Abstract
Aim
Contrast harmonic endosonography (CHEUS) is not widely available. This study assessed the utility of CHEUS using DEFINITY™, a second generation ultrasonic contrast agent, in the evaluation of suspected pancreatic and peri-ampullary malignancies.
Methods
Prospectively enrolled patients with suspected pancreatic and peri-ampullary malignancies underwent EUS followed by CHEUS. The incremental yield of CHEUS over EUS was analyzed. The gold standard for diagnosis of malignancy was positive cytology or histology; a negative diagnosis for malignancy was based on negative cytology or histology and benign clinical course.
Results
Twenty-nine patients were enrolled and underwent CHEUS. The final diagnoses were: pancreatic adenocarcinoma (16/29); metastases to pancreas (4/29); pancreatitis with inflammatory mass (4/29); normal pancreas with focal fat sparing (1/29); ampulla adenocarcinoma (2/29); serous cystic neoplasm (1/29); peri-pancreatic lymph node due to lymphoma (1/29). One bengin case of chronic pancreatitis had calcification casting artifacts that prevented accurate EUS examination and was excluded, leaving 28 cases for comparative analysis between EUS and CHEUS. CHEUS enhanced tumor margins. CHEUS detected vascular invasion missed by EUS in 2/16 patients with pancreatic adenocarcinoma. Masses appeared hypoechoic with EUS. With CHEUS malignant masses had an inhomogeneous hypoechoic pattern associated with abnormal vessels while lesions due to focal pancreatitis or fat sparing were characterized by diffuse enhancement (p<0.001).
Conclusion
CHEUS improved the visualization of tumor margins and vascular invasion, and differentiated benign from malignant masses.
Key words: contrast endosonography, pancreatic malignancies, pancreatitis
Introduction
Computer tomography (CT), magnetic resonance imaging (MRI) and endoscopic ultrasound (EUS) are routinely used in the evaluation of suspected pancreatic and peri-ampullary malignancies. EUS is considered the most sensitive technique for evaluation and one can obtain tissue diagnosis at the same setting via fine needle aspiration (EUSFNA)1. In spite of the good performance characteristics of EUS and EUSFNA, differentiation of malignant from inflammatory masses and assessment of tumor extent remain challenging. Unlike CT and MRI examinations, contrast agents are not routinely used to enhance images during EUS.
In transabdominal ultrasound, ultrasonic contrast agents are routinely used to characterize focal hepatic lesions2. These ultrasonic contrast agents consist of a solution of microbubbles injected intravenously. When microbubbles are hit by an ultrasonic wave, the vibration creates a strong backscattered acoustic shadow that is detected and reproduced as an opacification. Vascular structures are thus highlighted. The degree of signal enhancement is related to the magnitude of microbubble oscillation around its equilibrium radius. The magnitude of oscillation in turn is primarily dependent on its size, the surface tension, and the gas characteristics. Contrast harmonic EUS (CHEUS) was previously not possible due to instability of the microbubbles and a lack of appropriate hardware and software. CHEUS has become possible due to the development of second generation contrast agents containing inert gases with greater stability due to low solubility in water and the availability of appropriate EUS processors and endoscopes3. Sonovue, the most frequently used agent, is constituted by phospholipid-stabilized microbubbles of sulfurhexafluoride, a poorly soluble and totally innocuous gas. Sonazoid is another contrast agent that is constituted by lipid-stabilized microbubbles of another inert gas, perfluoropentane. Preliminary studies showed that second generation contrast agents such as Sonovue4 and Sonazoid5 improved T staging of pancreatic tumors. Sonazoid was also useful for differentiating pancreatic cancer from chronic pancreatitis6.
DEFINITY™ (Lantheus Medical Imaging, Massachusetts, United States of America) is a second generation contrast agent made of perfluopentane surrounded by a phospholipid outer shell commonly used in contrast echocardiography7. It is not clear whether it will also be useful in the context of CHEUS for evaluating pancreatic masses. This pilot study assessed the utility of CHEUS using DEFINITY™ in the evaluation of suspected pancreatic and peri-ampullary malignancies.
Patients and methods
Study overview
This prospective study was conducted at the Department of Gastroenterology, Changi General Hospital, Singapore. It was approved by the institutional review board and conducted in accordance with guidelines of good clinical practice. All patients gave informed consent for the study before enrollment.
Patient population
Inclusion criteria: Consecutive patients referred for EUS evaluation due to clinical suspicion of pancreatic or periampullary malignancies. Exclusion criteria included clinical conditions that precluded the use of DEFINITY™ and when the lesion could not be visualized by EUS. These exclusion criteria were: (1) known right-to-left, bi-directional, or transient right-to-left cardiac shunts; (2) worsening or clinically unstable congestive heart failure; (3) acute myocardial infarction or acute coronary syndromes; (4) serious ventricular arrhythmias or high risk for arrhythmias due to prolongation of the QT interval; (5) respiratory failure; (6) severe emphysema, pulmonary emboli or other conditions associated with pulmonary hypertension; (7) known hypersensitivity to DEFINITY™ or its components; (8) anatomical abnormalities or distortions precluding complete EUS examination of the pancreaticobiliary region.
Equipment and technique
The electronic radial echoendoscope GF-UE160-AL5 (Olympus, Tokyo, Japan) and Aloka Prosound α10 processor (Aloka, Tokyo, Japan) were used. CHEUS settings were: (1) ExPHD (THE): Extended Pure Harmonic Detection for Tissue Harmonic Echo; (2) ExPHD (CHE): Extended Pure Harmonic Detection for Contrast Harmonic Echo. ExPHD was specific to contrast enhanced harmonic ultrasonography which combined receiving frequencies of filtered fundamental and second harmonic components with transmitting frequency of 5 or 6 MHz. ExPHD (CHE) was more specific for contrast enhanced vascular imaging compared to ExPHD (THE). ExPHD (THE) provided better images of the entire anatomical structures of the pancreaticobiliary system, especially in areas far from the echoendoscope transducer. Once the focal lesion was identified, imaging was done with the ExPHD (CHE) mode because it accentuated the vascular enhancement after contrast injection. The mechanical index used for CHEUS was 0.3. The mechanical index (MI) represents the transmitted acoustic power of the ultrasound wave3. A MI of 0.3 was chosen based on personal experience and published data5 that a MI of 0.3 provided the optimal balance between contrast brightness and duration of effect of the contrast. We used intermittent mode for visualizing the pancreatic parenchyma and continuous mode for vessel assessment when needed. CE-EUS was performed using frequency of 5 MHz.
Patients were sedated using intravenous midazolam and fentanyl. A standard EUS examination of the pancreaticobiliary system was performed. The area of interest was re-evaluated using CHEUS after intravenous bolus injection of DEFINITY™ at a dose of 10 ul/kg body weight and the image immediately after enhancement was analyzed. EUSFNA was performed for focal lesions.
Statistical analysis
For this pilot study, a sample size of 25 was targeted. EUS and CHEUS were compared in terms of ability to clearly visualize a lesion, demarcation of margins, assessment of vascular invasion and prediction of malignancy. The assessment of differences between benign and malignant groups was based on overall impression before and after contrast injection. More vascular areas of the mass would light up from the contrast, whereas less vascular areas of the mass would not. The results were corroborated against reference standards of surgical histology when available, cytology from EUSFNA and clinical course. A positive diagnosis of malignancy was based on positive cytology or surgical histology. A negative diagnosis for malignancy was based on negative cytology with benign clinical course over 6 months follow up, or benign surgical histology. Categorical data were analyzed using chi-square or Fisher exact test, while continuous data were analyzed using student's t test. A p value of < 0.05 was taken as statistically significant.
Results
During the period from October 2009 to February 2011, 29 patients met study criteria and were recruited. The mean age was 62 years (range: 40 – 85 years) and 20/29 were males. The clinical presentations were jaundice (13/29), abdominal pain with weight loss (7/29), abdominal pain (3/29), weight loss (2/29) and incidental CT scan findings (4/29). The eventual clinical diagnoses were: pancreatic adenocarcinoma (16/29); non pancreatic malignancies with metastases to the pancreas (4/29), focal or chronic pancreatitis with inflammatory mass (4/29), normal pancreas with focal fat sparing (1/29), ampulla adenocarcinoma (2/29), serous cystic neoplasm (1/29) and peri-pancreatic head lymph node due to lymphoma (1/29). One patient with surgically confirmed chronic pancreatitis had extensive pancreatic parenchymal calcification that interfered with EUS examinations due to acoustic shadows from pancreatic calculi. This patient was excluded from comparative analysis of the EUS and CHEUS images, leaving 28 patients for analysis.
With EUS examination, the pancreatic and peri-ampullary lesions showed a hypoechoic appearance while the case of serous cystic neoplasm had a microcystic appearance. Compared to EUS, CHEUS enhanced the margins of the cases of pancreatic adenocarcinoma (Fig. 1A, 1B, 2A, 2B), metastases to pancreas, ampulla adenocarcinoma, serous cystic neoplasm and peripancreatic head lymph node due to lymphoma (Fig. 3A, 3B). Focal inflammatory masses due to pancreatitis and focal fat sparing that appeared hypoechoic on EUS exhibited diffuse enhancement with CHEUS (Fig. 4A, 4B), similar to the normal adjacent pancreatic parenchyma. In addition to enhancing the margins of tumors, with the use of CHEUS malignant pancreatic masses demonstrated hypoechoic inhomogeneous appearance with abnormal fine tumor vessels (Fig. 1B, 2B) while lesions due to pancreatitis or fat sparing were characterized by diffuse enhancement as mentioned earlier (p<0.001). The microcystic appearance of the serous cystic neoplasm was clearly seen with EUS but CHEUS enhanced the septa further. Among the 15 patients with pancreatic adenocarcinoma, 2 patients (13.3%) had upstaging of the T stage by CHEUS due to the detection of vascular invasion not seen on EUS. The results of T staging were concordant between EUS and CHEUS in the other cases.
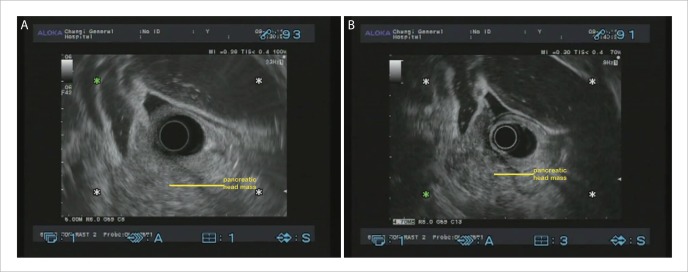
Figure 1.
A: EUS image of pancreatic head adenocarcinoma; B: CHEUS [ExPHD (THE)] image of pancreatic head adenocarcinoma showed distinct margins, hypoechoic inhomogeneous pattern and abnormal fine intra-tumoral vessels.
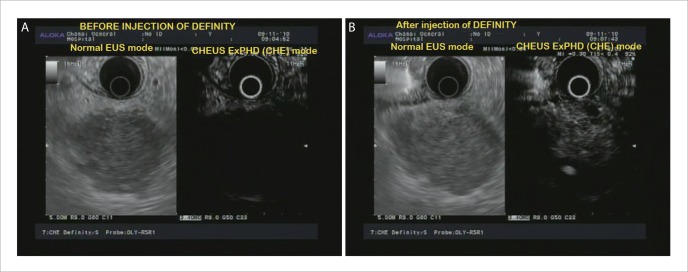
Figure 2.
A: EUS image of pancreatic body adenocarcinoma; B: CHEUS [ExPHD (CHE)] image of pancreatic body adenocarcinoma showed hypoechoic inhomogeneous pattern and abnormal fine intra-tumoral vessels.
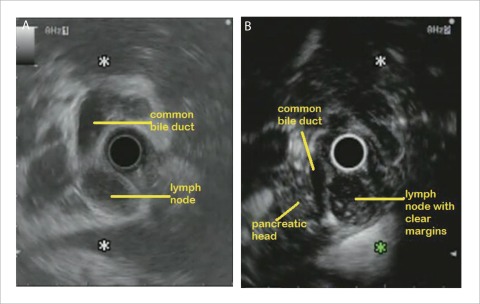
Figure 3.
A: EUS image of peri-pancreatic head lymph node due to lymphoma which was difficult to distinguish from adjacent pancreatic head; B: CHEUS [ExPHD (CHE)] image of peri-pancreatic head lymph node due to lymphoma showed clear margins.
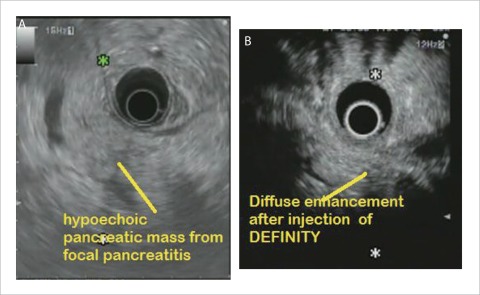
Figure 4.
A: EUS image of focal inflammatory mass due to pancreatitis; B: CHEUS [ExPHD (CHE)] image of focal inflammatory mass due to pancreatitis showed diffuse enhancement.
Discussion
Despite the excellent performance characteristics of EUS, differentiation between malignant and non-malignant pancreatic and peri-ampullary masses, and accurate assessment of T stage, remain a challenge. It may be argued that the use of EUSFNA will permit cytological or histological diagnosis but nonetheless, there are still concerns with regards to whether a negative EUSFNA result represents a false or true negative. Correct assessment of vascular invasion is crucial when considering surgical resectability and it may be difficult to distinguish between tumor abutting the vessel and early vascular invasion using EUS. This pilot study showed promising results for CHEUS using DEFINITY™. Tumor margins and vascular involvement could be seen clearly. In addition, the pattern of enhancement appeared different between malignant tumors and inflammatory masses, allowing differentiation between malignant and inflammatory masses.
The use of contrast agents in endosonography has lagged behind CT, MRI and transabdominal ultrasound. Intravascular contrast agents enhance vascular structures and now with the availability of both equipment and stable ultrasonic contrast agents, it is now possible to enhance EUS images. During CHEUS, vascular structures will become hyperechoic and thus be highlighted when the injected solution of microbubbles are hit by ultrasonic waves due to the vibration creating a strong backscattered acoustic shadow. In this way, the interface between a major vessel, the vascular wall and adjacent mass becomes clearer and the absence or presence of vascular invasion can be more clearly determined. The differences in the vascularity and microvascular pattern of different lesions will result in differences in CHEUS appearance thus facilitating diagnosis. A vascular lesion will be expected to enhance uniformly rapidly, whereas a hypovascular lesion, such as pancreatic adenocarcinoma, which is rather fibrotic, may be expected to enhance less, and even exhibit abnormal tumor vessels. Conversely, inflammatory masses are more hyperemic and hence would be expected to appear hyperechoic with CHEUS.
When this study was first started, published data were limited but very promising. Since 2010, additional data have been published but CHEUS is still not widely used. The contrast agents used included Sonazoid5, SonoVue4,9 and Levovist10,11 which are all second generation contrast agents that produce harmonic signals at lower acoustic power, making it suitable for EUS unlike first generation ultrasonic contrast agents. Data suggest that these agents could increase the accuracy of detection, differentiation and staging of pancreaticobiliary malignancies. Hocke et al performed CHEUS with SonoVue to investigate its role in distinguishing between chronic pancreatitis and pancreatic cancer based on perfusion characteristics of the microcirculation. The gold standard was histological diagnosis by EUSFNA or surgery. Compared to EUS, CHEUS increased the diagnostic sensitivity for pancreatic cancer from 79.3% to 91.7%, and increased the specificity for chronic pancreatitis from 82.2% to 95.9%9. Sakamoto et al used the sonographic contrast agent Levovist and found that CHEUS was significantly more sensitive than EUS and CT for differentiating ductal carcinomas from other tumours11. Kasono et al found that CHEUS with Levovist improved the preoperative localization of insulinomas12. Imazu et al used another sonographic contrast agent Sonazoid and found that the depth of invasion of biliary cancer and vascular invasion of pancreatic and biliary cancer could be demonstrated more clearly with CHEUS compared to EUS6. CHEUS with Sonazoid was found to be useful in differentiating pancreatic cancer from chronic pancreatitis in a more recent study7. Fusaroli et al examined 90 patients who were suspected of having pancreatic solid neoplasm using CHEUS with Sonovue. The final diagnosis was obtained based on results of surgical pathology and/or EUSFNA. It was found that a hypo-enhancing mass with an inhomogeneous pattern was a sensitive and accurate identifier of patients with adenocarcinoma (96% and 82% respectively) and this was more accurate than the finding of a hypoechoic lesion using EUS. In particular, hyper-enhancement specifically excluded adenocarcinoma (98%), although sensitivity was low (39%). These results were similar to our study. In addition, CHEUS allowed detection of small lesions in 7 patients who had uncertain standard EUS findings because of biliary stents or chronic pancreatitis12. Ishikawa et al recently evaluated the role of CHEUS in the differential diagnosis of malignant versus benign and preoperative localization of pancreatic endocrine tumors (PET). EUS showed high sensitivity (95.1%) in identifying PETs compared with multi-detector CT (80.6%) and transabdominal ultrasound (45.2%). Multivariable logistic regression analysis showed that heterogeneous ultrasonographic texture was the most significant factor for malignancy13. Recently a new linear echoendoscope with capability for CHEUS has been developed and preliminary data showed it to be useful in distinguishing adenocarcinoma from other pancreatic masses based on microvascular patterns.14,15 Compared with the pre-existent technique of performing CHEUS using an electronic radial echoendoscope, it allows the possibility of diagnostic evaluation with CHEUS and tissue acquisition by EUSFNA simultaneously, without a need to change echoendoscopes.
The focus of our study was on the role of CHEUS in the evaluation of pancreatic and peri-ampullary masses but another possible indication for CHEUS has emerged. Sakamoto et al evaluated gastrointestinal stromal tumors (GIST) using CHEUS and found that the visualization of intra-tumoral vessels with CHEUS could predict the malignant potential with sensitivity, specificity and accuracy of 100%, 63% and 83% respectively, compared to EUSFNA which had sensitivity, specificity and accuracy of 63%, 92% and 81% respectively.16
We acknowledge the limitations of our study. This was single center, and all examinations were performed by a single expert endosonographer (ATL). The patient population was small, and there was no randomized comparison with other imaging modalities or contrast agents. Nonetheless, all patients had undergone prior CT and conventional EUS, and CHEUS, a targeted examination technique, did provide additional information over CT and EUS. Although surgical pathology was not available in all cases, the case definition for benign and malignant disease should address it.
To conclude, CHEUS using DEFINITY™ was useful for evaluating suspected pancreatic and peri-ampullary malignancies. It improved visualization of tumor margins and vascular invasion, and demonstrated a hypoechoic inhomogeneous pattern and fine abnormal vessels in malignancies and diffuse enhancement in benign focal lesions. CHEUS, once routinely available, should become part of a standard EUS examination, similar to EUSFNA, when clinically indicated.
Acknowledgements
This study was supported by a research grant from the National Medical Research Council, Ministry of Health, Singapore.
Abbreviations
- CHEUS
contrast harmonic endosonography
- CT
computer tomography
- MRI
magnetic resonance imaging
- EUS
endoscopic ultrasound
- FNA
fine needle aspiration
Footnotes
Previously published online: www.landesbioscience.com/journals/jig
References
- 1.Gan SI, Rajan E, Adler DG, et al. Role of EUS. Gastrointest Endosc. 2007;66:425–434. doi: 10.1016/j.gie.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Molins IG, Font JM, Alvaro JC, Navarro JL, Gil MF, Rodríguez CM. Contrast-enhanced ultrasound in diagnosis and characterization of focal hepatic lesions. World J Radiol. 2010;2:455–462. doi: 10.4329/wjr.v2.i12.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez MV, Varadarajulu S, Napoleon B. EUS contrast agents: what is available, how do they work, and are they effective? Gastrointest Endosc. 2009;69:S71–S77. doi: 10.1016/j.gie.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Kitano M, Sakamoto H, Matsui U, Ito Y, Maekawa K, von Schrenck T, et al. A novel perfusion imaging technique of the pancreas: contrast-enhanced harmonic EUS (with video) Gastrointest Endosc. 2008;67:141–150. doi: 10.1016/j.gie.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 5.Imazu H, Uchiyama Y, Matsunaga K, Ikeda K, Kakutani H, Sasaki Y, et al. Contrast-enhanced harmonic EUS with novel ultrasonographic contrast (Sonazoid) in the preoperative T-staging for pancreaticobiliary malignancies. Scand J Gastroenterol. 2010;45:732–738. doi: 10.3109/00365521003690269. [DOI] [PubMed] [Google Scholar]
- 6.Imazu H, Uchiyama Y, Ikeda K, Ang TL, Tajiri H. Novel quantitative perfusion analysis with contrast-enhanced harmonic EUS facilitates distinguishing chronic pancreatitis from pancreatic cancer. Gut. 2010;(suppl):A69. [Google Scholar]
- 7.Wei K, Mulvagh SL, Carson L, Davidoff R, Gabriel R, Grimm RA, et al. The safety of definity and optison for ultrasound image enhancement: a retrospective analysis of 78,383 administered contrast doses. J Am Soc Echocardiogr. 2008;21:1202–1206. doi: 10.1016/j.echo.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Dolan MS, Gala SS, Dodla S, Abdelmoneim SS, Xie F, Cloutier D, et al. Safety and efficacy of commercially available ultrasound contrast agents for rest and stress echocardiography a multicenter experience. J Am Coll Cardiol. 2009;53:32–38. doi: 10.1016/j.jacc.2008.08.066. [DOI] [PubMed] [Google Scholar]
- 9.Hocke M, Schulze E, Gottschalk P, Topalidis T, Dietrich CF. Contrast-enhanced endoscopic ultrasound in discrimination between focal pancreatitis and pancreatic cancer. World J Gastroenterol. 2006;12:246–250. doi: 10.3748/wjg.v12.i2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakamoto H, Kitano M, Suetomi Y, Maekawa K, Takeyama Y, Kudo M. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound Med Biol. 2008;34:525–532. doi: 10.1016/j.ultrasmedbio.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Kasono K, Hyodo T, Suminaga Y, Sugiura Y, Namai K, Ikoma A, et al. Contrast-enhanced endoscopic ultrasonography improves the preoperative localization of insulinomas. Endocr J. 2002;49:517–522. doi: 10.1507/endocrj.49.517. [DOI] [PubMed] [Google Scholar]
- 12.Fusaroli P, Spada A, Mancino MG, Caletti G. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin Gastroenterol Hepatol. 2010;8:629–634. doi: 10.1016/j.cgh.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa T, Itoh A, Kawashima H, Ohno E, Matsubara H, Itoh Y, et al. Usefulness of EUS combined with contrast-enhancement in the differential diagnosis of malignant versus benign and preoperative localization of pancreatic endocrine tumors. Gastrointest Endosc. 2010;71:951–959. doi: 10.1016/j.gie.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Romagnuolo J, Hoffman B, Vela S, Hawes R, Vignesh S. Accuracy of contrast-enhanced harmonic EUS with a second-generation perflutren lipid microsphere contrast agent (with video) Gastrointest Endosc. 2011;73:52–63. doi: 10.1016/j.gie.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Napoleon B, Alvarez-Sanchez MV, Gincoul R, Pujol B, Lefort C, Lepilliez V, et al. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: results of a pilot study. Endoscopy. 2010;42:564–570. doi: 10.1055/s-0030-1255537. [DOI] [PubMed] [Google Scholar]
- 16.Sakamoto H, Kitano M, Matsui S, Kamata K, Komaki T, Imai H, et al. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos) Gastrointest Endosc. 2011;73:227–237. doi: 10.1016/j.gie.2010.10.011. [DOI] [PubMed] [Google Scholar]