Abstract
Objective. Test-retest reliability of the myotonometer was investigated in patients with subacute stroke. Methods. Twelve patients with substroke (3 to 9 months poststroke) were examined in standardized testing position twice, 60 minutes apart, with the Myoton-3 myometer to measure tone, elasticity, and stiffness of relaxed bilateral biceps and triceps brachii muscles. Intrarater reliability of muscle properties was determined using intraclass correlation coefficient (ICC), the standard error of measurement (SEM), and the minimal detectable change (MDC). Results. Intrarater reliability of muscle properties of bilateral biceps and triceps brachii muscles were good (ICCs = 0.79–0.96) except for unaffected biceps tone (ICC = 0.72). The SEM and MDC of bilateral biceps and triceps brachii muscles indicated small measurement error (SEM% <10%, MDC% <25%). Conclusion. The Myoton-3 myometer is a reliable tool for quantifying muscle tone, elasticity, and stiffness of the biceps and triceps brachii in patients with subacute stroke.
1. Introduction
Abnormalities in muscle structure and properties are a common feature after stroke [1–3] and lead to poor controlled movement and functional disability [4]. Examining the mechanical properties of muscle is important in monitoring the stage of the pathologic processes of muscles [5, 6] and for assessing efficacy of therapeutic interventions [7]. The most widely used clinical assessment of muscle tone is the modified Ashworth scale (MAS), which assesses muscular resistance to passive movement [8, 9]. Nevertheless, the MAS uses subjective grading [9, 10], has poor reliability [9] and clustering of scores [11, 12], and lacks significant correlation with muscular stiffness after stroke [13, 14]. Therefore, an objective measurement tool with an excellent reliability and small measurement error for assessing the mechanical properties of muscle is necessary. Researchers have reported a new approach, the myotonometric measure, which was more sensitive and precise than the MAS to quantify muscle properties [15].
The prerequisites of a proper measurement are validity and reliability. Validity ensures that a measurement actually evaluates what it is intended to measure, and reliability is the extent of a consistent measurement outside of measurement error [16]. The validity of the myotonometer has been established in healthy individuals [17, 18], in patients with chronic pain in the anterior leg or dorsal forearm [19], in patients with upper motoneuron disorders [12], and in stroke survivors [20]. Myotonometric measurements of muscle stiffness showed an approximately linear increase with increasing electromyographic measurements of muscle activation and contractile force during voluntary isometric contraction, indicating tissue displacement during contracted conditions provided an indirect measure of muscle strength [17–19]. The linear relationship between muscle stiffness and force output suggested that the myotonometer was giving a valid recording of the muscle stiffness rather than that of the subcutaneous tissue [18]. The myotonometer quantified spasticity of the biceps brachii muscle and correlations between the myotonometric measurements of muscle tone and MAS were moderate to high in subjects with upper motoneuron disorders [12]. Differences of myotonometric measurements in relaxed and active muscle contraction were significantly related to total ankle stiffness quantified using a torque motor [20]. The significance of the association between these outcomes indicates that they measure similar constructs.
Previous studies have shown that myotonometry is reliable for healthy adults [18, 21, 22] and for various patient populations, including those with Parkinson's disease [23, 24], cerebral palsy [25, 26], musculoskeletal disorders [27, 28], and chronic stroke [29]. To date, however, only one study has examined the test-retest reliability of the myotonometer on the forearm muscles in patients with chronic stroke [29], which limits its use in patients with stroke. Pathologic progressions in muscles may differ across various diseases and stage of disease; thus, the reliability of the myotonometer should be established for patients with subacute stroke.
Patients with stroke have increased passive biceps brachii tone [12] and stiffness [14]. Biceps and triceps brachii muscle paresis and biceps brachii cocontraction during voluntary reaching have shown significant correlations to decreased motor performance, indicating that these two muscles are good predictors of the motor performance of the upper extremity [14]. Therefore, it is important to explore the reliability of the myotonometer on the biceps brachii and triceps brachii muscles.
The present pilot study investigated the intrarater reliability of a hand-held myotonometry device (Myoton-3) for measuring muscle properties of bilateral biceps brachii and triceps brachii muscles in patients who had experienced a first-ever stroke within 3 to 9 months before enrollment. This time window is the period in which most available standard therapeutic interventions have been completed and the opportunity for spontaneous recovery to occur is attenuated [30]. Findings from the present study can contribute to a better understanding of mechanical properties of elbow muscles in patients with subacute stroke and may also provide diagnostic and therapeutic implications.
2. Methods
2.1. Participants
We recruited 12 participants (8 men and 4 women) with a mean age of 51.19 years. Table 1 summarizes participant characteristics. Inclusion criteria were (1) a first-ever stroke of 3 to 9 months before recruitment, (2) Brunnstrom stage III or above in the proximal and distal part of the arm [31], (3) no severe spasticity in the paretic arm (MAS ≤ 2) [8], (4) no cognitive deficits (Mini-Mental State Examination score ≥ 24) [32], and (5) no other neurologic, neuromuscular, or orthopedic disease. Institutional Review Board approval was obtained from the participating sites and written informed consent was obtained from all participants before data collection.
Table 1.
Characteristics of the participants (n = 12).
| Characteristic | |
|---|---|
| Sex, n | |
| Male | 8 |
| Female | 4 |
| Age, mean (SD), year | 51.19 (11.02) |
| Side of hemiplegia, n | |
| Right | 7 |
| Left | 5 |
| Months after stroke onset, mean (SD) | 6.58 (1.38) |
| Brunnstrom stage of upper limb, median (range) | |
| Proximal part | 4.5 (3.5–5) |
| Distal part | 4.5 (3.5–5.5) |
| Fugl-Meyer Assessment for upper limb, mean (SD) | 47.92 (6.33) |
| Mini Mental State Exam scores, mean (SD) | 27.50 (3.26) |
SD: standard deviation.
2.2. Testing Procedures
Myotonometric measurements in bilateral biceps and triceps brachii muscles were performed at rest, using the Myoton-3 myometer (Muomeetria AS, Tallinn, Estonia) by a senior occupational therapist (Figure 1) [33]. Before measurement, participants were informed standard measurement procedure. Measurements were done with the participant lying supine for biceps brachii and side lying for triceps brachii in a relaxed manner, with the participants' arms at their sides and forearms between pronation and supination. The location of the measured muscles was first determined on the unafffected side, thereafter on the affected side. The participant was requested to make an effort by applying resistance with the biceps brachii or triceps brachii to the therapist's hand and at the same time the measuring points for the biceps brachii and triceps brachii were identified by the therapist according to bone prominence and palpation. The middle part of the muscle belly is suggested as the particular measuring point [18, 34], which was marked with a marker in order to replicate the positioning for the subsequent hour used for the reliability measures. For example, the measuring point for the biceps brachii was at the long head, lateral part of muscle, in the middle of arm; and that for the triceps brachii was at the medial head of muscle, in the middle part of arm [35]. The muscles of the unaffected side of the body were measured first. After participants were instructed to relax their muscles maximally, the testing end of the Myoton-3 was placed perpendicular on the skin surface overlying the measuring points of the respective bilateral biceps brachii and triceps brachii. Three consecutive measurements with roughly 1 second in between were taken in each muscle, and the average value was used for later analysis. The entire test session was repeated 60 minutes after the first session with the same procedure, same position, and same measuring point.
Figure 1.

The Myoton-3 myometer.
The Myoton-3 myometer exerts a short mechanical pulse on the tested muscle, which causes muscle to be deformed for a short interval. The muscle responds to the mechanical stimulus in the form of damped oscillations recorded by an acceleration transducer on the testing end, and 3 parameters are calculated from the curve (Figure 2). Three mechanical properties of the muscle tissue are (1) the natural oscillation frequency (Hz), (2) the logarithmic decrement of damping oscillations, and (3) the stiffness (N/m) [15, 27, 34].
Figure 2.
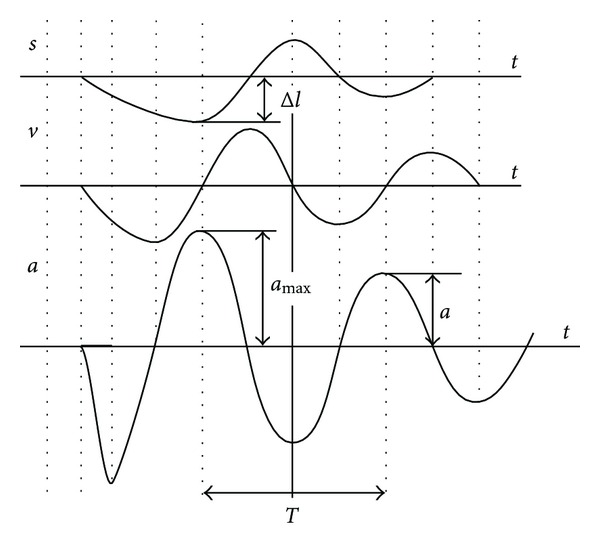
Damped oscillations of the muscle show displacement (s), velocity (v), and acceleration (a) in myotonometric measurements. T is the oscillation period, amax is the maximal amplitude of oscillation, and Δl is the maximal deformation depth of the muscle.
The frequency of the damped oscillations characterizes the muscle tone, the mechanical tension in a relaxed muscle. The higher the value, the more tense is the muscle. The frequency of the damping was calculated as (Frequency (Hz) = 1/T), where T is the oscillation period in seconds (Figure 2). The range of values of the oscillation frequency is usually 11 to 16 Hz in the functional state of relaxation and 18 to 40 Hz in contraction, depending on the muscle [34]. The logarithmic decrement of the damping oscillations characterizes muscle elasticity. The logarithmic decrement of damping was calculated as (Decrement = ln(amax/a)), where amax is the maximal amplitude of oscillation and a is the oscillation amplitude (Figure 2). The decrement values are usually 1.0 to 1.2, depending on the muscle. At the point of maximum compression of the muscle being measured, the corresponding acceleration (amax) characterizes the resistance of the muscle to the force deforming the muscle [28]. Stiffness was calculated as (Stiffness = amax × m/Δl), where m is the mass of the testing end of myometer (kg); amax is the maximal acceleration of oscillation (m/s2); Δl is the deformation depth of the muscle mass (Figure 2) [18]. The usual range of stiffness values is 150 to 300 N/m for resting muscle and may exceed 1000 N/m for contracted muscles [34].
Operational Definition and Functional Role of Muscle Tone, Elasticity, and Stiffness —
Muscle tone, elasticity, and stiffness quantify the functional state of the muscle [27, 28]. Muscle tone involves active nervous-system-stimulated tone and passive (resting) intrinsic viscoelastic tone [21, 36, 37]. From the biomechanics perspective, muscle tone is a mechanical tension in the relaxed muscle [34]. Passive muscle tone is defined as the passive muscle tonus or tension that derives from its intrinsic viscoelastic properties without contractile activity [36, 37]. The functional roles of passive muscle tone are maintaining balance, stability, and posture, providing adequate blood circulation to the muscle and achieving energy-efficient costs for prolonged duration without fatigue [34, 36]. Increased muscle tone disturbs the blood supply in the muscle to diminish oxygen transportation, which might relate to pain, lowered motor performance, overload, and so on [34].
Muscle elasticity is the ability of the muscle to restore its initial shape after contraction, which is inversely proportional to the decrement [34]. Muscle elasticity increases as the decrement decreases. Muscle elasticity is important in using muscle energy and increasing blood circulation volume during the effort. Decreased muscle elasticity brings on easier fatigability and limited speed of movement [34].
Muscle stiffness is a muscle's ability to resist the deformation caused by external forces [36–38]. The speed and ease of the movement performed by the agonist muscle is associated with the stiffness of the antagonist muscle. When a muscle becomes more stiff, greater force is required from the antagonist, which decreases the energy expenditure economy of movement [34].
2.3. Data Analysis
Results of myotonometric measurements are presented as mean and standard deviation (SD). Intrarater reliability was analyzed through the intraclass correlation coefficient (ICC), standard error of measurement (SEM), SEM%, minmal detectable change (MDC), and MDC%. The Kolmogorov-Smirnov test was used to examine whether the tested parameters satisfied conditions for normal distribution. The ICC was calculated using a 2-way mixed-effect model, with an agreement coefficient and average measure. The ICC determines the degree of consistency and agreement between repeated measurements [39], with an ICC exceeding 0.75 indicating excellent reliability [16]. The SEM represents the smallest change between 2 time points that provides an indication of within-subject variability in repeated tests and determines the extent of measurement error. The MDC represents the magnitude of change necessary to exceed the measurement error of test-retest measures that indicates a true statistical change at a certain confidence interval (CI) level for a single individual [39–41]. The SEM was calculated as (), where SDpooled is the SD for all observations from test sessions 1 and 2, and ICC is the test-retest reliability coefficient [42]. The SEM% indicates the relative amount of measurement error independent of the units of measurement and represents the threshold for the smallest change that shows a real change for a group of participants, which was defined as (SEM% = (SEM/mean) × 100), where mean is the mean for all observations from the 2 sessions [43, 44].
The MDC90 was used to determine whether the change score of a participant is real at the 90% confidence level, which was calculated as (MDC90 = 1.65 × × SEM = 1.65 × × SDpooled × ), where 1.65 is the two-tailed tabled z value for the 90% CI, and represents the variance of two measures [42]. The MDC90% represents the relative amount of measurement error and a relative true difference between repeated measurements over time in a participant, which was defined as (MDC90% = (MDC90/mean) × 100), where mean is the mean for all measurements from 2 sessions [43, 44].
Generally, differences between 2 measurements that are larger than the SEM and MDC90 can be attributed to a real change or beyond measurement error [45]. The smaller the SEM and MDC90, the greater the reliability [41].
3. Results
The study participants were 12 patients who met the selection criteria. Participants were a mean age of 51.19 years, and the average time after stroke onset was 6.58 months. Detailed characteristics of the participants are reported in Table 1. Descriptive statistics of the myotonometric measurements in the 2 test sessions are reported in Table 2. The values of muscle tone and stiffness in both biceps and triceps brachii muscles were within the range in the functional state of relaxation. Results of the Kolmogorov-Smirnov test demonstrated the myotonometric measurements were normal distribution.
Table 2.
Myotonometric measurements of the biceps and tricpes brachii musclesa.
| Muscle | Variable | First session | Second session | ||
|---|---|---|---|---|---|
| Affected | Unaffected | Affected | Unaffected | ||
| Biceps brachii | Tone (Hz) | 11.72 (1.83) | 12.03 (1.65) | 11.44 (1.63) | 11.82 (1.32) |
| Elasticity | 1.70 (0.30) | 1.52 (0.38) | 1.63 (0.30) | 1.46 (0.32) | |
| Stiffness (N/m) | 223.42 (31.68) | 225.50 (30.36) | 217.08 (27.19) | 217.25 (26.64) | |
| Triceps brachii | Tone (Hz) | 11.23 (2.05) | 11.75 (2.42) | 10.76 (2.53) | 11.18 (3.25) |
| Elasticity | 1.78 (0.51) | 1.64 (0.37) | 1.80 (0.50) | 1.73 (0.43) | |
| Stiffness (N/m) | 197.08 (28.23) | 192.08 (33.22) | 195.50 (28.79) | 189.08 (55.06) | |
aValues are reported as means (standard deviations).
As detailed in Table 3, the ICCs for bilateral biceps and triceps brachii muscles exceeded 0.75 (ICCs = 0.79–0.96), except for unaffected biceps brachii tone (ICC = 0.72), indicating that the myotonometric measurements had excellent intrarater reliability.
Table 3.
Test-retest reliability of myotonometric measurements of the biceps and tricpes brachii muscles.
| Muscle | Variable | ICC (95% CI) | SEM (SEM%) | MDC90 (MDC90%) | |||
|---|---|---|---|---|---|---|---|
| Affected | Unaffected | Affected | Unaffected | Affected | Unaffected | ||
| Biceps brachii | Tone (Hz) | 0.96 (0.86–0.99) | 0.72 (0.25–0.92) | 0.34 (2.93%) | 0.77 (6.45%) | 0.79 (6.82%) | 1.79 (15.01%) |
| Elasticity | 0.85 (0.54–0.96) | 0.94 (0.80–0.98) | 0.12 (6.92%) | 0.09 (6.04%) | 0.27 (16.26%) | 0.20 (13.42%) | |
| Stiffness (N/m) | 0.91 (0.70–0.97) | 0.87 (0.59–0.96) | 8.81 (4.00%) | 10.34 (4.67%) | 20.52 (9.31%) | 24.09 (10.88%) | |
| Triceps brachii | Tone (Hz) | 0.90 (0.68–0.97) | 0.89 (0.65–0.97) | 0.70 (6.36%) | 0.92 (8.04%) | 1.63 (14.83%) | 2.14 (18.67%) |
| Elasticity | 0.93 (0.76–0.98) | 0.93 (0.76–0.98) | 0.13 (7.26%) | 0.10 (5.95%) | 0.30 (16.75%) | 0.23 (13.69%) | |
| Stiffness (N/m) | 0.79 (0.40–0.94) | 0.79 (0.40–0.94) | 12.69 (6.46%) | 20.44 (10.72%) | 29.56 (15.05%) | 47.62 (24.98%) | |
ICC: intraclass correlation coefficient; CI: confidence interval; SEM: standard error of measurement; SEM%: SEM divided by the mean of all measurements from the two sessions and multiplied by 100%; MDC: minimal detectable change; MDC%: MDC divided by the mean of all measurements from the two sessions and multiplied by 100%.
The SEM (SEM%) of the bilateral biceps and triceps brachii muscles was from 0.34 to 0.92 Hz (2.93%–8.04%) for the tone, 0.09 to 0.13 (6.04%–7.26%) for the elasticity, and 8.81 to 20.44 N/m (4.00%–10.72%) for the stiffness, with affected biceps brachii tone being the smallest and unaffected triceps brachii stiffness being the largest. The MDC90 (MDC90%) of the bilateral biceps and triceps brachii muscles was from 0.79 to 2.14 Hz (6.82%–18.67%) for the tone, 0.20 to 0.30 (13.42%–16.75%) for the elasticity, and 20.52 to 47.62 N/m (9.31%–24.98%) for the stiffness (Table 3). Generally, the SEM% values were below 10% and the MDC90% values were below 25% in the muscle properties of the biceps brachii and triceps brachii, except for the SEM% of the unaffected triceps brachii stiffness, representing a small amount of measurement error [44]. The SEM (SEM%) and MDC90 (MDC90%) of the biceps brachii appeared to be smaller than those of the triceps brachii muscle.
4. Discussion
This study investigated intrarater reliability of the Myoton-3 myometer for the elbow muscles in patients with subacute stroke. The results showed good intrarater reliabilities of the myotonometric measurements, with high agreement and small measurement error in repeated tests.
This pilot study showed that the myotonometer was highly reliable for measuring biceps and triceps brachii muscles in patients with subacute stroke. The ICC values were high, indicating excellent reproducibility of the Myoton-3 between successive sessions of assessment. This was in agreement with results of previous interday reliability studies in different muscle groups and study populations [18, 21, 22, 24–27]. In a previous study, looking at the interday reliability of the Myoton-3 myometer in 10 healthy young volunteers who were retested after 2 days, the relaxed biceps femoris muscle exhibited a moderate ICC score (0.54–0.73) [21]. Another interday reliability study by Bizzini and Mannion repeated the same tests of day 1 on day 2 for measuring relaxed muscle stiffness of the rectus femoris, vastus lateralis, biceps femoris, and gastrocnemius using the Myoton-2 myometer in 10 healthy volunteers. The results showed good to excellent test-retest reliability for all muscles (ICCs 0.80–0.93), except for the vastus lateralis (ICC 0.40) [18].
The ICC cannot detect systematic errors [39], however, and assessments of within-subject variability in the test-retest measurements are necessary to evaluate reliability comprehensively [26]. A good myotonometric measure should present small measurement errors and be sensitive to identify the smallest real changes in repeated measurements. Establishing reliability of measures is important not only for repeated measurements with sound stability but also to identify changes over time [46].
The SEM and MDC90 provide the values of the measurement error between repeated tests for a group and for an individual, respectively. Clinicians and researchers can use the SEM and MDC90 values to determine whether a change in a group or in an individual is statistically significantly real [47, 48]. That is, the real change in a patient should exceed the MDC90 of the measure. The SEM (SEM%) and MDC90 (MDC90%) of the bilateral biceps and triceps brachii muscles in this study were small, indicating small measurement error [44]. It should be noted that the SEMs of tone and stiffness in the biceps and triceps brachii muscles were consistently higher in unaffected side compared to affected side. The ICCs of tone and stiffness in the unaffected biceps and triceps muscles were lower than those in the affected ones. This was similar to lower intrarater reliability of measurements of the unaffected biceps than that of the affected biceps brachii in children with spastic-type cerebral palsy [26] and lower intrarater reliability of the relaxed biceps brachii than the isometrically contracted biceps brachii in healthy adults [22]. The reasons for this were not clear, but might be that the participants had difficulty in remaining relaxed when the testing end of the Myoton-3 myometer was first placed on the unaffected muscles.
The SEM% and MDC90% are independent of the units of measurement, which are more easily interpreted and appropriately compares the amount of random error among muscle groups and properties [44]. The results of SEM and MDC in the present study can be used as a reference for the Myoton-3 to help clinicians and researchers identify small, real changes of muscle properties of the biceps and triceps brachii muscles between repeated measurements for patients with substroke.
This study needs to account for the following limitations. First, a variety of factors that may affect resting muscle tone in patients with subacute stroke include the location of stroke lesion, the severity and type of stroke, body positioning, level of tension in synergic and antagonist muscles, level of test anxiety, and time when the test was administered. Only 12 patients with subacute stroke who demonstrated a low level of spasticity and were without cognitive impairment were included in this pilot study, which may limit the generalizability of our findings. Future studies that consider possible factors that may affect test performance using a larger and more diverse group of patients with stroke are needed to validate our findings and to promote the clinical utility of the myotonometer.
Second, passive muscle tone as measured by the electromyography or isokinetic dynamometer has not been assessed and this is an acknowledged limitation of the present study. Additionally, passive muscle properties in the relaxed state cannot represent functional evaluation during contracted state. The concurrent measurement of muscle properties in relaxation and under contraction with a myotonometer and electromyography or dynamometer is suggested for future studies.
Third, the myotonometry method is not applicable for the following conditions: thin muscle, muscle with small mass, obese persons (BMI > 30 kg·m−2), patients suffering from severe pain, muscle which are palpable in small volume, and muscles which are located under other muscles [34]. In this pilot study, we did not record the arm girth, BMI, and fatty tissue to consider the obesity; therefore, future study is recommended to take this issue into account.
Finally, to enhance the applicability and interpretability of the myotonometric measurements, future studies to estimate minimal clinical important differences are warranted to determine the degree of meaningful change to patients with stroke.
5. Conclusion
Our pilot study showed that the Myoton-3 myometer has good intrarater reliability in measuring the mechanical properties of bilateral biceps brachii and triceps brachii muscles with high agreement and low thresholds to detect real changes in patients with stroke. The findings indicate that the Myoton-3 myometer is a reliable tool for quantifying the muscle tone, elasticity, and stiffness of elbow flexor and extensor muscles in patients with subacute stroke. Further research with larger and divergent groups of patients with stroke is needed to confirm the findings of our study.
Acknowledgments
This project was supported in part by the National Science Council (NSC 97-2314-B-002-008-MY3 and NSC 99-2314-B-182-014-MY3), the National Health Research Institutes (NHRI-EX100-10010PI and NHRI-EX100-9920PI), and the Healthy Aging Research Center at Chang Gung University (EMRPD1A0891) in Taiwan.
References
- 1.Sjostrom M, Fugl-Meyer AR, Nordin G, Wahlby L. Post-stroke hemiplegia; crural muscle strength and structure. Scandinavian Journal of Rehabilitation Medicine. 1980;7(supplement):53–67. [PubMed] [Google Scholar]
- 2.Svantesson U, Takahashi H, Carlsson U, Danielsson A, Sunnerhagen KS. Muscle and tendon stiffness in patients with upper motor neuron lesion following a stroke. European Journal of Applied Physiology. 2000;82(4):275–279. doi: 10.1007/s004210000216. [DOI] [PubMed] [Google Scholar]
- 3.de Vlugt E, de Groot JH, Schenkeveld KE, Arendzen JH, Van Der Helm FC, Meskers CG. The relation between neuromechanical parameters and Ashworth score in stroke patients. Journal of Neuro Engineering and Rehabilitation. 2010;7(1, article 35) doi: 10.1186/1743-0003-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naghdi S, Ansari NN, Mansouri K, Hasson S. A neurophysiological and clinical study of Brunnstrom recovery stages in the upper limb following stroke. Brain Injury. 2010;24(11):1372–1378. doi: 10.3109/02699052.2010.506860. [DOI] [PubMed] [Google Scholar]
- 5.Haas BM, Crow JL. Towards a clinical measurement of spasticity? Physiotherapy. 1995;81(8):474–479. [Google Scholar]
- 6.Rätsep T, Asser T. Changes in viscoelastic properties of skeletal muscles induced by subthalamic stimulation in patients with Parkinson's disease. Clinical Biomechanics. 2011;26(2):213–217. doi: 10.1016/j.clinbiomech.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Harburn KL, Hill KM, Vandervoort AA, et al. Spasticity measurement in stroke: a pilot study. Canadian Journal of Public Health. 1992;83(supplement 2):S41–S45. [PubMed] [Google Scholar]
- 8.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Physical Therapy. 1987;67(2):206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 9.Pandyan AD, Johnson GR, Price CI, Curless RH, Barnes MP, Rodgers H. A review of the properties and limitations of the Ashworth and modified Ashworth Scales as measures of spasticity. Clinical Rehabilitation. 1999;13(5):373–383. doi: 10.1191/026921599677595404. [DOI] [PubMed] [Google Scholar]
- 10.Brashear A, Zafonte R, Corcoran M, et al. Inter- and intrarater reliability of the Ashworth scale and the disability assessment scale in patients with upper-limb poststroke spasticity. Archives of Physical Medicine and Rehabilitation. 2002;83(10):1349–1354. doi: 10.1053/apmr.2002.35474. [DOI] [PubMed] [Google Scholar]
- 11.Katz RT, Rymer WZ. Spastic hypertonia: mechanisms and measurement. Archives of Physical Medicine and Rehabilitation. 1989;70(2):144–155. [PubMed] [Google Scholar]
- 12.Leonard CT, Stephens JU, Stroppel SL. Assessing the spastic condition of individuals with upper motoneuron involvement: validity of the myotonometer. Archives of Physical Medicine and Rehabilitation. 2001;82(10):1416–1420. doi: 10.1053/apmr.2001.26070. [DOI] [PubMed] [Google Scholar]
- 13.Alibiglou L, Rymer WZ, Harvey RL, Mirbagheri MM. The relation between Ashworth scores and neuromechanical measurements of spasticity following stroke. Journal of Neuro Engineering and Rehabilitation. 2008;5, article 18 doi: 10.1186/1743-0003-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leonard CT, Gardipee KA, Koontz JR, Anderson JH, Wilkins SA. Correlation between impairment and motor performance during reaching tasks in subjects with spastic hemiparesis. Journal of Rehabilitation Medicine. 2006;38(4):243–249. doi: 10.1080/16501970600609808. [DOI] [PubMed] [Google Scholar]
- 15.Ianieri G, Saggini R, Marvulli R, et al. New approach in the assessment of the tone, elasticity and the muscular resistance: nominal scales vs MYOTON. International Journal of Immunopathology and Pharmacology. 2009;22(supplement 3):21–24. doi: 10.1177/03946320090220S304. [DOI] [PubMed] [Google Scholar]
- 16.Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. 3rd edition. Upper Saddle River, NJ, USA: Pearson/Prentice Hall; 2009. [Google Scholar]
- 17.Gubler-Hanna C, Laskin J, Marx BJ, Leonard CT. Construct validity of myotonometric measurements of muscle compliance as a measure of strength. Physiological Measurement. 2007;28(8):913–924. doi: 10.1088/0967-3334/28/8/013. [DOI] [PubMed] [Google Scholar]
- 18.Bizzini M, Mannion AF. Reliability of a new, hand-held device for assessing skeletal muscle stiffness. Clinical Biomechanics. 2003;18(5):459–461. doi: 10.1016/s0268-0033(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 19.Korhonen RK, Vain A, Vanninen E, Viir R, Jurvelin JS. Can mechanical myotonometry or electromyography be used for the prediction of intramuscular pressure? Physiological Measurement. 2005;26(6):951–963. doi: 10.1088/0967-3334/26/6/006. [DOI] [PubMed] [Google Scholar]
- 20.Rydahl SJ, Brouwer BJ. Ankle stiffness and tissue compliance in stroke survivors: a validation of Myotonometer measurements. Archives of Physical Medicine and Rehabilitation. 2004;85(10):1631–1637. doi: 10.1016/j.apmr.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Ditroilo M, Hunter AM, Haslam S, De Vito G. The effectiveness of two novel techniques in establishing the mechanical and contractile responses of biceps femoris. Physiological Measurement. 2011;32(8):1315–1326. doi: 10.1088/0967-3334/32/8/020. [DOI] [PubMed] [Google Scholar]
- 22.Leonard CT, Deshner WP, Romo JW, Suoja ES, Fehrer SC, Mikhailenok EL. Myotonometer intra- and interrater reliabilities. Archives of Physical Medicine and Rehabilitation. 2003;84(6):928–932. doi: 10.1016/s0003-9993(03)00006-6. [DOI] [PubMed] [Google Scholar]
- 23.Marusiak J, Jaskólska A, Budrewicz S, Koszewicz M, Jaskólski A. Increased muscle belly and tendon stiffness in patients with Parkinson's disease, as measured by myotonometry. Movement Disorders. 2011;26(11):2119–2122. doi: 10.1002/mds.23841. [DOI] [PubMed] [Google Scholar]
- 24.Marusiak J, Kisiel-Sajewicz K, Jaskólska A, Jaskólski A. Higher muscle passive stiffness in Parkinson’s disease patients than in controls measured by myotonometry. Archives of Physical Medicine and Rehabilitation. 2010;91(5):800–802. doi: 10.1016/j.apmr.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Lidström A, Ahlsten G, Hirchfeld H, Norrlin S. Intrarater and interrater reliability of myotonometer measurements of muscle tone in children. Journal of Child Neurology. 2009;24(3):267–274. doi: 10.1177/0883073808323025. [DOI] [PubMed] [Google Scholar]
- 26.Aarestad DD, Williams MD, Fehrer SC, Mikhailenok E, Leonard CT. Intra- and interrater reliabilities of the myotonometer when assessing the spastic condition of children with cerebral palsy. Journal of Child Neurology. 2004;19(11):894–901. doi: 10.1177/08830738040190110801. [DOI] [PubMed] [Google Scholar]
- 27.Viir R, Laiho K, Kramarenko J, Mikkelson M. Repeatability of trapezius muscle tone assessment by a myometric method. Journal of Mechanics in Medicine and Biology. 2006;6(2):215–228. [Google Scholar]
- 28.Roja Z, Kalkis V, Vain A, Kalkis H, Eglite M. Assessment of skeletal muscle fatigue of road maintenance workers based on heart rate monitoring and myotonometry. Journal of Occupational Medicine and Toxicology. 2006;1(1, article 20) doi: 10.1186/1745-6673-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chuang LL, Wu CY, Lin KC. Reliability, validity, and responsiveness of Myotonometric measurement of muscle tone, elasticity, and stiffness in patients with stroke. Archives of Physical Medicine and Rehabilitation. 2012;93(3):532–540. doi: 10.1016/j.apmr.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34(9):2181–2186. doi: 10.1161/01.STR.0000087172.16305.CD. [DOI] [PubMed] [Google Scholar]
- 31.Brunnstrom S. Movement Therapy in Hemiplegia. New York, NY, USA: Harper & Row; 1970. [Google Scholar]
- 32.Folstein MF, Folstein SE, McHugh PR. “Mini mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 33.Vain A. Estimation of the functional state of skeletal muscle. In: Veltink PH, Boom HBK, editors. Control of Ambulation Using Functional Neuromuscular Stimulation. Enschede, The Netherlands: University of Twente Press; 1995. pp. 51–55. [Google Scholar]
- 34.Gapeyeva H, Vain A. Methodical Guide: Principles of Applying Myoton in Physical Medicine and Rehabilitation. Tartu, Estonia: Muomeetria; 2008. [Google Scholar]
- 35.Vain A, Gapeyeva H. Myoton-3: Examples of Patterns, Reports and Results' Visualization for Studies of Muscle Tone, Elasticity and Stiffness. Tartu, Estonia: Muomeetria; 2007. [Google Scholar]
- 36.Masi AT, Hannon JC. Human resting muscle tone (HRMT): narrative introduction and modern concepts. Journal of Bodywork and Movement Therapies. 2008;12(4):320–332. doi: 10.1016/j.jbmt.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Simons DG, Mense S. Understanding and measurement of muscle tone as related to clinical muscle pain. Pain. 1998;75(1):1–17. doi: 10.1016/S0304-3959(97)00102-4. [DOI] [PubMed] [Google Scholar]
- 38.Panjabi MM. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. Journal of Spinal Disorders. 1992;5(4):383–389. doi: 10.1097/00002517-199212000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Bruton A, Conway JH, Holgate ST. Reliability: what is it, and how is it measured? Physiotherapy. 2000;86(2):94–99. [Google Scholar]
- 40.Goldsmith CH, Boers M, Bombardier C, Tugwell P. Criteria for clinically important changes in outcomes: development, scoring and evaluation of rheumatoid arthritis patient and trial profiles. Journal of Rheumatology. 1993;20(3):561–565. [PubMed] [Google Scholar]
- 41.Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Medicine. 1998;26(4):217–238. doi: 10.2165/00007256-199826040-00002. [DOI] [PubMed] [Google Scholar]
- 42.Schmitt JS, Di Fabio RP. Reliable change and minimum important difference (MID) proportions facilitated group responsiveness comparisons using individual threshold criteria. Journal of Clinical Epidemiology. 2004;57(10):1008–1018. doi: 10.1016/j.jclinepi.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Wagner JM, Rhodes JA, Patten C. Reproducibility and minimal detectable change of three-dimensional kinematic analysis of reaching tasks in people with hemiparesis after stroke. Physical Therapy. 2008;88(5):652–663. doi: 10.2522/ptj.20070255. [DOI] [PubMed] [Google Scholar]
- 44.Flansbjer UB, Holmbäck AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. Journal of Rehabilitation Medicine. 2005;37(2):75–82. doi: 10.1080/16501970410017215. [DOI] [PubMed] [Google Scholar]
- 45.Haley SM, Fragala-Pinkham MA. Interpreting change scores of tests and measures used in physical therapy. Physical Therapy. 2006;86(5):735–743. [PubMed] [Google Scholar]
- 46.Beckerman H, Roebroeck ME, Lankhorst GJ, Becher JG, Bezemer PD, Verbeek ALM. Smallest real difference, a link between reproducibility and responsiveness. Quality of Life Research. 2001;10(7):571–578. doi: 10.1023/a:1013138911638. [DOI] [PubMed] [Google Scholar]
- 47.Hopkins WG. Measures of reliability in sports medicine and science. Sports Medicine. 2000;30(1):1–15. doi: 10.2165/00007256-200030010-00001. [DOI] [PubMed] [Google Scholar]
- 48.Lexell JE, Downham DY. How to assess the reliability of measurements in rehabilitation. American Journal of Physical Medicine and Rehabilitation. 2005;84(9):719–723. doi: 10.1097/01.phm.0000176452.17771.20. [DOI] [PubMed] [Google Scholar]


