Although the mechanisms regulating the nuclear export of eukaryotic mRNAs remain poorly understood, it has been known for some time that pre-mRNAs, i.e., mRNAs that retain functional splice sites, are actively retained in the nucleus by what are termed splicing commitment factors (1, 2). The subsequent nuclear export of mature mRNAs therefore could be perceived as being largely caused by the removal, concomitantly with the completion of splicing, of these nuclear retention factors. However, in a recent issue of PNAS, Luo and Reed (3) provide evidence that nuclear mRNA export also involves the selective recruitment to fully spliced transcripts of factors that target these mRNAs for nuclear export (Fig. 1).
Figure 1.
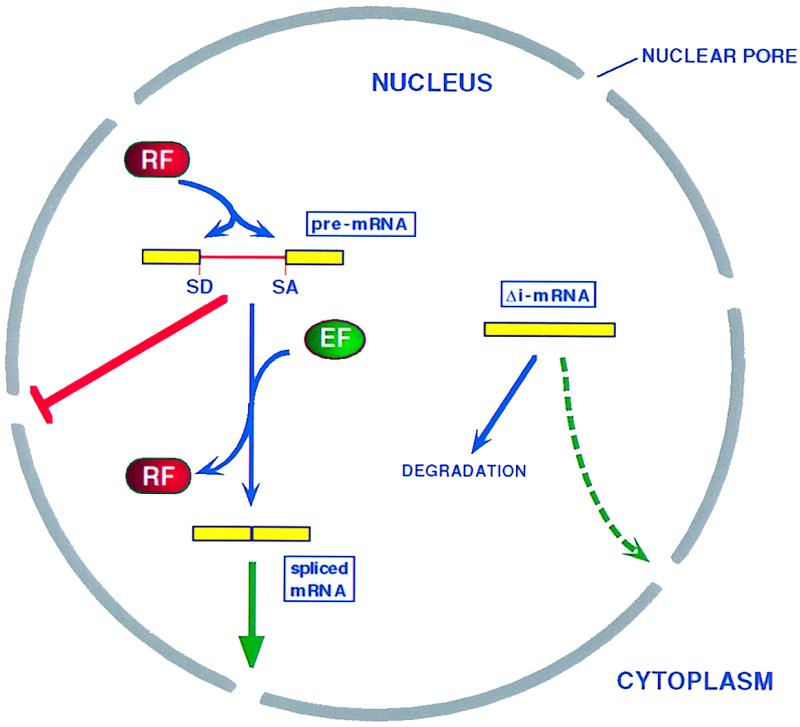
RNA splicing and nuclear RNA export are functionally linked. A pre-mRNA, whether transcribed in vivo or microinjected into the nucleus, is recognized by splicing commitment factors that both function as nuclear retention factors (RF) and initiate the process of mRNA splicing. As shown by Luo and Reed (3), completion of mRNA splicing results not only in the removal of these retention factors but also in the recruitment of nuclear export factors (EF) that target the resultant mature mRNA to the cytoplasm. In contrast, a microinjected mRNA lacking any intron (Δi-mRNA) is exported only very inefficiently, apparently because export factor recruitment is also inefficient. SD, splice donor; SA, splice acceptor.
Although the identification of cellular factors involved in mediating nuclear RNA export has been the subject of intense research interest, this issue is at best partly resolved (reviewed by refs. 4 and 5). However, it has been known for some time, based on in vivo competition assays, that different classes of RNAs, i.e., tRNAs, U small nuclear RNAs (snRNA), ribosomal RNAs (rRNAs), and mRNAs, exit the nucleus via distinct pathways that depend on different cofactors (6). Although rRNA nuclear export, which occurs as part of assembled ribosomal subunits, has not been extensively characterized, both tRNA export and U snRNA export are believed to depend on distinct members of the importin β family of nucleocytoplasmic factors, i.e., exportin t in the case of tRNA and Crm1 in the case of U snRNAs (5). Although Crm1 is capable of mediating nuclear mRNA export, and is indeed recruited for this purpose by retroviral RNA export factors such as the HIV-1 Rev protein, neither Crm1 nor exportin t appears to play any role in cellular mRNA export (5, 7). Although it initially was thought that mRNA export therefore might depend on yet a third, unidentified member of the importin β protein family, it now appears likely that an entirely unrelated export mechanism might be involved.
The first proteins that were proposed to play a role in mRNA export were the heterogenous nuclear RNA-binding proteins (hnRNPs). The hnRNPs are a highly abundant family of proteins that associate with pre-mRNAs during transcription and remain associated with nuclear mRNAs after splicing is completed (4). Although some hnRNPs, such as hnRNP C, are removed from mRNA molecules at the nuclear pore during export, others, including particularly hnRNP A1 and hnRNP K, leave the nucleus with mRNAs and then dissociate from the mRNA in the cytoplasm, whereupon they are reimported into the nucleus. This nucleocytoplasmic shuttling is not passive, as both hnRNP A1 and hnRNP K contain nuclear export signals that can mediate protein nuclear export in the absence of RNA binding (8, 9). This observation therefore led to the suggestion that these shuttling hnRNPs might in fact be directly required for nuclear mRNA export (4), a hypothesis that gained support from data in yeast showing that the mutational inactivation of Npl3p, a likely functional homolog of vertebrate hnRNP A1, resulted in a defect in mRNA export (10). However, binding to hnRNP A1 and/or hnRNP K is clearly not sufficient for mRNA export as these proteins are also bound to pre-mRNAs that are not capable of leaving the nucleus. The finding that a fusion of hnRNP C, a nonshuttling hnRNP, to hnRNP A1 blocked hnRNP A1 nuclear export raised the possibility that mRNA export was regulated by the interplay of hnRNPs that promoted nuclear retention (e.g., hnRNP C) and hnRNPs that could mediate nuclear export (e.g., hnRNP A1) (4).
Although this scenario remains an attractive one, recent data have strongly implicated yet another protein in the mRNA export pathway, i.e., the Tap nuclear RNA export factor. Tap first was identified as the target for a retroviral constitutive transport element (CTE), an RNA element that permits the nuclear export of pre-mRNAs when present in cis (11). The Tap protein contains not only a CTE-specific RNA binding domain but also an essential carboxyl-terminal sequence that functions both as a nuclear export signal and as a binding domain for nucleoporins, a group of proteins that constitute the nuclear pores, the portal used for all nuclear import and export (5, 12, 13). Two lines of evidence implicate Tap as a critical constituent in global nuclear mRNA export. First, titration of Tap by nuclear microinjection of high levels of CTE RNA inhibits nuclear mRNA export but does not affect the export of other RNAs, such as tRNAs or U snRNAs (14, 15). Second, the yeast homolog of Tap, termed Mex67, is clearly essential for poly(A)+ RNA export (13). Remarkably, human Tap can rescue mRNA export in Mex67-deficient yeast cells if expressed together with an essential human cofactor termed p15, thus strongly suggesting that a critical role for Tap in nuclear mRNA export has been conserved across a considerable evolutionary distance (13). It therefore has been suggested that recruitment of a Tap/p15 complex might be the critical, final step that induces cellular mRNA export from the nucleus (11). However, how Tap/p15 is recruited to mature mRNAs, and how access to Tap/p15 is regulated to prevent an inappropriate interaction with pre-mRNAs, remains unknown.
This important issue, i.e., how do mRNAs receive an export license from the nucleus, is addressed by the Luo and Reed manuscript (3). These authors report two novel findings. First, injection of intron containing pre-mRNAs into the Xenopus oocyte nucleus results in the efficient splicing and subsequent nuclear export of these mRNAs. In contrast, however, nuclear injection of the fully spliced forms of these same mRNAs, what the authors term Δi-mRNAs, results in very inefficient nuclear mRNA export and primarily leads to the nuclear degradation of the Δi-mRNA (Fig. 1). Second, these authors present compelling evidence that mRNA splicing can result not only in the removal of nuclear retention factors but also in the selective recruitment of nuclear RNA export factors. Specifically, Luo and Reed (3) demonstrate that incubation of their pre-mRNAs in a nuclear extract in vitro results, as expected, in splicing and in assembly of the resultant mature mRNA into a ribonucleoprotein (RNP) complex. However, the RNP complex containing the mRNA spliced in vitro was found to migrate significantly more slowly, when examined on a nondenaturing polyacrylamide gel, than the RNP formed on the identical Δi-mRNA. More importantly, microinjection of these isolated RNP complexes, containing identical mRNA molecules, into Xenopus oocyte nuclei resulted in the efficient nuclear export of the spliced mRNA whereas the Δi-mRNA remained almost entirely nuclear. Therefore, the factors assembled onto the spliced mRNA in vitro, during the process of splicing, were able to target this mRNA for nuclear export. In contrast, the equivalent Δi-mRNA was apparently unable to assemble these factors and therefore remained confined to the nucleus. Thus, the process of splicing, either in vivo or in vitro, was able to confer an export license on the spliced mRNAs that is somehow not available to the, in principle, identical Δi-mRNA.
Although the slower mobility of the RNP formed by the spliced mRNAs in vitro, when compared with the RNP containing the Δi-mRNAs, is most consistent with the idea that splicing somehow promotes the recruitment of RNA export factors, it remains possible that splicing also has facilitated the removal of retention factors. Given that the former possibility is in fact correct, then the next, obvious question is the identity of these critical export factors. As noted above, these appear unlikely to be hnRNPs, as these assemble relatively promiscuously onto both pre-mRNAs and mature mRNAs (4). An alternative, intriguing possibility is the Tap nuclear RNA export factor and its cofactor, p15. In the case of CTE-containing RNAs, Tap/p15 recruitment appears, as noted above, to be both necessary and sufficient for nuclear export and there is good evidence that the Tap/p15 complex is also critical for global mRNA export (13–15). It is therefore possible that recruitment of Tap/p15 is the penultimate nuclear step in the mRNA export pathway, i.e., the final step before nuclear pore binding. In this scenario, splicing therefore would either enhance Tap/p15 recruitment directly or, perhaps more probably, allow assembly of a protein(s) onto the mature mRNA that then would act to recruit Tap/p15. This presently unidentified protein(s) then would constitute the final export license for the mRNA.
A final important point is that the Luo and Reed manuscript (3) does not demonstrate that splicing is invariably required for mRNA export. In fact, a significant number of mRNAs in human cells, and the bulk of mRNAs synthesized in yeast, lack introns yet are exported from the nucleus and translated perfectly efficiently. At least in human cells, it is possible that this reflects the existence of specific cis-acting RNA sequences that can selectively promote the expression of intronless mRNAs. However, a recent functional analysis of such an element suggests that this sequence predominantly enhances polyadenylation and likely affects nuclear RNA export only indirectly (16). More importantly, however, it is apparent that many, but certainly not all, human genes can be efficiently expressed, in the context of a mammalian expression vector, in the absence of their normal complement of introns. This apparently contradictory observation may be explained by the fact that these active Δi-mRNAs are transcribed in vivo whereas the defective Δi-mRNAs tested by Luo and Reed (3) were transcribed in vitro and then microinjected as RNA molecules. Transcription in vivo is known to facilitate the efficient recruitment of factors involved in a range of RNA posttranscriptional modifications including mRNA capping, polyadenylation, and splicing (17). It is therefore possible that the recruitment of factors involved in mRNA export also is rendered more efficient when the mRNA is actually transcribed, capped, and polyadenylated in the nucleus from which it will be exported. However, this possibility does not detract from the significance of the observation by Luo and Reed (3) that splicing per se promotes the assembly of an mRNA-containing RNP complex that can efficiently access the cell's nuclear mRNA export pathway. The discovery of how RNA splicing is functionally linked to RNA export, and which export factors are recruited during RNA processing, would represent a major step forward in understanding the posttranscriptional regulation of eukaryotic gene expression.
Footnotes
See companion article on page 14937 in issue 26 of volume 96.
References
- 1.Chang D D, Sharp P A. Cell. 1989;59:789–795. doi: 10.1016/0092-8674(89)90602-8. [DOI] [PubMed] [Google Scholar]
- 2.Legrain P, Rosbash M. Cell. 1989;57:573–583. doi: 10.1016/0092-8674(89)90127-x. [DOI] [PubMed] [Google Scholar]
- 3.Luo M-j, Reed R. Proc Natl Acad Sci USA. 1999;96:14937–14942. doi: 10.1073/pnas.96.26.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakielny S, Fischer U, Michael W M, Dreyfuss G. Annu Rev Neurosci. 1997;20:269–301. doi: 10.1146/annurev.neuro.20.1.269. [DOI] [PubMed] [Google Scholar]
- 5.Görlich D, Kutay U. Annu Rev Cell Dev Biol. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- 6.Jarmolowski A, Boelens W C, Izaurralde E, Mattaj I W. J Cell Biol. 1994;124:627–635. doi: 10.1083/jcb.124.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fornerod M, Ohno M, Yoshida M, Mattaj I W. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 8.Michael W M, Choi M, Dreyfuss G. Cell. 1995;83:415–422. doi: 10.1016/0092-8674(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 9.Michael W M, Eder P S, Dreyfuss G. EMBO J. 1997;16:3587–3598. doi: 10.1093/emboj/16.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee M S, Henry M, Silver P A. Genes Dev. 1996;10:1233–1246. doi: 10.1101/gad.10.10.1233. [DOI] [PubMed] [Google Scholar]
- 11.Grüter P, Tabernero C, von Kobbe C, Schmitt C, Saavedra C, Bachi A, Wilm M, Felber B K, Izaurralde E. Mol Cell. 1998;1:649–659. doi: 10.1016/s1097-2765(00)80065-9. [DOI] [PubMed] [Google Scholar]
- 12.Kang Y, Cullen B R. Genes Dev. 1999;13:1126–1139. doi: 10.1101/gad.13.9.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katahira J, Straβer K, Podtelejnikov A, Mann M, Jung J U, Hurt E. EMBO J. 1999;18:2593–2609. doi: 10.1093/emboj/18.9.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saavedra C, Felber B, Izaurralde E. Curr Biol. 1997;7:619–628. doi: 10.1016/s0960-9822(06)00288-0. [DOI] [PubMed] [Google Scholar]
- 15.Pasquinelli A E, Ernst R K, Lund E, Grimm C, Zapp M L, Rekosh D, Hammarskjöld M-L, Dahlberg J E. EMBO J. 1997;16:7500–7510. doi: 10.1093/emboj/16.24.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y, Wimler K M, Carmichael G G. EMBO J. 1999;18:1642–1652. doi: 10.1093/emboj/18.6.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCracken S, Fong N, Yankulov K, Ballantyne S, Pan G, Greenblatt J, Patterson S D, Wickens M, Bentley D L. Nature (London) 1997;385:357–361. doi: 10.1038/385357a0. [DOI] [PubMed] [Google Scholar]


