Abstract
Background
Probe-based confocal laser endomicroscopy (pCLE) is an emerging method for in-vivo imaging of the gastrointestinal tract and requires a contrast agent. Fluorescein is the most commonly used agent. The optimal dose of fluorescein for pCLE in colon is unknown.
Objective
Exploration of optimal dose of fluorescein for pCLE in colon.
Design
Comparative, prospective pilot trail.
Setting
Tertiary-care center.
Patients
18 participants underwent colonoscopy without complications.
Interventions
pCLE videos were recorded in normal cecum, using 10% fluorescein intravenously.
Main Outcome Measurements
For subjective analysis, pCLE videos were scored for quality, by 2 observers, independently and blinded to fluorescein dose. For objective analysis, signal-to-noise ratios (SNR) were calculated for each video by an expert.
Results
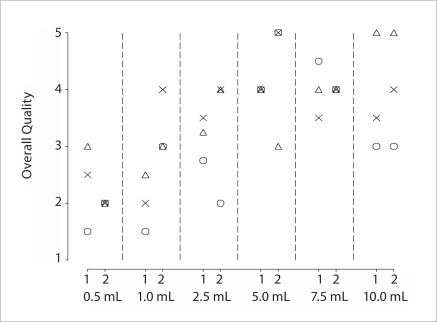
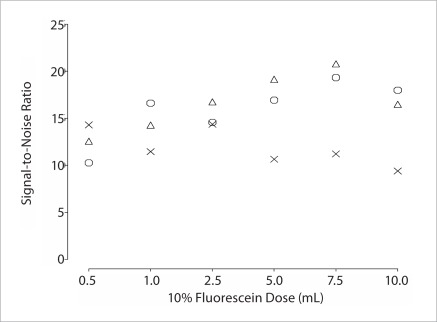
6 fluorescein doses were used, including 0.5 mL, 1 mL, 2.5 mL, 5 mL, 7.5 mL and 10 mL and each dose was used in three patients. For each dose, median image quality score was 2.5, 2.0, 3.25, 4.0, 4.0 and 3.5 by first observer and 2.0, 3.0, 4.0, 5.0, 4.0 and 4.0 by second observer, respectively. The subjective quality scores increased from 0.5 mL to 5.0 mL, with no evidence of further improved quality at 7.5 mL and 10 mL doses. SNR were not significantly different between doses but trended higher for higher doses.
Limitations
Small sample size. The results can not be applied to other parts of gastrointestinal tract i.e. duodenum, esophagus with different blood supply.
Conclusion
This preliminary study suggests that the optimal dose of fluorescein for high quality pCLE imaging in colon is approximately 5.0 mL.
Key words: confocal, colon, fluorescein dose, contrast
Introduction
Confocal laser endomicroscopy (CLE) is a new method for gastrointestinal endoscopy that allows cross-sectional imaging of epithelium at resolution of approximately 1 micron. It is capable of in-vivo visualization of cellular and sub-cellular structures as well as capillaries and red blood cells, and in the case of pCLE, video imaging of blood flow at a capillary level. Clinically this has the potential to facilitate true in-vivo “virtual biopsy.” allowing real-time guidance of endoscopic therapy, as well as reducing the need for random biopsy.1 The various potential applications of this technology include detection of neoplasia in colon1–3, duodenum4 and Barrett's esophagus5 allowing improved targeting of biopsies, avoidance of random biopsy by confirmation of non-diseased tissue, reducing the need for histopathological confirmation of small non-advanced colon polyps, and detection of inflammatory bowel disease, celiac sprue, and microscopic colitis.
Currently, there are two systems for CLE; one which is integrated into an endoscope (eCLE, Pentax corporation, Ft Wayne, NJ), and second that is integrated into a small caliber probe that can be passed through the accessory channel of a standard endoscope (pCLE, Mauna Kea Technologies, Paris, France). Both systems are optimized with the use of a contrast agent. The most common contrast agents currently being studied or used in clinical practice include fluorescein sodium and acriflavine hydrochloride. Acriflavine can only be used topically (0.05% in saline) but is not approved for use in humans due to a small probability of mutagenic potential.6 The fluorescein sodium is a Food and Drug Administration (FDA) class IIa drug which has been approved by the FDA for ophthalmic angiography or angioscopy of the retina and iris vasculature in conjunction with a confocal scanning laser ophthalmoscope. It is a useful diagnostic technique for anatomic and physiologic evaluation of ocular structures.7–11 The standard adult dose of Fluorescite® (fluorescein injection, USP) 10% containing fluorescein sodium, used in ophthalmic practice, is 500 mg (100 mg/mL) via intravenous administration.with rapid onset of action within 6 to 15 second.7,8 Fluorescein undergoes rapid metabolism to fluorescein monoglucuronide in liver.12 The fluorescein monoglucuronide has fluorescent properties and contributes about 20% of fluorescence as compared to unbound fluorescein.12 Fluorescein and its metabolites are mainly eliminated via renal excretion. After IV administration, the urine remains slightly fluorescent for 24 to 36 hours. A renal clearance of 1.75 mL/min/kg and a hepatic clearance (due to conjugation) of 1.50 mL/min/kg have been estimated. The systemic clearance of fluorescein was essentially complete by 48 to 72 hours after administration of 500 mg fluorescein.
The safety of fluorescein sodium in gastrointestinal CLE has been investigated recently. A large multi-center study led by our group including 2272 patients (excluding pregnant and breast feeding females), evaluated the safety of intravenous use of 2.5 mL to 5 mL of 10% fluorescein sodium. This study showed very low rates of mild (1.4%) and serious (0%) side effects during immediate post procedure period.13 In addition to its good safety profile and rare side effects, fluorescein is inexpensive, easy to use, and has excellent fluorescent properties.
The intravenous dose of fluorescein sodium, which has been used for colorectal CLE, is in the range of 0.5 mL to 10 mL of 10% fluorescein in the US and 0.5 mL to 10 mL of 1% fluorescein in various centers around the world.1,5,14 However, the optimal dose of the fluorescein for colorectal CLE use is unknown.
We conducted the current study as a preliminary exploration of the effectiveness of a range of doses of 10% fluorescein in a small number of patients. We included the range of fluorescein doses currently used in clinical/research procedures such as ophthalmologic angioscopy, and gastrointestinal CLE in various international research or clinical centers. Furthermore, this study attempted to explore the optimal fluorescein dose needed for imaging in colon only. Both objective and subjective measures of quality were used in examining confocal images in the colon (cecum) of human subjects.
Material and methods
Patients
Participants in this study were patients undergoing surveillance or screening colonoscopy. The study was approved by Mayo Clinic Institutional Review Board and all participants provided informed consent. Exclusion criteria were patients with non-corrected coagulopathy, women suspected of being pregnant or breast feeding, documented allergy to fluorescein, and patients with no colorectal lesions found during a study colonoscopy. Twenty four hours prior to the procedure patients were prepped satisfactorily with two to four liters of polyethylene glycol solution. Conscious sedation was performed with intravenous administration of midazolam, meperidine and/or fentanyl or with propofol.
Endoscopy equipment and procedure
All procedures and examinations were performed by two experts in advanced endoscopic imaging methods and pCLE (MBW, AMB). Each procedure was performed using a high-definition colonoscope (Olympus CFH180, Olympus, Center Valley, NY). A 4 mm transparent cap was attached to the tip of the endoscope to stabilize pCLE probe on the mucosa.
The pCLE system consists of three items. The first part of the system was a confocal high resolution probe (ColoFlex, type UHD Confocal Miniprobes, Cellvizio®-GI, Mauna Kea Technologies, Paris, France) made of 30000 optical fibers bundled together. The external diameter of probe is 2.5 mm and is compatible with the accessory channel of any standard endoscope. The second part of the system is a proximal laser scanning unit (LSU, Cellvizio-GI, Mauna Kea Technologies, Paris, France) that combines laser light illumination and rapid laser scanning providing a frame rate of 12 images per second signal detection. The third part of the system is a control and acquisition software for real time image reconstruction; immediate sequences display and post procedure analysis with editing tools available (Cellvizio Software, Mauna Kea Technologies, Paris, France). The images obtained have a lateral resolution of 1.0 µm, axial resolution of 5.0 µm and a total field of view of 240 µm in diameter.
During each procedure, the colonoscope was advanced from anal verge to cecum which was recognized by the presence of illeocecal valve and appendiceal orifice. The confocal miniprobe was passed through the scope and gently placed at the randomly selected normal appearing spot in the cecum after overlying mucosal secretions were cleared with sterile water lavage. The single dose of fluorescein was administered by rapid I.V. push and, simultaneously, recording of confocal images was started. The confocal sequences were recorded for at least 2 minutes while keeping the probe at the same point. Although pCLE imaging is possible for up to 15 minutes after fluorescein injection (elimination half life of fluorescein is 24 minutes), we did not attempt to standardize the analysis of image quality/SNR as a function of time. In our clinical experience we have previously observed that, stable good quality images can be obtained within first two to three minutes of injection of fluorescein dose. Time dependant studies with pCLE have also shown that optimal images are obtained beginning at 30 seconds to 8 minutes after injection.15 The different doses which were evaluated for image quality included 0.5 mL, 1 mL, 2.5 mL, 5 mL, 7.5 mL and 10 mL. Each dose was tested on three patients and each patient received only one dose of fluorescein during this portion of the study. Following the imaging with each dose above, for patients who received < 5 mL, we completed the injection up to our standard dose of 5 mL to 10 mL while imaging polyps. Only the normal mucosal imaging at the starting dose was analyzed as part of this study.
Hemodynamic parameters and overall side effects were monitored continuously during the whole procedure and for up to 3 hours afterwards until the patient was awake, alert, and fit for discharge from the endoscopy laboratory.
Subjective analysis
Each video sequence, at least two minutes duration, was assessed for quality from beginning to end. Before subjective assessment, all eighteen video sequences were randomized irrespective of the subject or fluorescein dose. Each sequence was viewed in its original format (.mkt, proprietary format, Mauna Kea Technologies) and quality analysis was performed after the completion of acquisition, in “offline” fashion, by two observers (MW, AM). The observers were blinded to the dose of fluorescein and each other's assessment. Each video clip was scored on a scale of 1 to 5 (1 being worst, 3 acceptable and 5 being equal to the histology) by each observer.
Objective analysis
The objective analysis of the pCLE video sequences was performed by calculating and using the signal-to-noise ratio (SNR) as an objective contrast parameter. It is an electrical engineering term, defined as the ratio of signal power to the noise power corrupting the signal. A ratio higher than 1:1 indicates more signal than noise. In less technical terms, signal-to-noise ratio compares the level of a desired signal to the level of background noise. The higher the ratio, the less obtrusive the background noise is.
The standard method to evaluate the signal-to-noise ratio is by processing images acquired on a medium with a constant signal.16 For CLE signal-to-noise ratio, we chose areas on tissue image that had a very high probability of having homogeneous signal and computed the signal and noise measurements only on these areas. This method is based on the assumption of a constant signal over the area; which was confirmed by visual inspection of the video image.
Each two minutes video sequence was de-identified and analyzed for the signal-to-noise ratio (SNR) in a blinded, randomized fashion to actual dose of fluorescein used and subjective analysis of the observers. Each sequence was comprised of repeated still frames acquired at 12 frames/second. For evaluation of SNR, each frame or image was divided into 32−32 pixels square blocks. The pixel is the smallest addressable screen element; it is the smallest unit of picture that can be controlled. The location of a pixel corresponds to its coordinates. Each homogenous square block of pixels corresponds to tissue areas where fluorescein had constant concentration and was used to calculate the SNR. The SNR, here, was the ratio between the mean pixel value and the standard deviation of the pixel values in a homogenous square block of pixels. It was calculated mathematically using the following formula.
SNR = Signal Power / Noise Power
Where,
Signal Power = Sum over all pixels of the area of the intensities
Noise Power = Standard deviation of the intensities of all pixel over the area.
Each single SNR value was then used to calculate the average of all measurements to represent accurately the quality of the whole sequence.
Statistical considerations
As an exploratory study, the sample size of 3 patients per dose was decided upon as the minimum to obtain initial estimates of image quality at difference doses. The formal statistical tests were not performed; however, both SNRs and observer quality ratings were described as means, medians, and standard errors and displayed in tabular and graphical format. The purpose was to provide sufficiently robust estimates in order to plan larger more definitive studies, and to refine the selection of dose ranges for such studies.
Results
General characteristics of patients
Between September 2008 and June 2009 eighteen patients were enrolled in the study. Among 18 participants, there were 8 males and 10 females. The median age at the time of colonoscopy was 70 years (range 43–87). One patient was African American; all others were Caucasians. None of the patients experienced any endoscopic complications or adverse reaction to sodium fluorescein with the exception of transient yellow discoloration of the skin and urine which resolved by the time of discharge from the recovery room (skin) or within 24 hours (urine).
Subjective assessment of the image quality
The ratings of the two observers are shown on Figure 1. This figure indicates that although the two raters assigned variable quality scores to the same pCLE video, that differed by up to 2 points on the 1 to 5 scale, there is a clear overall upward trend in quality scores from 0.5 mL to 5 mL with scores remaining relatively stable for higher doses of fluorescein. Across the two raters, quality scores at the three lower doses ranged from 1.5 to 4 with a median of 2.6; scores at the higher three doses ranged from 3 to 5 with a median of 4. As a reference, a score of 5 was to be assigned when the quality was considered to be equal to that of histology, and a score of 3 represented quality that was considered adequate for interpretation. The Figure 2 shows the confocal images of the colonic mucosa at 6 respective doses of 10% fluorescein sodium.
Figure 1.
The subjective quality ratings by the two observers: The 1–5 quality score is such that: 1=poor, 3=adequate, 5=equivalent to histology. At each dose, each patient has the same symbol for the two raters, and the same symbol is used for the same patient in Figure 2.
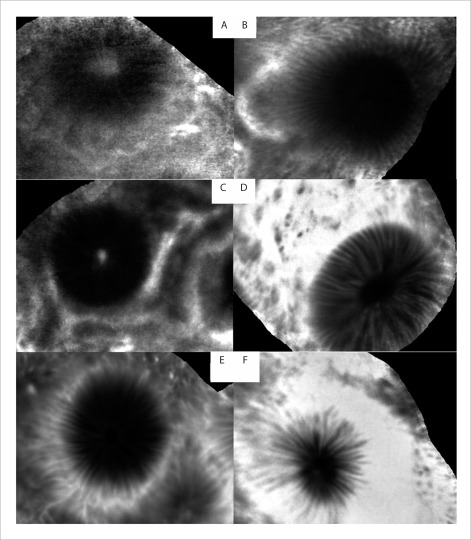
Figure 2.
The pCLE images (after mosaicing) of normal cecum obtained using various doses of 10% IV fluorescein. A: 0.5 mL; B: 1 mL; C: 2.5 mL; D: 5 mL; E: 7.5 mL; F: 10mL.
Objective evaluation - signal-to-noise ratio (SNR)
A total of 24892 images from the video sequences of the 18 patients were analyzed. The SNRs were calculated for all study patients and are shown in Figure 3. Although there were some low SNRs at the three higher doses that might affect the credibility of SNR as an objective measure of image quality with fluorescein dose, SNRs tend to increase from the 0.5 mL to 7.5 mL dose; showing a clear upward trend overall.
Figure 3.
Signal to noise ratio calculated on the confocal images when three patients each were assigned to six different doses of fluorescein. At each dose, each patient has the same symbol as in Figure 1.
Discussion
The data from this preliminary study suggests that the optimal dose range for gastrointestinal CLE is approximately 5 mL of 10% sodium fluorescein, with lower doses resulting in lower quality and higher doses yielding no further gain in quality. At the lower doses of fluorescein the images had less contrast and increased background noise.
Intravenous fluorescein has been used extensively in humans for ophthalmologic angiography. Safety and efficacy are well established for its use in ophthalmologic angiography. In a dose ranging study of 150 patients undergoing ophthalmoscopy with the same formulation of 10% I.V. fluorescein at a 2 mL and 5 mL dose,17 superior image quality was observed at the higher (5 mL) dose, although side effects (mild nausea and vomiting) were reported in 2 patients at the higher dose. In the ophthalmologic literature, mild nausea and vomiting occurred in up to 2–10% of individuals.18,19 Severe adverse reactions such as cardiovascular, respiratory, or neurologic compromise were reported in 0.05% of individuals. In another study, the frequency rate for a moderate reaction was 1.6%, for a severe reaction 0.05%, and for death 0.0005% (1 in 222000).20 In a recent study of more than 11000 patients undergoing fluorescein ophthalmologic angiography, minor adverse events were reported in 1.1% and none had severe adverse events.21
Confocal laser endomicroscopy is a new imaging technique and currently, is being evaluated for its application in diagnosis of mucosal diseases of various organs. Recent studies suggest that CLE is can be used for diagnosis of neoplasia in Barrett's esophagus5,22–26, colon polyps1,3,27–30, colitis associated neoplasia31–34, and even inflammatory conditions such as H. pylori35, and celiac sprue36–38. However, the optimal dose of fluorescein to be used during confocal laser endomicroscopy in gastroenterology has not been determined. This is the first prospective study, to our knowledge, to assess the optimal dose of fluorescein in human subjects for gastrointestinal CLE.
Limitations of this study include the small size of patient population enrolled for each dose of 10% fluorescein. However this was a preliminary study and further larger studies could now be conducted that focus on doses in the vicinity of 5 mL. In addition, our study was volume based dosing and was not based on the weight of the patients. Although this is standard for adult patients, weight-based dosing may allow further refinement in dose optimization.
The optimal fluorescein dose for pCLE images may vary from one luminal organ of GI to another as the blood supply, type of epithelial lining, mucosal thickness and permeability of each part of GI is different. These factors can affect the distribution of fluorescein and resultant fluorescence released at the site of CLE imaging. For example the duodenum, being more vascular, may require lower dose of fluorescein for CLE imaging than esophagus, which is lined with tightly adherent squamous cells. As our study primarily attempted to explore optimal fluorescein dose during colorectal CLE, the results might not be applicable to other areas of gastrointestinal tract.
By exploring the effect of fluorescein dose on both of objective and subjective measures of image quality, we have taken a first step in attempting to standardize an important aspect of the protocol for confocal endomicroscopy image acquisition. Ultimately studies of this kind may lead to an improvement in the in-vivo diagnostic accuracy and facilitate real-time in vivo interpretation of histology.
Abbreviations
- pCLE
Probe-based confocal laser endomicroscopy
- SNR
Signal to Noise Ratios
- CLE
Confocal Laser Endomicroscopy
- eCLE
Confocla laser endomicroscopy integrated into an endoscope
- FDA
Food and Drug Administration
- LSU
Laser Scanning Unit
Footnotes
Previously published online: www.landesbioscience.com/journals/jig
Disclosures
Dr. Wallace received research funding from Mauna Kea Technologies to support this and other related studies.
Dr. Perchant is an employee of Mauna Kea Technologies and an expert in image analysis systems. He performed the signal to noise ratio evaluation for the study.
References
- 1.Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706–713. doi: 10.1053/j.gastro.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 2.Meining A, Saur D, Bajbouj M, Becker V, Peltier E, Höfler H, et al. In vivo histopathology for detection of gastrointestinal neoplasia with a portable, confocal miniprobe: an examiner blinded analysis. Clin Gastroenterol Hepatol. 2007;5:1261–1267. doi: 10.1016/j.cgh.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Buchner AM, Shahid MW, Heckman MG, Krishna M, Ghabril M, Hasan M, et al. Comparison of probe-based confocal laser endomicroscopy with virtual chromoendoscopy for classification of colon polyps. Gastroenterology. 2010;138:834–842. doi: 10.1053/j.gastro.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 4.Shahid MW, Buchner AM, Hasan MK, Gomez V, Wallace MB. The role of probe-based confocal laser endomicroscopy (pCLE) in detection of dysplasia in duodenal polyps. Gastrointest Endosc. 2009;69:AB369. [Google Scholar]
- 5.Wallace MB, Sharma P, Lightdale C, Wolfsen H, Coron E, Buchner A, et al. Preliminary accuracy and interobserver agreement for the detection of intraepithelial neoplasia in Barrett's esophagus with probe-based confocal laser endomicroscopy. Gastrointest Endosc. 2010;72:19–24. doi: 10.1016/j.gie.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwamoto Y, Itoyama T, Yasuda K, Morita T, Shimizu T, Masuzawa T, et al. Photodynamic DNA strand breaking activities of acridine compounds. Biol Pharm Bull. 1993;16:1244–1247. doi: 10.1248/bpb.16.1244. [DOI] [PubMed] [Google Scholar]
- 7.Anand R. Fluorescein angiography. Part 2: Clinical applications. J Ophthalmic Nurs Technol. 1989;8:102–107. [PubMed] [Google Scholar]
- 8.Anand R. Fluorescein angiography. Part 1: Technique and normal study. J Ophthalmic Nurs Technol. 1989;8:48–52. [PubMed] [Google Scholar]
- 9.Wykes WN, Livesey SJ. Review of fluorescein angiograms performed in one year. Br J Ophthalmol. 1991;75:398–400. doi: 10.1136/bjo.75.7.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipson BK, Yannuzzi LA. Complications of intravenous fluorescein injections. Int Ophthalmol Clin. 1989;29:200–205. doi: 10.1097/00004397-198902930-00011. [DOI] [PubMed] [Google Scholar]
- 11.Holz FG, Bellmann C, Rohrschneider K, Burk RO, Völcker HE. Simultaneous confocal scanning laser fluorescein and indocyanine green angiography. Am J Ophthalmol. 1998;125:227–236. doi: 10.1016/s0002-9394(99)80095-6. [DOI] [PubMed] [Google Scholar]
- 12.Chahal PS, Neal MJ, Kohner EM. Metabolism of fluorescein after intravenous administration. Invest Ophthalmol Vis Sci. 1985;26:764–768. [PubMed] [Google Scholar]
- 13.Wallace MB, Meining A, Canto MI, Fockens P, Miehlke S, Roesch T, et al. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment Pharmacol Ther. 2010;31:548–552. doi: 10.1111/j.1365-2036.2009.04207.x. [DOI] [PubMed] [Google Scholar]
- 14.Becker V, Vercauteren T, von Weyhern CH, Prinz C, Schmid RM, Meining A. High-resolution miniprobe-based confocal microscopy in combination with video mosaicing (with video) Gastrointest Endosc. 2007;66:1001–1007. doi: 10.1016/j.gie.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Becker V, von Delius S, Bajbouj M, Karagianni A, Schmid RM, Meining A. Intravenous application of fluorescein for confocal laser scanning microscopy: evaluation of contrast dynamics and image quality with increasing injection-to-imaging time. Gastrointest Endosc. 2008;68:319–323. doi: 10.1016/j.gie.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez RC, Woods RE. Digital image processing. 3rd ed. Upper Saddle River, NJ: Pearson/Prentice Hall; 2008. p. 954. [Google Scholar]
- 17.Moosbrugger KA, Sheidow TG. Evaluation of the side effects and image quality during fluorescein angiography comparing 2 mL and 5 mL sodium fluorescein. Can J Ophthalmol. 2008;43:571–575. doi: 10.3129/i08-122. [DOI] [PubMed] [Google Scholar]
- 18.Jennings BJ, Mathews DE. Adverse reactions during retinal fluorescein angiography. J Am Optom Assoc. 1994;65:465–471. [PubMed] [Google Scholar]
- 19.Lopez-Saez MP, Ordoqui E, Tornero P, Baeza A, Sainza T, Zubeldia JM, et al. Fluorescein-induced allergic reaction. Ann Allergy Asthma Immunol. 1998;81:428–430. doi: 10.1016/S1081-1206(10)63140-7. [DOI] [PubMed] [Google Scholar]
- 20.Yannuzzi LA, Rohrer KT, Tindel LJ, Sobel RS, Costanza MA, Shields W, et al. Fluorescein angiography complication survey. Ophthalmology. 1986;93:611–617. doi: 10.1016/s0161-6420(86)33697-2. [DOI] [PubMed] [Google Scholar]
- 21.Kwan AS, Barry C, McAllister IL, Constable I. Fluorescein angiography and adverse drug reactions revisited: the Lions Eye experience. Clin Experiment Ophthalmol. 2006;34:33–38. doi: 10.1111/j.1442-9071.2006.01136.x. [DOI] [PubMed] [Google Scholar]
- 22.Kiesslich R, Gossner L, Goetz M, Dahlmann A, Vieth M, Stolte M, et al. In vivo histology of Barrett's esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2006;4:979–987. doi: 10.1016/j.cgh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Kaise M, Kato M, Urashima M, Arai Y, Kaneyama H, Kanzazawa Y, et al. Magnifying endoscopy combined with narrow-band imaging for differential diagnosis of superficial depressed gastric lesions. Endoscopy. 2009;41:310–315. doi: 10.1055/s-0028-1119639. [DOI] [PubMed] [Google Scholar]
- 24.Pohl H, Rosch T, Vieth M, Koch M, Becker V, Anders M, et al. Miniprobe confocal laser microscopy for the detection of invisible neoplasia in patients with Barrett's oesophagus. Gut. 2008;57:1648–1653. doi: 10.1136/gut.2008.157461. [DOI] [PubMed] [Google Scholar]
- 25.Miehlke S, Morgner A, Aust D, Madisch A, Vieth M, Baretton G. Combined use of narrow-band imaging magnification endoscopy and miniprobe confocal laser microscopy in neoplastic Barrett's esophagus. Endoscopy. 2007;39:E316. doi: 10.1055/s-2007-966797. [DOI] [PubMed] [Google Scholar]
- 26.Becker V, Vieth M, Bajbouj M, Schmid RM, Meining A. Confocal laser scanning fluorescence microscopy for in vivo determination of microvessel density in Barrett's esophagus. Endoscopy. 2008;40:888–891. doi: 10.1055/s-2008-1077718. [DOI] [PubMed] [Google Scholar]
- 27.Hurlstone DP, Baraza W, Brown S, Thomson M, Tiffin N, Cross SS. In vivo real-time confocal laser scanning endomicroscopic colonoscopy for the detection and characterization of colorectal neoplasia. Br J Surg. 2008;95:636–645. doi: 10.1002/bjs.5988. [DOI] [PubMed] [Google Scholar]
- 28.Buchner AM, Shahid MW, Wallace MB. The learning curve of probe based confocal laser endomicroscopy (pCLE) for detection neoplasia in colon polyps. Gastroenterology. 2010;138:S95. doi: 10.1053/j.gastro.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 29.Buchner AM, Shahid MW, Raimondo M, Woodward TA, Wallace MB. The role of a high definition, probe based confocal laser endomicroscopy (pCLE) system) in diagnosing smaller indeterminate colorectal polyps in vivo. Gastroenterology. 2010;138:S114. [Google Scholar]
- 30.Buchner AM, Shahid MW, Dekker E, Gomez V, Fockens P. Accuracy of probe based confocal laser endomicroscopy (pCLE) in predicting recurrence of colorectal neoplasia after endoscopic mucosal resection. Gastrointest Endosc. 2010;71:AB189. [Google Scholar]
- 31.Watanabe O, Ando T, Maeda O, Hasegawa M, Ishikawa D, Ishiguro K, et al. Confocal endomicroscopy in patients with ulcerative colitis. J Gastroenterol Hepatol. 2008;23:S286–S290. doi: 10.1111/j.1440-1746.2008.05559.x. [DOI] [PubMed] [Google Scholar]
- 32.Kiesslich R, Goetz M, Lammersdorf K, Schneider C, Burg J, Stolte M, et al. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology. 2007;132:874–882. doi: 10.1053/j.gastro.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 33.Kiesslich R, Hoffman A, Goetz M, Biesterfeld S, Vieth M, Galle PR, et al. In vivo diagnosis of collagenous colitis by confocal endomicroscopy. Gut. 2006;55:591–592. doi: 10.1136/gut.2005.084970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurlstone DP, Thomson M, Brown S, Tiffin N, Cross SS, Hunter MD. Confocal endomicroscopy in ulcerative colitis: differentiating dysplasia-associated lesional mass and adenoma-like mass. Clin Gastroenterol Hepatol. 2007;5:1235–1241. doi: 10.1016/j.cgh.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Kiesslich R, Goetz M, Burg J, Stolte M, Siegel E, Maeurer MJ, et al. Diagnosing Helicobacter pylori in vivo by confocal laser endoscopy. Gastroenterology. 2005;128:2119–2123. doi: 10.1053/j.gastro.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 36.Trovato C, Sonzogni A, Ravizza D, Fiori G, Rossi M, Tamayo D, et al. Celiac disease: in vivo diagnosis by confocal endomicroscopy. Gastrointest Endosc. 2007;65:1096–1099. doi: 10.1016/j.gie.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 37.Zambelli A, Villanacci V, Buscarini E, Lupinacci G, De Grazia F, Brambilla G, et al. Confocal laser endomicroscopy in celiac disease: description of findings in two cases. Endoscopy. 2007;39:1018–1020. doi: 10.1055/s-2007-966783. [DOI] [PubMed] [Google Scholar]
- 38.Leong RW, Nguyen NQ, Meredith CG, Al-Sohaily S, Kukic D, Delaney PM, et al. In vivo confocal endomicroscopy in the diagnosis and evaluation of celiac disease. Gastroenterology. 2008;135:1870–1876. doi: 10.1053/j.gastro.2008.08.054. [DOI] [PubMed] [Google Scholar]