Abstract
Expression of low molecular weight (LMW) isoforms of cyclin E is a strong predictor of poor outcome in patients with breast cancer. The purpose of this study was to examine the expression of full-length and LMW cyclin E in bladder cancer cell lines and patient tumors. We used western blotting, immunoprecipitation and kinase assays to examine the expression and activity of key cell cycle-regulatory proteins in various human bladder cell lines, both tumorigenic and non-tumorigenic. We also analyzed cyclin E expression, kinase activity and immune complex binding partners in 43 tissue samples from grade 2 and 3 transitional cell carcinomas. Cyclin E was overexpressed and LMW isoforms were present only in bladder cancer cells. Overexpression of LMW isoforms of cyclin E and increased cyclin E kinase activity were both significantly associated with tumorigenicity of the bladder cell lines (p = 0.005 and 0.022, respectively). Binding of the cyclin-dependent kinase inhibitors p21 and p27 to LMW cyclin E did not inhibit the kinase activity of cyclin E and cyclin-dependent kinase 2 in primary tumor samples overexpressing LMW cyclin E. Full-length and LMW cyclin E were significantly overexpressed in grade 3 tumors compared with grade 2 tumors (p = 0.004). Finally, LMW cyclin E levels were significantly associated with a non-papillary growth pattern (p = 0.031) and invasiveness (p = 0.021) of the bladder tumors and poor overall survival (p = 0.06). These results suggest that LMW cyclin E can be used as a new prognostic marker for bladder cancer.
Key words: cyclin E, p27, Cdk2 kinase, bladder cancer, cell cycle
Introduction
Urinary bladder cancer is the fifth most common cancer in the western world and is responsible for about 3% of all cancer-related deaths. Approximately 55,000 new patients are diagnosed with bladder cancer annually in the United States, and 15,000 of them die of the disease each year.1 Transitional cell (urothelial) carcinoma (TCC) is the most common urinary bladder neoplasm in the western world. Current pathogenetic concepts postulate that common urothelial neoplasms of the bladder arise via two distinct but somewhat overlapping pathways: papillary and nonpapillary.2 Approximately 80% of urothelial tumors of the bladder are superficially growing exophytic papillary lesions that may recur but usually do not invade and metastasize. They originate from hyperplastic urothelial changes. The remaining 20% of urothelial tumors are highly aggressive, solid, nonpapillary carcinomas with a strong propensity to invade and metastasize. The vast majority of invasive bladder cancers occur in patients without a prior history of papillary tumors and originate from clinically occult mild to moderate dysplasia (low-grade intraurothelial neoplasia) progressing to severe dysplasia and carcinoma in situ (high-grade intra-urothelial neoplasia) and invasive cancer.2
Cyclin E is a G1 cyclin that is overexpressed in many human cancers.3 Cyclin E and cyclin-dependent kinase (Cdk)-2 have been implicated in a number of processes at the G1/S cell cycle-transition that influence the fidelity of chromosome segregation, including licensing of origins of DNA replication, centrosome duplication or coordinate replication with gene transcription (in the case of histone biosynthesis).4 Deregulation of these processes can lead to chromosome instability. In some bladder cancer cell lines, for example, the combination of cyclin E overexpression and p53 loss induces centrosome amplification and chromosome instability.5
More generally, in tumor cells, cyclin E can be deregulated by a number of mechanisms, including gene amplification,6 downregulation of p277 or downregulation of the F-box protein Fbw7 (also called hCDC4), which tags phosphorylated cyclin E for proteosomal degradation.8 Mutations in the gene encoding hCDC4 have been found in breast, ovarian, endometrial9 and colorectal cancers10 and are associated with elevated levels of cyclin E protein. More recently, the overexpression of miR-27a in pediatric acute lymphoblastic leukemia (ALL) has been shown to suppress Fbw7 expression, leading to improper cell cycle progression and DNA replication stress, consistent with dysregulation of cyclin E expression.11 We have found that deregulation of cyclin E can also occur through post-translational processing of the full-length cyclin E by an elastase-like protease to generate low molecular weight (LMW) isoforms.12,13 Expression of these LMW isoforms in tumor cells leads to increased genomic instability12 due to premature activation of CDC25C14 and shortening of the length of mitosis from nuclear envelope breakdown to prometaphase.14
In a previous study, we compared cyclin E levels in 395 breast cancer patients to other frequently used clinical, pathological and biological prognostic factors, including tumor size, nodal status, clinical stage, Her-2/neu expression, DNA ploidy, proliferative index, estrogen and progesterone receptor expression and cyclin D1 and D3 levels.15 Cyclin E levels in tumor tissue associated strongly with disease-specific and overall survival in patients with stage I, II and III disease but had no impact on outcome in patients with stage IV disease. The prognostic significance of cyclin E was particularly striking in patients with stage I disease: only 12 patients (of 114) whose tumors overexpressed cyclin E died of disease, with a median time to death of 4.1 years. We have also found that cyclin E expression is deregulated in ovarian cancer16 as well as melanomas.17
Studies of cyclin E deregulation in bladder cancer have shown that the gene encoding cyclin E is amplified in about 2% of bladder tumors, and that strong immunostaining for cyclin E is seen in about 12% of samples.18 Invasive transitional-cell carcinomas (TCCs) have greater expression of cyclin E mRNA than do superficial TCCs or normal bladder cells.19 Del Pizzo et al. showed that p27(Kip1) expression and cyclin E expression are downregulated as the stage of disease advances.20 These studies of the role of cyclin E in bladder cancer examined the genomic alterations, mRNA and protein expression as measured by immunohistochemistry; however, none of these studies has established an association between cyclin E deregulation and prognosis. The absence of association between cyclin E expression and recurrence, progression or tumor-specific survival could be because the assays used by the aforementioned studies to examine cyclin E expression do not detect the LMW isoforms of cyclin E, which are detected by western blot analysis. Our overall goal in this study was to establish if there is a correlation between LMW cyclin E overexpression and their associated kinase activity with bladder tumorigenesis. We used western blotting, immunoprecipitation and kinase assays to examine the activity of full-length and LMW cyclin E in bladder cancer cell lines and tumor samples. We also examined the impact of p21 and p27 on cyclin E kinase activity in the cell lines and tumor samples. Collectively, our results revealed that the presence of LMW isoforms of cyclin E is tumor-specific, associated with higher grade, stage and invasiveness of bladder cancer and correlated with poor survival. Furthermore, the LMW cyclin E are refractory to p21 and p27 inhibition, conferring a growth advantage to the tumor.
Results
Overexpression of LMW cyclin E in human bladder cancer cell lines.
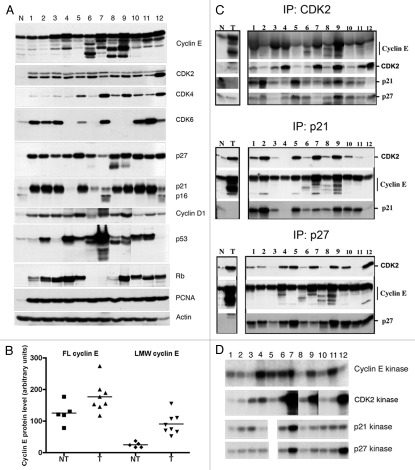
To address whether overexpression of cyclin E and its LMW isoforms are implicated in bladder cancer tumorigenesis, we examined their expression and that of major cell cycle regulatory proteins in a set of cultured human bladder-derived cells representing different stages of bladder carcinogenesis (Fig. 1). We used one primary culture of normal urothelial cells, three immortalized cell lines and nine TCC cell lines (all but one of which were tumorigenic in nude mice); the characteristics of these cell lines (tumor grade, tumorigenicity, Rb, p53 and cyclin E status) are listed in Table 1. Cell extracts were evaluated for protein expression by immunoblotting using the indicated antibodies (Fig. 1A) and for cyclin E and p27 kinase activity by in vitro kinase assays (Fig. 1D).
Figure 1.
LMW cyclin E overexpression and cyclin E kinase activity in bladder cell lines. (A) Western blot analysis of cyclin E, Cdk2, Cdk4, Cdk6, p27, p21, p16, cyclin D1, p53, Rb, PCNA and actin in bladder cell extracts. (B) Comparison of full-length (FL) and LMW cyclin E protein levels in non-tumorigenic (NT) and tumorigenic (T) bladder cell lines. Mean values ± SEM for NT and T, respectively, were 125.0 ± 15.8 and 176.3 ± 16.8 (p = 0.063) for FL cyclin E and 24.6 ± 3.8 and 90.2 ± 11.6 (p = 0.001) for LMW cyclin E. (C) Immune complexes of Cdk2, p21 and p27 in cell extracts were immunoprecipitated (IP) with polyclonal antibodies to Cdk2, p21 and p27 coupled to protein A-coated beads. Immune complexes were then subjected to western blot (WB) analysis using antibodies to cyclin E, Cdk2, p21 and p27. Normal mammary epithelial cells (N) and breast cancer cells (T) were used as standards. (D) Kinase assays were performed on cell extracts by immunoprecipitation with monoclonal antibodies to cyclin E coupled to protein G-coated beads or polyclonal antibodies to Cdk2, p21 and p27 coupled to protein A-coated beads. Histone H1 was used as the substrate. Lane designations correspond to sample numbers given in Table 1.
Table 1.
Rb, p53, and LMW cyclin E status in five non-tumorigenic and eight tumorigenic bladder cell lines
| Sample no. | Cell line | Tumor type/grade | Tumorigenicity | Rb status | p53 status# | FL Cyclin E* | LMW cyclin E* | LMW/FL Ratio (%) | Cyclin E kinase activity** | Cdk2 kinase activity** |
| N | Normal | Normal | NT | + | + (WT) | 78 | 17 | 21.8 | ND | ND |
| 1 | Alpha-E6-1 | Immortalized | NT | Inactive | + (WT) | 130 | 28 | 21.5 | 1.2 | 0.3 |
| 2 | Alpha-E6-2 | Immortalized | NT | Inactive | ++ (WT) | 119 | 17 | 14.3 | 1.1 | 0.7 |
| 3 | Alpha-E7 | Immortalized | NT | + | Inactive | 177 | 37 | 20.9 | 0.6 | 2.0 |
| 4 | UM-UC9 | TCC/G3 | T | + | ++++ | 200 | 66 | 33.0 | 5.2 | 2.7 |
| 5 | UM-UC11 | TCC/G3 | NT | + | ++ | 121 | 24 | 19.8 | 2.7 | 1.4 |
| 6 | UM-UC14 | TCC/G4 | T | − | +++ | 117 | 110 | 94.0 | 3.4 | 3.7 |
| 7 | HTB9 | TCC/G2 | T | − | +++++ (MT) | 153 | 88 | 57.5 | 4.2 | 5.9 |
| 8 | RT4P | TCC/G1 | T | + | ++ (WT) | 145 | 107 | 73.8 | 0.5 | 1.8 |
| 9 | RT4-V6 | TCC/metastatic | T | + | ++ | 177 | 155 | 87.6 | 2.4 | 4.9 |
| 10 | 253J | TCC/G3 | T | + | ++ (WT) | 155 | 52 | 33.5 | 1.2 | 1.1 |
| 11 | 253J B-V | TCC/metastatic | T | + | ++ (WT) | 189 | 70 | 37.0 | 2.7 | 2.0 |
| 12 | KU7/GFP | TCC/metastatic | T | + | + | 274 | 74 | 27.0 | 0.8 | 5.4 |
Abbreviations: NT, non-tumorigenic; T, tumorigenic; WT, wild type; MT, mutant; ND, not determined.
+ and ++ is wild type, > ++ is likely to be mutant p53.
Arbitrary values derived from the relative intensity of the bands in western blots.
Cyclin E and Cdk2 kinase activities relative to the mean value obtained from the three immortalized cell lines.
The expression of both full-length cyclin E and the LMW forms were measured by densitometric analysis of the corresponding bands on western blots (Fig. 1A and B and Table 1). Actin densitometric values were used to standardize protein loading. For protein kinase assays, the protein bands corresponding to histone H1 were excised, and the radioactivity of each band was measured by Cerenkov counting. Values shown in Table 1 and Figure 1B represent the mean of two independent experiments. The results revealed that the overexpression of the LMW isoforms of cyclin E was significantly associated with the tumorigenic cell lines only (p = 0.001), whereas overexpression of full-length cyclin E was not (p = 0.063). Furthermore, the quantitation of LMW-E/FL-E ratio shown in Table 1 (and Fig. S1A) was higher in tumorigenic vs. non-tumorigenic cell lines (55.4% vs. 19.7%, p = 0.0067 for student's t test). Furthermore, the level of endogenous full-length cyclin E did not correlate with the LMW-E isoforms levels in our panel of cell lines (Fig. S1B and Pearson's r = 0.3329, p = 0.2665) or even when exogenous flag-tagged, full-length cyclin E was introduced by adenoviral gene transfer (Fig. S1C). These observations demonstrate that the level of LMW isoforms of cyclin E1 in bladder cell lines is dependent on the activity of the protease(s) cleaving full-length cyclin E1 rather than on the full-length cyclin E protein levels. Five of the eight tumorigenic cells lines showed a 2.4- to 5.2-fold increase in cyclin E kinase activity relative to the mean value for the three immortalized cell lines (Fig. 1D, lanes 4, 6, 7, 9 and 11). We also observed a parallel increase in Cdk2 kinase activity except in the KU7/GFP cell line (Fig. 1D, lane 12), which is tumorigenic and metastatic in severe combined immunodeficient mice.21 In KU7/GFP cells, the strong Cdk2 kinase activity (5.4-fold greater than that of immortalized cells) was not associated with increased cyclin E kinase activity. This discrepancy is most likely caused by high levels of Cdk2 protein (3-fold greater than those of immortalized cell lines; Fig. 1A, lane 12), which can also form a complex with cyclin A.
To further investigate the role of LMW cyclin E in bladder cancer progression, we compared LMW cyclin E expression in cultured bladder cancer cells before and after implantation in nude mice. For these studies, we used the parental bladder cancer cell lines RT4 and 253J, which initially had low tumorigenic potential (Fig. 1 and Table 1). The metastatic variants RT4-V6 and 253J B-V were obtained by orthotopic implantation of the parental lines in athymic nude mice as described in a previous study in reference 22. We found that RT4-V6 and 253J B-V had 4.8- and 2.2-fold greater cyclin E kinase activity, respectively, than the parental lines. This increased cyclin E kinase activity was paralleled by increased expression of full-length and LMW cyclin E, as detected by western blot analysis.
We also examined the expression of the major cell cycle-regulatory proteins p53 and Rb in the bladder cancer cell lines. The cell lines UC14 and HTB9 showed a complete absence of Rb expression (Fig. 1A, lanes 6 and 7, respectively). p53 was stabilized in cell lines UC9, UC14 and HTB9 (Fig. 1A, lanes 4, 6 and 7, respectively), indicative of a mutation in the p53 gene. Consistent with these alterations in the p53 and Rb pathways, the cell lines UC14 and HTB9 had higher cyclin E and Cdk2 kinase activities and lower expression of p21 than did immortalized cell lines.
The results from the analyses of the bladder cell line model system showed a strong association between the presence of LMW isoforms of cyclin E, higher cyclin E kinase activity and greater tumorigenicity.
Interactions between LMW cyclin E, p21, p27 and Cdk2 kinase activity.
Numerous studies have shown that binding of p21 and p27 to cyclin E/Cdk2 complexes inhibits the Cdk2 kinase activity.23 However, we recently found that breast cancer cells overexpressing LMW cyclin E become resistant to p21 and p27 inhibition.12 To determine whether the LMW isoforms of cyclin E are found in complex with p21 and p27 in our bladder cell lines, and whether these complexes were still active, we immunoprecipitated cell lysates with anti-p21 and anti-p27 antibodies and analyzed them for activity and binding to cyclin E. These experiments revealed a strong association between the presence of LMW cyclin E in p21/p27 complexes and higher kinase activity of cyclin E, Cdk2, p21 and p27 (lanes 7, 9 and 12 of Fig. 1C). These results suggested that the LMW forms of cyclin E remained refractory to the Cdk inhibitors p21 and p27 despite being in complexes with them.
In five of the eight tumorigenic cell lines, the presence and overexpression of LMW cyclin E were associated with a parallel increase in cyclin E and Cdk2 kinase activities compared with those of the immortalized cell lines. However, the tumorigenic cell lines HTB9, RT4-V7 and KU7/GFP had much higher Cdk2 kinase activity than the immortalized cell lines (Fig. 1C, lanes 7, 9 and 12, respectively), which might have been caused by a combination of deregulation of cell cycle regulators and overexpression of LMW cyclin E.
For cell line HTB9, the combination of mutant p53, loss of Rb expression, low p21 and p27 expression, overexpression of full-length cyclin E and presence of LMW isoforms of cyclin E that bind to p21 and p27 (lane 7 of Fig. 1A and C) likely accounted for the 5.9-fold higher Cdk2 kinase activity in that cell line than in the immortalized lines. The highly tumorigenic line RT4-V6 had 4.8-fold higher cyclin E kinase activity, 2.2-fold higher Cdk2 kinase activity and 2-fold higher p21 and p27 kinase activity than its poorly tumorigenic parental line RT4. The most notable changes in RT4-V6 were increased expression of full-length and LMW cyclin E and greater binding of p21 and p27 to LMW isoforms (lane 9 of Fig. 1A and C), despite having no significant changes in p21 and p27 expression levels (compare lanes 8 and 9 of Fig. 1A). We believe the increased expression of LMW cyclin E in RT4-V6 led to increased binding of p21 and p27, which, in turn, led to increased cyclin E and Cdk2 kinase activity. In the KU7/GFP cell line, as mentioned earlier, the 5.4-fold increase in Cdk2 kinase activity was caused mainly by the 3-fold higher expression of Cdk2 and 4-fold lower expression of p21 than in immortalized cell lines. The greater p27 kinase activity reflected increased binding of LMW cyclin E to p27 (lane 12 of Fig. 1C).
Collectively, the biochemical data suggested that the presence and overexpression of LMW isoforms of cyclin E were accompanied by increased cyclin E activity, increased Cdk2 activity and decreased inhibitory function of p21 and p27 in most of the tumorigenic cell lines examined.
Association of LMW cyclin E overexpression with high-grade, high-stage, invasive tumors and poor overall survival in bladder cancer patients.
Next, we addressed the role of LMW cyclin E in bladder tumorigenesis. To this end, we examined the association between the presence and overexpression of LMW cyclin E, levels of p21 and p27 protein and histopathological characteristics of primary tumors from 43 patients with TCC of the bladder. Table 2 lists clinico-pathological characteristics of the 43 patients, including tumor grade and stage, patient age at time of surgery (median 69 y for those with grade 2 tumors and 68 y for those with grade 3 tumors) and follow-up time (mean 41.1 mo).
Table 2.
Patient characteristics and tumor sample results
| Sample no. | Age | Sex | Grade | Stage (pT) | Death (mo)* | LMW cyclin E | p27 | p21 |
| 11 | 65 | M | 2 | 1 | No (77.8) | Low | ND | Low |
| 12 | 76 | F | 2 | A | Yes (21.1) | Low | High | Low |
| 13 | 72 | M | 2 | A | No (80.5) | Low | Low | Low |
| 14 | 76 | F | 2 | 1 | No (12.4) | Low | High | High |
| 15 | 83 | M | 2 | 2 | No (83.1) | High | ND | High |
| 65 | 70 | M | 2 | A | No (127.5) | Low | ND | ND |
| 67 | 43 | M | 2 | A | No (71.7) | Low | ND | ND |
| 68 | 53 | M | 2 | 1 | No (76.0) | Low | ND | ND |
| 69 | 67 | M | 2 | A | No (81.5) | Low | ND | ND |
| 84 | 68 | M | 2 | A | No (11.5) | Low | ND | ND |
| 1 | 53 | M | 3 | 2 | No (91.9) | Low | High | Low |
| 2 | 50 | M | 3 | 3 | Yes (50.7) | High | High | Low |
| 3 | 64 | M | 3 | 4 | Yes (7.6) | Low | ND | High |
| 4 | 76 | M | 3 | 3 | Yes (1.3) | High | Low | High |
| 5 | 65 | M | 3 | 3 | No (79.8) | High | High | Low |
| 6 | 67 | M | 3 | 3 | No (3.8) | High | ND | High |
| 7 | 88 | M | 3 | 2 | Yes (15.1) | High | High | High |
| 8 | 76 | M | 3 | 4 | No (0.0) | Low | ND | High |
| 9 | 68 | M | 3 | 3 | No (28.2) | High | High | High |
| 10 | 82 | M | 3 | 2 | Yes (2.0) | High | ND | ND |
| 62 | 80 | M | 3 | 1 | Yes (30.0) | High | Low | ND |
| 64 | 76 | F | 3 | 3B | Yes (17.9) | High | Low | ND |
| 66 | 51 | M | 3 | 3 | Yes (23.0) | Low | High | ND |
| 85 | 55 | M | 3 | 3 | No (17.3) | Low | Low | Low |
| 56 | 68 | M | 3 | 3 | No (12.9) | High | Low | Low |
| 57 | 56 | M | 3 | 4A | No (0.2) | High | High | Low |
| 58 | 75 | M | 3 | 2 | No (81.2) | High | Low | Low |
| 59 | 88 | M | 3 | 2B | No (3.4) | High | Low | High |
| 60 | 65 | M | 3 | 2 | Yes (7.3) | High | High | Low |
| 61 | 54 | M | 3 | 3 | No (23.1) | High | Low | Low |
| 71 | 65 | F | 3 | 3B | No (8.1) | High | High | Low |
| 72 | 67 | F | 3 | 3B | No (5.2) | Low | Low | Low |
| 73 | 77 | M | 3 | 4A | No (9.9) | High | Low | Low |
| 74 | 51 | M | 3 | 4A | No (137.0) | Low | High | High |
| 75 | 70 | M | 3 | ND | No (17.2) | Low | Low | High |
| 76 | 61 | M | 3 | ND | No (5.0) | Low | Low | Low |
| 77 | 68 | F | 3 | ND | No (3.0) | High | Low | Low |
| 78 | 77 | F | 3 | 3B | Yes (107.9) | Low | Low | Low |
| 79 | 76 | F | 3 | 3B | Yes (124.0) | High | Low | Low |
| 80 | 66 | M | 3 | 3B | No (121.4) | High | High | Low |
| 81 | 78 | F | 3 | 3A | No (4.1) | Low | High | Low |
| 82 | 73 | M | 3 | 3A | No (69.6) | Low | Low | Low |
| 83 | NA | NA | 3 | 3A | Yes (15.0) | High | Low | Low |
Abbreviations: NA, not available; ND, not determined.
Time in months from operation to death or last follow-up.
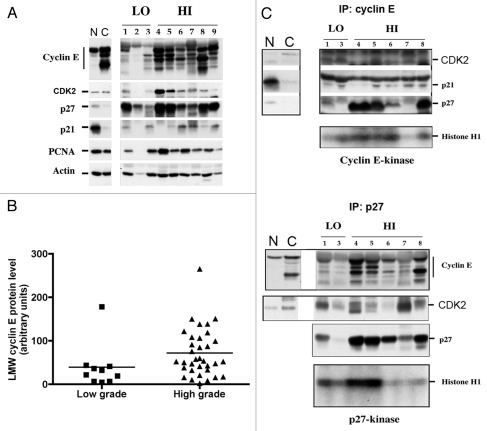
Tumor tissues were assayed for full-length, LMW and total cyclin E levels by western blotting; representative results are shown in Figure 2A, and all results are summarized in Table 2. Protein expression was measured by densitometric analysis of the corresponding bands on western blots, and actin densitometric values were used to standardize protein loading. Densitometric values for the full-length and LMW forms of cyclin E, p21 and p27 were scored as low (less than or equal to the mean level of protein found in non-tumorigenic bladder cell lines) or high (higher than in non-tumorigenic bladder cell lines). Normal mammary epithelial cells (MCF-10A) and breast cancer cells (MDA-MB157) were used as laboratory standards for western blot analysis of cyclin E. Values shown in Figure 2B represent the mean of two independent experiments. Total cyclin E protein levels were higher in grade 3 tumors than in grade 2 tumors. Specifically, 21 (64%) of 33 grade 3 tumors overexpressed full-length and LMW cyclin E compared with 1 (10%) of 10 grade 2 tumors (p = 0.004; Fig. 2B). The results from the tumor samples confirmed the association between LMW cyclin E overexpression and higher tumorigenicity of the bladder cancer cell lines. In addition, high levels of p21 protein were associated with papillary growth patterns (p = 0.032). No significant association was found between high levels of p27 and any of the parameters listed in Table 2.
Figure 2.
LMW cyclin E protein levels and responsiveness to p27 inhibition in primary tumor samples. (A) Whole-cell lysates were extracted from low-grade (LO) and high-grade (HI) human bladder cancer tissues. Cell extracts were analyzed by western blotting for cyclin E, Cdk2, p27, p21, PCNA and actin. Normal mammary epithelial cells (N) and breast cancer cells (C) were used as standards. (B) Overexpression of LMW cyclin E protein in low- and high-grade tumors. Mean values ± SEM were 39.1 ± 16.1 for grade 2 tumors (n = 10) and 71.6 ± 9.5 for grade 3 tumors (n = 33). (C) Immunoprecipitation (IP) of cyclin E and p27 and western blot (WB) analysis or kinase assays were performed as described in Figure 1.
We also found high p27 protein levels in eight (38%) of 21 tumors overexpressing LMW cyclin E (Table 2). Overexpression of p27 should suppress cyclin E kinase activity, but biochemical analysis of p27 in the tumor samples showed otherwise. Lysates from the tumor samples were immunoprecipitated with cyclin E and analyzed by western blotting with antibodies to cyclin E, Cdk2, p21 and p27 (Fig. 2C). These experiments showed that p27 was in complexes with cyclin E and Cdk2 in all samples. However, when the LMW isoforms were present, cyclin E kinase activity was not suppressed by the presence of p27 in the cyclin E complexes (lanes 4, 5 and 8 of Fig. 2C). We also performed the reverse experiment by first immunoprecipitating with p27 and then western blotting with antibodies to cyclin E, Cdk2 and p27 (Fig. 2C, lower part). We found that the LMW forms of cyclin E were in complex with p27 and that the complexed p27 retained high kinase activity, suggesting that the LMW forms of cyclin E are refractory to p27-mediated inactivation.
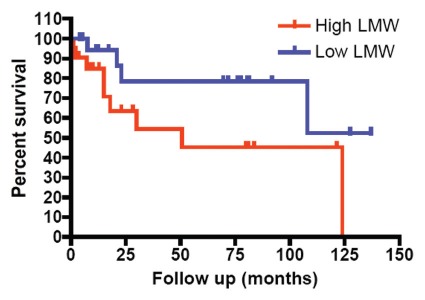
The in vivo results described in this report indicate that the overexpression of the LMW isoforms of cyclin E in bladder cell lines was not a consequence of tissue culture conditions, as it was also observed in tumors from bladder cancer patients. The presence and overexpression of LMW isoforms of cyclin E in bladder cancer cells were associated with increased stage of disease (p = 0.021, Table 3) and muscle-invasive disease (p = 0.031, Table 3). We next stratified the 43 bladder tumors as a function of LMW cyclin E expression, and found that those patients who had high levels of LMW cyclin E protein expression had worse outcome compared with those patients whose tumors had low levels of LMW cyclin E expression (p = 0.06; Fig. 3).
Table 3.
Association between expression of LMW cyclin E, p21 and p27 protein and pathologic features of primary bladder cancers
| LMW cyclin E | p21 | p27 | ||||
| Expression | P | Expression | P | Expression | P | |
| Growth pattern | ||||||
| Papillary | 5/17 | 0.031 | 9/15 | 0.032 | 7/18 | NS |
| Non-papillary | 17/26 | 4/20 | 9/25 | |||
| Histological grade | ||||||
| Low (1–2) | 1/10 | 0.004 | 5/9 | NS | 3/11 | NS |
| High (3) | 21/33 | 8/26 | 13/32 | |||
| Stage | ||||||
| Superficial (Ta-T1a) | 1/8 | 0.021 | 4/8 | NS | 1/8 | NS |
| Invasive (T1b-T4) | 21/35 | 9/27 | 15/35 | |||
Abbreviations: NS, not significant.
Figure 3.
Relationship between high LMW cyclin E protein expression and poor survival in bladder cancer. Bladder tumor specimens from 43 patients were assessed for cyclin E expression by western blot. Patients were stratified by LMW cyclin E expression. Patients with high LMW cyclin E had markedly decreased overall survival (median OS, 30 mo) compared with those with low levels of LMW cyclin E (median OS not reached; p = 0.06).
Discussion
Currently, clinical and pathological stages are the most reliable prognostic markers for bladder cancer, but they are insufficient to accurately predict the evolution of most invasive bladder cancers. Our study has shown that cyclin E and its LMW forms might be better prognostic markers. Our data showed that cyclin E was overexpressed, and its LMW isoforms were present only in tumor cells, not normal urothelial, cells. The presence and overexpression of LMW isoforms of cyclin E were associated with tumorigenicity of the bladder cell lines and with high-grade, high-stage, invasive tumors. Analysis of the activity of cyclin E complexes in cell lines and tumor samples revealed that binding of the Cdk inhibitors p21 and p27 to LMW cyclin E did not inhibit the kinase activity of cyclin E and Cdk2. We also demonstrated that p27 was upregulated and associated with cyclin E complexes in high-grade bladder tumor tissues overexpressing cyclin E. However, the binding of p27 did not suppress cyclin E kinase activity in those complexes, which resulted in phosphorylation of cyclin E/Cdk2 downstream substrate targets and progression of cells through the cell cycle. We also show that the subgroup of patients with high LMW cyclin E protein expression had a poor prognosis, with a median survival time of only 30 mo. These findings indicate that LMW cyclin E might have a key role in the oncogenesis and biological behavior of TCC of the bladder.
Accelerated proteolysis and cytoplasmic accumulation of p27 protein are common events in a majority of human malignancies and lead to a reduced ability of p27 to bind and inhibit nuclear cyclin E/Cdk2 complexes. In many human cancers, p27 proteolysis is the result of activation of receptor tyrosine kinases24 and the ras signaling pathways.25 Activation of the phosphatidylinositol-3-kinase pathway can also lead to cytoplasmic accumulation of p27 as a result of impaired nuclear import in breast cancer cells.26 Interestingly in a mouse model of Sonic hedgehog (Shh)-mediated medulloblastoma, mice heterozygous for p27Kip1 have decreased survival latency compared with p27Kip1-null animals, because a single copy of p27Kip1 is sufficient to recruit cyclin D/Cdk4/6 to promote cell cycle progression yet insufficient to inhibit cyclin E/Cdk2.27 We have shown here that bladder cancer cells escape p27 inhibition through the generation of p27-resistant LMW cyclin E. Resistance of LMW cyclin E to p21 and p27 inhibition is a recently described mechanism for failure of endocrine therapy in breast cancer patients12 and increased resistance to lovastatin-induced G1 arrest in an ovarian cancer cell line model.16
The observation that p27 does not necessarily lead to growth inhibition if LMW cyclin E is overexpressed may explain why the predictive potential of p27 levels has been controversial. Some studies have found that high p27 levels are associated with better clinical outcome,28,29 while others have not found any prognostic power of p27 expression.30,31 These contradictory studies could be reconciled by the fact that p27 is upregulated but cannot function as a Cdk inhibitor when LMW cyclin E isoforms are overexpressed, as these isoforms are refractory to p27 inhibition. In the subgroup of tumors that overexpress LMW isoforms of cyclin E, high p27 would thus be predictive of poor clinical outcome, especially at an early stage of the disease;30 in contrast, high p27 would be predictive of good clinical outcome in tumors without LMW cyclin E overexpression.
Our study should encourage a large-scale evaluation of the prognostic potential of LMW cyclin E in terms of recurrence, progression, tumor-specific survival and response to therapy of patients with bladder cancer.
Materials and Methods
Cell cultures.
Primary human urothelial cells were initiated and cultured on thick rat-tail collagen in a 50:50 mixture of Ham's F12 (Invitrogen, 11765-054) and serum-free keratinocyte medium (Sigma Chemical, S0196) supplemented with 25 µg/ml bovine pituitary extract and 25 ng/ml epidermal growth factor (Invitrogen, E3476). The cells were collected at 75–90% confluence for protein extraction (these cells were maintained in culture for fewer than six passages).
Dr. Barton Grossman (The University of Texas MD Anderson Cancer Center) kindly provided three immortalized cell lines (α-E6-1, α-E6-2, α-E7) and seven TCC cell lines [UM-UC9, UM-UC11, UM-UC14, HTB9 (5637), RT4, 253J and KU7/GFP]. These were maintained in a humidified incubator with 5% CO2 and 95% air at 37°C. The UM-UC9, UM-UC11, UM-UC14 and KU7/GFP cell lines were cultured in NEAA Earl's Salt (Invitrogen, 10370-070); the HTB9 cell line was grown in RPMI 1640 (Life Technologies, 11875-093), and the α-E6-1, α-E6-2 and α-E7 cell lines were grown in Ham's F12 medium (Invitrogen, 11765-054). Dr. Colin P.N. Dinney (MD Anderson) kindly provided the RT4, RT4-V6, 253J and 253J B-V TCC cell lines. These were cultured in modified Eagle's medium (Invitrogen, 12491-015).
All media were supplemented with 10% fetal calf serum and 100 units/ml penicillin-streptomycin. Modified Eagle's medium was further supplemented with 1 mM sodium pyruvate, vitamins and 0.1 mM nonessential amino acids. Proteins were extracted from cells at passage 3.
Western blot analysis.
Cell lysates were prepared and subjected to western blot analysis as previously described in reference 12. We used primary antibodies against cyclin E (HE-12; Santa Cruz Biotechnology, SC-247); Cdk6 (H-11; Santa Cruz Biotechnology, SC-271364); Cdk2, (BD Biosciences-Transduction Laboratories, 010146) and Cdk4 (BD Biosciences-Transduction Laboratories, 559693); p21 (Oncogene Research Products, OP64); p27 (BD Biosciences-Transduction Laboratories, K25020); p16 (C-20; Santa Cruz Biotechnology, SC-468); cyclin D1 (Zymed Laboratories, AHF0082); p53 (Ab-6; EMD Biosciences, OP43T); Rb (Becton Dickinson Biosciences PharMingen, 554144); PCNA (PC-10; Santa Cruz Biotechnology, SC-56); and actin (Chemicon International, MAB1501R) at dilutions recommended by the manufactures. Densitometry was done using IPLab software (Scanalytics). Statistical analysis of the densitometry values was done with GraphPad Prism software version 4.0 (GraphPad Software).
Immunoprecipitation and immunoblotting.
We used 250 µg of cell extract for each immunoprecipitation with polyclonal antibodies to cyclin E, Cdk2,13,15,32 p21 (C-19, sc-397; Santa Cruz Biotechnology) and p27 (C-19, sc-528; Santa Cruz Biotechnology) coupled to protein A- or protein G-coated beads. After being washed, the immunoprecipitates were subjected to electrophoresis on 13% gels, transferred to Immobilon P membranes, blocked and incubated with the antibodies used above.
Protein kinase assays.
For histone H1 kinase assays, the immunoprecipitates were incubated with kinase assay buffer containing 60 µM cold ATP, 5 µCi of [32P]ATP and 5 µg of histone H1 (Roche Diagnostics, 10223549001) in a final volume of 30 µl at 37°C for 30 min. The products of the reaction were analyzed on 13% SDS-PAGE gels that were stained, destained, dried and exposed to X-ray film.
Tumor tissue analysis.
Expression of cyclin E and its LMW isoforms and other cell cycle regulators were evaluated by western blot analysis of lysates prepared from frozen tumor tissue specimens as described previously in reference 15. Approximately 0.1 to 0.5 mg of the tumor tissue was added to one volume of sonication buffer containing protease and phosphatase inhibitors. The tumor tissue was then homogenized in a MicroMincer tissue press (BioSpec products, 2941) and sonicated at 4°C using a cup-horn adaptor. Homogenates were centrifuged at 100,000x -g for 45 min at 4°C. The supernatants were assayed for protein content, aliquoted and stored at -70°C until use.
Statistical methods.
Comparisons of cyclin E protein levels between immortalized, non-tumorigenic and tumorigenic cell lines and between grade 2 and 3 tumors were subjected to two-tailed t-tests. p < 0.05 was considered significant. Fisher's exact test was used to evaluate the association between cyclin E, p21 and p27 protein levels and pathological growth pattern, grade and stage of the tumors. Overall survival (OS) was calculated from the date of surgical excision of the primary tumor to the date of death or last follow-up. OS curves were computed by the Kaplan-Meier method. Univariate analyses of OS survival according to levels of LMW cyclin E were done with the use of a two-sided log-rank test.
Acknowledgments
This work was supported by the GU SPORE Career Development Award P50 CA091846 from the National Institute of Health to S.A., grant (R01-CA87548) from the National Cancer Institute to K.K. and the MDACC Cancer Center Grant P30-CA16672 from the National Institute of Health. We thank Ms. Jessica Marino for her critical reading and editing of this manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
References
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. American Cancer Society. Cancer statistics. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Dinney CP, McConkey DJ, Millikan RE, Wu X, Bar-Eli M, Adam L, et al. Focus on bladder cancer. Cancer Cell. 2004;6:111–116. doi: 10.1016/j.ccr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Akli S, Keyomarsi K. Cyclin E and its low molecular weight forms in human cancer and as targets for cancer therapy. Cancer Biol Ther. 2003;2:38–47. [PubMed] [Google Scholar]
- 4.Hwang HC, Clurman BE. Cyclin E in normal and neoplastic cell cycles. Oncogene. 2005;24:2776–2786. doi: 10.1038/sj.onc.1208613. [DOI] [PubMed] [Google Scholar]
- 5.Kawamura K, Izumi H, Ma Z, Ikeda R, Moriyama M, Tanaka T, et al. Induction of centrosome amplification and chromosome instability in human bladder cancer cells by p53 mutation and cyclin E overexpression. Cancer Res. 2004;64:4800–4809. doi: 10.1158/0008-5472.CAN-03-3908. [DOI] [PubMed] [Google Scholar]
- 6.Schraml P, Bucher C, Bissig H, Nocito A, Haas P, Wilber K, et al. Cyclin E overexpression and amplification in human tumours. J Pathol. 2003;200:375–382. doi: 10.1002/path.1356. [DOI] [PubMed] [Google Scholar]
- 7.Bloom J, Pagano M. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin Cancer Biol. 2003;13:41–47. doi: 10.1016/S1044-579X(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 8.Koepp DM, Schaefer LK, Ye X, Keyomarsi K, Chu C, Harper JW, et al. Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science. 2001;294:173–177. doi: 10.1126/science.1065203. [DOI] [PubMed] [Google Scholar]
- 9.Spruck CH, Strohmaier H, Sangfelt O, Müller HM, Hubalek M, Müller-Holzner E, et al. hCDC4 gene mutations in endometrial cancer. Cancer Res. 2002;62:4535–4539. [PubMed] [Google Scholar]
- 10.Rajagopalan H, Jallepalli PV, Rago C, Velculescu VE, Kinzler KW, Vogelstein B, et al. Inactivation of hCDC4 can cause chromosomal instability. Nature. 2004;428:77–81. doi: 10.1038/nature02313. [DOI] [PubMed] [Google Scholar]
- 11.Lerner M, Lundgren J, Akhoondi S, Jahn A, Ng HF, Moqadam FA, et al. MiRNA-27a controls FBW7/hCDC4-dependent cyclin E degradation and cell cycle progression. Cell Cycle. 2011;10:2172–2183. doi: 10.4161/cc.10.13.16248. [DOI] [PubMed] [Google Scholar]
- 12.Akli S, Zheng PJ, Multani AS, Wingate HF, Pathak S, Zhang N, et al. Tumor-specific low molecular weight forms of cyclin E induce genomic instability and resistance to p21, p27 and antiestrogens in breast cancer. Cancer Res. 2004;64:3198–3208. doi: 10.1158/0008-5472.CAN-03-3672. [DOI] [PubMed] [Google Scholar]
- 13.Porter DC, Zhang N, Danes C, McGahren MJ, Harwell RM, Faruki S, et al. Tumor-specific proteolytic processing of cyclin E generates hyperactive lower-molecular-weight forms. Mol Cell Biol. 2001;21:6254–6269. doi: 10.1128/MCB.21.18.6254-69.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagheri-Yarmand R, Biernacka A, Hunt KK, Keyomarsi K. Low molecular weight cyclin E overexpression shortens mitosis, leading to chromosome missegregation and centrosome amplification. Cancer Res. 2010;70:5074–5084. doi: 10.1158/0008-5472.CAN-09-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keyomarsi K, Tucker SL, Buchholz TA, Callister M, Ding Y, Hortobagyi GN, et al. Cyclin E and survival in patients with breast cancer. N Engl J Med. 2002;347:1566–1575. doi: 10.1056/NEJMoa021153. [DOI] [PubMed] [Google Scholar]
- 16.Bedrosian I, Lu KH, Verschraegen C, Keyomarsi K. Cyclin E deregulation alters the biologic properties of ovarian cancer cells. Oncogene. 2004;23:2648–2657. doi: 10.1038/sj.onc.1207408. [DOI] [PubMed] [Google Scholar]
- 17.Bales E, Mills L, Milam N, McGahren-Murray M, Bandyopadhyay D, Chen D, et al. The low molecular weight cyclin E isoforms augment angiogenesis and metastasis of human melanoma cells in vivo. Cancer Res. 2005;65:692–697. [PubMed] [Google Scholar]
- 18.Richter J, Wagner U, Kononen J, Fijan A, Bruderer J, Schmid U, et al. High-throughput tissue microarray analysis of cyclin E gene amplification and overexpression in urinary bladder cancer. Am J Pathol. 2000;157:787–794. doi: 10.1016/S0002-9440(10)64592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGarvey TW, Tait E, Tomaszewski JE, Malkowicz SB. Expression of Transforming Growth Factorbeta Receptors and Related Cell-Cycle Components in Transitional-Cell Carcinoma of the Bladder. Mol Urol. 1999;3:371–380. [PubMed] [Google Scholar]
- 20.Del Pizzo JJ, Borkowski A, Jacobs SC, Kyprianou N. Loss of cell cycle regulators p27(Kip1) and cyclin E in transitional cell carcinoma of the bladder correlates with tumor grade and patient survival. Am J Pathol. 1999;155:1129–1136. doi: 10.1016/S0002-9440(10)65216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibayama T, Tachibana M, Deguchi N, Jitsukawa S, Tazaki H. SCID mice: a suitable model for experimental studies of urologic malignancies. J Urol. 1991;146:1136–1137. doi: 10.1016/s0022-5347(17)38025-4. [DOI] [PubMed] [Google Scholar]
- 22.Dinney CP, Fishbeck R, Singh RK, Eve B, Pathak S, Brown N, et al. Isolation and characterization of metastatic variants from human transitional cell carcinoma passaged by orthotopic implantation in athymic nude mice. J Urol. 1995;154:1532–1538. doi: 10.1016/S0022-5347(01)66923-4. [DOI] [PubMed] [Google Scholar]
- 23.Vidal A, Koff A. Cell cycle inhibitors: three families united by a common cause. Gene. 2000;247:1–15. doi: 10.1016/S0378-1119(00)00092-5. [DOI] [PubMed] [Google Scholar]
- 24.Lenferink AE, Busse D, Flanagan WM, Yakes FM, Arteaga CL. ErbB2/neu kinase modulates cellular p27(Kip1) and cyclin D1 through multiple signaling pathways. Cancer Res. 2001;61:6583–6591. [PubMed] [Google Scholar]
- 25.Liu X, Sun Y, Ehrlich M, Lu T, Kloog Y, Weinberg RA, et al. Disruption of TGFbeta growth inhibition by oncogenic ras is linked to p27Kip1 mislocalization. Oncogene. 2000;19:5926–5935. doi: 10.1038/sj.onc.1203991. [DOI] [PubMed] [Google Scholar]
- 26.Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, et al. PKB/Akt mediates cell cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8:1145–1152. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- 27.Bhatia B, Malik A, Fernandez-L A, Kenney AM. p27(Kip1), a double-edged sword in Shh-mediated medulloblastoma: Tumor accelerator and suppressor. Cell Cycle. 2010;9:4307–4314. doi: 10.4161/cc.9.21.13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korkolopoulou P, Christodoulou P, Konstantinidou AE, Thomas-Tsagli E, Kapralos P, Davaris P. Cell cycle regulators in bladder cancer: a multivariate survival study with emphasis on p27Kip1. Hum Pathol. 2000;31:751–760. doi: 10.1053/hupa.2000.8227. [DOI] [PubMed] [Google Scholar]
- 29.Lacoste-Collin L, Gomez-Brouchet A, Escourrou G, Delisle MB, Levade T, Uro-Coste E. Expression of p27(Kip1) in bladder cancers: immunohistochemical study and prognostic value in a series of 95 cases. Cancer Lett. 2002;186:115–120. doi: 10.1016/S0304-3835(02)00319-1. [DOI] [PubMed] [Google Scholar]
- 30.Franke KH, Miklosi M, Goebell P, Clasen S, Steinhoff C, Anastasiadis AG, et al. Cyclin-dependent kinase inhibitor p27(KIP1) is expressed preferentially in early stages of urothelial carcinoma. Urology. 2000;56:689–695. doi: 10.1016/S0090-4295(00)00678-6. [DOI] [PubMed] [Google Scholar]
- 31.Santos LL, Amaro T, Pereira SA, Lameiras CR, Lopes P, Bento MJ, et al. Expression of cell cycle regulatory proteins and their prognostic value in superficial low-grade urothelial cell carcinoma of the bladder. Eur J Surg Oncol. 2003;29:74–80. doi: 10.1053/ejso.2002.1371. [DOI] [PubMed] [Google Scholar]
- 32.Keyomarsi K, O'Leary N, Molnar G, Lees E, Fingert HJ, Pardee AB. Cyclin E, a potential prognostic marker for breast cancer. Cancer Res. 1994;54:380–385. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.