Abstract
Recent clinical trials aimed at improved treatment of AML by administration of vitamin D derivatives showed unremarkable results, suggesting development of vitamin D resistance in patients' AML blasts. Since mechanisms of vitamin D resistance are not clear, we studied 40AF cells, a subline of HL60 cells that can proliferate in the presence of 1α,25-dihydroxyvitamin D3 (1,25D). We found that mRNA and protein levels of HPK1, an upstream MAP4 kinase, are dramatically increased in 40AF cells, and HPK1 protein is further increased when the 1,25D resistance of 40AF cells is partially reversed by the addition of carnosic acid and p38 MAPK inhibitor SB202190 (DCS cocktail). Knockdown of HPK1 reduces 1,25D/DCS-induced differentiation of both 1,25D-sensitive HL60 and U937 cells and 1,25D-resistant 40AF cells, but the effect of HPK1 knockdown on differentiation-associated G1 arrest is more apparent in the resistant than the sensitive cells. To explain why 40AF and the intrinsically vitamin D-resistant KG-1a cells can proliferate in the presence of vitamin D, we found that the cleaved HPK1 fragment (HPK1-C) level is high in 40AF and KG-1a cells, but when differentiation is induced by DCS, HPK1-C decreases, while full-length (FL)-HPK1 increases. Accordingly, inhibition of proteolysis with the pan-caspase inhibitor Q-VD-OPh reduced HPK1 cleavage and enhanced DCS-induced differentiation of 40AF cells. The results indicate that FL-HPK1 is a positive regulator of vitamin D-induced differentiation in AML cells, but the cleaved HPK1 fragment inhibits differentiation. Thus, high HPK1 cleavage activity contributes to vitamin D resistance, and HPK1 has a dual role in AML cell differentiation.
Key words: HPK1, vitamin D, AML differentiation, cell cycle arrest, differentiation resistance, caspase inhibition, signaling pathways
Introduction
It has been known for three decades that 1α,25-dihydroxyvitamin D3 (1,25D) can effectively overcome the blocked differentiation of acute myeloid leukemia (AML) cells,1–3 and it is evident that clinical exploitation of this action may lead to improved differentiation therapy of AML subtypes non-responsive to ATRA.1,4–6 However, the clinical use of 1,25D and its analogs (vitamin D derivatives, VDDs) for treatment of AML has not been possible so far due to the danger that VDDs will produce life-threatening hypercalcemia or ineffectiveness because of the development of 1,25D resistance.7,8 Thus, an increased understanding of the mechanisms of 1,25D resistance is needed to reveal new insights for translating the in vitro results with VDDs to the clinic.
We previously established a series of 1,25D-resistant cell lines from HL60, an AML cell line, by long-term culture in the presence of increasing concentrations of 1,25D.9 Studies of these 1,25D-resistant cells showed their altered cell cycle regulation, associated with the increased CDK2 and CDK6 activity, and a shortened G1 phase.10 The more rapid proliferation rate of the resistant cells can also be explained by the lower level of p27Kip1 following development of 1,25D resistance.11 In addition, a partial explanation for the 1,25D resistance of 40AF cells, one of the resistant cell lines developed from HL60 cells by growing in 40 nM 1,25D, is the reduced transcriptional activity and nuclear localization of the vitamin D receptor (VDR).12
More recently, it has been shown that in 40AF cells cJun N-terminal kinase 2 (JNK2) antagonizes signaling of differentiation by JNK1 and contributes to 1,25D resistance, revealing the importance of MAPK signaling in this form of resistance.13 MAPK signaling, along with PI3K/Akt/mTOR, Src kinase, PKC and JAK-STATs are among the major networks that respond to various environmental stimuli and participate in the actions of vitamin D to regulate cell survival, proliferation, differentiation and apoptosis.7,14–20 Several components of MAPK pathways, such as MEKs and ERKs as well as the β-catenin pathway, interact with the classical 1,25D-mediated pathway through direct binding of VDR and then cross-activation of transcription of its target genes.21,22 Other genes also play key roles in cell differentiation; for instance, the KSR-MAPK-C/EBP pathway is critical to the VDD-induced monocytic differentiation in HL60 cells.23 In a translationally relevant ex vivo study, JNK pathway was shown to play an important role in monocytic differentiation of human AML cells induced by 1,25D, its analogs or by the combination DCS, consisting of 1,25D, carnosic acid (CA), an antioxidant, and SB202190 (SB), a p38 MAPK inhibitor.24 Different isoforms of p38 MAPK, including gamma and delta, which are not inhibited by SB, also contribute to the differentiation of HL60 and U937 cells.25 The downstream target genes of MAPK signaling cascades include the differentiation-related transcription factors (TFs) Jun-ATF2/AP1, C/EBPβ and Egr-1, which are known to be important for myeloid differentiation.26–29 However, despite numerous reports on the role of major MAPK cascade kinases and their target genes in hematopoiesis,30 it is not clear what regulates the MAPK cascades in VDD-induced cell differentiation.
To investigate the upstream control of MAPKs in this system, we profiled the signaling networks using MAPK/cell cycle mRNA arrays. The 1,25D-sensitive HL60 cells were compared with 1,25D-resistant 40AF cells, and this identified, for the first time, hematopoietic progenitor kinase 1 (HPK1, or MAP4K1) as the most upregulated MAPK gene in the 1,25D-resistant cells. Subsequent studies in several AML cell lines revealed that HPK1 signaling can provide a dual function, both as a regulator of AML cell differentiation/cell cycle and as a mediator of resistance to vitamin D derivatives.
Results
MAPK mRNA profiles differ between vitamin D-sensitive HL60 and vitamin D-resistant 40AF cells.
We initiated this study by investigating gene alterations in vitamin D-resistant 40AF cells compared with their parental vitamin D-sensitive HL60-G cells, which may explain the acquisition of resistance. We determined at mRNA level the expression of 84 genes participating in the MAPK signaling network and cell cycle regulation using “Human MAP kinase RT2-Profile PCR Array”. The majority of genes studied increased their expression in 40AF cells, and the 27 genes upregulated more than 2-fold, 10 with statistical significance, are listed in Table 1. Note that MAP4K1 (HPK1) mRNA upregulation is highest (54-fold) and highly significant (p < 0.01). Nine genes were downregulated, three with statistical significance, but in this report we describe the role of the dramatically upregulated HPK1.
Table 1.
MAPK mRNA profiles differ between vitamin D-sensitive HL60 and vitamin D-resistant 40AF cells
| Gene symbol | Gene name | Fold regulation | p-value |
| BRAF | V-raf murine sarcoma viral oncogene homolog B1/BRAF1 | 2.53 | 0.02 |
| CCNA2 | Cyclin A2 | 2.52 | 0.10 |
| CCNB1 | Cyclin B1 | 2.18 | 0.04 |
| CCNB2 | Cyclin B2 | 3.01 | 0.00 |
| CCNE1 | Cyclin E1 | 2.00 | 0.22 |
| CDKN2A | Cyclin-dependent kinase inhibitor 2A (melanoma, p16, inhibits CDK4) | 3.98 | 0.01 |
| CDKN2B | Cyclin-dependent kinase inhibitor 2B (p15, inhibits CDK4) | 2.66 | 0.05 |
| CHUK | Conserved helix-loop-helix ubiquitous kinase/IKBKA/IKK-α | 3.23 | 0.01 |
| DLK1 | Delta-like 1 homolog | 119.61 | 0.27 |
| EGR1 | Early growth response 1 | 18.36 | 0.31 |
| ELK1 | Elk1, member of ETS oncogene family | 2.89 | 0.11 |
| KRAS | V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog | 2.26 | 0.05 |
| MAP2K1 | MEK1 | 2.32 | 0.17 |
| MAP2K4 | JNKK1 | 3.67 | 0.03 |
| MAP2K6 | MEK6 | 2.91 | 0.18 |
| MAP4K1 | HPK1 | 54.33 | 0.01 |
| MAPK11 | P38BETA2 | 2.25 | 0.15 |
| MAPK6 | ERK3 | 2.14 | 0.11 |
| MAPK7 | BMK1/ERK4 | 3.53 | 0.00 |
| MAPK8IP2 | MAPK8 interacting protein 2/JIP2 | 3.92 | 0.23 |
| MST1 | Macrophage stimulating 1 | 3.67 | 0.01 |
| NFATC4 | Nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 4 | 48.07 | 0.08 |
| NRAS | Neuroblastoma RAS viral (v-ras) oncogene homolog | 2.14 | 0.14 |
| PAK1 | p21 protein (Cdc42/Rac)-activated kinase 1 | 2.06 | 0.05 |
| PRDX6 | Peroxiredoxin 6/1-Cys/AOP2 | 2.35 | 0.05 |
| RB1 | Retinoblastoma 1/OSRC/RB | 2.58 | 0.07 |
| SFN | Stratifin/YWHAS | 2.85 | 0.09 |
(0.00 means p < 0.01). Comparison of the expression of 84 genes known to participate in MAPK signaling network and cell cycle regulation determined at mRNA level using “Human MAP kinase RT2- Profile PCR Array.” The majority of genes increased their expression in 40AF cells, and the genes upregulated more than two times are listed. The altered genes with statistical significance (p < 0.05) are in bold font. Note that MAP4K1 (HPK1) mRNA level was the highest upregulated-(54.33), with statistical significance. The three genes which were downregulated are not shown.
Upregulation of HPK1 protein by 1,25D, alone or with enhancers of its action, parallels differentiation of 1,25D-sensitive, but not resistant AML cells.
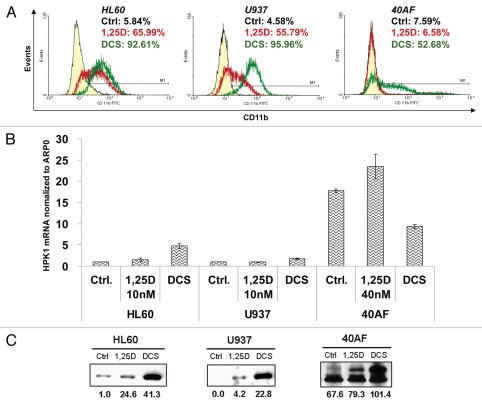
The resistance of 40AF cells to 1,25D can be overcome by enhancing 1,25D action by simultaneous addition to 1,25D of carnosic acid, a plant-derived antioxidant, and SB202910, a selective inhibitor of isoforms α and β of p38 MAPK,25 the combination, referred to as DCS. This is illustrated in Figure 1A, which also shows that HL60-G cells used in our studies are more sensitive to 1,25D than U937 cells, but treatment with DCS combination results in similarly enhanced differentiation in both cell lines. Figure 1B shows that in untreated 40AF cells HPK1 mRNA levels are markedly higher than in untreated parental HL60 cells, validating the results of the RT2-PCR array presented in Table 1. Interestingly, DCS increased HPK1 mRNA levels in 1,25D-sensitive HL60 and U937 cells, but reduced the high mRNA levels in 1,25D-resistant 40AF cells. These levels were also consistently reduced in the 1,25D-resistant sublines of U937 cells (data not shown). This is in contrast to the protein levels illustrated in Figure 1C, which showed a marked increase in DCS-treated 40AF cells, indicating a major role for post-transcriptional control of HPK1 protein levels.
Figure 1.
Upregulation of HPK1 protein by 1,25D, alone or in combination with enhancers of its action (DCS) parallels the differentiation of AML cells. HL60 (derived from AML-M2) and U937 (derived from AML-M5) represent 1,25D-sensitive cells; 40AF (derived from HL60 maintained in 40 nM 1,25D) represent 1,25D-resistant cells. The cells were treated with 1,25D or DCS (1,25D 10 nM + CA 10 µM + SB 5 µM) for 48 h (sensitive cells) or 72 h (resistant cells). (A) The typical pattern of response to differentiating agents determined by FC for the differentiation marker CD11b. The percentage of positive cells in different treatment groups is indicated in the right top corner of each FC image. (Left peak: control; middle peak: 1,25D; right peak: DCS). Note that in 40AF cells, ‘1,25D’ peak overlaps ‘control peak.’ (B) HPK1 mRNA levels relative to ARP0 as control in different cell lines were determined by real-time RT-PCR. (C) FL-HPK1 protein levels in different cell lines determined by western blots. The OD of each band relative to HL60 untreated (control) cells are displayed below each band. The same amount of total protein extract (40 µg) was loaded for each cell type.
Knockdown of HPK1 in 1,25D-sensitive HL60 and U937 cells decreases 1,25D-induced differentiation and HPK1 signaling through the JNK pathway.
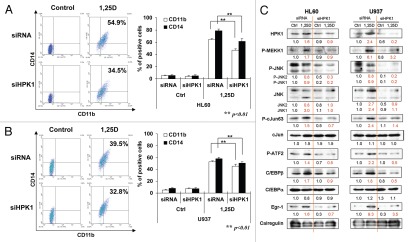
We have confirmed the requirement of HPK1 function for 1,25D-induced differentiation by reducing the levels of HPK1 protein with siHPK1: Figure 2A and B show highly significant inhibition of differentiation of HL60 and U937 cells when HPK1 protein levels are reduced (Fig. 2C). As reported in other systems,31–33 HPK1, a MAP4 level kinase, signals downstream to target TFs, and this cascade includes the signaling to JNK1/2 (Fig. 2C). We also identified cJun, ATF2, Egr-1 and C/EBPβ but not C/EBPα, as TFs regulated by HPK1 in HL60 and U937 cells (Fig. 2C). Since the basal level of HPK1 protein is low in untreated HL60 and U937 cells, the knockdown effect is more obvious in 1,25D-treated cells which have higher levels of induced HPK1. Also, when HPK1 protein is knocked-down in U937 cells, the reduction of differentiation effect is less marked than in HL60 cells. This may be due to a different stage of differentiation block in these two cell lines. U937 cells are derived from promonocytic subtype of AML cells, while HL60 cells are derived from myeloblastic AML cells. This suggests that HPK1 signaling more effectively regulates differentiation in HL60 cells, because they are derived from a less well-differentiated sub-type of AML cells.
Figure 2.
Knockdown of HPK1 in 1,25D-sensitive HL60 and U937 cells decreases 1,25D-induced differentiation, downstream signaling and differentiation-related TFs. Cells were pre-treated with siRNA-HPK1 (siHPK1) or scrambled-siRNA (siRNA) for 24 h, then 10 nM 1,25D was added for another 48 h. Differentiation markers CD11b and CD14 were detected by FC, mean ± SD values are shown, n = 3. Representative images of CD11b (x axis) and CD14 (y axis) determinations are shown in the left side of each figure. (A) HL60 cells; (B) U937 cells. (C) Demonstration of HPK1 decrease, activation of several components of MEKK1-JNK-AP1 signaling cascade and expression of differentiation-related TFs as detected by western blots. Calregulin is a loading control.
HPK1 activates the JNK pathway in DCS-treated 40AF cells, but JNK activation does not strictly correlate with AP-1 signaling and differentiation.
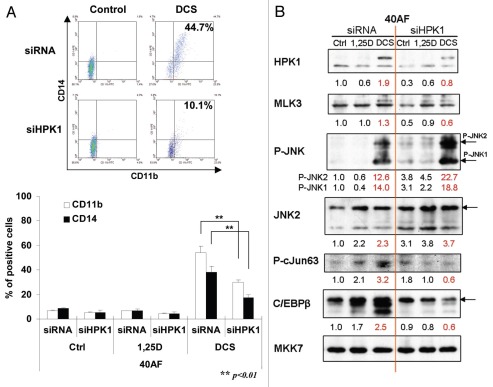
Knockdown of HPK1 also inhibited differentiation induced by DCS in 40AF cells (Fig. 3A), but in contrast to the sensitive cells, the 40AF cells showed paradoxically increased activation of JNK1/2 when HPK1 expression was reduced (Fig. 3B). Also surprising was the reduced activation of cJun while JNK1/2 was activated by siHPK1, suggesting that in 40AF cells, the cascade of signaling is altered during the development of resistance to vitamin D. It should be noted, however, that JNK2 activation exceeded the activation of JNK1, and the abundance of the differentiation-related transcription factor C/EBPβ correlated with the reduced HPK1 levels and inhibition of differentiation (Fig. 3C). This is consistent with our previously reported observation that JNK2 activity is inhibitory to differentiation of 40AF cells.13 Thus, in 1,25D-resistant 40AF cells HPK1 does not appear to signal differentiation through the JNK pathway.
Figure 3.
HPK1 is required for optimal differentiation of DCS-treated 40AF cells, activates the JNK pathway and leads to increased expression of differentiation-related transcription factors. Cells were pre-treated by siRNA-HPK1 (siHPK1) or scrambled-siRNA (siRNA) for 24 h, 40 nM 1,25D or DCS (1,25D 10 nM + CA 10 µM + SB 5 µM) was added for another 48 h. (A) Expression of CD11b and CD14; Mean ± SD values are shown, n=3. (B) Western blots illustrating HPK1 signaling, with MKK7 serving as loading control, and the effect of HPK1 knockdown on JNK1/2 and the differentiation-related TFs.
Cell cycle arrest accompanies DCS-induced differentiation of 40AF cells.
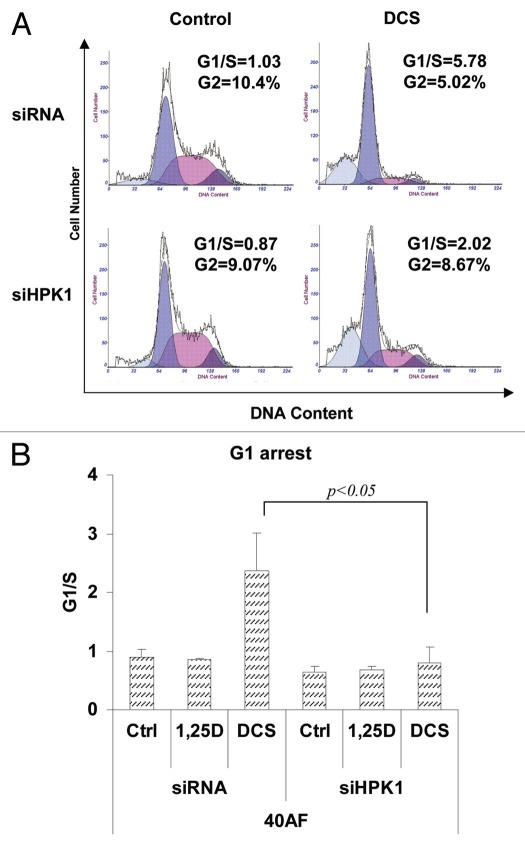
Examination of cell cycle parameters showed that the DCS-induced block in the G1 phase and reduced occupancy of the G2 compartment is dependent on optimum levels of HPK1, as siHPK1 abrogated these effects (Fig. 4). This confirms that HPK1 participates in terminal differentiation in this system. The sub-G1 peaks, which represent necrosis/apoptosis, are higher in DCS-treated 40AF cells compared with the control group. This appears to be due to the cytotoxic effect of the DCS cocktail combination that may aid eradication of the malignant cells.
Figure 4.
Knockdown HPK1 in vitamin D-resistant 40AF cells abrogates the DCS-induced increase in the G1 cell cycle block. Knockdown of HPK1 signaling is described in Figure 3 legend. The cells were stained by propidium iodide and cell cycle distribution was assessed by flow cytometry. (A) Representative images of cell cycle distribution. X-axis: DNA content; y-axis: cell number. The peaks from left to right are: sub-G1, G1, S and G2/M. Note also the abrogation by HPK1 knockdown of DCS-induced G2 compartment depletion. (B) Summary of experiments presented as the G1/S ratio. Mean ± SD values are shown, n = 3.
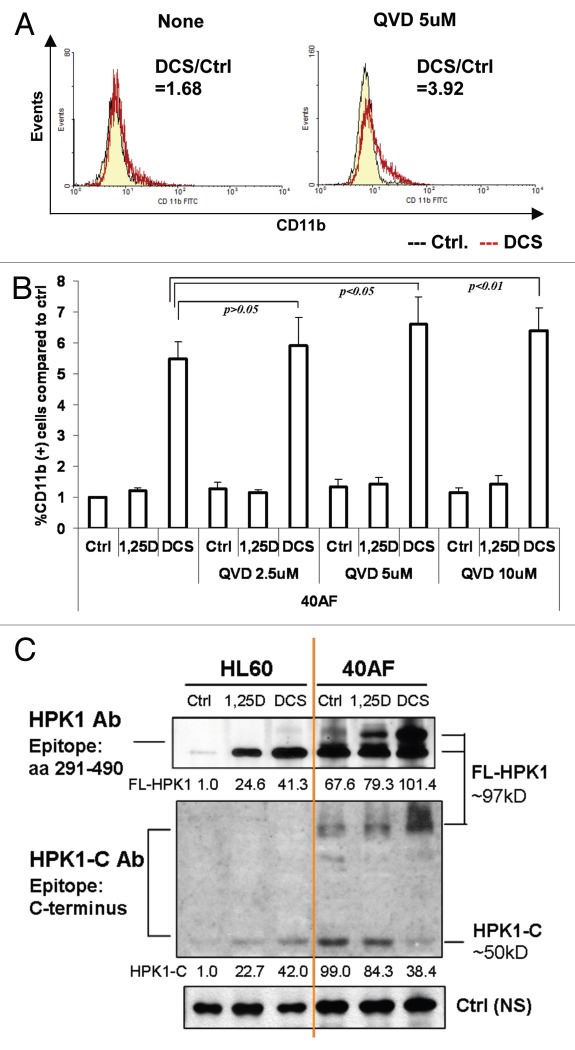
The pan-caspase inhibitor Q-VD-OPh further enhances DCS-induced differentiation of 40AF cells by inhibition of HPK1 cleavage.
To explore the mechanism by which DCS reverses resistance of 40AF cells to 1,25D, we asked if HPK1 signaling is enhanced by the inhibition of its proteolytic cleavage, known to take place in other systems.33–35 The pan-caspase inhibitor Q-VD-OPh (QVD) significantly increases differentiation of DCS-treated 40AF cells (Fig. 5A and B). Interestingly, the maximal effect on differentiation is 5 µM, a concentration lower than the 10 µM minimum reported to block apoptosis.36 This indicates that the previously documented non-apoptotic functions of caspases37 may contribute to the effects of QVD on AML cells, similar to the antitumor effects of other protease inhibitors.38 Consistent with the increased differentiation, G1 arrest (G1/S ratio) also increases when QVD is used to inhibit HPK1 cleavage in DCS-treated 40AF cells (data not shown). A comparison of the abundance of the C-terminal cleaved fragment of HPK1 (HPK1-C), between parental 1,25D-sensitive HL60 cells and the 40AF cells with acquired resistance to 1,25D, is shown in Figure 5C. It demonstrates that while 40AF cells have a higher level of the fragment, treatment with 1,25D or DCS, particularly the latter, decreases the levels of the cleaved fragment HPK1-C and concurrently increases the level of the full-length HPK1 (FL-HPK1). Thus, the cleaved fragment may play a role in the resistance, while FL-HPK1 allows differentiation.
Figure 5.
The pan-caspase inhibitor Q-VD-OPh further enhances DCS-induced differentiation of 40AF cells by inhibition of HPK1 cleavage. (A) Representative images of the changes of CD11b expression in DCS-treated 40AF cells induced by adding 5 µM QVD for 48 h. The ratio of CD11b positive cells between DCS-treated cells and control group is indicated in the right top corner of each image (straight line peak, control; jagged line peak, DCS). (B) Summary of experiments showing the increased expression of CD11b differentiation marker in 1,25D or DCS-treated 40AF cells following addition of increasing concentrations of QVD for 72 h. Mean ± SD values are shown, n = 3. (C) Western blots of HPK1 protein levels in HL60 and 40AF cells treated by 1,25D or DCS for 48 h. HPK1 Ab (epitope: aa 290–490) detects FL-HPK1. HPK1-C Ab (epitope: C-terminus) detects cleaved HPK1-C fragment and FL-HPK1.
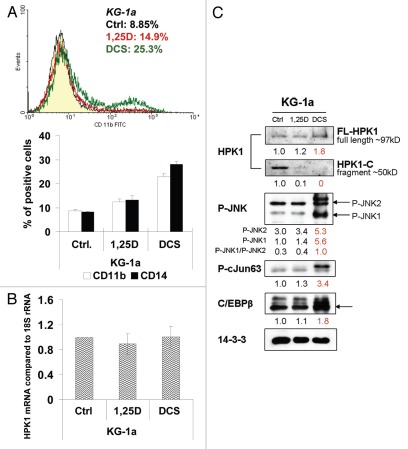
KG-1a cells with innate resistance to 1,25D also express HPK1-C fragments, which are reduced by treatment DCS.
Vitamin D resistance of KG-1a, AML-M1-type cells (early myeloblastic without maturation),39 can also be attenuated by treatment with DCS (Fig. 6A), and, as in adaptively resistant 40AF cells, this is associated with the disappearance of the cleaved fragment and concurrent increase in the level of the FL-HPK1 (Fig. 6C). The similarity between KG-1a and 40AF cells extends to the finding that the regulation of HPK1 protein levels is largely post-transcriptional, as HPK1 mRNA levels are unaltered by 1,25D or DCS in KG-1a cells (Fig. 6B). Also, activation of JNK1/2 and downstream TFs correlates with increased levels of FL-HPK1, though the significance of this requires further study (Fig. 6C).
Figure 6.
DCS treatment of KG-1a cells with innate resistance to 1,25D also reduces the expression of HPK1-C fragments and increases differentiation. (A) CD11b and CD14 expression in KG-1a cells treated by 1 nM 1,25D or DCS (with 1 nM 1,25D) for 72 h. A typical FC image of CD11b (treated for only 48 h) is shown above the summary bar-graph. The left-side peaks overlap and show the negative cell population. The one right-side peak resulting from DCS exposure is apparent. (B) Real-time RT-PCR for HPK1 mRNA in 1,25D or DCS-treated KG-1a cells relative to 18S rRNA as control. There was no significance (p > 0.05) of the mRNA level between control group and 1,25D/DCS group. (C) Western blots of HPK1-JNK-AP-1 signaling pathway in KG-1a cells.
Discussion
There are several novel findings in this report. HPK1 is identified as an upstream MAPK required for optimal monocytic differentiation induced in AML cells by 1,25D, and its cleavage by caspases or caspase-like enzymes produces a HPK1 C-terminal fragment that contributes to vitamin D resistance. Thus, HPK1 plays a dual role in the control of differentiation of AML cells.
HPK1 is principally expressed in hematopoietic cells32 and is known to regulate stress responses, apoptosis and cell proliferation in cancer cells,40 though in contrast to the current report, most previous studies focused on lymphoid cells.35,41 An activation of cell membrane receptors forms a membrane-proximal complex that includes several small adaptor proteins, such as Grb2 and SLP-76 families containing the SH2 domain,42,43 and HPK1 is subject to phosphorylation by this complex. An example is that in B lymphocytes, the ligation of BCR induces tyrosine phosphorylation of HPK1 by Syk and Lyn, resulting in its association with the B-cell adaptor and catalytic activation of HPK1.44 Upstream regulation of HPK1 has also been suggested to take place by Src.45 While the mechanism of the upregulation of HPK1 expression in AML cells by 1,25D is currently not clear, a possible explanation is that HPK1 signaling is increased by the MAPK scaffold proteins, such as KSR1/2,46,47 upregulated by the exposure of AML cells to 1,25D.
As a MAP4 kinase, HPK1 is an upstream kinase in the MAPK phosphorylation cascade and can activate MAP3 kinases, such as MLK3 or MEKK1.31,32,45 In several systems, HPK1 is a potent activator of the SAPK/JNK MAPK pathway, in some cases via the SH3-containing MLK3,32,45 while regulation of MEKK1 by HPK1 is considered to be important for cellular decisions regarding survival or apoptosis.48 Here, we show that MEKK1 activation is regulated by HPK1 and correlates with differentiation (Fig. 2). Of note, while the knockdown of HPK1 has the expected negative effect further downstream on the activation of JNK in 1,25D-sensitive HL60 and U937 cells (Fig. 2C), in the 1,25D-resistant cells 40AF cells HPK1 appears to have a suppressive effect on JNK activation (Fig. 3B), perhaps an adaptation that contributes to the resistance evoked by the presence of excessive concentration of the hormone 1,25D or dominant expression of JNK2 over JNK1.13 Nonetheless, the effect of HPK1 knockdown on cJun activation and C/EBPβ levels was the expected decrease, indicating that the transcription factors can be controlled by alternate pathways in the resistant cells.
In addition to cJun and C/EBPβ, several transcription factors are firmly linked to 1,25D-induced monocytic differentiation, including ATF-2 and Egr-1. Our laboratory has previously reported that AP-1 transcription factor is essential for 1,25D-induced differentiation, and its principal components are cJun and ATF-2, with minor contributions from JunB and Fos-B.13,27,49 Also, several isoforms of C/EBPβ were shown to increase during 1,25D-induced monocytic differentiation in HL60 cells,28 and there is evidence that C/EBPs can form heterodimers with cJun, JunB and cFos during monopoiesis.50 The data shown in Figure 2 confirm that HPK1 is required for the MEKK1-JNK-AP1 or -C/EBPβ sequence of events. Importantly, there was no effect of HPK1 knockdown on C/EBPα levels (Fig. 2C), which is principally required for granulopoiesis rather than monopoiesis.51
We also found that knockdown of HPK1 in both 1,25D-sensitive (Fig. 2C) and -resistant cells (Fig. 3B) reduced the 1,25D/DCS-enhanced expression of Egr-1. Since previous work showed that Egr-1 upregulates the Cdk5/p35 complex and contributes to 1,25D-induced terminal differentiation of HL60 cells,26 this suggests that Egr-1 serves to mediate proliferation control of AML cells by HPK1. Indeed, we found that the knockdown of HPK1 reduces the DCS-induced G1 arrest in 40AF cells (Fig. 4). The involvement of HPK1 in cell cycle regulation is also supported by the recent report that restoration of wild-type HPK1 in pancreatic ductal carcinoma cells increases p21 and p27 expression and leads to cell cycle arrest.40 This finding adds to the known control by 1,25D of cell cycle regulators, which include MAPK influence on pRb,52 the AKT pathway15 and the regulation of p27/Kip1 by the Cot1/Tpl2 oncogene53 and microRNA181.54
The caspase-mediated cleavage of HPK1 in 1,25D-resistant cells demonstrated in Figures 5C and 6C has been observed in previous studies, but not as the basis for cell resistance to treatment. It is known that HPK1 protein contains a proline-rich domain between the N-terminal serine/threonine kinase domain and the C-terminal citron homology domain,55 and caspase-mediated cleavage of this domain leads to the functional changes of HPK1 first observed in Fas-ligation-induced apoptosis.34 Also, the cleavage converts HPK1 from an activator to an inhibitor of NFκB and sensitizes primary T cells to activation-induced cell death. Thus, HPK1 becomes a negative regulator of leukocyte activation.56,57
HPK1 signaling in monocytic differentiation has only been previously studied in primary mouse progenitor cells, where promotion of differentiation was attributed to a constitutively active cleavage fragment of HPK1 resulting from proteolytic cleavage of HPK1 by activated caspases.33 In direct contrast, we find that high levels of full-length HPK1 protein and its downstream MAPK signaling are required for optimal induction of differentiation by 1,25D or DCS in either 1,25D-sensitive or -resistant AML cell lines. It is possible that the cell context is responsible for this difference, due to normal vs. malignant nature of the cells or mouse vs. human species differences.
While the main focus of this report is on the adaptive resistance of AML cells to 1,25D, we also found that the innately 1,25D-resistant KG-1a cells display a similar basis for the resistance (Fig. 6). KG-1a cells were established as a cell line from very early myeloblasts and were described to have poor response to 1,25D-induced differentiation.3,39 We observed similar effects of enhanced differentiation by DCS as in 40AF cells (compare Fig. 1A with 6A): DCS-induced increase in FL-HPK1 with concomitant decrease of the cleaved fragment (Figs. 5C and 6C) as well as increased activation of cJun and increased levels of C/EBPβ (Fig. 6C). Also of note, the DCS-induced increases in FL-HPK1 protein were observed while HPK1 mRNA levels were decreased in 40AF (Fig. 1B) or unchanged in KG-1a (Fig. 6B) cells. Thus, post-transcriptional control of HPK1 expression appears to be the key feature of resistance-related phenomena.
The results with both 40AF and KG-1a cells complement the findings of our concurrent ex vivo study of different subtypes of human AML blasts (MS in submission). In these cells in primary culture, caspase inhibition increased VDD-induced differentiation at least in part by a reduction of the proteolytic cleavage of HPK1 and thus restored the level of FL-HPK1. Together, these studies document that caspase or caspase-like activity is important in the mechanisms of resistance to differentiation therapy that utilize VDDs, and suggest that this should be considered in the design of therapeutic trials.
Materials and Methods
AML cells.
HL60, U937 and KG-1a cell lines represent different subtypes of AML cells. The HL60-G subclone used in this study is highly sensitive to 1,25D.58 The 40AF subclone of HL60 cells, derived by cultivation in the presence of 40 nM 1,25D to make these cells resistant to 1,25D,9 was also used. The cells were maintained in RPMI 1640 medium supplemented with 10% bovine calf serum and, following treatment with the indicated compounds, were harvested for determination of differentiation markers, cell cycle distribution, viability and modulation of differentiation-related signaling pathways and transcription factors.
Reagents and antibodies.
1,25D was a kind gift from Dr. Milan Uskokovic (BioExcell). Carnosic acid was obtained from Alexis Biochemicals (Enzo Life Sciences, ALX-270-264), SB202190 from Calbiochem (EMD Chemicals, 559388). Antibodies that detect surface differentiation markers Mo1-FITC (CD11b) and MY4-RD1 (CD14) and isotype controls were from Beckman Coulter (6602573 and 6603262). Q-VD-OPh was purchased from R&D Systems (OPH001). For western blotting, the following antibodies were obtained from Santa Cruz Biotechnology: HPK1 (full-length, sc-25414), HPK1-C (sc-6230), P-MEK kinase-1 (P-MEKK-1, Thr 1402, sc-130202), JNK2 (sc-827), cJun (sc-1694), C/EBPβ (sc-746), C/EBPα (sc-61), Egr-1 (sc-189), 14-3-3 (sc-628), Calregulin (sc-11398). The following antibodies were obtained from Cell Signaling Technologies: MLK3 (2817), MKK7 (4172), Phospho-JNK (Thr183/Tyr185, 4668), JNK (9252), Phospho-cJun (Ser63, 9261) and Phospho-ATF2 (Thr69/71, 9225).
Knockdown of HPK1.
HL60, U937 or 40AF cells were transfected with 5 µM of HPK1 siRNA (siHPK1, Ambion, s22080) or scrambled siRNA (siRNA, Ambion, AM4611) as control using Amaxa nucleofector according to the manufacturer's protocol. Following nucleofection, the cells were allowed to recover in RPMI 1640 medium with 10% BCS for 24 h then were exposed to the indicated compounds for 48 h. The experiments were performed with the most effective siRNA compared with the effects of scrambled siRNA as control.
Determination of differentiation markers and cell cycle distribution.
Monocytic differentiation was determined by the expression of differentiation markers CD14 and CD11b, using EPICS XL Flow Cytometer (Beckman Coulter). The acquisition parameters were set for an isotype control. Data analysis was performed using EPICS XL and WinMDI software. Cell cycle distribution was determined by staining with propidium iodide (Sigma-Aldrich, P4170), following the analysis on flow cytometer and using Multicycle software (Phoenix Flow Systems).
Determination of the expression and activity of cell signaling pathways.
The different profiles of MAPK signaling networks in HL60 or 40AF cells were compared by the Human MAP Kinase Signaling Pathway RT2 Profiler™ PCR Array (SABiosciences, PAHS-061A). The mRNA levels of HPK1 were detected by Taqman RT-PCR (primers obtained from Applied Biosystems, MAP4K1, Hs01018250_m1). The protein levels of HPK1 and downstream targets related to differentiation were detected by western blotting using 40 µg whole-cell extracts. Representative images of western blots are shown in the figures. The optical density of each western blot band was quantified using ImageQuant 5.0 software (Molecular Dynamics) and is labeled under the corresponding band.
Statistical methods.
Each experiment was repeated at least three times. The results of PCR array were acquired using web based data analysis software supplied by SABiosciences. Significance of the differences between mean values was assessed by Student t-test. The p-values are reported in the figures and in the figure legends.
Acknowledgments
This work was supported by NIH grant 2RO1-044722-21 from the National Cancer Institute to G.P.S.
Abbreviations
- AML
acute myeloid leukemia
- 1,25D
1α, 25-dihydroxyvitamin D
- VDD
vitamin D derivatives
- CA
carnosic acid
- SB
SB202190
- DCS
1,25D + CA + SB
- HPK1
hematopoietic progenitor kinase 1
- FL-HPK1
full length HPK1
- HPK1-C
HPK1 C-terminus fragment
- siRNA
scrambled siRNA
- siHPK1
HPK1 siRNA
- QVD
Q-VD-OPh
- TF
transcription factors
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Nowak D, Stewart D, Koeffler HP. Differentiation therapy of leukemia: 3 decades of development. Blood. 2009;113:3655–3665. doi: 10.1182/blood-2009-01-198911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyaura C, Abe E, Kuribayashi T, Tanaka H, Konno K, Nishii Y, et al. 1Alpha,25-Dihydroxyvitamin D3 induces differentiation of human myeloid leukemia cells. Biochem Biophys Res Commun. 1981;102:937–943. doi: 10.1016/0006-291X(81)91628-4. [DOI] [PubMed] [Google Scholar]
- 3.Koeffler HP. Induction of differentiation of human acute myelogenous leukemia cells: therapeutic implications. Blood. 1983;62:709–721. [PubMed] [Google Scholar]
- 4.Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–572. [PubMed] [Google Scholar]
- 5.Castaigne S, Chomienne C, Daniel MT, Ballerini P, Berger R, Fenaux P, et al. All-trans retinoic acid as a differentiation therapy for acute promyelocytic leukemia. I. Clinical results. Blood. 1990;76:1704–1709. [PubMed] [Google Scholar]
- 6.Raffoux E, Cras A, Recher C, Boëlle PY, de Labarthe A, Turlure P, et al. Phase 2 clinical trial of 5-azacitidine, valproic acid, and all-trans retinoic acid in patients with high-risk acute myeloid leukemia or myelodysplastic syndrome. Oncotarget. 2010;1:34–42. doi: 10.18632/oncotarget.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 8.Trump DL, Deeb KK, Johnson CS. Vitamin D: considerations in the continued development as an agent for cancer prevention and therapy. Cancer J. 2010;16:1–9. doi: 10.1097/PPO.0b013e3181c51ee6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wajchmann HJ, Rathod B, Song S, Xu H, Wang X, Uskokovic MR, et al. Loss of deoxcytidine kinase expression and tetraploidization of HL60 cells following long-term culture in 1,25-dihydroxyvitamin D3. Exp Cell Res. 1996;224:312–322. doi: 10.1006/excr.1996.0141. [DOI] [PubMed] [Google Scholar]
- 10.Wang QM, Luo X, Kheir A, Coffman FD, Studzinski GP. Retinoblastoma protein-overexpressing HL60 cells resistant to 1,25-dihydroxyvitamin D3 display increased CDK2 and CDK6 activity and shortened G1 phase. Oncogene. 1998;16:2729–2737. doi: 10.1038/sj.onc.1201803. [DOI] [PubMed] [Google Scholar]
- 11.Wang QM, Chen F, Luo X, Moore DC, Flanagan M, Studzinski GP. Lowering of p27Kip1 levels by its antisense or by development of resistance to 1,25-dihydroxyvitamin D3 reverses the G1 block but not differentiation of HL60 cells. Leukemia. 1998;12:1256–1265. doi: 10.1038/sj.leu.2401088. [DOI] [PubMed] [Google Scholar]
- 12.Garay E, Donnelly R, Wang X, Studzinski GP. Resistance to 1,25D-induced differentiation in human acute myeloid leukemia HL60-40AF cells is associated with reduced transcriptional activity and nuclear localization of the vitamin D receptor. J Cell Physiol. 2007;213:816–825. doi: 10.1002/jcp.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen-Deutsch X, Garay E, Zhang J, Harrison JS, Studzinski GP. c-Jun N-terminal kinase 2 (JNK2) antagonizes the signaling of differentiation by JNK1 in human myeloid leukemia cells resistant to vitamin D. Leuk Res. 2009;33:1372–1378. doi: 10.1016/j.leukres.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcinkowska E, Wiedlocha A, Radzikowski C. Evidence that phosphatidylinositol-3-kinase and p70S6K protein are involved in differentiation of HL-60 cells induced by calcitriol. Anticancer Res. 1998;18:3507–3514. [PubMed] [Google Scholar]
- 15.Zhang Y, Zhang J, Studzinski GP. AKT pathway is activated by 1,25-dihydroxyvitamin D3 and participates in its anti-apoptotic effect and cell cycle control in differentiating HL60 cells. Cell Cycle. 2006;5:447–451. doi: 10.4161/cc.5.4.2467. [DOI] [PubMed] [Google Scholar]
- 16.Clark CS, Konyer JE, Meckling KA. 1Alpha,25-dihydroxyvitamin D3 and bryostatin-1 synergize to induce monocytic differentiation of NB4 acute promyelocytic leukemia cells by modulating cell cycle progression. Exp Cell Res. 2004;294:301–311. doi: 10.1016/j.yexcr.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J Bone Miner Res. 2006;21:37–47. doi: 10.1359/JBMR.050908. [DOI] [PubMed] [Google Scholar]
- 18.Hughes PJ, Brown G. 1Alpha,25-dihydroxyvitamin D3-mediated stimulation of steroid sulphatase activity in myeloid leukaemic cell lines requires VDRnuc-mediated activation of the RAS/RAF/ERK-MAP kinase signalling pathway. J Cell Biochem. 2006;98:590–617. doi: 10.1002/jcb.20787. [DOI] [PubMed] [Google Scholar]
- 19.Altman JK, Sassano A, Platanias LC. Targeting mTOR for the treatment of AML. New agents and new directions. Oncotarget. 2011;2:510–517. doi: 10.18632/oncotarget.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu WD, Ma Y, Flynn G, Muindi JR, Kong RX, Trump DL, et al. Calcitriol enhances gemcitabine anti-tumor activity in vitro and in vivo by promoting apoptosis in a human pancreatic carcinoma model system. Cell Cycle. 2010;9:3022–3029. doi: 10.4161/cc.9.15.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narayanan R, Sepulveda VA, Falzon M, Weigel NL. The functional consequences of cross-talk between the vitamin D receptor and ERK signaling pathways are cell-specific. J Biol Chem. 2004;279:47298–47310. doi: 10.1074/jbc.M404101200. [DOI] [PubMed] [Google Scholar]
- 22.Shah S, Islam MN, Dakshanamurthy S, Rizvi I, Rao M, Herrell R, et al. The molecular basis of vitamin D receptor and betacatenin crossregulation. Mol Cell. 2006;21:799–809. doi: 10.1016/j.molcel.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 23.Studzinski GP, Wang X, Ji Y, Wang Q, Zhang Y, Kutner A, et al. The rationale for deltanoids in therapy for myeloid leukemia: role of KSR-MAPK-C/EBP pathway. J Steroid Biochem Mol Biol. 2005;97:47–55. doi: 10.1016/j.jsbmb.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q, Harrison JS, Uskokovic M, Kutner A, Studzinski GP. Translational study of vitamin D differentiation therapy of myeloid leukemia: effects of the combination with a p38 MAPK inhibitor and an antioxidant. Leukemia. 2005;19:1812–1817. doi: 10.1038/sj.leu.2403916. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Harrison JS, Studzinski GP. Isoforms of p38MAPK gamma and delta contribute to differentiation of human AML cells induced by 1,25-dihydroxyvitamin D3. Exp Cell Res. 2011;317:117–130. doi: 10.1016/j.yexcr.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen F, Wang Q, Wang X, Studzinski GP. Upregulation of Egr1 by 1,25-dihydroxyvitamin D3 contributes to increased expression of p35 activator of cyclin-dependent kinase 5 and consequent onset of the terminal phase of HL60 cell differentiation. Cancer Res. 2004;64:5425–5433. doi: 10.1158/0008-5472.CAN-04-0806. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Studzinski GP. The requirement for and changing composition of the activating protein-1 transcription factor during differentiation of human leukemia HL60 cells induced by 1,25-dihydroxyvitamin D3. Cancer Res. 2006;66:4402–4409. doi: 10.1158/0008-5472.CAN-05-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcinkowska E, Garay E, Gocek E, Chrobak A, Wang X, Studzinski GP. Regulation of C/EBPbeta isoforms by MAPK pathways in HL60 cells induced to differentiate by 1,25-dihydroxyvitamin D3. Exp Cell Res. 2006;312:2054–2065. doi: 10.1016/j.yexcr.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones JE, Wang L, Kropf PL, Duan R, Johnson DE. Src family kinase gene targets during myeloid differentiation: identification of the EGR-1 gene as a direct target. Leukemia. 2009;23:1933–1935. doi: 10.1038/leu.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geest CR, Coffer PJ. MAPK signaling pathways in the regulation of hematopoiesis. J Leukoc Biol. 2009;86:237–250. doi: 10.1189/jlb.0209097. [DOI] [PubMed] [Google Scholar]
- 31.Hu MC, Qiu WR, Wang X, Meyer CF, Tan TH. Human HPK1, a novel human hematopoietic progenitor kinase that activates the JNK/SAPK kinase cascade. Genes Dev. 1996;10:2251–2264. doi: 10.1101/gad.10.18.2251. [DOI] [PubMed] [Google Scholar]
- 32.Kiefer F, Tibbles LA, Anafi M, Janssen A, Zanke BW, Lassam N, et al. HPK1, a hematopoietic protein kinase activating the SAPK/JNK pathway. EMBO J. 1996;15:7013–7025. [PMC free article] [PubMed] [Google Scholar]
- 33.Arnold R, Frey CR, Müller W, Brenner D, Krammer PH, Kiefer F. Sustained JNK signaling by proteolytically processed HPK1 mediates IL-3 independent survival during monocytic differentiation. Cell Death Differ. 2007;14:568–575. doi: 10.1038/sj.cdd.4402042. [DOI] [PubMed] [Google Scholar]
- 34.Chen YR, Meyer CF, Ahmed B, Yao Z, Tan TH. Caspase-mediated cleavage and functional changes of hematopoietic progenitor kinase 1 (HPK1) Oncogene. 1999;18:7370–7377. doi: 10.1038/sj.onc.1203116. [DOI] [PubMed] [Google Scholar]
- 35.Brenner D, Golks A, Becker M, Müller W, Frey CR, Novak R, et al. Caspase-cleaved HPK1 induces CD95L-independent activation-induced cell death in T and B lymphocytes. Blood. 2007;110:3968–3977. doi: 10.1182/blood-2007-01-071167. [DOI] [PubMed] [Google Scholar]
- 36.Caserta TM, Smith AN, Gultice AD, Reedy MA, Brown TL. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis. 2003;8:345–352. doi: 10.1023/A:1024116916932. [DOI] [PubMed] [Google Scholar]
- 37.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarez-Díaz S, Larriba MJ, López-Otín C, Muñoz A. Vitamin D: Proteases, protease inhibitors and cancer. Cell Cycle. 2010;9:32–37. doi: 10.4161/cc.9.1.10266. [DOI] [PubMed] [Google Scholar]
- 39.Koeffler HP, Billing R, Lusis AJ, Sparkes R, Golde DW. An undifferentiated variant derived from the human acute myelogenous leukemia cell line (KG-1) Blood. 1980;56:265–273. [PubMed] [Google Scholar]
- 40.Wang H, Song X, Logsdon C, Zhou G, Evans DB, Abbruzzese JL, et al. Proteasome-mediated degradation and functions of hematopoietic progenitor kinase 1 in pancreatic cancer. Cancer Res. 2009;69:1063–1070. doi: 10.1158/0008-5472.CAN-08-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han A, Saijo K, Mecklenbräuker I, Tarakhovsky A, Nussenzweig MC. Bam32 links the B cell receptor to ERK and JNK and mediates B cell proliferation but not survival. Immunity. 2003;19:621–632. doi: 10.1016/S1074-7613(03)00275-9. [DOI] [PubMed] [Google Scholar]
- 42.Liu SK, Smith CA, Arnold R, Kiefer F, McGlade CJ. The adaptor protein Gads (Grb2-related adaptor downstream of Shc) is implicated in coupling hemopoietic progenitor kinase-1 to the activated TCR. J Immunol. 2000;165:1417–1426. doi: 10.4049/jimmunol.165.3.1417. [DOI] [PubMed] [Google Scholar]
- 43.Yu J, Riou C, Davidson D, Minhas R, Robson JD, Julius M, et al. Synergistic regulation of immunoreceptor signaling by SLP-76-related adaptor Clnk and serine/threonine protein kinase HPK-1. Mol Cell Biol. 2001;21:6102–6112. doi: 10.1128/MCB.21.18.6102-6112.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsuji S, Okamoto M, Yamada K, Okamoto N, Goitsuka R, Arnold R, et al. B cell adaptor containing src homology 2 domain (BASH) links B cell receptor signaling to the activation of hematopoietic progenitor kinase 1. J Exp Med. 2001;194:529–539. doi: 10.1084/jem.194.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li T, Yu XJ, Zhang GY. Tyrosine phosphorylation of HPK1 by activated Src promotes ischemic brain injury in rat hippocampal CA1 region. FEBS Lett. 2008;582:1894–1900. doi: 10.1016/j.febslet.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Wang TT, White JH, Studzinski GP. Induction of kinase suppressor of RAS-1(KSR-1) gene by 1,alpha25-dihydroxyvitamin D3 in human leukemia HL60 cells through a vitamin D response element in the 5′-flanking region. Oncogene. 2006;25:7078–7085. doi: 10.1038/sj.onc.1209697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Wang TT, White JH, Studzinski GP. Expression of human kinase suppressor of Ras 2 (hKSR-2) gene in HL60 leukemia cells is directly upregulated by 1,25-dihydroxyvitamin D(3) and is required for optimal cell differentiation. Exp Cell Res. 2007;313:3034–3045. doi: 10.1016/j.yexcr.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlesinger TK, Fanger GR, Yujiri T, Johnson GL. The TAO of MEKK. Front Biosci. 1998;3:1181–1186. doi: 10.2741/a354. [DOI] [PubMed] [Google Scholar]
- 49.Kasukabe T, Okabe-Kado J, Hozumi M, Honma Y. Inhibition by interleukin 4 of leukemia inhibitory factor-, interleukin 6- and dexamethasone-induced differentiation of mouse myeloid leukemia cells: role of c-myc and junB proto-oncogenes. Cancer Res. 1994;54:592–597. [PubMed] [Google Scholar]
- 50.Hong S, Skaist AM, Wheelan SJ, Friedman AD. AP-1 protein induction during monopoiesis favors C/EBP: AP-1 heterodimers over C/EBP homodimerization and stimulates FosB transcription. J Leukoc Biol. 2011;90:643–651. doi: 10.1189/jlb.0111043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paz-Priel I, Friedman A. C/EBPα dysregulation in AML and ALL. Crit Rev Oncog. 2011;16:93–102. doi: 10.1615/critrevoncog.v16.i1-2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ji Y, Kutner A, Verstuyf A, Verlinden L, Studzinski GP. Derivatives of vitamins D2 and D3 activate three MAPK pathways and upregulate pRb expression in differentiating HL60 cells. Cell Cycle. 2002;1:410–415. doi: 10.4161/cc.1.6.269. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Gocek E, Novik V, Harrison JS, Danilenko M, Studzinski GP. Inhibition of Cot1/Tlp2 oncogene in AML cells reduces ERK5 activation and upregulates p27Kip1 concomitant with enhancement of differentiation and cell cycle arrest induced by silibinin and 1,25-dihydroxyvitamin D(3) Cell Cycle. 2010;9:4542–4551. doi: 10.4161/cc.9.22.13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Gocek E, Liu CG, Studzinski GP. MicroRNAs181 regulate the expression of p27Kip1 in human myeloid leukemia cells induced to differentiate by 1,25-dihydroxyvitamin D3. Cell Cycle. 2009;8:736–741. doi: 10.4161/cc.8.5.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boomer JS, Tan TH. Functional interactions of HPK1 with adaptor proteins. J Cell Biochem. 2005;95:34–44. doi: 10.1002/jcb.20401. [DOI] [PubMed] [Google Scholar]
- 56.Arnold R, Liou J, Drexler HC, Weiss A, Kiefer F. Caspase-mediated cleavage of hematopoietic progenitor kinase 1 (HPK1) converts an activator of NFkappaB into an inhibitor of NFkappaB. J Biol Chem. 2001;276:14675–14684. doi: 10.1074/jbc.M008343200. [DOI] [PubMed] [Google Scholar]
- 57.Brenner D, Golks A, Kiefer F, Krammer PH, Arnold R. Activation or suppression of NFkappaB by HPK1 determines sensitivity to activation-induced cell death. EMBO J. 2005;24:4279–4290. doi: 10.1038/sj.emboj.7600894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Studzinski GP, Reddy KB, Hill HZ, Bhandal AK. Potentiation of 1-beta-D-arabinofuranosylcytosine cytotoxicity to HL-60 cells by 1,25-dihydroxyvitamin D3 correlates with reduced rate of maturation of DNA replication intermediates. Cancer Res. 1991;51:3451–3455. [PubMed] [Google Scholar]