Abstract
Endoscopic ultrasonography (EUS) is highly accurate for assessing the pancreatic parenchyma and ductal system. Currently, it is the most sensitive imaging procedure for detecting small solid pancreatic masses. EUS-guided fine needle aspiration cytology (EUS-FNA) is a safe and highly accurate tool for the diagnosis of pancreatic malignancy. Prior to perform an EUS-FNA one should wonder whether the benefits outweigh the potential risks of the procedure. Therefore, it is important to take into account whether the procedure will influence patient management. The diagnostic yield and success rate of EUS-FNA in pancreatic lesions varies greatly depending on many factors including: the characteristics of the lesion itself (location of the mass and consistency of the lesion), technical factors (type of needle size, use of stylet, use of suction and number of needle passes performed) and the availability of immediate cytological assessment of the specimen. The aim of this review is to analyze all these factors for optimizing specimen collection and diagnostic efficiency in dealing with solid pancreatic masses.
Key words: endoscopic ultrasound guided fine needle aspiration cytology, pancreatic lesions, diagnosis
Introduction
EUS has an excellent imaging resolution of the gastrointestinal wall and the surrounding organs. EUS-FNA allows obtaining adequate material for cytological diagnosis of different types of lesions.1,2
It has the advantage over other imaging techniques that it allows real-time imaging. In addition, the proximity of the needle to the lesion reduces the risk of complication specifically the risk of tumor dissemination in the needle tract.3
EUS-FNA is able to provide a cytological diagnosis in between 80% and 95% with a sensitivity and specificity of 90% and 100% respectively.4,5 Pancreatic tumors represent the most challenging lesions with the lowest diagnostic yield (76%–90%) and a false negative rate up to 15%.5,7 EUS-FNA accuracy rests on several factors: first, it may be related to the characteristics of the lesion itself; second, technical and equipment related factors; third, the availability of rapid on-site evaluation of the sample by a cytopathologist and lastly, skills and experience of the endoscopist as well as the cytopathologist. The present review gives a practical approach of EUS-FNA in solid pancreatic lesions and analyzes the technical factors related to EUS-FNA accuracy in pancreatic lesions
Decision of biopsy
Before taking the decision of EUS-FNA, some clinical considerations should be taken into account. Whether or not the biopsy will affect the management and if benefits outweigh potential risks (i.e. risk of seeding or haemorrhage) are two important questions. Patients with non-resectable pancreatic tumors will need cytological diagnosis before initiation of chemotherapy. In advanced tumors (i.e. with liver metastasis, mediastinal lymph nodes or suspicion of peritoneal carcinomatosis) it is useful to undergo EUS-FNA as a proof of malignancy.8
Although percutaneous biopsy is an option in these patients, diagnostic accuracy is probably lower and needle tract metastasis is more frequent, painful and difficult to treat.9 In addition, a higher frequency of peritoneal carcinomatosis after chemotherapy has been described in patients who undergone a pancreatic CT biopsy compared with those who had EUS-FNA.10
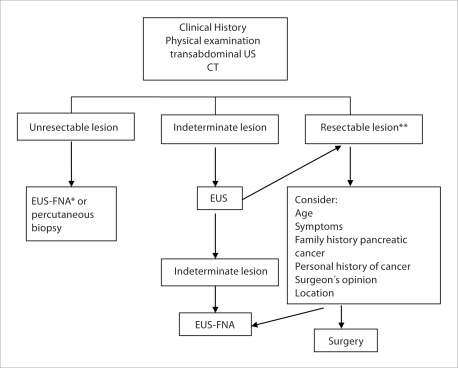
In resectable lesions, the main concern with EUS-FNA is the risk of seeding. As a general rule, EUS-FNA should be avoided in resectable body and tail pancreatic masses, since the biopsy tract will not be included in the surgical specimen. Seeding is not of great importance in head or uncinate lesions, since the biopsy tract would be removed as part of the surgical specimen. Clinical management may be different depending on the clinical suspicion. In fact, although pancreatic adenocarcinoma, accounts for 90% of the solid pancreatic neoplasms11, in the other 10% the differential diagnosis includes a heterogenous group of lesions such as, islet cell tumors12, pancreatic metastasis13, and lymphomas14. Therefore, the correct diagnosis of these "unusual pancreatic lesions" is crucial as the prognosis and management are completely different. Factors such as the patient's age, family history of pancreatic cancer, past personal history of cancer and location of the tumor, are key elements to establish the diagnostic suspicion. EUS-FNA could also be useful in patients with indeterminate lesions by CT/EUS (i.e. focal pancreatitis, biliary obstruction but without obvious mass, and atypical clinical presentation). The opinion of the surgeon should be taken into account prior to undergo a EUS-FNA. In view of these data it is important to remember that EUS is a part of the diagnostic scheme and that the endosonographer is a part of one team along with the surgeon, radiologist and oncologist (Fig. 1).
Figure 1.
EUS-FNA in the diagnosis of pancreatic neoplasms. * Preferable EUS-FNA; ** Avoid EUS-FNA as a general rule in body and tail lesions. Reconsider EUS FNA when a different lesion to a pancreatic adenocarcinoma is suspected (i.e. pancreatic mass in young patients, atypical symptoms, personal history of tumors).
Prioritizing lesions sampling
When prioritizing lesion sampling, highest yield result from first targeting the lesion that stages the patient higher. This sequence spares the pancreas for the last attempt. It is rational to start with those lesions which involve advanced disease, such as mediastinal malignant - appearing LN, followed by ascetic fluid, distant abdominal malignant-appearing LN and liver metastasis. The second step should be to evaluate for local regional disease (malignant - appearing LN) and finally the pancreas. In this stepwise FNA protocol, it is crucial to have a cytopathologist to avoid multiple passes and to minimize the risk of seeding.
Precautions should be taken when the endosonographer deals with a concominant adrenal mass. Although pancreatic cancer can metastasize to the adrenal gland, it is a rarity.15 On the other hand, FNA is currently not recommended in primary adrenal malignancies (i.e. adrenocortical carcinoma, pheochromocytoma) because it is rarely informative and potentially hazardous.16 In this setting is preferred to biopsy other lesion which involves the same stage disease. If the adrenal mass is the lesion with the highest stage disease, it is mandatory to perform an appropriate workup prior to performing an EUS-FNA to rule out primary adrenal tumors.
Considerations by site
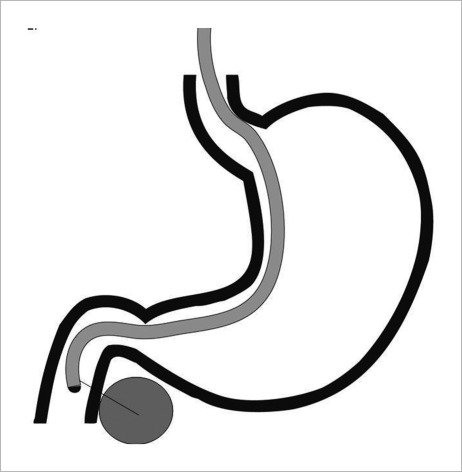
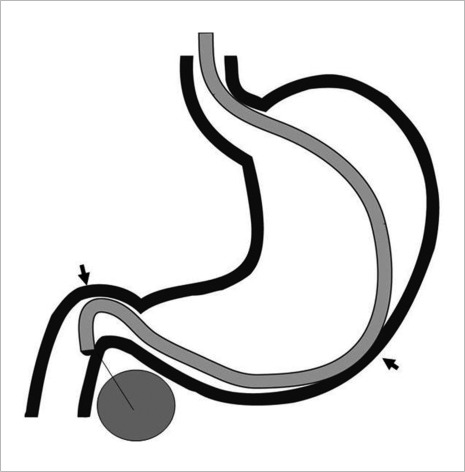
The pancreas can be accessed either transgastric (usually body and tail lesions) or transduodenal (head and uncinate process). In general transgastric approach is easier as the scope is straighter. Pancreatic head or uncinate masses are technically more challenging as the bending of the scope tip makes the needle exit more difficult. It is important to take into account that the natural scope position, it means, avoiding large amount of tip deflections or the use of the elevator, increases FNA success. To overcome the difficulties of FNA in head or uncinate pancreatic masses, it is better to place the scope in a short position whenever possible (Fig. 2). Although the scope is more unstable, the short position facilitates easier needle exit.17 However, when the target is an indurate pancreatic lesion, wedging the scope in the duodenal bulb (long position) offers a more stable position, but more resistance is encountered to get the needle out (Fig. 3). Air suction also helps bring the wall closer to the probe.
Figure 2.
Echoendoscope in short loop position. Notice that there is no fulcurm during fine needle aspiration cytology.
Figure 3.
Echoendoscope in long loop position. The gastric and duodenal walls work as fulcurm during the fine needle aspiration cytology (arrowheads).
The transgastric approach may be hampered by the fact that the stomach has a generous lumen that tends to yield away in response to the needle movement against it. In this situation, thinner needles (25 Gauge needle) and quick needle movements could facilitate the FNA. Another manoeuvre is to push the scope through the stomach to form a long loop that positions the scope to the opposite gastric wall and acts as a hinge. Additionally, this problem can be salvaged by targeting the pancreatic lesion in two steps; first traversing the gastric wall, and second, puncture the pancreatic lesion. EUS-FNA success in patients with surgically altered upper gastrointestinal anatomy has been poorly studied.18,19 The largest study19, retrospectivly, studied the feasibility of performing a complete pancreatobiliatry examination in patients with Billroth I (n=13), Billroth II (n=39), Roux en Y (n=25), Whipple (n=45), Puestow (n=17), Nissen funduplication (n=31) and esophaghectomy (n=18). Of the 109 patients in whom a pancreaticobiliary examination was indicated, in 48 (41%) the examination was not complete. The length of the Roux limb , inability of intubating the afferent limb in Billroth II and Whipple surgeries were the most frequent limitations for precluding a complete examination.
Nature and consistency of the lesion
Indurate lesions are difficult to penetrate. In order to overcome this problem first the endosonographer should check the needle which might have lost its sharpness or became bent because of previous sampling. If the needle is functioning properly, either increasing upward tip deflection against the luminal wall or using more force during penetration is useful. One other important issue is the size of the lesion. Necrotic areas within larger tumors often provide low yield samples whereas targeting the edges on the lesion tend to produce more adequate material.20
Choosing the appropriate needle
Currently, there are three available needle sizes for cytological diagnosis, 19 G, 22 G and 25 G and two needles for histological analysis, 22 G and 19 G. Large needles have been frequently used in lesions in which additional techniques other than cytological analysis are necessary for achieving a definitive diagnosis.21 The 19 G needle is able to provide a core biopsy useful for histological.22 However, technical difficulty because of its stiffness is a problem when the transduodenal approach is needed. Besides, contrary to intuitive thinking, larger samples are not always better samples for cytological diagnosis as they could be associated with more bloody specimens.23 Smaller needles (22 G and 25 G) are the most frequently used in clinical practice as they are more flexible, can produce less tissue trauma and decrease the risk of potential complications. The 25 G needle could be useful for targeting small lesions far from the transducer and penetrate through the tissue more easily in hard pancreatic masses due to the smaller diameter of the needle.24 This needle can also provide less hypocellular or acellular specimens as well as a less bloody sample and sometimes a better sampling.24,25 Currently, seven comparative studies are available in the literature comparing 22 G and 25 G needles.24–30 Four are randomized controlled studies (RCT) (Table 1).25,29,30 In general, available RCT have not found significant differences between both needles, but suggest that 25 G could be better in very fibrotic lesions and those located in the head or uncinate process of the pancreas.
Table 1.
Comparative studies: 22 G vs. 25 G needle
| Author (year) | Needle | Patients (n) | Passes (n) | Ease (%) | Failure (%) | Adequacy (%) | Yield (%) |
| Yusuf (2009) | 22G | 540 | NR | NR | NR | NR | 84 |
| 25G | 302 | NR | NR | NR | 92 | 92 | |
| Sakamoto (2009) | 22G | 24 | 2 | 79 | 21 | NR | 75 |
| 25G | 24 | 2 | 100 | 0 | NR | 92 | |
| Imazu (2009) | 22G | 43 | 2 | 1.3 | 0.2 | 1.64 | 81 |
| 25G | 43 | 2 | 1.9 | 0 | 1.5 | 77 | |
| Lee* (2009) | 22G | 12 | 2 | NR | 25 | NR | NR |
| 25G | 12 | 2 | NR | 0 | NR | NR | |
| Siddiqui* (2009) | 22G | 64 | 2.6 | 79.7 | 17 | NR | 87 |
| 25G | 67 | 2.6 | 85.1 | 15 | NR | 95 | |
| Fabri* (2011) | 22G | 50 | 2 | NR | 0 | 2.1** | 86 |
| 25G | 50 | 2 | NR | 0 | 2.4 | 94 | |
| Camellini* (2011) | 22G | 64 | 3.6 | NR | NR | 6.25 | 87 |
| 25G | 63 | 3.5 | NR | NR | 3.17 | 89 |
Randomized controlled trials;
Results expressed in a as a mean in a 5 points numerical score scale; NR: no reported.
EUS disposable needles are comercialized by Cook Endoscopy (Echotip™; 19, 22 and 25 G), Mediglobe (Sonotip™; 19, 22 and 25 G), Olympus (EZ-Shot™; 22G) and Boston Scientific (Expect™; 19, 22 and 25 G). Cook Endoscopy, Mediglobe and Boston Scientific needles have a comfortable ergonomic handle and can be adaptable to the length of the scope. The new Expect™ needle looks sharper compared with other needles and offers greater hardness and higher needle penetration. Visible echogenic pattern has also been improved. However, the handle may seem a bit bulky for some endosonographers and the needle sheath is not as slippery as that of the Cook Endoscopy needle. A 19 G (replacing Quick-Core needle, Cook Endoscopy, Winston Salem, NC.) and 22 G Procore needles (Cook Endoscopy, Winston Salem, NC.) have recently commercialized. Although the experience is low they seem to be principally indicated in those endoscopy units in which immediate cytological evaluation is not available.31
Concerns about the use of stylet
The usual practice is to perform the EUS-FNA with an internal stylet, reinserting it before each FNA pass. It is felt that the stylet prevents blockage of the needle with surrounding nonlesional tissue which may result in misdiagnosis, decreasing the EUS-FNA diagnostic accuracy. Stylet would also appear to have several potential advantages as providing stability to the needle or for carefully expressing the material in a controlled manner. However, this recommendation has an empirical basis. Recently, two prospective studies32,33 and one retrospective study34 (Table 2) suggest that use of the stylet does not offer any benefit in increasing diagnostic accuracy. All the studies used the 22 G conventional needle in a variety of lesions, so that, the role of the stylet for different needles sizes or lesions is currently unknown. Although large prospective randomized studies are necessary to find out the role of the stylet, it seems to be logical to recommend FNA without stylet at least in difficult sites (i.e. lesions located in the uncinate process) in which the stylet can hinder the FNA.
Table 2.
Studies with and without stylet
| Author | Needle | Stylet (n) | Lesions (n) | Adequate sample (%) | Bloody specimen(%) | Diagnostic yield (%) | Sensitivity (%) |
| Sahai (2010) | 22G | Yes | 46 | 75* | 75** | 89 | 87 |
| No | 46 | 87 | 52 | 87 | 83 | ||
| Wani (2011) | 22G | Yes | 106 | 94 | 41 | 39 | NR |
| No | 122 | 91 | 46 | 36 | NR | ||
| Rastogi (2011) | 22G | Yes | 118 | 57 | 17 | 23 | NR |
| No | 118 | 62 | 14 | 28 | NR |
P=0.013;
P<0.0001.
Number of fna passes
Endosonographic findings do not predicts the number of passes required for adequate sampling. The number of passes required is related to the degree of differentiation of the tumor. Although, on site cytologist evaluation is of great value in these cases, immediate assistance of cytologist during EUS guided FNA is not universal. Retrospective and prospective studies suggest that 5 to 7 passes should be made for achieving the maximum diagnostic yield.35,36 In one study, increasing the number of passes more than 7 did not increase diagnostic accuracy.36
Recently, it has been suggested that 19 G and 22 G core needles could be useful in those units without immediate cytological assessment.31
EUS-FNA with or without suction
Few studies have compared EUS-FNA with and without negative pressure. In theory, suction could increase the amount of material for diagnosis although also bloodiness of the specimen, especially in vascular lesions. Two meta analytical studies carried out in other organs did not find any advantage of capillary sampling as compared with the suction techniques.37,38 Only, one EUS-FNA randomized study including small number of pancreatic lesions (n=8) has been performed thus far.39 The authors observed a higher sensitivity and negative predictive values for malignancy in the suction group (85.7% and 85.7% respectively) compared with the non-suction group (66.6% and 66.7% respectively, p=0.05). In addition, bloodiness or contamination was not increased in the suction group. Thus, given the scarce data regarding this aspect, firm recommendations cannot be given. In clinical practice, the first pass could be made with suction changing to the non-suction if the aspirate is bloody.
On-site cytologic evaluation
On site cytological assessment of the EUS-FNA specimen has been shown to be beneficial for rapid clinical diagnosis and decision making.40 Several studies have demonstrated that specimen adequacy is higher than 90% when on site interpretation by a cytopathologist is available, precluding the necessity of a second procedure.40–43 In addition, EUS-FNA studies have reported a higher diagnostic yield when compared with non-onsite assessment as well as higher sensitivity and diagnostic accuracy for malignancy.40–43 It has also been suggested this could reduce FNA related complications because the lower number of needle passes.43 Unfortunately, resources and staff limitations frequently preclude the availability of an on-site cytopathologist. In these cases, a cytotechnologist could be a good option to assess sample adequacy.41,42
Summary
EUS-FNA in pancreatic solid masses remains a diagnostic challenge. Indication of EUS-FNA should be considered carefully taken into account risks and benefits of the procedure. Ranges in diagnostic accuracy are wide because of the influence of multiple related factors. Although, the importance of each factor in the diagnostic accuracy and adequacy of the sample is unknown, knowledge of these factors and a proper FNA technique are warranted for increasing the efficiency of the procedure.
Acknowledgements
This work was supported by a grant from the Fundación Alfonso Martín Escudero (convocatoria 2010) and by a grant from the Egyptian government “Post doctoral scientific mession scheme 2010”. Funding sources had no involvement in study design, collection, analysis, interpretation of data, in the writing of the report or in the decision to submit the paper for publication.
The authors wish to thank Dr. Nadim Jafri (Baylor College of Medicine, Houston. Texas) for critical comments of the manuscript.
Abbreviations
- EUS
endoscopic ultrasonography
- EUS-FNA
EUS-guided fine needle aspiration cytology
Footnotes
Previously published online: www.landesbioscience.com/journals/jig
References
- 1.Crowe DR, Eloubeidi MA, Chhieng DC, Jhala NC, Jhala D, Eltoum IA. Fine-needle aspiration biopsy of hepatic lesions: computerized tomographic-guided versus endoscopic ultrasound-guided FNA. Cancer. 2006;108:180–185. doi: 10.1002/cncr.21912. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen P, Feng JC, Chang KJ. Endoscopic ultrasound (EUS) and EUS-guided fineneedle aspiration (FNA) of liver lesions. Gastrointest Endosc. 1999;50:357–361. doi: 10.1053/ge.1999.v50.97208. [DOI] [PubMed] [Google Scholar]
- 3.Paquin SC, Gariepy G, Lepanto L, Bourdages R, Raymond G, Sahai AV. A first report of tumor seeding because of EUS-guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc. 2005;61:610–611. doi: 10.1016/s0016-5107(05)00082-9. [DOI] [PubMed] [Google Scholar]
- 4.Eloubeidi MA, Chen VK, Eltoum IA, Jhala D, Chhieng DC, Jhala N, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98:2663–2668. doi: 10.1111/j.1572-0241.2003.08666.x. [DOI] [PubMed] [Google Scholar]
- 5.Williams DB, Sahai AV, Aabakken L, Penman ID, van Velse A, Webb J, et al. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut. 1999;44:720–726. doi: 10.1136/gut.44.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puli SR, Batapati Krishna Reddy J, Bechtold ML, Ibdah JA, Antillon D, Singh S, et al. Endoscopic ultrasound: it's accuracy in evaluating mediastinal lymphadenopathy? A meta-analysis and systematic review. World J Gastroenterol. 2008;14:3028–3037. doi: 10.3748/wjg.14.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiersema MJ, Kochman ML, Cramer HM, Tao LC, Wiersema LM. Endosonography-guided real-time fine-needle aspiration biopsy. Gastrointest Endosc. 1994;40:700–707. [PubMed] [Google Scholar]
- 8.Levy MJ. Know when to biopsy 'em, know when to walk away. Gastrointest Endosc. 2006;63:630–634. doi: 10.1016/j.gie.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Horwhat JD, Paulson EK, McGrath K, Branch MS, Baillie J, Tyler D, et al. A randomized comparison of EUS-guided FNA versus CT or US-guided FNA for the evaluation of pancreatic mass lesions. Gastrointest Endosc. 2006;63:966–975. doi: 10.1016/j.gie.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 10.Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690–695. doi: 10.1016/s0016-5107(03)02009-1. [DOI] [PubMed] [Google Scholar]
- 11.Shami V, Srinivason I, Kahaleh M. The role of EUS in pancreatic cancer. In: Shami VM, Kahaleh M, editors. Endoscopic Ultrasound. Springer Science: New York; 2010. pp. 283–298. [Google Scholar]
- 12.Gines A, Vazquez-Sequeiros E, Soria MT, Clain JE, Wiersema MJ. Usefulness of EUS-guided fine needle aspiration (EUS-FNA) in the diagnosis of functioning neuroendocrine tumors. Gastrointest Endosc. 2002;56:91–96. doi: 10.1016/s0016-5107(02)70196-x. [DOI] [PubMed] [Google Scholar]
- 13.Gimeno-Garcia AZ, Fernandez-Esparrach G, Sendino O, Gines A. Diagnosis of pancreatic metastasis through endoscopic ultrasonography-guided aspiration puncture. Med Clin (Barc) 2007;129:119. doi: 10.1157/13107384. [DOI] [PubMed] [Google Scholar]
- 14.Gimeno-Garcia AZ, Alonso MM, Garcia Castro C, Nicolas Perez D, Quintero E. Primary pancreatic lymphoma diagnosed by endoscopic ultrasound-guided fine needle aspiration biopsy. Gastroenterol Hepatol. 33:638–642. doi: 10.1016/j.gastrohep.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Lam KY, Lo CY. Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin Endocrinol (Oxf) 2002;56:95–101. doi: 10.1046/j.0300-0664.2001.01435.x. [DOI] [PubMed] [Google Scholar]
- 16.McLean K, Lilienfeld H, Caracciolo JT, Hoffe S, Tourtelot JB, Carter WB. Management of isolated Adrenal Lesions in Cancer Patients. Cancer Control. 18:113–126. doi: 10.1177/107327481101800206. [DOI] [PubMed] [Google Scholar]
- 17.Sahai A, Paquin SC. How to perform Eus-guided fine-needle aspiration and biopsy. In: Hawes RH, Fockens P, editors. Endosonography. 2nd edition. 2010. pp. 224–234. Expert Consult. [Google Scholar]
- 18.Lee JH, Topazian M. Pancreatic endosonography after Billroth II gastrectomy. Endoscopy. 2004;36:972–975. doi: 10.1055/s-2004-825867. [DOI] [PubMed] [Google Scholar]
- 19.Wilson JA, Hoffman B, Hawes RH, Romagnuolo J. EUS in patients with surgically altered upper GI anatomy. Gastrointest Endosc. 72:947–953. doi: 10.1016/j.gie.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Binmoeller KF, Rathod VD. Difficult pancreatic mass FNA: tips for success. Gastrointest Endosc. 2002;56:S86–S91. doi: 10.1016/s0016-5107(02)70093-x. [DOI] [PubMed] [Google Scholar]
- 21.Binmoeller KF, Thul R, Rathod V, Henke P, Brand B, Jabusch HC, et al. Endoscopic ultrasound-guided, 18-gauge, fine needle aspiration biopsy of the pancreas using a 2.8 mm channel convex array echoendoscope. Gastrointest Endosc. 1998;47:121–127. doi: 10.1016/s0016-5107(98)70343-8. [DOI] [PubMed] [Google Scholar]
- 22.Vilmann P, Puri R. Endoscopic ultrasound guided fine needle aspiration and tru-cut biopsy. Tech Gastrointest Endosc. 2007;9:2–19. [Google Scholar]
- 23.Jhala D, Jhala N. A cytology primer for endosonographers. In: Hawes R, Fockens P, editors. Endosonography. 2nd Edition. Philadelphia: Elsevier; 2011. [Google Scholar]
- 24.Yusuf TE, Ho S, Pavey DA, Michael H, Gress FG. Retrospective analysis of the utility of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) in pancreatic masses, using a 22-gauge or 25-gauge needle system: a multicenter experience. Endoscopy. 2009;41:445–448. doi: 10.1055/s-0029-1214643. [DOI] [PubMed] [Google Scholar]
- 25.Siddiqui UD, Rossi F, Rosenthal LS, Padda MS, Murali-Dharan V, Aslanian HR. EUS-guided FNA of solid pancreatic masses: a prospective, randomized trial comparing 22-gauge and 25-gauge needles. Gastrointest Endosc. 2009;70:1093–1097. doi: 10.1016/j.gie.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto H, Kitano M, Komaki T, Noda K, Chikugo T, Dote K, et al. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge Trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol. 2009;24:384–390. doi: 10.1111/j.1440-1746.2008.05636.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, Stewart J, Ross WA, Anandasabapathy S, Xiao L, Staerkel G. Blinded prospective comparison of the performance of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of the pancreas and peri-pancreatic lesions. Dig Dis Sci. 2009;54:2274–2281. doi: 10.1007/s10620-009-0906-1. [DOI] [PubMed] [Google Scholar]
- 28.Imazu H, Uchiyama Y, Kakutani H, Ikeda K, Sumiyama K, Kaise M, et al. A prospective comparison of EUS-guided FNA using 25-gauge and 22-gauge needles. Gastroenterol Res Pract. 2009;2009:546390. doi: 10.1155/2009/546390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fabbri C, Polifemo AM, Luigiano C, Cennamo V, Baccarini P, Collina G, et al. Endoscopic ultrasound-guided fine needle aspiration with 22- and 25-gauge needles in solid pancreatic masses: A prospective comparative study with randomisation of needle sequence. Dig Liver Dis. 2011;43:647–652. doi: 10.1016/j.dld.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Camellini L, Carlinfante G, Azzolini F, Iori V, Cavina M, Sereni G, et al. A randomized clinical trial comparing 22G and 25G needles in endoscopic ultrasound-guided fineneedle aspiration of solid lesions. Endoscopy. 2011;43:709–715. doi: 10.1055/s-0030-1256482. [DOI] [PubMed] [Google Scholar]
- 31.Iglesias-Garcia J, Poley JW, Larghi A, Giovannini M, Petrone MC, Abdulkader I, et al. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189–1196. doi: 10.1016/j.gie.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 32.Rastogi A, Wani S, Gupta N, Singh V, Gaddam S, Reddymasu S, et al. A prospective, single-blind, randomized, controlled trial of EUS-guided FNA with and without a stylet. Gastrointest Endosc. 2011;74:58–64. doi: 10.1016/j.gie.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Sahai AV, Paquin SC, Gariepy G. A prospective comparison of endoscopic ultrasoundguided fine needle aspiration results obtained in the same lesion, with and without the needle stylet. Endoscopy. 2009;42:900–903. doi: 10.1055/s-0030-1255676. [DOI] [PubMed] [Google Scholar]
- 34.Wani S, Gupta N, Gaddam S, Singh V, Ulusarac O, Romanas M, et al. A comparative study of endoscopic ultrasound guided fine needle aspiration with and without a stylet. Dig Dis Sci. 2011;56:2409–2414. doi: 10.1007/s10620-011-1608-z. [DOI] [PubMed] [Google Scholar]
- 35.Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–190. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 36.LeBlanc JK, Ciaccia D, Al-Assi MT, McGrath K, Imperiale T, Tao LC, et al. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475–481. doi: 10.1016/s0016-5107(03)02863-3. [DOI] [PubMed] [Google Scholar]
- 37.Pothier DD, Narula AA. Should we apply suction during fine needle cytology of thyroid lesions? A systematic review and meta-analysis. Ann R Coll Surg Engl. 2006;88:643–645. doi: 10.1308/003588406X149147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vetrani A, Fulciniti F, Di Benedetto G, Zeppa P, Troncone G, Boscaino A, et al. Fine-needle aspiration biopsies of breast masses. An additional experience with 1153 cases (1985 to 1988) and a meta-analysis. Cancer. 1992;69:736–740. doi: 10.1002/1097-0142(19920201)69:3<736::aid-cncr2820690321>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 39.Puri R, Vilmann P, Saftoiu A, Skov BG, Linnemann D, Hassan H, et al. Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scand J Gastroenterol. 2009;44:499–504. doi: 10.1080/00365520802647392. [DOI] [PubMed] [Google Scholar]
- 40.Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289–1294. doi: 10.1111/j.1572-0241.2003.07472.x. [DOI] [PubMed] [Google Scholar]
- 41.Savoy AD, Raimondo M, Woodward TA, Noh K, Pungpapong S, Jones AD, et al. Can endosonographers evaluate on-site cytologic adequacy? A comparison with cytotechnologists. Gastrointest Endosc. 2007;65:953–957. doi: 10.1016/j.gie.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Alsohaibani F, Girgis S, Sandha GS. Does onsite cytotechnology evaluation improve the accuracy of endoscopic ultrasound-guided fine-needle aspiration biopsy? Can J Gastroenterol. 2009;23:26–30. doi: 10.1155/2009/194351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, Larino-Noia J, Eugenyeva E, Lozano-Leon A, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705–1710. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]