||||||
| Quadriceps | 48.0 | 11.9 | 51.0 | 13.2 | −5.4 | 7.7 | 0.00001* |
| Hamstrings | 31.9 | 7.9 | 32.0 | 7.3 | −0.2 | 10.4 | 0.84 |
| Adductors | 11.1 | 5.0 | 11.8 | 5.6 | −1.8 | 23.8 | 0.13 |
| Middle slice | |||||||
| Quadriceps | 49.7 | 12.2 | 52.6 | 13.3 | −4.9 | 8.0 | 0.00008* |
| Hamstrings | 31.9 | 8.0 | 32.0 | 7.4 | −0.1 | 9.8 | 0.86 |
| Adductors | 13.9 | 5.5 | 14.2 | 5.6 | 0.1 | 21.7 | 0.58 |
| Distal slice | |||||||
| Quadriceps | 51.1 | 12.5 | 54.2 | 13.8 | −5.2 | 7.8 | 0.00004* |
| Hamstrings | 31.5 | 8.0 | 31.3 | 7.4 | 0.6 | 9.9 | 0.76 |
| Adductors | 17.2 | 6.2 | 17.3 | 13.3 | 0.7 | 18.3 | 0.83 |
SD = standard deviation. The mean % and SD % of the difference (painful vs. painless knees) was determined across the individual pairwise differences between both knees of all participants. Negative differences refer to lower values in painful vs. painless (contralateral) knees.
DISCUSSION
The objective of this study was to take a step in disentangling the relationship between knee pain, thigh muscle strength, muscle MCSAs, and radiographic knee OA. This was done by determining whether thigh MCSAs and muscle strength differ between painful and (contra-lateral) painless OA knees with the same radiographic disease stage, and whether specific muscle strength (strength/MCSAs) and the correlation between strength and MCSAs of the quadriceps and hamstrings differ between these knees. Key findings were that quadriceps MCSAs and maximal isometric force were significantly lower in painful knees with the same radiographic disease stage than in contra-lateral knees without pain, whereas the hamstrings and adductors did not show significant side differences. The specific muscles strength (strength per unit MCSA) also was lower in painful than in painless knees, but the difference did not reach statistical significance. Correlations between the MCSAs and the maximal isometric force did not exhibit significant side differences between painful and painless (contra-lateral) OA knees.
To eliminate confounding in pain perception and other inter-person differences, a between-knee, within-person approach was chosen 4. This design represents a distinct strength of the study, as it has been shown to be more sensitive to identifying associations between structural changes and symptoms than between-person comparisons 4. Another advantage of this particular approach is that it circumvents the need to normalize the MCSAs and muscle strength to body weight or other anthropometric measures, as this can pose conceptional difficulties, particularly when including participants with a large variation in body mass index 16,48.
Within-person, between-knee comparisons do not account for between-knee confounding: Pain is known to increase with radiographic disease stage 4, and quadriceps strength is also known to be significantly reduced in participants with radiographic knee OA 26,28,50–55. To eliminate confounding by this co-linear relationship, the current analysis was confined to participants with the same KL grade in both knees. A limitation of this study design is that only a limited number of participants show differences in pain frequency status (frequent versus none) between contra-lateral knees with the same KL grade, despite selection from a larger cohort. However, this approach permits one to explore the relationship between pain and muscle status “over and above” femorotibial radiographic disease stage, and thus to disentangle the relationship between pain and muscle status from that between radiographic disease and muscle status.”
Another limitation is that no further parameters such as limb alignment or limb length were used in the selection process or as covariates, because these measures are currently available only for very few subjects from the OAI cohort. Malalignment has been shown to mediate the effect of quadriceps strengthening on knee adduction moments, pain and function in knee OA49, but the difference in alignment between both (contralateral) knees should be relatively small, particularly given that they were at the same radiographic disease stage. Likewise, femoro-patellar disease status was not taken into account, because no radiographic or semiquantitative MRI readings of this compartment are currently available for the sample studied.
In our study, both muscle isometric forces and MCSAs were compared between painful and painless knees. Measuring MCSAs permitted inclusion of the adductors, for which no force measurements were available. Further, this allowed us to investigate whether or not potential differences in muscle strength between painful and painless OA knees result from morphological differences in thigh muscles (i.e. differences in MCSAs), or from inability to activate (existing) muscle fibers in knee OA, with the latter being potentially affected by anxiety, motivation and other covariates 16. Previous studies have reported that the extent of maximal voluntary muscle activation was reduced in subjects with knee OA 15,26–29,31. We find a slightly lower “specific” maximal isometric force in painful versus contra-lateral painless knees, and although the difference did not attain statistical significance (when accounting for the differences in MCSAs), between-knee percent difference of extensor muscle strength were larger compared with quadriceps MCSAs in painful vs. painless knees. These findings indicate that, in addition to reductions in quadriceps MCSAs, pain may also provide a source of inhibition in the ability to voluntarily activate muscles surrounding arthritic joints 16 and in reducing the central activation ratio 31. This is in agreement with a recent cross-sectional study in 1344 OAI participants, which reported a weak association between moderate to severe (but not mild) pain occurring during muscle strength testing (WOMAC pain subscale scores) with reduced isometric quadriceps strength 30.
The correlation coefficients between MCSAs and strength observed here (r=0.44 to 0.66) compare well to correlations reported in the literature, e.g. that between quadriceps MCSA and extension force in 19 young healthy participants (r=0.73)56, and in 28 athletes (r=0.55)57, or the correlation between hamstring MCSA and flexor force (r=0.81) in 28 athletes 57. A weaker correlation between muscle strength and MCSAs was observed in knees with unilateral end-stage knee osteoarthritis (r=0.52) compared with contralateral knees without OA (r=0.64) 31. According to our current findings, the presence of symptoms does not appear to introduce increased variability in the relationship between the MCSAs and the strength that can be generated in OA knees.
Care was taken, to measure MCSAs at anatomically corresponding locations across participants 40. A recent study used the same slice (number) of the OAI acquisitions in all participants and found a greater ratio between the medial versus lateral vastus in men than in women48. Because men are larger and have longer femora than women, the measurements in this study very likely had a more distal location in men48. As the medial vastus extends further distally than the lateral vastus, the reported sex-difference in the medial/lateral vastus ratio 48 is potentially due to failure to account for differences in femoral length, when using the same slice number from the OAI protocol across participants. Also, failure to account for differences in body height and measurement at variable anatomical locations would likely attenuate the correlation between muscle cross-sectional areas and strength. Although no measurements of femoral length are currently available in OAI participants, slice selection by body height has been shown to substantially reduce the variability in measurement location of MCSAs 40. Sensitivity analyses performed in the current study indicate that, if the slice selection considers variation in body size, analysis of a single MR image is sufficient in identifying relevant relationships between MCSAs and pain, and that analysis of several images may not be necessary.
In a previous study, quadriceps and hamstring weakness was observed in subjects with knee pain but without radiographic knee OA13. Our results highlight that, in participants with radiographic OA, the association between muscle weakness and loss of MCSAs is limited to the quadriceps and cannot be identified in other muscle groups of the thigh (i.e. the hamstrings or adductors). Further, knee extensor strength was previously found to protect against the onset of symptomatic (albeit not radiographic) knee OA 58, and quadriceps strengthening represents an established approach of OA exercise therapy 16,59. Thus, quadriceps weakness appears to be of particular importance in symptomatic knee OA, potentially due to the lack of providing sufficient joint stability during physiological activity, and may hence be a primary therapeutic target. Based upon our study, small differences in muscle strength and size are related to substantive differences in pain status, and could be potent targets to improve symptom control. Although our findings support the use of quadriceps strengthening exercise in the symptomatic treatment of knee OA, it has to be kept in mind that our study is cross-sectional. Future longitudinal studies will have to explore the causal relationship and temporal sequence of pain onset (or progression) and changes and muscle status. In particular, these studies should identify whether pain leads to loss of muscle mass and strength, or whether muscle weakness precedes the onset of symptoms.
In conclusion, knees with frequent knee pain demonstrate significantly lower quadriceps MCSAs and strength compared with contra-lateral knees without knee pain with same radiographic OA stage. Other muscles of the thigh, in contrast, did not differ between painful and painless knees. The presence of frequent pain does not appear to affect the correlations between MCSAs and strength in OA knees. The findings indicate that quadriceps strengthening exercise may be useful in treating symptomatic knee OA, and future interventional studies will have to demonstrate to what extent quadriceps strengthening programs can reduce the onset of progression of pain in knee OA.
ACKNOWLEDGEMENTS
We would like to thank the OAI participants, OAI investigators and OAI Clinical Center’s staff for generating this publicly available image data set. The study and image acquisition was supported by the Osteoarthritis Initiative (OAI). The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Pfizer, Inc.; Novartis Pharmaceuticals Corporation; Merck Research Laboratories; and GlaxoSmithKline. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation. The image analysis was supported by the Paracelsus Medical University (PMU) Forschungsfond.
ROLE OF THE FUNDING SOURCE
The funding sources took no active part of influence on the analysis of the data and in drafting or revising the article. However, the manuscript received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR’S CONTRIBUTION
All authors have made substantial contributions to (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
- the conception and design of the study: MS, TD, WW, AS, FE
- acquisition of data: MS, TD, MH, KK
- analysis and interpretation of data: MS, TD, MH, WW, AS, KK, DH, FE
- Drafting the article: MS, TD, FE
- Revising the article critically for important intellectual content: MS, TD, MH, WW, AS, KK, DH, FE
- Final approval of the version submitted: MS, TD, MH, WW, AS, KK, DH, FE
- Statistical expertise: KK, DH, FE
- Obtaining of funding: MH, FE
- Collection and assembly of data: MS, TD, MH
FE takes responsibility for the integrity of the work as a whole, from inception to finished article. FE was involved in conception and design of the study, obtaining of funding, analysis and interpretation of the data, drafting of the article, critical revision of the article for important intellectual content, and final approval of the article.
DECLARATION OF POTENTIALLY COMPETING INTERESTS
Felix Eckstein is CEO and co-owner of Chondrometrics GmbH, a company providing MR image analysis services. He provides consulting services to MerckSerono, Novartis, and Sanofi Aventis. Martin Hudelmaier and Torben Dannhauer have part time appointments with Chondrometrics GmbH. Wolfgang Wirth has a part-time appointment with Chondrometrics GmbH and is co-owner of Chondrometrics GmbH. Martina Sattler and Kent Kwoh have no competing interests. Dr Hunter is funded by an Australian Research Council Future Fellowship.
References
- 1.Lane NE, Brandt K, Hawker G, Peeva E, Schreyer E, Tsuji W, et al. OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthritis Cartilage. 2011;19:478–482. doi: 10.1016/j.joca.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman BN, Aliabadi P, et al. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1995;38:1500–1505. doi: 10.1002/art.1780381017. [DOI] [PubMed] [Google Scholar]
- 3.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neogi T, Felson D, Niu J, Nevitt M, Lewis CE, Aliabadi P, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. doi: 10.1136/bmj.b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134:541–549. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 6.Hill CL, Gale DG, Chaisson CE, Skinner K, Kazis L, Gale ME, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol. 2001;28:1330–1337. [PubMed] [Google Scholar]
- 7.Conaghan PG, Felson D, Gold G, Lohmander S, Totterman S, Altman R. MRI and non-cartilaginous structures in knee osteoarthritis. Osteoarthritis Cartilage. 2006;14(Suppl 1):87–94. doi: 10.1016/j.joca.2006.02.028. Epub;%2006 May;%19.:87-94. [DOI] [PubMed] [Google Scholar]
- 8.Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56:2986–2992. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 9.Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–1603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score) Ann Rheum Dis. 2008;67:206–211. doi: 10.1136/ard.2006.066183. [DOI] [PubMed] [Google Scholar]
- 11.Javaid MK, Lynch JA, Tolstykh I, Guermazi A, Roemer F, Aliabadi P, et al. Pre-radiographic MRI findings are associated with onset of knee symptoms: the most study. Osteoarthritis Cartilage. 2010;18:323–328. doi: 10.1016/j.joca.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter DJ, Zhang W, Conaghan PG, Hirko K, Menashe L, Li L, et al. Systematic review of the concurrent and predictive validity of MRI biomarkers in OA. Osteoarthritis Cartilage. 2011;19:557–588. doi: 10.1016/j.joca.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandt KD, Heilman DK, Slemenda C, Katz BP, Mazzuca S, Braunstein EM, et al. A comparison of lower extremity muscle strength, obesity, and depression scores in elderly subjects with knee pain with and without radiographic evidence of knee osteoarthritis. J Rheumatol. 2000;27:1937–1946. [PubMed] [Google Scholar]
- 14.McAlindon TE, Cooper C, Kirwan JR, Dieppe PA. Determinants of disability in osteoarthritis of the knee. Ann Rheum Dis. 1993;52:258–262. doi: 10.1136/ard.52.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Reilly SC, Jones A, Muir KR, Doherty M. Quadriceps weakness in knee osteoarthritis: the effect on pain and disability. Ann Rheum Dis. 1998;57:588–594. doi: 10.1136/ard.57.10.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennell KL, Hunt MA, Wrigley TV, Lim BW, Hinman RS. Muscle and exercise in the prevention and management of knee osteoarthritis: an internal medicine specialist's guide. Med Clin North Am. 2009;93:161–177. doi: 10.1016/j.mcna.2008.08.006. xii. [DOI] [PubMed] [Google Scholar]
- 17.Mikesky AE, Meyer A, Thompson KL. Relationship between quadriceps strength and rate of loading during gait in women. J Orthop Res. 2000;18:171–175. doi: 10.1002/jor.1100180202. [DOI] [PubMed] [Google Scholar]
- 18.Brandt KD, Heilman DK, Slemenda C, Katz BP, Mazzuca SA, Braunstein EM, et al. Quadriceps strength in women with radiographically progressive osteoarthritis of the knee and those with stable radiographic changes. J Rheumatol. 1999;26:2431–2437. [PubMed] [Google Scholar]
- 19.Sharma L, Dunlop DD, Cahue S, Song J, Hayes KW. Quadriceps strength and osteoarthritis progression in malaligned and lax knees. Ann Intern Med. 2003;138:613–619. doi: 10.7326/0003-4819-138-8-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 20.Amin S, Baker K, Niu J, Clancy M, Goggins J, Guermazi A, et al. Quadriceps strength and the risk of cartilage loss and symptom progression in knee osteoarthritis. Arthritis Rheum. 2009;60:189–198. doi: 10.1002/art.24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roos EM, Herzog W, Block JA, Bennell KL. Muscle weakness, afferent sensory dysfunction and exercise in knee osteoarthritis. Nat Rev Rheumatol. 2011;7:57–63. doi: 10.1038/nrrheum.2010.195. [DOI] [PubMed] [Google Scholar]
- 22.Glasberg MR, Glasberg JR, Jones RE. Muscle pathology in total knee replacement for severe osteoarthritis: a histochemical and morphometric study. Henry Ford Hosp Med J. 1986;34:37–40. [PubMed] [Google Scholar]
- 23.Ikeda S, Tsumura H, Torisu T. Age-related quadriceps-dominant muscle atrophy and incident radiographic knee osteoarthritis. J Orthop Sci. 2005;10:121–126. doi: 10.1007/s00776-004-0876-2. [DOI] [PubMed] [Google Scholar]
- 24.Fink B, Egl M, Singer J, Fuerst M, Bubenheim M, Neuen-Jacob E. Morphologic changes in the vastus medialis muscle in patients with osteoarthritis of the knee. Arthritis Rheum. 2007;56:3626–3633. doi: 10.1002/art.22960. [DOI] [PubMed] [Google Scholar]
- 25.Beattie KA, Macintyre NJ, Ramadan K, Inglis D, Maly MR. Longitudinal changes in intermuscular fat volume and quadriceps muscle volume in the thighs of women with knee osteoarthritis. Arthritis Care Res (Hoboken) 2012;64:22–29. doi: 10.1002/acr.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurley MV, Scott DL, Rees J, Newham DJ. Sensorimotor changes and functional performance in patients with knee osteoarthritis. Ann Rheum Dis. 1997;56:641–648. doi: 10.1136/ard.56.11.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens JE, Mizner RL, Snyder-Mackler L. Quadriceps strength and volitional activation before and after total knee arthroplasty for osteoarthritis. J Orthop Res. 2003;21:775–779. doi: 10.1016/S0736-0266(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 28.Lewek MD, Rudolph KS, Snyder-Mackler L. Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthop Res. 2004;22:110–115. doi: 10.1016/S0736-0266(03)00154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Childs JD, Sparto PJ, Fitzgerald GK, Bizzini M, Irrgang JJ. Alterations in lower extremity movement and muscle activation patterns in individuals with knee osteoarthritis. Clin Biomech (Bristol, Avon) 2004;19:44–49. doi: 10.1016/j.clinbiomech.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Riddle DL, Stratford PW. Impact of pain reported during isometric quadriceps muscle strength testing in people with knee pain: data from the osteoarthritis initiative. Phys Ther. 2011;91:1478–1489. doi: 10.2522/ptj.20110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petterson SC, Barrance P, Buchanan T, Binder-Macleod S, Snyder-Mackler L. Mechanisms underlying quadriceps weakness in knee osteoarthritis. Med Sci Sports Exerc. 2008;40:422–427. doi: 10.1249/MSS.0b013e31815ef285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Felson DT, Nevitt MC. Epidemiologic studies for osteoarthritis: new versus conventional study design approaches. Rheum Dis Clin North Am. 2004;30:783–797. doi: 10.1016/j.rdc.2004.07.005. vii. [DOI] [PubMed] [Google Scholar]
- 33.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nevitt MC, Peterfy C, Guermazi A, Felson DT, Duryea J, Woodworth T, et al. Longitudinal performance evaluation and validation of fixed-flexion radiography of the knee for detection of joint space loss. Arthritis Rheum. 2007;56:1512–1520. doi: 10.1002/art.22557. [DOI] [PubMed] [Google Scholar]
- 36.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(Suppl A):1–56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Spector TD, Hart DJ, Byrne J, Harris PA, Dacre JE, Doyle DV. Definition of osteoarthritis of the knee for epidemiological studies. Ann Rheum Dis. 1993;52:790–794. doi: 10.1136/ard.52.11.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider E, NessAiver M, White D, Purdy D, Martin L, Fanella L, et al. The osteoarthritis initiative (OAI) magnetic resonance imaging quality assurance methods and results. Osteoarthritis Cartilage. 2008;16:994–1004. doi: 10.1016/j.joca.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cotofana S, Hudelmaier M, Wirth W, Himmer M, Ring-Dimitriou S, Sanger AM, et al. Correlation between single-slice muscle anatomical cross-sectional area and muscle volume in thigh extensors, flexors and adductors of perimenopausal women. Eur J Appl Physiol. 2010 doi: 10.1007/s00421-010-1477-8. [DOI] [PubMed] [Google Scholar]
- 40.Dannhauer T, Wirth W, Eckstein F. Selection of comparable anatomical locations of muscle cross-sectional images in the Osteoarthritis Initiative MRI data. Osteoarthritis Cartilage. 2010;18:195. [abstract] [Google Scholar]
- 41.Hudelmaier M, Wirth W, Himmer M, Ring-Dimitriou S, Sanger A, Eckstein F. Effect of exercise intervention on thigh muscle volume and anatomical cross-sectional areas- Quantitative assessment using MRI. Magn Reson Med. 2010 doi: 10.1002/mrm.22550. [DOI] [PubMed] [Google Scholar]
- 42.Prescott JW, Best TM, Swanson MS, Haq F, Jackson RD, Gurcan MN. Anatomically anchored template-based level set segmentation: application to quadriceps muscles in MR images from the Osteoarthritis Initiative. J Digit Imaging. 2011;24:28–43. doi: 10.1007/s10278-009-9260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prescott JW, Priddy M, Best TM, Pennell M, Swanson MS, Haq F, et al. An automated method to detect interstitial adipose tissue in thigh muscles for patients with osteoarthritis. Conf Proc IEEE Eng Med Biol. Soc. 2009;2009:6360–6363. doi: 10.1109/IEMBS.2009.5333260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hudelmaier M, Glaser C, Englmeier KH, Reiser M, Putz R, Eckstein F. Correlation of knee-joint cartilage morphology with muscle cross-sectional areas vs. anthropometric variables. Anat Rec. 2003;270A:175–184. doi: 10.1002/ar.a.10001. [DOI] [PubMed] [Google Scholar]
- 45.Rantanen T, Era P, Heikkinen E. Physical activity and the changes in maximal isometric strength in men and women from the age of 75 to 80 years. J Am Geriatr Soc. 1997;45:1439–1445. doi: 10.1111/j.1532-5415.1997.tb03193.x. [DOI] [PubMed] [Google Scholar]
- 46.Rantanen T, Era P, Heikkinen E. Maximal isometric strength and mobility among 75-year-old men and women. Age Ageing. 1994;23:132–137. doi: 10.1093/ageing/23.2.132. [DOI] [PubMed] [Google Scholar]
- 47.Curb JD, Ceria-Ulep CD, Rodriguez BL, Grove J, Guralnik J, Willcox BJ, et al. Performance-based measures of physical function for high-function populations. J Am Geriatr Soc. 2006;54:737–742. doi: 10.1111/j.1532-5415.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 48.Pan J, Stehling C, Muller-Hocker C, Schwaiger BJ, Lynch J, McCulloch CE, et al. Vastus lateralis/vastus medialis cross-sectional area ratio impacts presence and degree of knee joint abnormalities and cartilage T2 determined with 3T. Osteoarthritis Cartilage. 2011;19:65–73. doi: 10.1016/j.joca.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lim BW, Hinman RS, Wrigley TV, Sharma L, Bennell KL. Does knee malalignment mediate the effects of quadriceps strengthening on knee adduction moment, pain, and function in medial knee osteoarthritis? A randomized controlled trial. Arthritis Rheum. 2008;59:943–951. doi: 10.1002/art.23823. [DOI] [PubMed] [Google Scholar]
- 50.Jan MH, Lai JS, Tsauo JY, Lien IN. Isokinetic study of muscle strength in osteoarthritic knees of females. J Formos Med Assoc. 1990;89:873–879. [PubMed] [Google Scholar]
- 51.Messier SP, Loeser RF, Hoover JL, Semble EL, Wise CM. Osteoarthritis of the knee: effects on gait, strength, and flexibility. Arch Phys Med Rehabil. 1992;73:29–36. [PubMed] [Google Scholar]
- 52.Slemenda C, Brandt KD, Heilman DK, Mazzuca S, Braunstein EM, Katz BP, et al. Quadriceps weakness and osteoarthritis of the knee. Ann Intern Med. 1997;127:97–104. doi: 10.7326/0003-4819-127-2-199707150-00001. [DOI] [PubMed] [Google Scholar]
- 53.Slemenda C, Heilman DK, Brandt KD, Katz BP, Mazzuca SA, Braunstein EM, et al. Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998;41:1951–1959. doi: 10.1002/1529-0131(199811)41:11<1951::AID-ART9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 54.Petterson SC, Raisis L, Bodenstab A, Snyder-Mackler L. Disease-specific gender differences among total knee arthroplasty candidates. J Bone Joint Surg Am. 2007;89:2327–2333. doi: 10.2106/JBJS.F.01144. [DOI] [PubMed] [Google Scholar]
- 55.Palmieri-Smith RM, Thomas AC, Karvonen-Gutierrez C, Sowers MF. Isometric quadriceps strength in women with mild, moderate, and severe knee osteoarthritis. Am J Phys Med Rehabil. 2010;89:541–548. doi: 10.1097/PHM.0b013e3181ddd5c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blazevich AJ, Coleman DR, Horne S, Cannavan D. Anatomical predictors of maximum isometric and concentric knee extensor moment. Eur J Appl Physiol. 2009;105:869–878. doi: 10.1007/s00421-008-0972-7. [DOI] [PubMed] [Google Scholar]
- 57.Gratzke C, Hudelmaier M, Hitzl W, Glaser C, Eckstein F. Knee cartilage morphologic characteristics and muscle status of professional weight lifters and sprinters: a magnetic resonance imaging study. Am J Sports Med. 2007;35:1346–1353. doi: 10.1177/0363546507299746. [DOI] [PubMed] [Google Scholar]
- 58.Segal NA, Torner JC, Felson D, Niu J, Sharma L, Lewis CE, et al. Effect of thigh strength on incident radiographic and symptomatic knee osteoarthritis in a longitudinal cohort. Arthritis Rheum. 2009;61:1210–1217. doi: 10.1002/art.24541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bennell KL, Hinman RS. A review of the clinical evidence for exercise in osteoarthritis of the hip and knee. J Sci Med Sport. 2011;14:4–9. doi: 10.1016/j.jsams.2010.08.002. [DOI] [PubMed] [Google Scholar]