Abstract
Endoscopic ampullectomy is a minimally invasive method of treating superficial lesions of the ampulla of Vater. With careful patient selection and lesion assessment it is a safe and efficacious therapeutic procedure that can obviate the need for potentially major surgical intervention. Strategies for safe and successful endoscopic ampullectomy with a focus on resection technique and recognition and management of complications are presented.
Key words: ampullectomy, ampullary adenoma, endoscopic resection, papillectomy
Introduction
Endoscopic ampullectomy offers a minimally invasive method of effectively treating mucosal (and sometimes superficial submucosal) lesions of the ampulla of Vater and surrounding peri-ampullary region with high success and relative safety.1 These lesions would otherwise require surgical, intervention including pancreatico-duodenectomy (Whipple's procedure). This paper provides a practical guide to undertaking safe endoscopic ampullectomy and highlights some of the common difficulties and pitfalls with this technique as well as offering strategies to deal with these challenges.
Lesion assessment and staging
Whilst small, obviously benign, less than 20 mm adenomas may be resected without extensive work up, complete multi-modality tumour staging is essential before endoscopic resection in more substantial lesions. For large complex lesions this should include endoscopic appearance, histology, endoscopic ultrasound (EUS), magnetic resonance cholangio-pancreatography (MRCP) and endoscopic retrograde cholangio-pancreatography (ERCP) at the time of planned ampullectomy. These modalities are used to define the type and extent of the lesion, in particular assessing for evidence of submucosal invasion or lymph node metastasis and the degree of intraductal extension (IDE), if any.2,3 Optical image enhancement systems, such as narrow band imaging may be useful in assessing the margins of the lesion.4
At present a widely accepted classification system for ampullary adenomas does not exist. In general they may be macroscopically classified into three types.
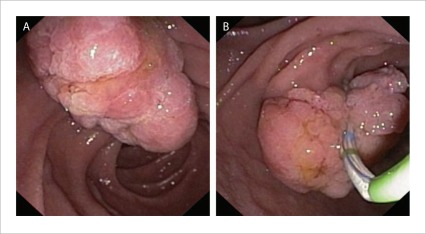
Granular or villiform exophytic lesions are most commonly encountered and are usually benign. They may also have a laterally spreading component (termed laterally spreading tumor of the papilla or LST-P), extending onto the duodenal wall beyond the papilla (Fig. 1)
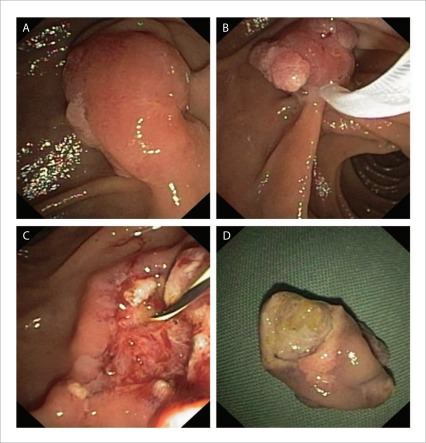
Smooth elevated lesions are less frequent but have a greater risk for invasive disease5 (Fig. 2)
Umbilicated lesions are uncommon. They are characterised by neoplastic tissue at the biliary orifice that may be exophytic and a swollen papilla (with usually overlying featureless, uninvolved mucosa) due to intraductal extension of the neoplasm within the intrapapillary portion of the common bile duct (CBD) and possibly beyond the duodenal wall. An unknown proportion of these lesions are primary distal biliary epithelial neoplasms.
Figure 1.
A granular or villiform exophytic ampullary adenoma.
Figure 2.
A smooth elevated lesion with a small amount of granular tissue at the lateral edge (A). Complete en-bloc ampullectomy (B, C). Papillary mechanism evident on undersurface of resected specimen (D).
Endoscopic appearances are less reliable in predicting deep invasion than in other mucosal sites, probably reflecting the absence of large scale systematic study including enhanced imaging techniques given the comparative infrequency of the problem and the comparatively limited utility of current duodenoscopic imaging technology. Most ampullary lesions are adenomas but occasionally submucosal lesions are encountered including carcinoid tumours6 and gangliocytic paraganglioma7. Routine biopsies of the lesion should be obtained to define the histology and degree of dysplasia, however care should be taken to avoid sampling in close proximity to the pancreatic orifice (located in the 5 o'clock position when the papilla is viewed en-face) as there is a small risk of causing pancreatitis, particularly with small lesions such as those seen in familial adenomatous polyposis.8 If there is discordance between the anticipated pathology based on endoscopic appearances and the results of biopsies consideration should be given to further biopsies to confirm favourable histology.
MRCP allows for non-invasive assessment of the distal CBD and pancreatic duct (PD) to detect for ductal dilatation, IDE and anatomical variants such as pancreas divisum which has a prevalence of between 1.5% and 6%9 (in which case attempts at post resection PD stenting may prove frustratingly futile!). Its accuracy is reported at 81%3, though this is in the hands of expert pancreatico-biliary radiologists. It is also subject to significant artefact if the patient cannot undertake a prolonged breath hold.
EUS can detect IDE, assess depth of invasion and identify pathological lymphadenopathy. It can be used for tissue sampling in cases where the diagnosis remains unclear. Its accuracy at T staging has been reported at between 63% and 72.7% with accuracy improving at higher T stages.3,10 EUS assessment should be considered for large lesions or those where there is concern for invasive cancer.
Despite multi-modality staging as outlined above, on occasion IDE may only be identified at endoscopic cholangiography at the time of resection. If IDE is limited to the papilla (and not beyond the duodenal wall) then en-bloc excision can still be achieved.
Endoscopic resection technique
General principles
Complete en-bloc excision of the entire neoplasm should be the goal with conventional papillary adenomas. To this end complete papillectomy to the plane of the duodenal wall should always be considered to minimise recurrence due to small IDE within the papilla.5 For lesions with extrapapillary extension the goal should be to remove the lesion in as few pieces as safely possible and again the papilla itself should be excised as one. En-bloc resection has many advantages including more accurate histological assessment and negligible recurrence.5 It should be remembered that endoscopic ampullectomy is an advanced therapeutic intervention and that the endoscopist must have sufficient training and expertise to undertake the procedure. Repeat intervention for partially resected lesions is often difficult with an increased risk due to submucosal fibrosis and disruption of the underlying anatomy.
Equipment
We recommend the use of a thin wire snare of approximately 0.3 mm diameter. Snare size should be closely adapted to the size of the target. The thin wire maximizes current density for swift transection of the papillary mechanism, minimizing the risk of “stalling” and limiting dispersion of the energy which may cause unnecessary injury to the pancreatic orifice, increasing the risk of a late stenosis. Oval or hexagonal snares of approximately 15 mm x 30 mm are ideal for most conventional adenomas. The close approximation of lesion and snare size affords precision in tissue capture, ensuring an R0 excision where this is technically feasible. Procedures are performed with a duodenoscope allowing visualisation of the papilla and also pancreatic and biliary orifices post resection to places stents.
Microprocessor-controlled electrosurgical generators capable of delivering alternating cycles of high frequency short pulse cutting with more prolonged coagulation current are required. [Erbe VIO 300 (Tübingen, Germany); Olympus ESG-100 (Tokyo, Japan)]. These generators sense tissue impedance via signals from the return electrode and adjust power output accordingly. As the tissue to be transected usually includes the muscle of the papillary mechanism which may be resistant to division, these generators minimize the likelihood of “stalling”. For endoscopic ampullectomy we use Erbe electrosurgical generators with the setting of Endocut Q, effect 3.
Resection technique
Endoscopic cholangiopancreatography is important for staging and should be undertaken prior to endoscopic ampullectomy. On occasion deep cannulation may be difficult, especially with giant laterally spreading lesions. In this case it may be best to obtain the cholangiogram and pancreatogram post resection.
After careful lesion assessment, an endoscopic resection plan is formulated. In most cases this is a direct en-bloc papillectomy, resulting in a R0 resection with free lateral and deep margins. This minimises the risk of recurrence.
Orientate the lesion so that an en-face view is obtained with a stable scope position.
For small lesions (<20mm) complete, en-bloc resection of the entire papilla without submucosal injection should be performed. This is also feasible for larger (>30 mm) lesions where the point of attachment does not exceed the boundaries of the papillary mound.
To resect the papilla the snare should be opened in a line corresponding to the long axis of the mound. Ideally, the snare tip is anchored above the apex of the papilla and the snare carefully opened and drawn down over the papilla, whilst the tip maintains its contact where it was impacted above. This has been termed the fulcrum technique. Once the snare has been placed over the papilla in this manner, it is closed maximally, as tight as possible without losing contact with the point of impaction above. The entrapped papilla should be independently mobile relative the duodenal wall behind. If it is comparatively fixed this implies entrapment of deeper structures or invasive disease. You may elect to release (even momentarily) and re-capture (Fig. 3).
The snare is closed maximally and the papilla divided by continuous application of current (as described above). This takes approximately 2 to 3 times (sometimes disconcertingly) as long as a polyp stalk of comparable size.
After each resection the snare catheter should be used to lift the specimen above the papilla. If the patient is in the prone (“Swimmers”) ERCP position it will then drop into the duodenal cap. Anti-peristaltic agents such as hyoscine butylbromide 10mg or glucagon 1mg should be given just prior to ampullectomy to prevent distal migration.
-
For larger complex lesions, or LST-P, submucosal injection of the extra-papillary portion is generally required. Submucosal injection to elevate the lesion should be reserved for extension to the duodenal mucosa beyond the margins of the papilla. The role of injection for the papillary resection is controversial1. We do not recommend papillary injection as it may cause a sunken papillary region because of elevation of adjacent mucosa making subsequent papillectomy dif.cult. The optimum resection technique is dependent on the morphology of the extra-papillary portion of the lesion (Fig. 4).
Lesions with predominant vertical extrapapillary extension (usually Paris 0-Is + IIa)11 should be treated by initial maximal papillectomy in the vertical plane and beyond the inferior aspect of the true papilla. Submucosal injection should be used if it is thought possible to perform an en bloc excision of the entire lesion (extent < 30–35 mm), in which case only the extrapapillary component should be elevated.
Lesions with predominant lateral extrapapillary extension (LST-P, usually Paris 0-IIa + Is)11 should be treated with submucosal injection and endoscopic mucosal resection (EMR) at 1 edge, working sequentially from the distal aspect on one side and then the other to isolate the papilla, allowing subsequent en bloc papillectomy
Submucosal injection should not be placed directly into the papillary region before adjacent adenoma is removed.
Most centres use an injection solution based on normal saline. We prefer succinylated gelatin, which is a widely available (in Australia) inexpensive, safe colloidal solution that is commonly used for intravenous fluid resuscitation. It has been demonstrated to greatly improve technical outcomes compared to normal saline in colonic EMR12, although no evidence exists to quantify the magnitude of benefit in the duodenum. A biologically inert blue dye such as indigo carmine in a concentration of 0.04% is used in the injection solution to define the perimeter of the lesion, delineate the extent of the submucosal cushion and to confirm that one is working in the correct tissue plane. Dilute epinephrine in a concentration of 1:100000 is also added to the injection solution.
The PD should be accessed and stented as the first priority after the papillectomy. Level 1 evidence confirms that PD stent placement greatly reduces the risk of pancreatitis.13 After an initial hiatus post ampullectomy bleeding of varying intensity (mild venous oozing or major arterial bleeding) often ensues. This will often obscure the PD orifice and frustrate attempts at PD cannulation. The bile duct should then be cannulated. If there is concern of IDE on cholangiogram, sweeping with a stone extraction balloon can be used to try and expose this tissue, though a biliary sphincterotomy is usually required. Further snare resection of this tissue may then be performed.14
Intra-procedural bleeding is common15 and can hamper visualisation which may be a particular challenge for stent placement. The endoscopist should have available, and be familiar with a range of haemostatic devices including injectors, haemostatic clips and coagulating forceps. Major bleeding is often best treated using coagulating forceps, but several therapeutic modalities may be required.
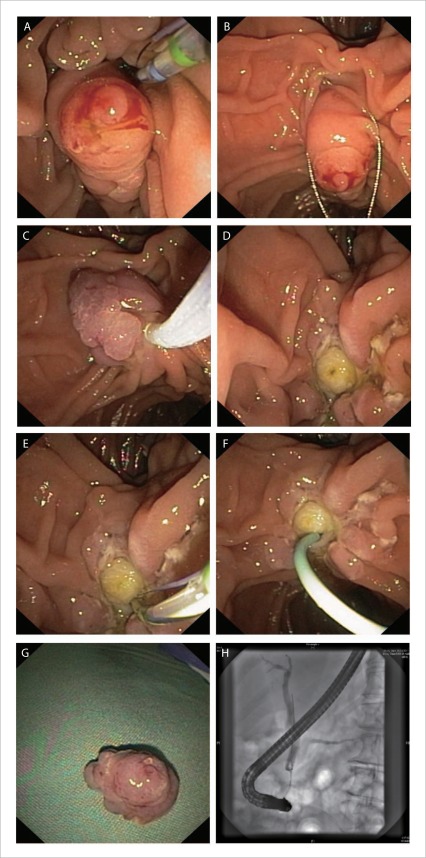
Figure 3.
Technique of en-bloc ampullectomy of a granular/villiform exophytic ampullary adenoma. A: lesion is identified and margins carefully assessed; B: with the snare tip anchored above the papillary mound the entire papilla is ensared; C: check mobility and ensure the snare is firmly closed; D: en-bloc ampullary resection is performed; E: pancreatic orifice is identified and cannulated; F: short, 5Fr pancreatic stent placed; G: ampullectomy specimen; H: cholangiogram with no evidence of IDE.
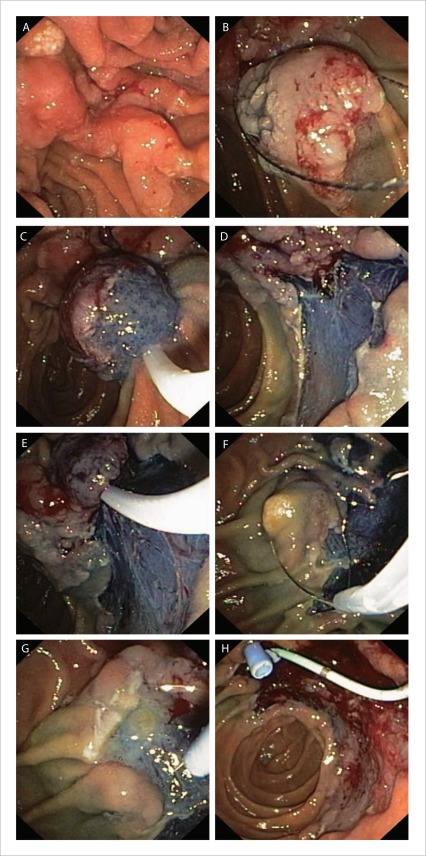
Figure 4.
Resection of an adenoma with a significant laterally spreading component (LST-P). A: the lesion's margins are closely examined; B–E: standard EMR techniques are used to resect the extra-papillary portion of the lesion; F: the papilla is excised en-bloc; G: needle knife used to gain deep biliary access; H: pancreatic stenting is performed prior to bleeding that may hamper visualisation.
Pancreatic stenting
The first priority after resection the papilla is to place a prophylactic pancreatic stent to reduce the risk of post ampullectomy pancreatitis.13 It is advisable to place a short, 5 French pancreatic stent as the first manoeuvre after ampullectomy so that the pancreatic orifice can be protected. See Box 1 for tips on achieving pancreatic cannulation.
Box 1.
Tips for successful pancreatic cannulation post ampullectomy
| If possible, cannulate PD (and CBD) prior to ampullectomy and obtain a pancreatogram to determine the location of the pancreatic orifice |
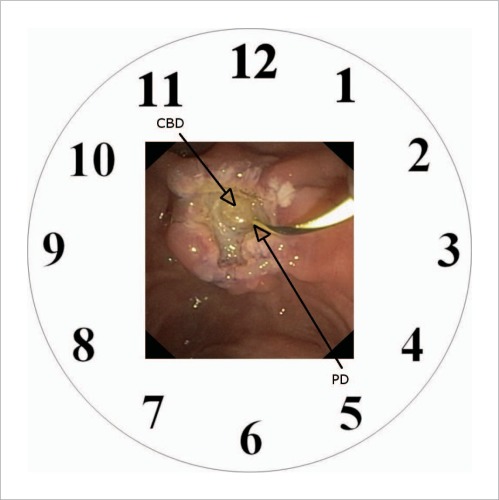
| Remember the anatomical relationship between biliary and pancreatic orifices. If imagined on a clock face, the CBD is at 11 o'clock and PD at 5 o'clock (Fig. 5) |
| Attempt PD cannulation as the first manoeuvre after ampullectomy whilst visualisation is optimal |
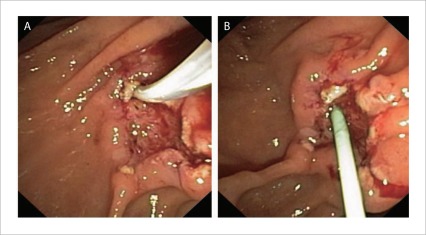
| If the PD cannot be identified then cannulate the distal aspect of the CBD with a sphincterotome and lift it up to ‘stretch’ the pancreatic orifice and improve visibility (Fig. 6) |
| Be wary of underlying pancreas divisum. The benefit of stenting the ventral duct is limited and attempts to do so can be frustrating |
Biliary stenting/sphincterotomy
Prophylactic biliary stenting is generally not necessary. It may be beneficial in major bleeding to reduce the risk of ascending cholangitis from haemobilia or to ensure correct bile drainage if concern over retroduodenal perforation exists.16 Biliary sphincterotomy may be considered if there is concern for stenosis of the orifice (eg a slow transection of the papilla) though evidence to recommend routine sphincterotomy is lacking. In contrast to PD stenting the evidence for routine biliary stent placement after ampullectomy is weak and the decision to proceed with biliary sphincterotomy and/or stenting should be individualised.
Thermal ablation
We do not routinely apply thermal ablation (APC) during ampullectomy as complete tissue destruction cannot be ensured.17 Instead we prefer to completely resect all neoplastic tissue including a small cuff of normal tissue in laterally to ensure clear margins are obtained. We limit the use of these techniques to instances where there is a concern of residual lesion.
Specimen processing, post procedural care and endoscopic follow up
All specimens should be retrieved for histological assessment. Commercially available 2–3 cm nets are the best option for multiple specimen retrieval.
The papillary resection and specimens larger than 15 mm should be flattened on a cork board and their margins pinned. Pinning of specimens (particularly after en-bloc excision) prevents curling of the tissue within the formalin and facilitates more accurate histological assessment allowing the histopathologist to report on lateral and deep margins of excision.
After complex ampullectomy patients are kept in first stage recovery for two hours with abdominal examination before moving to second stage recovery. They remain fasting for four hours post procedure and then commence on a clear liquid diet. If they are well at that point, they are discharged home on a clear fluid diet overnight to commence a normal diet in the morning provided they remain well. We perform all our ampullectomy procedures in the morning and retain the patients in the second stage recovery for most of the day allowing for outpatient management. Where there is clinical concern, patients are assessed and admitted to hospital as required. All patients receive twice daily proton pump inhibitors.
After ampullectomy, pancreatic stent removal should be undertaken within within two weeks to minimize the risk of pancreatic ductal injury. In our unit surveillance of the ampullectomy site with careful examination of the biliary orifice for possible residual is undertaken at 4 months with photodocumentation and biopsy. If there is a residual, this is generally diminutive and easily excised (or ablated if excision is not possible).5,18,19 Subsequent to that endoscopy with a side viewing endoscope is repeated annually for the next three to five years, with direct visualization and biopsy of the site, with surveillance intervals gradually lengthening subsequent to that.
Complications and management
The most serious complications are perforation, delayed bleeding and pancreatitis.20 Identification of high risk patients, early recognition of complications and aggressive management ameliorates their frequency and severity. The incidences of the most concerning complications are summarized in Table 1.
Table 1.
Incidence of post ampullectomy complications16
| Complication | Incidence |
| Perforation | 0%–8% |
| Bleeding | 2%–30% |
| Pancreatitis | 3%–25% |
| Cholangitis | 0%–5% |
| Papillary stenosis in follow up | 0%–8% |
Perforation
A careful inspection of the resection defect must always be undertaken to assess for areas of deep resection. Endoscopic features are less reliable at determining deep resection than in other sites and so a high clinical index of suspicion must be maintained during post-procedural clinical assessment.
Ongoing pain should prompt radiological assessment and, if required, surgical review. The absence of free intra-peritoneal or sub diaphragmatic air on plain x-rays does not exclude perforation which is usually retroperitoneal. Computed tomography (preferably with oral contrast) is more sensitive and is required if symptoms continue. Multi-disciplinary management between medical and surgical teams is necessary to achieve the best possible clinical outcome. Not all cases of perforation require surgical intervention and select cases may be managed with gut rest and intravenous antibiotics.6,15
Bleeding
The duodenum is highly vascular and is at high risk of both early and delayed bleeding.21 Clinically significant delayed bleeding is common (see Table 1) and should be managed as for bleeding after endoscopic sphincterotomy. If major bleeding is anticipated then temporary biliary stenting is helpful to prevent obstructing haemobilia and localize the ampullectomy bed (and the likely bleeding site) amidst the brisk bleeding at the time of urgent endoscopy. For focal arterial bleeding coagulating forceps using soft coagulation (80 Watts, Effect 4–6 on the Erbe generator) is preferred. Clipping may be useful but can be difficult over the elevator of the duodenoscope. Consider removing the outer sheath of the clipping catheter. Aspirin may be continued or used instead of another agent if there is a high risk of cardiovascular events, however all other anti-platelet agents and anti-coagulants should be ceased prior.
Pancreatitis
Prophylactic PD stenting is associated with a marked decrease in the risk of post ampullectomy pancreatitis13 and is the accepted standard. Prompt placement of a PD stent immediately after ampullectomy is necessary as brisk bleeding may swiftly ensue making PD localization difficult. Prior MRCP may demonstrate pancreas divisum, in which case PD stenting is usually not necessary. Management of post ampullectomy pancreatitis is generally the same as post-ERCP pancreatitis.
Conclusion
The optimal technique for endoscopic ampullectomy is dependent on the lesions size and and the presence and extent of extrapapillary extension. Lesions confined to the papilla should be resected en-bloc. Larger lesions require piecemeal resection and if there is significant extension onto the duodenal wall then the resection will include an EMR component.5
The endoscopist must be mindful of both early and delayed complications and in particular the importance of early placement of a pancreatic duct stent cannot be overstated.
Endoscopic ampullectomy is a safe and effective therapy for papillary adenomas, LST-P and some submucosal ampullary lesions when performed by experienced endoscopists however there is a substantial incidence of moderate to severe complications that the endoscopist must be prepared to identify and manage.
Figure 5.
The anatomical relationship of the pancreatic and biliary orifices as visualised on a clock face.
Figure 6.
A: Elevating the biliary orifice to expose the pancreatic orifice; B: placement of a pancreatic stent.
Abbreviations
- CBD
common bile duct
- ERCP
endoscopic retrograde cholangio-pancreatography
- EUS
endoscopic ultrasound
- IDE
intraductal extension
- LST-P
laterally spreading tumor of the papilla
- MRCP
magnetic resonance cholangiopancreatography
- PD
pancreatic duct
Footnotes
Previously published online: www.landesbioscience.com/journals/jig
References
- 1.Adler DG, Qureshi W, Davila R, Gan SI, Lichtenstein D, Rajan E, et al. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2006;64:849–854. doi: 10.1016/j.gie.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 2.Manta R, Conigliaro R, Castellani D, Messerotti A, Bertani H, Sabatino G, et al. Linear endoscopic ultrasonography vs magnetic resonance imaging in ampullary tumors. World J Gastroenterol. 2010;16:5592–5597. doi: 10.3748/wjg.v16.i44.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CH, Yang CC, Yeh YH, Chou DA, Nien CK. Reappraisal of endosonography of ampullary tumors: Correlation with transabdominal sonography, CT, and MRI. J Clin Ultrasound. 2009;37:18–25. doi: 10.1002/jcu.20523. [DOI] [PubMed] [Google Scholar]
- 4.Itoi T, Tsuji S, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, et al. A novel approach emphasizing preoperative margin enhancement of tumor of the major duodenal papilla with narrow-band imaging in comparison to indigo carmine chromoendoscopy (with videos) Gastrointest Endosc. 2009;69:136–141. doi: 10.1016/j.gie.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 5.Hopper AD, Bourke MJ, Williams SJ, Swan MP. Giant laterally spreading tumors of the papilla: endoscopic features, resection technique, and outcome (with videos) Gastrointest Endosc. 2010;71:967–975. doi: 10.1016/j.gie.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 6.Cheng CL, Sherman S, Fogel EL, McHenry L, Watkins JL, Fukushima T, et al. Endoscopic snare papillectomy for tumors of the duodenal papillae. Gastrointest Endosc. 2004;60:757–764. doi: 10.1016/s0016-5107(04)02029-2. [DOI] [PubMed] [Google Scholar]
- 7.Lytras D, Kalaitzakis E, Hatfield AR, Vyas S, Imber CJ, Webster GJ, et al. Recurrent acute pancreatitis as primary manifestation of gangliocytic paraganglioma of the ampulla. Pancreas. 2011;40:985–986. doi: 10.1097/MPA.0b013e31821fd9df. [DOI] [PubMed] [Google Scholar]
- 8.Morales TG, Hixson LJ. Acute pancreatitis following endoscopic biopsy of the ampulla in a patient with Gardner's syndrome. Gastrointest Endosc. 1994;40:367–369. doi: 10.1016/s0016-5107(94)70076-1. [DOI] [PubMed] [Google Scholar]
- 9.Gonoi W, Akai H, Hagiwara K, Akahane M, Hayashi N, Maeda E, et al. Pancreas divisum as a predisposing factor for chronic and recurrent idiopathic pancreatitis: initial in vivo survey. Gut. 2011;60:1103–1108. doi: 10.1136/gut.2010.230011. [DOI] [PubMed] [Google Scholar]
- 10.Ito K, Fujita N, Noda Y, Kobayashi G, Horaguchi J, Takasawa O, et al. Preoperative evaluation of ampullary neoplasm with EUS and transpapillary intraductal US: a prospective and histopathologically controlled study. Gastrointest Endosc. 2007;66:740–747. doi: 10.1016/j.gie.2007.03.1081. [DOI] [PubMed] [Google Scholar]
- 11.The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–S43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 12.Moss A, Bourke MJ, Metz AJ. A randomized, double-blind trial of succinylated gelatin submucosal injection for endoscopic resection of large sessile polyps of the colon. Am J Gastroenterol. 2010;105:2375–2382. doi: 10.1038/ajg.2010.319. [DOI] [PubMed] [Google Scholar]
- 13.Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc. 2005;62:367–370. doi: 10.1016/j.gie.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Moon JH, Choi HJ, Lee HS, Kim HK, Cheon YK, et al. Endoscopic snare papillectomy by using a balloon catheter for an unexposed ampullary adenoma with intraductal extension (with videos) Gastrointest Endosc. 2009;69:1404–1406. doi: 10.1016/j.gie.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 15.Norton ID, Gostout CJ, Baron TH, Geller A, Petersen BT, Wiersema MJ. Safety and outcome of endoscopic snare excision of the major duodenal papilla. Gastrointest Endosc. 2002;56:239–243. doi: 10.1016/s0016-5107(02)70184-3. [DOI] [PubMed] [Google Scholar]
- 16.Ito K, Fujita N, Noda Y. Endoscopic diagnosis and treatment of ampullary neoplasm (with video) Dig Endosc. 2011;23:113–117. doi: 10.1111/j.1443-1661.2010.01101.x. [DOI] [PubMed] [Google Scholar]
- 17.Moss A, Bourke MJ, Williams SJ, Hourigan LF, Brown G, Tam W, et al. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology. 2011;140:1909–1918. doi: 10.1053/j.gastro.2011.02.062. [DOI] [PubMed] [Google Scholar]
- 18.Yamao T, Isomoto H, Kohno S, Mizuta Y, Yamakawa M, Nakao K, et al. Endoscopic snare papillectomy with biliary and pancreatic stent placement for tumors of the major duodenal papilla. Surg Endosc. 2010;24:119–124. doi: 10.1007/s00464-009-0538-8. [DOI] [PubMed] [Google Scholar]
- 19.Moon JH, Cha SW, Cho YD, Ryu CB, Cheon YK, Kwon KW, et al. Wire-guided endoscopic snare papillectomy for tumors of the major duodenal papilla. Gastrointest Endosc. 2005;61:461–466. doi: 10.1016/s0016-5107(04)02649-5. [DOI] [PubMed] [Google Scholar]
- 20.Irani S, Arai A, Ayub K, Biehl T, Brandabur JJ, Dorer R, et al. Papillectomy for ampullary neoplasm: results of a single referral center over a 10-year period. Gastrointest Endosc. 2009;70:923–932. doi: 10.1016/j.gie.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Alexander S, Bourke MJ, Williams SJ, Bailey A, Co J. EMR of large, sessile, sporadic nonampullary duodenal adenomas: technical aspects and long-term outcome (with videos) Gastrointest Endosc. 2009;69:66–73. doi: 10.1016/j.gie.2008.04.061. [DOI] [PubMed] [Google Scholar]