Abstract
Autoimmune pancreatitis (AIP) is a rare disorder frequently manifesting as a mass-like lesion that may lead to obstructive jaundice. We report here a case of pancreatic obstruction with painless jaundice, and elevation of CA 19-9 without elevation of serum IgG4. Contrast enhanced ultrasonography (CE US) revealed the possibility of AIP, and the final pathological findings confirmed the diagnosis.
Key words: AIP, CEUS, IgG4, diagnosis, case report
Introduction
Autoimmune pancreatitis (AIP) is a rare, but increasingly recognized disorder. However, distinguishing AIP from pancreatic carcinoma is challenging or even impossible when the disease manifests as a mass-like lesion that may lead to obstructive jaundice.1 Contrast enhanced ultrasonography (CEUS), with real-time continuous visualization of blood perfusion of the pancreas and its masses, has recently been used in the evaluation and diagnosis of solid pancreatic lesions.2
Case report
A 40 year-old man with a history of diabetes mellitus presented with a 2 week history of painless jaundice. He had no history of alcohol use or abdominal symptoms related to other pancreatic disease. The work-up revealed the following: total bilirubin, 117.8 µmol/l (2–18 µmol/l) with 87.3 µmol/L of direct bilirubin (<7 µmol/l); globulins, 39 g/l (20–30 g/l); alkaline phosphatase, 1020 U/l (40–150 U/l); γ-glutamyl transpeptidase, 2148 U/l (<47 U/l); glucose (fasting), 19.9 mmol/l (3.6–6.1 mmol/l); CA-19-9, 208.3 U/ml (<37 U/ml); IgG, 11.30 g/l (7.51–15.6 g/l) with IgG4 0.002 g/l (0.03–2 g/l); anti-smooth muscle antibody, weakly positive; antinuclear antibody, negative. Transabdominal US revealed a dilated common duct, and a focal mass in the head of pancreas.
A pancreatic CT scan with contrast showed focal enlargement and inhomogeneous hypo-attenuation of the pancreatic head, and a dilated intra- and extrahepatic biliary tract. MRCP revealed dilation of common bile duct and main pancreatic duct. T1-weighted fat-suppressed MRI showed a mixed lower signal in the head of the pancreas (Fig. 1). EUS demonstrated an ill-defined hypoechoic mass located in the head of the pancreas (Fig. 2). ERCP showed a long-segment of smooth narrowing of the common bile duct. An endoscopic plastic bile duct stent was inserted.
Figure 1.
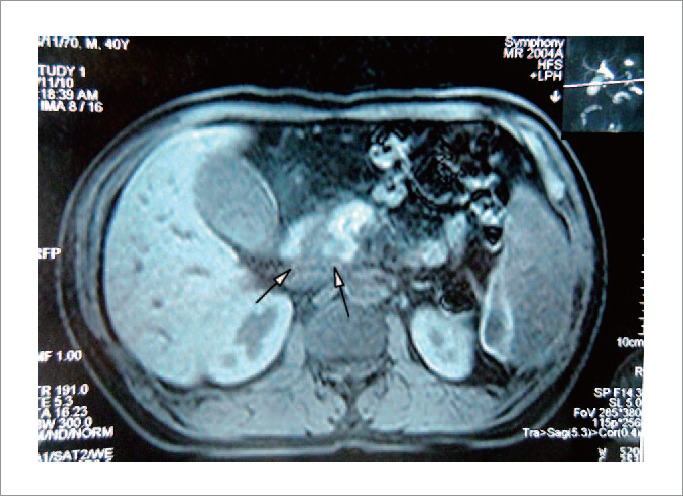
T1-weighted fat suppressed MRI showed a mixed lower signal in the head of the pancreas (arrows).
Figure 2.
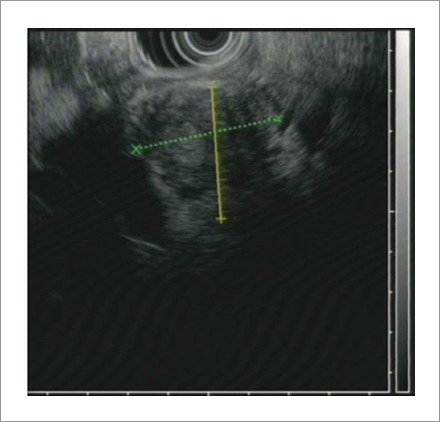
EUS demonstrated an ill-defined hypoechoic mass located in the head of the pancreas.
CEUS revealed the hypoechoic mass of 4.7×3.4 cm in the pancreatic head, with blurred delineated margins (Fig. 3). During the perfusion phase, harmonic imaging demonstrated enhancement of the mass in the arterial phase using an agent detection imaging mode approximately synchronized with the rest of the pancreas. The intermittent perfusion imaging showed homogeneous hyperenhancement of the mass with a longer regression time, which was notably different from that of pancreatic cancer. This represents a synchronism or delayed heterogeneous enhancement compared to surrounding tissue with early regression of hypo-enhancement.3 FNA was not feasible because complete prevention of seeding of the cancer by the needle could not be guaranteed. Concern for malignancy prompted surgical consultation, and subsequent radical pancreaticoduodenectomy.
Figure 3.
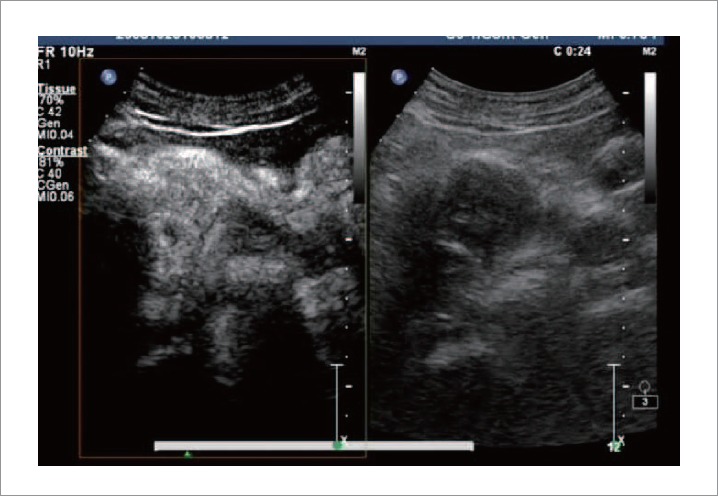
CE US revealed a hypoechoic mass of 4.7 cm×3.4 cm in the pancreatic head, with blurred delineated margins. In the perfusion image phase, harmonic imaging demonstrated enhancement of the mass in its arterial phase using agent detection imaging mode approximately synchronizing with the rest of the pancreas.
Gross examination of the specimen revealed enlargement of the head of pancreas to 6×5×2.5 cm, with a bulky major papilla. Histological examination showed peri-ductal infiltration by inflammatory cells (lymphocytes and plasma cells) with diffuse fibrosis in the pancreas. Immunohistochemical studies demonstrated large numbers of IgG4-positive plasma cells, with infiltrating fibrosis in the pancreas (Fig. 4), which is the gold standard for diagnosing AIP, according to the HISORt criteria 2006 reported by the Mayo Clinic.4
Figure 4.
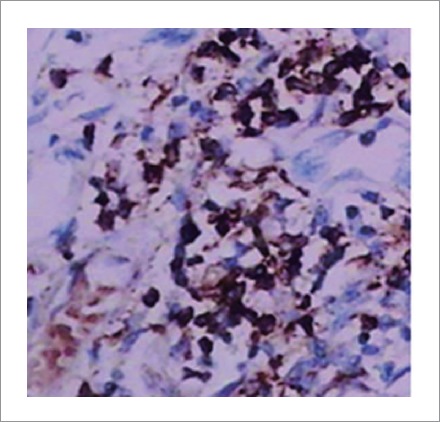
Immunohistochemical studies demonstrated high numbers of IgG4-positive plasma cells in pancreas (×400).
Discussion
AIP is a rare disorder that can mimic pancreatic neoplasia. AIP shares demographic, clinical, biochemical and imaging features with pancreatic cancer especially when it presents in focal forms. Current diagnostic criteria are too narrow, and do not capture the broad spectrum of presentations.5 In our case, the patient had painless jaundice, which is a typical manifestation of pancreatic cancer. In addition, there was an elevation of CA19-9 without elevation of serum IgG4, the latter a commonly used and sensitive marker for diagnosing AIP.5 The establishment of a correct diagnosis, upon which appropriate management of patient depended on, was thus especially difficult. US, CT, MRI, and ERCP features of AIP have been described. However, none of these modalities can provide an unequivocal diagnosis of AIP. EUS has been shown to be superior for detection of small pancreatic masses, but the sensitivity and specificity for determining whether or not a focal lesion is malignant is still poor. EUS-guided fine needle aspiration (EUS-FNA) providing only cellular material for microscopic examination or EUS-guided Tru-cut biopsy acquiring larger pieces of tissues have all been disappointing. Therefore, there is a clear need for improvement?6
CEUS has become a sensitive tool for observing the vascularity of liver tumors, as well as in the gastrointestinal tract.2 Signals from microbubbles obtained using a contrast agent enables the visualization of slow flow in microscopic vessels in normal and pathological tissues. The role of contrast-enhanced endoscopic ultrasound techniques in the differential diagnosis of chronic pancreatitis and ductal adenocarcinoma has been recently discussed with promising results, and seems to be a useful method in clinical practice.7 In ductal adenocarcinomas, only arterial vessels are displayed in contrast to chronic pancreatitis in which both arterial and venous vessels are displayed. CEUS of AIP shows a moderate to marked enhancement in the early contrastenhanced phase, although the images of thinning of the glandular vessels are frequently inhomogeneous due to thick lymphocytic infiltration and fibrosis. Contrast medium washout is usually slow, but progressive. For this reason, CEUS findings may be especially useful in the study of focal forms of AIP,3 and this was confirmed in our case. However, it is doubtful that contrast-enhanced imaging will replace tissue acquisition, especially for cancer management. In certain situations, however, CEUS may help decide if biopsy is warranted particularly if surgical treatment and outcomes would be affected. Furthermore, while the negative predictive value of EUS-FNA only reaches 30–44%, this often necessitates a second EUS procedure for repeat FNA or a percutaneous biopsy.8,9 With its high sensitivity and specificity, CEUS may reduce the need for repeat procedures if the initial FNA is negative.10 In addition, the use of contrast-enhanced techniques might prove to be useful for the follow-up of the patients during treatment because they are non-traumatic and safe.
In conclusion, CEUS technology may be useful in establishing the diagnosis of AIP with obstruction symptoms, especially when the serum IgG4 is negative.
Abbreviations
- AIP
Autoimmune pancreatitis
- CEUS
Contrast enhanced ultrasonography
- EUS-FNA
EUS-guided fine needle aspiration
Footnotes
Previously published online: www.landesbioscience.com/journals/jig
References
- 1.Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A, Kamata N. Clinical difficulties in the differentiation of autoimmune pancreatitis and pancreatic carcinoma. Am J Gastroenterol. 2003;98:2694–2699. doi: 10.1111/j.1572-0241.2003.08775.x. [DOI] [PubMed] [Google Scholar]
- 2.Kitano M, Kudo M, Maekawa K, Suetomi Y, Sakamoto H, Fukuta N, et al. Dynamic imaging of pancreatic diseases by contrast enhanced coded phase inversion harmonic ultrasonography. Gut. 2004;53:854–859. doi: 10.1136/gut.2003.029934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Numata K, Ozawa Y, Kobayashi N, Kubota T, Akinori N, Nakatani Y, et al. Contrast enhanced sonography of autoimmune pancreatitis: Comparison with pathologic findings. J Ultrasound Med. 2004;23:199–206. doi: 10.7863/jum.2004.23.2.199. [DOI] [PubMed] [Google Scholar]
- 4.Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010–1016. doi: 10.1016/j.cgh.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Nakazawa T, Ohara H, Sano H, Ando T, Imai H, Takada H, et al. Difficulty in diagnosing autoimmune pancreatitis by imaging findings. Gastrointest Endosc. 2007;65:99–108. doi: 10.1016/j.gie.2006.03.929. [DOI] [PubMed] [Google Scholar]
- 6.Farrell JJ. Diagnosing pancreatic malignancy in the setting of chronic pancreatitis: is there room for improvement? Gastrointest Endosc. 2005;62:737–741. doi: 10.1016/j.gie.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Hocke M, Schulze E, Gottschalk P, Topalidis T, Dietrich CF. Contrast-enhanced endoscopic ultrasound in discrimination between focal pancreatitis and pancreatic cancer. World J Gastroenterol. 2006;12:246–250. doi: 10.3748/wjg.v12.i2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savides TJ, Donohue M, Hunt G, Al-Haddad M, Aslanian H, Ben-Menachem T, et al. EUS-guided FNA diagnostic yield of malignancy in solid pancreatic masses: a benchmark for quality performance measurement. Gastrointest Endosc. 2007;66:277–282. doi: 10.1016/j.gie.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 9.LeBlanc JK, Ciaccia D, Al-Assi MT, McGrath K, Imperiale T, Tao LC, et al. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475–481. doi: 10.1016/s0016-5107(03)02863-3. [DOI] [PubMed] [Google Scholar]
- 10.Kitano M, Sakamoto H, Matsui U, Ito Y, Maekawa K, von Schrenck T, et al. A novel perfusion imaging technique of the pancreas: contrast-enhanced harmonic EUS (with video) Gastrointest Endosc. 2008;67:141–150. doi: 10.1016/j.gie.2007.07.045. [DOI] [PubMed] [Google Scholar]


