Abstract
House dust mites (HDM) are the most common source of indoor allergens and are associated with allergic diseases worldwide. To benefit allergic patients, safer and non-invasive mucosal routes of oral administration are considered to be the best alternative to conventional allergen-specific immunotherapy. In this study, transgenic rice was developed expressing derivatives of the major HDM allergen Der f 2 with reduced Der f 2-specific IgE reactivity by disrupting intramolecular disulphide bonds in Der f 2. These derivatives were produced specifically as secretory proteins in the endosperm tissue of seeds under the control of the endosperm-specific glutelin GluB-1 promoter. Notably, modified Der f 2 derivatives aggregated in the endoplasmic reticulum (ER) lumen and were deposited in a unique protein body (PB)-like structure tentatively called the Der f 2 body. Der f 2 bodies were characterized by their intracellular localization and physico-chemical properties, and were distinct from ER-derived PBs (PB-Is) and protein storage vacuoles (PB-IIs). Unlike ER-derived organelles such as PB-Is, Der f 2 bodies were rapidly digested in simulated gastric fluid in a manner similar to that of PB-IIs. Oral administration in mice of transgenic rice seeds containing Der f 2 derivatives encapsulated in Der f 2 bodies suppressed Der f 2-specific IgE and IgG production compared with that in mice fed non-transgenic rice seeds, and the effect was dependent on the type of Der f 2 derivative expressed. These results suggest that engineered hypoallergenic Der f 2 derivatives expressed in the rice seed endosperm could serve as a basis for the development of viable strategies for the oral delivery of vaccines against HDM allergy.
Keywords: Allergy vaccine, Der f 2, endosperm, immunotherapy, mite allergen, protein body, rice seed
Introduction
Allergen-specific immunotherapy is the only known curative treatment for allergic diseases. Conventional immunotherapy by subcutaneous injection of increasing doses of crude allergen has been used for the last 3–5 years, resulting in a reduction in clinical symptoms and prevention of disease progression (Frew, 2003; Larche et al., 2006). However, this therapeutic approach is complicated in many patients by safety issues and inconvenient treatment schedules. To overcome these drawbacks, alternative routes for immunotherapy administration such as nasal, sublingual, and oral treatments are being developed. An orally administered rice-based allergy vaccine is expected to be an alternative to subcutaneous injections, offering simple, convenient, and cost-effective immunotherapy (Takaiwa, 2011).
House dust mites (HDMs; Dermatophagoides spp.) are one of the most common indoor allergens associated with bronchial asthma, rhinitis, and atopic dermatitis. HDMs are responsible for more than 70% of childhood bronchial asthma cases and, to date, more than 20 HDM allergens have been identified and characterized (Kawamoto et al., 2002; Thomas et al., 2002). The major HDM allergens are classified into group I (Der f 1 and Der p 1, molecular mass 25 kDa) and group 2 (Der f 2 and Der p 2, molecular mass 14 kDa). Der f 2 is the main group 2 allergen derived from Dermatophagoides farinae, and comprises 129 aa with marked homology (87%) to Der p 2, the group 2 allergen from Dermatophagoides pteronyssinus (Haida et al., 1985; Yuuki et al., 1991; Chua et al., 1996; Thomas and Smith, 1998). More than 80% of mite-allergic patients are sensitized against group 2 mite allergens (Yuuki et al., 1990, 1991), which are present at high concentrations in mite faeces (Park et al., 2000). The group 2 mite allergens show similarities in sequence, size, and distribution of cysteine residues to a family of epididymal proteins (Thomas and Chua, 1995), which have recently been reported to bind to lipopolysaccharides in a similar manner to MD-2, a lipopolysaccharide-binding component of the Toll-like receptor 4 (TLR4) signalling complex, resulting in the promotion of allergenicity through TLR4 signalling.
Recognition of group 2 allergens by specific IgE (B-cell epitopes) is dependent on the conformational integrity of the protein rather than the contiguous sequence of amino acids. Der f 2 contains three disulphide bonds (Cys8–Cys119, Cys21–Cys27, and Cys73–Cys78) and comprises two anti-parallel β-sheets (Derewenda et al., 2002; Johannessen et al., 2005). Two of the disulphide bonds, Cys8–Cys119 and Cys73–Cys78, are critical for the IgE-binding capacity of Der f 2 (Nishiyama et al., 1995; Takai et al., 1997, 2000b). By contrast, the T-cell epitopes of Der p 2 and Der f 2 are distributed along the entire protein (O’Hehir et al., 1993; van Neerven et al., 1993; O’Brien et al., 1995; Fujii et al. 1997; Inoue et al., 1997). Understanding the structural characteristics associated with the reduced allergenicity of engineered allergen mutants is important for the development of effective strategies against these important allergens.
Mutant allergens with reduced allergenicity are a safer and more effective immunotherapy strategy because the clinical complications of allergic anaphylaxis can be avoided (Linhart and Valenta, 2005; Valenta and Niederberger, 2007; Valenta et al., 2010). Reduced allergenicity enables the administration of high doses of tolerogen, which results in a greater efficacy of allergen-specific immunotherapies. Der f 2 derivatives with reduced IgE reactivity have been developed by altering the structure of the allergen (Takai et al., 1999, 2000a, b).
In the present study, transgenic rice containing Der f 2 derivatives with reduced IgE reactivity was generated by mutating the cysteine residues involved in disulphide-bond formation. Notably, expression of Der f 2 derivatives in rice endosperm cells caused the appearance of a novel storage protein body (PB) designated the Der f 2 body, which differs from endoplasmic reticulum (ER)-derived PBs (PB-Is) and protein storage vacuoles (PB-IIs) observed in non-transgenic rice seeds. Despite being derived directly from the ER as PB-Is, Der f 2 bodies containing Der f 2 derivatives showed unique physico-chemical properties and sensitivity to digestive enzymes from the gastrointestinal tract.
When transgenic rice seeds containing Der f 2 derivatives were fed to mice via the oral route, immune tolerance against the Der f 2 antigen was induced. The inhibition of Der f 2-specific IgE and IgG production indicated that the efficacy of these derivatives was related to the resistance of Der f 2 bodies against digestive enzymes in the gastrointestinal tract.
Materials and methods
Plasmid construction and rice transformation
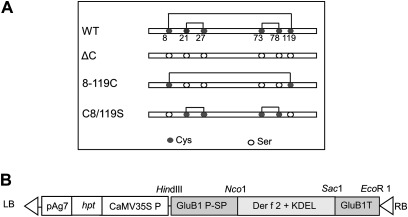
The gene encoding mature Der f 2 with the ER retention signal KDEL at its C-terminal end was optimized for translation based on codons frequently used in rice seed storage protein genes (Takaiwa, 2007), and was synthesized by GenScript Corp. (NJ, USA). Six cysteine residues are present in the Der f 2 sequence at positions 8, 21, 27, 73, 78, and 119, and there are three disulphide bonds in the native Der f 2 (Cys8–Cys119, Cys21–Cys27, and Cys73–Cys78). Three cysteine mutants of Der f 2 were made by replacing cysteines with serines at Cys8 and Cys119, at Cys21, Cys27, Cys73, and Cys78, or by replacing all six cysteine residues, generating the derivatives 8-119C, C8/119S, and ΔC, respectively (Fig.1A). The genes encoding Der f 2 and the three derivatives were ligated downstream of the 2.3 kb GluB-1 promoter (Fig.1B) (Qu and Takaiwa, 2004). A DNA sequence encoding the GluB-1 signal peptide was attached to the N terminus of genes that contained a KDEL ER retention signal at their C-terminal ends, followed by a 0.65 kb fragment of the GluB-1 terminator. The resulting four constructs were cloned into the HindIII and EcoRI sites of the binary vector pGPTV-35S-HPT (Goto et al., 1999) (Fig. 1B).
Fig. 1.
Construction of expression vectors for rice transformation. (A) Schematic representation of native wild-type (WT) Der f 2 and the three cysteine mutants, ΔC, 8-119C, and C8/119S. The three intramolecular disulphide bonds of Cys8–Cys119, Cys21–Cys27, and Cys73–Cys78 are shown. (B) The binary vector used for transformation. pAg7, Agrobacterium gene 7 terminator; hpt, hygromycin phosphotransferase coding region; CaMV35S P, Cauliflower mosaic virus 35S promoter; GluB1 P-SP, 2.3 kb glutelin B1 promoter and its signal sequence; Der f 2 + KDEL, coding region of Der f 2 and its mutants with KDEL added to the C terminus; GluB1T, glutelin B1 terminator; LB, left border; RB, right border.
Introduction of the expression vectors into rice (Oryza sativa cv. Kita-ake) by Agrobacterium tumefaciens-mediated transformation and regeneration of transgenic plants in a controlled green house were carried out as described previously (Goto et al., 1999).
Expression of wild-type and cysteine mutants of Der f 2 in Escherichia coli and in vitro IgE-binding capacities
The DNA sequences encoding native Der f 2 protein and the ΔC, 8-119C, and C8/119S derivatives were cloned into the expression plasmid pET23d (+) (Novagen, USA) at the Nco1 and Sac1 sites, and transformed into E. coli BL21(DE3) (Novagen). The expression and purification of proteins was carried out using a QIAexpressionist™ kit according to the manufacturer’s instructions (Qiagen, Tokyo, Japan).
Identical amounts (0.5 μg per lane) of the Der f 2 protein and derivatives isolated from E. coli culture were loaded onto 12% SDS-PAGE gels under reducing conditions and electroblotted onto a membrane as described previously (Yang et al., 2008). The blot was probed with sera from seven mite-sensitive patients (diluted 1:1000) and further detected with a horseradish peroxidase-conjugated anti-human IgE antibody (1:5000).
Antibodies
E. coli-expressed and purified mature Der f 2 was used to raise a rabbit anti-Der f 2 antibody (Qiagen). Antibodies to glutelins (GluA, GluB, GluC, and GluD) and 26K globulin peptides, 10K, 13K (RM1, RM2, RM4, and RM9), and 16K prolamins, RAG2, and the rice chaperones BiP1, BiP08g, PDI 1-1, PDI 2-3, and calnexin were prepared previously in our laboratory (Yasuda et al., 2009; Oono et al., 2010; Wakasa et al., 2011). The anti-maize BiP antibody was a kind gift from Dr R.S. Boston (North Carolina State University, NC, USA). Horseradish peroxidase-conjugated anti-human IgE(ϵ) and anti-rabbit IgG were purchased from KPL (MD, USA) and Cell Signaling Technology (Danvers, MA, USA), respectively.
SDS-PAGE and Western blot analysis
Mature seeds from independent transgenic rice plants were harvested and ground separately to a fine powder using a Multi-Beads shocker (Yasui Kikai, Tokyo, Japan). For total protein extraction, 600 μl urea/SDS buffer [50 mM Tris/HCl (pH 6.8), 8 M urea, 4% SDS, 5% 2-mercaptoethanol (2-ME), 20% glycerol] was used as described previously (Tada et al., 2003). After separation by 12% SDS-PAGE, the proteins were visualized by Coomassie Brilliant Blue (CBB) R250 staining or transferred to PVDF membranes (Millipore, Billerica, MA, USA) for immunodetection. Proteins were detected with the corresponding primary antibodies, followed by a goat anti-rabbit IgG secondary antibody (Cell Signaling Technology).
The recombinant protein yields were estimated on the basis of the intensity of bands that reacted with the Der f 2 antibody, using Der f 2 produced in E. coli as a standard. The band images were scanned into a computer and the corresponding bands were quantified using NIH image software (US National Institutes of Health, MD, USA).
Sequential protein extraction
Sequential extraction of proteins was performed according to the method of Tada et al. (2003). Briefly, glutelins were extracted from 20 mg of seed powder with 1% (v/v) lactic acid after the stepwise removal of albumins and globulins with 500 μl of saline buffer (0.5 M NaCl, 10 mM Tris/HCl pH 7.5), followed by removal of prolamins with 500 μl of 60% (v/v) n-propanol solution containing 5% (v/v) 2-ME. Each extraction step was accomplished by resuspending the seed powder in the solution and sonicating on ice for 1 min. After centrifugation (10 000 r.p.m., 10 min), the residues were extracted twice more with the same solution.
Der f 2 proteins were also extracted from transgenic seed powders with 20 mM Tris/HCl (pH 7.5). The extraction was repeated three times.
In vitro deglycosylation
Total seed proteins were extracted with urea/SDS buffer and precipitated using a chloroform/methanol method (Seigneurin-Berny et al., 1999). The proteins were then resuspended in reaction buffer and incubated in the presence or absence of endoglycosidase H (Endo H) or peptide N-glycosidase F (PNGase F) at 37 °C for 2 h according to the manufacturer’s protocol (New England Biolabs Japan, Tokyo, Japan). The treated samples were analysed by Western blotting with an anti-Der f 2 antibody.
Immunohistochemical electron and confocal laser-scanning microscopy
Immature transgenic rice seeds (15–20 days old) were collected and used for immunohistochemical electron and confocal laser-scanning microscopic analyses. The procedures for preparing both types of sample have been described previously (Yasuda et al., 2006; Takaiwa et al., 2009; Kawakatsu et al., 2010), except that the sections were reacted with primary anti-Der f 2 (diluted 1:200), GluA (1:3000), 13K cysteine-rich prolamin (RM1) (1:1000), PDI 1-1 (1:500) and maize BiP (1:500) antibodies, followed by secondary 20 nm gold particle-labelled goat anti-rabbit IgG (Fc-specific, 1:200; EY Laboratories, San Mateo, CA, USA).
In vitro digestion of transgenic rice seeds by gastric digestive enzymes
Transgenic rice seeds were subjected to proteolytic digestion by pepsin according to the method of Astwood et al. (1996), with some minor modifications. In brief, 150 μl of reaction buffer containing 0.01% (w/v) pepsin (Sigma, USA) and 30 mM NaCl (pH 1.2) was added to 5 mg of seed powder and incubated at 37 °C; the reaction was stopped by neutralization with NaOH at 0, 2, 5, 15, 30, 60, 120, and 180 min. For pancreatin digestion, 5 mg of seed powder was incubated at 37 °C in a buffer containing 1% (w/v) pancreatin (Nacalai Tesque, Japan) and 50 mM KH2PO4 (pH 7.5) at 0, 5, 15, 30, 60, 120, and 180 min. The reaction was terminated by heating at 100 °C for 5 min. After the addition of 150 μl of urea/SDS buffer, the digested samples were analysed by 12% SDS-PAGE, followed by Western blot analysis.
Oral vaccination of mice with transgenic rice
All experimental animal protocols were performed in accordance with guidelines approved by the animal use committee of the Tokyo Metropolitan Institute of Medical Science. For each construct, five or six 6–8-week-old female BALB/c mice (Japan Clea, Tokyo, Japan) were vaccinated orally by feeding ad libitum with a fine powder (1 g day−1) of transgenic rice seeds, or non-transgenic rice seeds as a control, for 7 days, as described previously (Suzuki et al., 2011a). Mice were given four intraperitoneal injections of 0.5 μg of recombinant Der f 2 (rDer f 2) adsorbed to alum (ImjectAlum; Pierce, Rockford, IL, USA) with a 1-week interval, beginning on days 7, 14, 21, and 28 after oral vaccination with the transgenic rice grains. Blood was collected from the mice on day 36 and allergen-specific serum IgE and IgG were assayed by ELISA.
Statistical analysis
Results were expressed as arithmetic means ±SD (standard deviation). Statistical analysis was performed by one-way analysis of variance and Dunnet’s method. P <0.05 was considered to be statistically significant.
Results
Overproduction of Der f 2 derivatives with reduced IgE reactivity by mature transgenic rice seeds
Genes encoding three different mutagenized Der f 2 derivatives were synthesized using rice seed storage protein gene sequences (Fig. 1A). To generate the Der f 2 derivative without cysteine (ΔC), all six cysteine residues were changed to serine residues. To generate the C8/119S Der f 2 derivative, the two cysteine residues at positions 8 and 119, which are involved in disulphide-bond formation, were substituted by serine residues. The 8-119C Der f 2 derivative was created by substituting four cysteine residues at positions 21, 27, 73, and 78, leaving two cysteine residues in the molecule. Genes encoding the wild-type sequence were also synthesized as controls.
Der f 2 derivative genes were first expressed in E. coli and binding to Der f 2-specific IgE was examined using sera from seven HDM allergy patients (see Supplementary Fig. S1 in JXB online). As reported by Takai et al. (2000b), removal of all three intramolecular disulphide bonds (ΔC construct) resulted in a marked reduction in specific IgE-binding compared with the wild type and the Der f 2 derivative 8-119C lacking two disulphide bonds (Cys21–Cys27 and Cys73–Cys78 ). The lower IgE-binding capacity of C8/119S compared with that of the 8-119C construct was probably due to disruption of the β-sheet structure and global folding, indicating that the disulphide bond between Cys8–Cys119 is the most critical for maintenance of the tertiary structure required for specific IgE recognition.
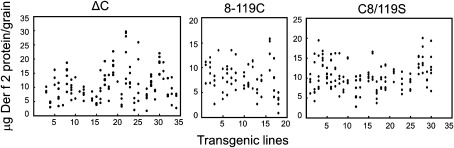
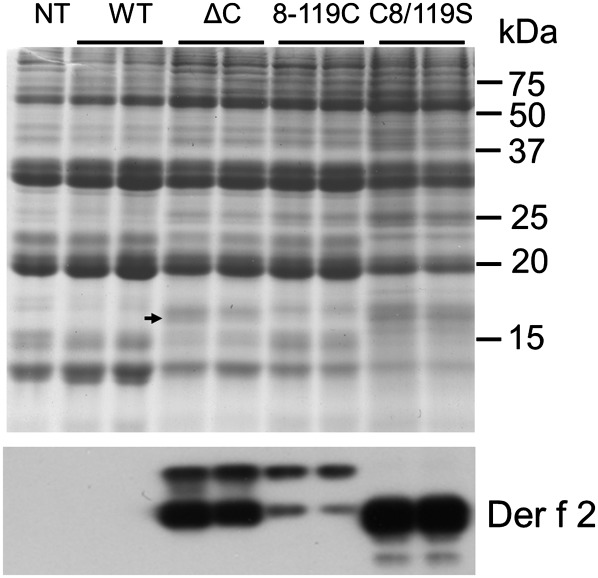
For expression of the Der f 2 derivatives in the endosperm tissue of rice seeds, proteins were engineered to contain the KDEL ER retention signal at the C terminus and the rice seed storage protein glutelin 2.3 kb GluB-1 promoter containing the signal peptide sequence (Fig. 1B). Introduction of these expression cassettes into rice by Agrobacterium-mediated transformation resulted in the accumulation of Der f 2 products in the endosperm of transgenic rice seeds. More than 20 independent transformants were generated from each individual construct (Fig. 2). Lines accumulating high levels of the Der f 2 derivatives were screened by immunoblot analysis of total seed proteins from mature grains using an anti-Der f 2 antibody. Mature seeds from the highest expression line contained 15–30 μg of Der f 2 derivative per grain (about 0.75–1.5 mg g−1 dry weight) (Fig. 2), detected as a weak CBB-stained band (Fig. 3). Although native Der f 2 contains no N-glycosylation sites (Thomas and Smith, 1998), a higher molecular weight band in addition to the major band of the estimated size was detected in the 8-119C and ΔC constructs by immunoblot analysis (Fig. 3). This may have been due to N-glycosylation at aa 71, because an N-glycosylation site (Asn-X-Ser) was accidentally introduced during mutagenesis of the cysteine residue. Endo H or PNGase F treatment led to the disappearance of the upper band, indicating the presence of N-glycan modification of the asparagine site in Der f 2 derivatives (see Supplementary Fig. S2 in JXB online).
Fig. 2.
Accumulation of Der f 2 derivatives in mature seeds of transgenic rice plants. The amounts of Der f 2 derivatives in mature seeds were examined by Western blot analysis in individual transgenic lines using Der f 2 produced in E. coli as a standard. Six seeds from each transgenic line were extracted with urea/SDS buffer and used for determination of the accumulation levels in ΔC, 8-119C, and C8/119S, and each filled dot represents the accumulation level of Der f 2 derivatives in mature seeds.
Fig. 3.
Expression of Der f 2 in transgenic rice seeds. Upper panel: 12% SDS-PAGE analysis of total proteins extracted from non-transgenic O. sativa cv. Kita-ake (NT) and transgenic rice seeds expressing native Der f 2 (WT) and its derivatives (ΔC, 8-119C, and C8/119S). The arrowhead indicates the faint bands of expressed Der f 2 derivatives. Molecular markers are shown on the right. Lower panel: immunodetection of Der f 2 derivatives with anti-Der f 2 antibody.
Subcellular localization of Der f 2 derivatives in rice endosperm
Two types of PB exist in the endosperm of wild-type rice seeds, namely, the ER-derived type I PB (PB-I), which has a smooth and spherical structure of 1–2 μm in diameter, and the type II PB (PB-II), which is a protein storage vacuole with an irregular shape. PB-Is and PB-IIs can also be distinguished by their electron density (PB-I: low density; PB-II: high density) (Tanaka et al., 1980). Glutelins and 26 kDa globulins are deposited in PB-II, whereas PB-I contains 10, 13, and 16 kDa prolamins. PB-I is derived from the ER, while PB-II is formed via the Golgi apparatus or by precursor-accumulating vesicles (Takahashi et al., 2005).
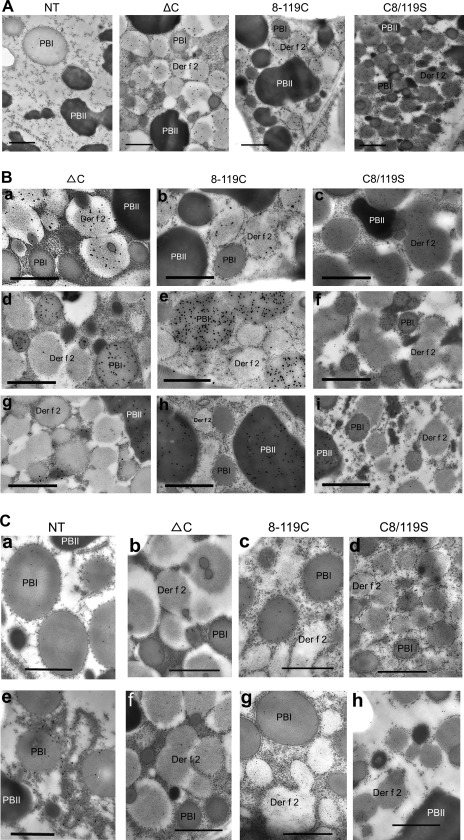
The intracellular localization of Der f 2 derivatives was examined by immunoelectron microscopy in developing endosperm cells of transgenic and non-transgenic rice 15 and 20 days after flowering using an anti-Der f 2 antibody. As shown in Fig. 4A, Der f 2 derivatives accumulated in PB-like structures in transgenic rice seed that differed from PB-I and PB-II in electron density and were tentatively named Der f 2 bodies. Der f 2 bodies had a mean size of 1–2 μm diameter and existed as lower-density organelles than PB-Is. Der f 2 bodies were distinguished from PB-Is by co-staining developing seed sections with rhodamine B and an anti-Der f 2 antibody and visualization by confocal laser-scanning microscopy. The merged images showed no overlapping signals, indicating that Der f 2 bodies exist as separate organelles (see Supplementary Fig. S3 in JXB online). Furthermore, immunogold particles specific to 13K cysteine-rich prolamin (RM1) and GluA were localized predominantly in PB-Is and PB-IIs, respectively, but not in Der f 2 bodies (Fig. 4B). Der f 2 bodies were labelled with gold particle-conjugated anti-Der f 2 antibody, indicating the accumulation of Der f 2 derivatives in Der f 2 bodies (Fig. 4A, B). Furthermore, immunoelectron microscopy observation showed that PDI or maize BiP chaperones, which are characteristic of the ER lumen and involved in folding and assembly of newly synthesized proteins, were localized predominantly in Der f 2 bodies as well as PB-Is (Fig. 4C). This finding indicated that Der f 2 bodies are derived from the ER. Notably, Der f 2 bodies were enclosed in ER-derived membranes, which are characterized by polysomes, and were also located within the ER lumen, indicating that Der f 2 bodies are ER-derived structures and may be formed as aggregates of Der f 2 derivatives synthesized on rough ER ribosomes and then transported into the ER lumen. It was interesting that immunogold particles specific to PDI and BiP were localized mainly to the peripheral region of PB-Is and within the high-density regions of Der f 2 bodies. This difference in their localization may reflect the role they play in the folding and assembly of Der f 2 derivatives deposited in Der f 2 bodies. Most Der f 2 bodies were observed to bud off from the ER as discrete spherical organelles surrounded by ribosomes, as shown in Fig. 4. In transgenic rice containing the ΔC and C8/119S Der f 2 constructs, certain Der f 2 bodies were associated with PB-Is but were not fused to PB-Is (see Supplementary Fig. S4 in JXB online). Der f 2 bodies exhibited variations in electron density and morphological structure among constructs. This variation may reflect differences in the physical properties of Der f 2 derivatives. Another explanation is that accumulation levels of Der f 2 derivatives may be involved in the intensity of electron density of individual Der f 2 bodies in light of the fact that the C8/119S Der f 2 derivative accumulated at a higher level than the ΔC and 8-119C derivatives. It is notable that the ΔC and 8-119C constructs were associated with empty spaces within Der f 2 bodies, although they normally exhibited a round structure (Fig. 4B).
Fig. 4.
Intracellular localization of Der f 2 derivatives in developing rice endosperm cells observed by immunoelectron microscopy. (A) PB-I (PBI), PB-II (PBII), and Der f 2 bodies are indicated. Anti-Der f 2 antibody labelled with 20 nm gold particles was distributed in the Der f 2 bodies. No Der f 2 bodies were detected in the control non-transgenic O. sativa cv. Kita-ake (NT) seeds. (B) Gold particles were labelled with anti-Der f 2 (a–c), anti-13K cysteine-rich prolamin (RM1) (d–f), and anti-GluA (g–i) antibodies. The Der f 2 derivatives were located specifically in Der f 2 bodies, while RM1 and GluA localized to PB-Is and PB-IIs, respectively. (C) Gold particles were labelled with anti-maize BiP (a–d) and ant-rice PDI 1-1 (e–h) antibodies, respectively. The transgenic constructs ΔC (b, f), 8-119C (c, g), and C8/119S (d, h) are indicated, while non-transgenic O. sativa cv. Kita-ake (NT) was used as control (a, e). Bars: 1 μm.
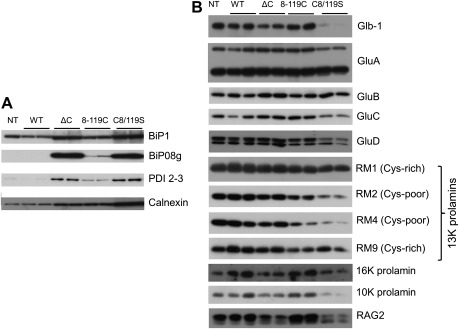
The increased expression of Der f 2 derivatives as secretory proteins altered the structure of PBs, particularly in seeds containing the C8/119S construct in which the mean size of the PB-Is and PB-IIs decreased to less than 0.5 μm in diameter. Changes in the structure and number of PB-Is and PB-IIs correlated with a decrease in prolamin and glutelin levels (Figs 3 and 5). By contrast, the size and structure of PB-Is and PB-IIs were not affected by the 8-119C construct, which could be due to the lower accumulation level of this Der f 2 derivative in the seed (Figs 3 and 5).
Fig. 5.
Effect of accumulation of Der f 2 derivatives on molecular chaperones and seed storage proteins. (A) Immunoblot analysis of the chaperones BiP1 (Os02g0115900), BiP08g, PDI 2-3, and calnexin. (B) Immunoblot analysis of glutelins (GluA, GluB, GluC, and GluD), 26K globulin (Glb-1), 13 kDa prolamins (RM1, RM2, RM4, and RM9), 10 and 16 kDa (10K and 16K, respectively) prolamins, and RAG2 allergen proteins.
Taken together, these results suggested that the progressive accumulation of abnormal Der f 2 derivatives in the ER lumen during seed development may induce the ER stress response or unfolded protein response, which may alter the structure and reduce the size of PBs.
ER stress response caused by accumulation of Der f 2 bodies
The effect of the accumulation of Der f 2 derivatives on the expression of chaperones and enzymes involved in disulphide-bond formation or glycosylation was examined by immunoblotting of mature seed extracts. Figure 5A shows that BiP1 and calnexin levels were 3–5-fold higher in ΔC, 8-119C, and C8/119S transgenic seeds than in non-transgenic seeds. BiP08g and PDI 2-3 were also upregulated in transgenic rice. These findings suggested that the abnormal morphology of ER-derived PBs (PB-Is) in transgenic seeds may be attributed to ER stress caused by accumulation of Der f 2 derivatives in the ER lumen.
Effect on synthesis of seed storage proteins
SDS-PAGE separation of total seed proteins showed that the accumulation of high levels of Der f 2 derivatives in the ER lumen caused a reduction in seed storage proteins (Fig. 5B), particularly in transgenic plants containing the C8/119S construct. Immunoblotting against specific seed storage proteins revealed that expression of 26 kDa globulin, 16 kDa prolamin, 13 kDa cysteine-poor prolamins (RM2 and RM4), and 10 kDa prolamin was significantly reduced in the mature seeds. Inhibition of these seed storage proteins is commonly observed in transformants that accumulate recombinant products in the seeds (Oono et al., 2010). Notably, the ΔC and C8/119S transgenics showed a decrease in 14–16 kDa RAG2 (α-amylase inhibitor, an allergenic protein), as reported previously for other transformants (Yang et al., 2008).
Solubility of Der f 2 derivatives
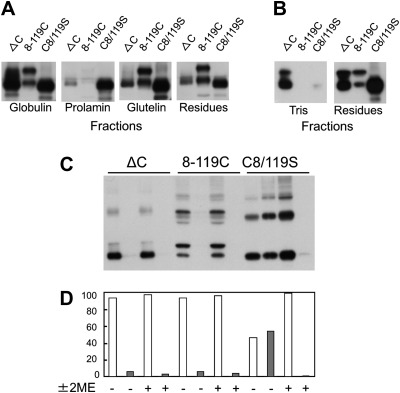
The Der f 2 derivatives in Der f 2 bodies were examined further by sequential extraction in different buffers. Surprisingly, significant amounts of Der f 2 derivatives (30–60%) were found in the globulin fraction extracted with 0.5 M NaCl, despite their aggregation as Der f 2 bodies surrounded by ER membranes similar to PB-Is (Fig. 6A). Notably, about 40% of Der f 2 derivatives lacking all six Cys residues (ΔC construct) were soluble in 20 mM Tris/HCl buffer (Fig. 6B). Furthermore, significant amounts (20–30%) of C8/119S Der f 2 derivatives were extracted with the prolamin fraction in isopropanol (Fig. 6A), suggesting their interaction with prolamins.
Fig. 6.
Solubility of Der f 2 derivatives in transgenic rice seeds analysed by sequential extraction and immunoblotting. (A) The glutelin fraction was extracted with 1% (v/v) lactic acid after pre-extraction of the globulin fraction with a saline buffer [0.5 M NaCl, 10 mMTris/Cl(pH 7.5)], followed by prolamin fraction extraction with 60% (v/v) n-propanol containing 5% (v/v) 2-ME. (B) Extraction of Der f 2 derivatives from transgenic seeds using 20mM Tris/HCl buffer (pH 7.5). (C) Extraction of Der f 2 derivatives from transgenic seeds using urea/SDS buffer with or without 2-ME. (D) Graphic depiction of the extraction efficiency of proteins in (C). Open bars represent the supernatant containing Der f 2 derivatives, while filled bars represent derivatives in the insoluble residue.
The sequential extraction experiment revealed the effect of disulphide-bond disruption on the solubility of the different Der f 2 derivatives (Fig. 6A). Der f 2 derivatives containing only one disulphide bond between positions 8 and 119 (8-119C) were soluble in an extraction buffer containing urea in the absence of 2-ME, while extraction of Der f 2 from transgenic seeds in which the disulphide bond was disrupted (C8/119S) required the addition of 2-ME (Fig. 6C, D). These findings suggested that four of the cysteine residues (Cys21, Cys27, Cys73, and Cys78) are likely to participate in the interaction between Der f 2 and cysteine-rich prolamines as a result of the alteration of tertiary structure caused by lack of this disulphide bond. The Cys8–Cys119 disulphide bond may therefore play a critical role in determining the physico-chemical properties of Der f 2, confirming that it is important in the determination of the conformational structure.
In vitro digestion of transgenic rice by gastric digestive enzymes
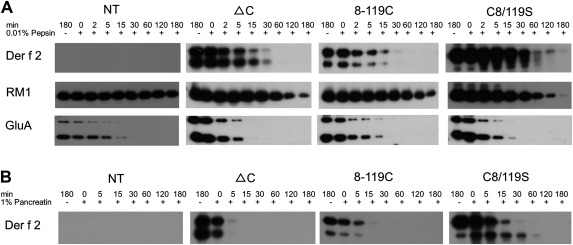
The digestibility of Der f 2 derivatives in Der f 2 bodies from transgenic seeds was compared with that of the PB-I and PB-II storage proteins from non-transgenic seeds by in vitro digestion with pepsin or pancreatin (Fig. 7). The ΔC and 8-119C Der f 2 derivatives were quickly digested within 30 min by pepsin, similar to PB-II glutelins, and in contrast to the 13K cysteine-rich prolamin (RM1) from ER-derived PB (PB-I), which required several hours for digestion (Fig. 7A). Digestibility by gastrointestinal enzymes was comparable for PB-II proteins and the ΔC and 8-119C Der f 2 derivatives. Notably, the C8/119S Der f 2 derivative was more resistant to pepsin than the ΔC and 8-119C Der f 2 derivatives. A similar result was obtained for pancreatin treatment, which required more than 30 min for digestion of the C8/119S Der f 2 derivatives, whereas the ΔC and 8-119C derivatives required 5 and 15 min for complete digestion, respectively (Fig. 7B). Interestingly, the 8-119C derivative was more resistant to pancreatin than the ΔC derivative. The differences in resistance to digestive enzymes correlated with the extraction properties (Fig. 6) and with the interaction with prolamins in the ER lumen.
Fig. 7.
In vitro digestibility of transgenic rice seed powder containing Der f 2 derivatives. Seed powder (5 mg) was added to reaction mixtures containing 0.01% pepsin (A) or 1% pancreatin (B) and incubated at 37 °C for up to 3 h. Total proteins in each reaction mixture were extracted with urea/SDS buffer and examined by immunoblotting with the corresponding antibodies.
Tolerogenic efficiency of transgenic rice seeds accumulating Der f 2 derivatives
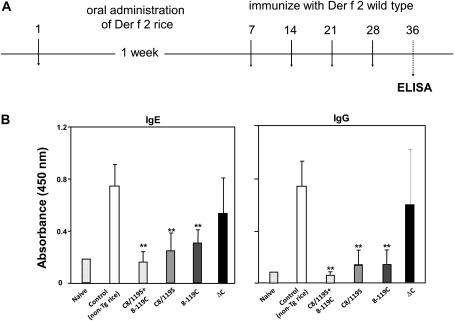
The efficacy of transgenic rice containing the Der f 2 derivatives as a tolerogen was evaluated by feeding them to model mice, as described previously (Takagi et al., 2010; Suzuki et al., 2011a). Der f 2-specific IgE and IgG responses were evaluated after oral administration of rice seeds containing the Der f 2 derivatives. First, sera from mice immunized with purified rDer f 2 were assessed for increased Der f 2-specific IgE and IgG levels compared with levels in non-immunized mice to confirm a positive reaction to vaccination in allergen-immunized mice (Fig. 8A). To investigate the preventative effect of transgenic rice seeds, mice were treated by oral administration of transgenic rice seeds containing Der f 2 derivatives for 7 consecutive days prior to immunization with the rDer f 2 antigen (0.5 μg per mouse). As shown in Fig. 8B, the rDer f 2-induced increase in allergen-specific IgE levels was significantly reduced by feeding mice with transgenic seeds containing C8/119S, 8-119C, or a mixture of both, but no effect was observed with non-transgenic seeds. Similar results were obtained for Der f 2-specific IgG. Notably, feeding of transgenic seeds containing ΔC slightly decreased Der f 2-specific IgE production, but the suppression was very low compared with that obtained with the C8/119S or 8-119C construct. The differences in the efficacy of transgenic seeds on the suppression of Der f 2-specific IgE levels may be attributed to differences in antigen delivery to gut-associated lymphoid tissue (GALT), caused by the different digestibility of ΔC-, C8/119S-, and 8-119C-containing derivatives.
Fig. 8.
Immunosuppressive effect of oral vaccination with transgenic rice seeds expressing ΔC, C8/119S, and 8-119C Der f 2 derivatives. (A) Feeding schedule and challenge with Der f 2. (B) Inhibition of Der f 2-specific serum IgE and IgG. BALB/c mice were vaccinated orally by feeding ad libitum with a diet containing the powder of transgenic rice grains and a commercial mouse diet mixed at a 1:1 ratio. After oral vaccination for 1 week with the transgenic rice grains, mice were given four intraperitoneal injections of 0.5 μg rDer f 2 adsorbed onto alum on days 7, 14, 21, and 28. Blood was collected from the mice on day 36 and allergen-specific serum IgE and IgG were assayed by ELISA. Data represent the mean ±SD of five or six animals in each group. **, P <0.05 compared with the non-transgenic rice group.
Discussion
Allergen-specific immunotherapy is usually administered by subcutaneous, sublingual, or nasal routes. The efficacy of oral vaccination has not yet been determined conclusively, although many investigations are ongoing. Seed-based allergy vaccines can be administered orally because the double barrier formed by the PB and cell wall encapsulates and protects antigens from proteolysis by digestive enzymes and ensures their delivery to GALT (Nochi et al., 2007; Takagi et al., 2010). Seed-based vaccines have additional advantages such as stability at ambient temperatures for several years and high production yields, in addition to plant-specific benefits such as mammalian pathogen-free safety and needle-free administration (Hiroi et al., 2011; Takaiwa, 2011).
To enhance the production of Der f 2 derivatives, a Der f 2 gene was synthesized using codons optimized for rice endosperm expression. The KDEL ER retrieval signal was attached to the C terminus of the Der f 2 derivatives (Schouten et al., 1996; Takagi et al., 2005) and the N-terminal glutelin GluB-1 signal peptide was added to express the Der f 2 derivatives as secretory proteins. Unexpectedly, codon-optimized native Der f 2 did not accumulate at levels comparable to those of the other Der f 2 derivatives (see Supplementary Fig. S5 in JXB online). Recently, Der f 2 was found to have homology with MD-2, a lipopolysaccharide-binding component of the TLR4 signalling complex (Ichikawa et al., 2009). As the lipopolysaccharide-binding property of Der f 2 is unlikely to be involved in determining its level of accumulation, the low level of accumulation of the native Der f 2 protein may be related to some structural property of this protein. Similar results were obtained with the native cedar pollen allergens Cry j 1 and Cry j 2 (Yang et al., 2007; Suzuki et al., 2011b). Importantly, deconstructed antigens such as shuffled or mosaic molecules, or artificial gene products such as T-cell epitope peptides, accumulate at higher levels when expressed in rice endosperm than native proteins and are not affected by ER-associated degradation (Takagi et al., 2005). As shown in Fig. 5A, chaperones such as BiP proteins and PDI were upregulated in transgenic rice seeds expressing Der f 2 derivatives, which may have caused the difference in the distribution of ER chaperones between Der f 2 bodies and PB-Is, as shown in Fig. 4C. The accumulation of several seed proteins, including 26 kDa globulin, 16 kDa prolamin, 13 kDa prolamin, and 10 kDa prolamin, was also decreased by Der f 2 derivatives (Fig. 5B), which could be a response to ER stress. Another possible explanation is that the aggregation of Der f 2 derivatives in the ER lumen in the absence of the ER-associated degradation response may inhibit the synthesis of other seed storage proteins to maintain a constant protein content in the seed endosperm (Tada et al., 2003; Kawakatsu et al., 2010).
Stable and high accumulation of engineered proteins with altered structure is restricted to endosperm tissue in rice. When produced under the control of a constitutive promoter in vegetative tissues such as the leaf or root, foreign products such as T-cell epitope peptides do not accumulate to detectable levels because these unfolded proteins are degraded by quality-control mechanisms (Takaiwa et al., 2007). Thus, the rice endosperm can provide a good production platform for many kinds of recombinant proteins because they accumulate stably. This may be related to the amounts of chaperone proteins required for the production of marked amounts of storage proteins during seed maturation. Because the seed is edible tissue, and T-cell epitopes produced in rice seeds are preserved even after heat treatment at 100 °C for 30 min (steaming), the production of antigens with reduced IgE-binding capacity by alteration of their conformational structure in transgenic rice seeds has many advantages.
Notably, production of modified Der f 2 derivatives as secretory proteins in the rice endosperm modified the intracellular structure of the endosperm compared with that in the wild type (Fig. 4A). Endosperm cells accumulated Der f 2 bodies, characterized by their low electron density compared with that of PB-Is and PB-IIs. Der f 2 bodies, which were identified as unique storage organelles derived from the ER, differed from PB-Is and PB-IIs in their morphological and physico-chemical properties. Der f 2 derivatives specifically accumulated at relatively higher amounts (15–30 μg per grain) in Der f 2 bodies of mature transgenic rice seeds (Fig. 2).
Der f 2 bodies were surrounded by ribosome-studded ER membranes, indicating their origin in the ER (Figs 4 and Supplementary S4). The ER origin of Der f 2 bodies was further confirmed by the localization of ER-specific BiP and PDI chaperones to Der f 2 bodies and PB-Is (Fig. 4C). Der f 2 derivatives aggregated in the ER lumen, resulting in the formation and budding of Der f 2 bodies from the cisternal ER into the cytoplasm, while remaining surrounded by ER membranes similar to other ER-derived PBs. The electron density of Der f 2 bodies, which was lower than that of PB-Is, varied between different Der f 2 derivative-containing bodies. This may be related to the concentration of Der f 2 derivatives or aggregation properties dependent on the number and type of disulphide bonds present, as well as the interaction with cysteine-rich prolamins. Interestingly, vacant space within Der f 2 bodies was found in transgenic rice expressing the ΔC and C8/119S constructs. Certain Der f 2 bodies incorporated PB-I within the Der f 2 body (Figs 4 and Supplementary S4).
Similar novel PB formation has been reported for maize prolamins (γ- and β-zeins) expressed in tobacco and Arabidopsis leaves (Geli et al., 1994; Bagga et al., 1995). Chimeric fusion of the proline-rich N-terminal domain of maize prolamin γ-zein (zera) or erastin-like polypeptide (ELP) induced PB formation in plant vegetative tissues (Conley et al., 2009; Torrent et al., 2009). Zeolin, a fusion of the phaseolin and γ-zein sequences, and GFP fused to rice prolamin, formed PBs in the ER of tobacco and rice vegetative tissues (Mainieri et al., 2004; Saito et al., 2009). Proline-rich regions, Pro-X motifs, and cysteine residues are involved in the biogenesis of PBs through aggregation and self-assembly (Geli et al., 1994; Llop-Tous et al., 2010). In the case of ELP, PB formation is related to the physical properties of the protein, which is rich in hydrophobic residues. The KDEL ER retention signal is indispensable for induction of PBs in ELP. When the ELP–intein–PAL (plant lectin) fusion was expressed in rice seeds, the formation of PB-Is was disturbed and the morphology of PB-Is was distorted (Tian and Sun, 2011). However, production of this ELP fusion did not result in the generation of a new PB. Furthermore, when amyloid proteins and cytokines (IL-10) were produced as secretory proteins in the rice endosperm, a large number of very small abnormal PBs containing amyloids and prolamins accumulated, in addition to the typical PB-I containing prolamins in the endosperm (Oono et al., 2010). By contrast, the HDM group 1 allergen Der p 1 was normally deposited in PB-Is, even if higher amounts of Der p 1 accumulated in endosperm cells than Der f 2 (Yang et al., 2008; Suzuki et al, 2011a). Further work will be required to elucidate the mechanisms involved in biogenesis of Der f 2 bodies.
Recombinant allergens with low specific IgE-binding capacity (allergenicity) but retaining T-cell epitopes (immunogenicity) hold promise as tolerogens for allergen-specific immunotherapy because their hypoallergenicity enables their administration at high doses, and the clinical efficacy of immunotherapy is generally dose dependent. Several hypoallergenic molecules have been created by alterations in conformation, site-directed mutagenesis, and deletion or shuffling of molecules (Valenta et al., 2010). The tertiary structure of group 2 allergens containing Der f 2 and Der p 2 is critical for recognition of specific IgEs, and disruption of disulphide bonds substantially reduces their allergenicity. Assessment of the immunological properties of Der f 2 derivatives by immunoblotting using sera from HDM allergy patients showed a marked decrease in specific IgE-binding in ΔC constructs, which were devoid of all six cysteine residues, and in C8/119S constructs (Supplementary Fig. S1; Takai et al., 2000b).
In contrast, the physico-chemical properties of Der f 2 bodies containing different derivatives were dependent on the type and number of disulphide bonds. When all six cysteine residues of Der f 2 were substituted by serine residues, the Der f 2 derivative (ΔC) was soluble in extraction buffer (Fig. 6B) and was rapidly digested by gastrointestinal enzymes in comparison with the other derivatives (Fig. 7). Although Der f 2 derivatives are bio-encapsulated in Der f 2 bodies, their resistance to digestive enzymes was weaker than that of PB-Is. Disruption of the C8/119S construct generated a Der f 2 that interacted with prolamins, resulting in higher resistance to digestive enzymes.
When transgenic rice seeds expressing ΔC derivatives were fed to mice that were subsequently challenged with native Der f 2, the reduction in specific IgE or IgG levels was not sufficient and its efficacy as a tolerogen was very low. In contrast, transgenic rice seeds containing C8/119S significantly suppressed specific IgE and IgG responses in mice (Fig. 8). This difference in the tolerogenic efficacy of the two types of transgenic rice may be associated with the resistance of Der f 2 bodies to digestion by gastrointestinal enzymes as a result of specific physico-chemical properties (Fig. 7). These findings suggest that digestibility of antigens may be one of the critical factors determining the efficacy of seed-based allergy vaccines containing Der f 2 derivatives.
Taken together, the present results showed the feasibility of engineered hypoallergenic derivatives expressed in the seed endosperm for use as orally administered allergy vaccines. The efficient delivery of these antigens to GALT, however, requires the development of derivatives resistant to digestive enzymes in the gastrointestinal tract.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Immunoblot analysis of IgE-binding reactivity of Der f 2 derivatives. A 0.5 mg aliquot of the E. coli culture-derived wild-type (WT) Der f 2 protein and its derivatives was analysed using sera of seven HDM-allergic patients (P1–P7). CBB staining was used to assess equal loading.
Fig. S2. Analysis of glycoproteins of Der f 2 derivatives (ΔC and 8-119C). Immunoblot analysis showed increased electrophoretic mobility after deglycosylation by treatment with (+) or without (–) Endo H or PNGase F compared with the controls. The arrow indicates the glycosylated Der f 2 band.
Fig. S3. Localization of Der f 2 derivatives in developing seeds. Red and green signals indicated PB-Is labelled with rhodamine and immunostained with anti-Der f 2 antibody, respectively. Bars: 10 μm.
Fig. S4. Immunoelectron microscopic analysis of Der f 2 derivatives in developing seeds. Gold particles labelled with anti-13K cysteine-rich prolamin (RM1) antibody were located specifically in small PB-Is, which were mixed but not fused with Der f 2 bodies (left and middle panels). The right panel shows a Der f 2 body surrounded by ribosomes. Bars: 1 μm.
Fig. S5. Immunoblotting analysis of native Der f 2 protein in transgenic seeds. Four independent lines of native Der f 2 (WT) transgenic rice seeds were assayed. Faint bands were visible only after overexposure of the film. C8/119S was used as a positive control. NT, non-transgenic O. sativa cv. Kita-ake.
Acknowledgments
We thank Dr R.S. Boston (North Carolina State University, NC, USA) for providing the anti-maize BiP antibody, and Ms M. Utsuno, Ms Y. Ikemoto, Ms H. Yajima, and Ms. Y Suzuki for technical assistance. This work was supported by an Agri-Health Translational Research Project grant from the Ministry of Agriculture Forestry and Fisheries of Japan to F.T. and T.H.
Glossary
Abbreviations
- ELP
erastin-like polypeptide
- Endo H
endoglycosidase H
- ER
endoplasmic reticulum
- GALT
gut-associated lymphoid tissue
- HDM
house dust mites
- 2-ME
2-mercaptoethanol
- PB
protein body
- PNGase F
peptide N-glycosidase F
- SD
standard deviation
References
- Astwood JD, Leach JN, Fuchs RL. Stability of food allergens to digestion in vitro. Nature Biotechnology. 1996;14:1269–1273. doi: 10.1038/nbt1096-1269. [DOI] [PubMed] [Google Scholar]
- Bagga S, Adams H, Kemp JD, Sengupta-Gopalan C. Accumulation of 15-kilodalton zein in novel protein bodies in transgenic tobacco. Plant Physiology. 1995;107:13–23. doi: 10.1104/pp.107.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua KY, Huang CH, Shen HD, Thomas WR. Analysis of sequence polymorphism of a major mite allergen, Der p 2. Clinical and Experiment Allergy. 1996;26:829–837. [PubMed] [Google Scholar]
- Conley AJ, Joensuu JJ, Menassa R, Brandle JE. Induction of protein body formation in plant leaves by elastin-like polypeptide fusions. BMC Biology. 2009;7:48. doi: 10.1186/1741-7007-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derewenda U, Li J, Derewenda Z, Dauter Z, Mueller GA, Rule GS, Benjamin DC. The crystal structure of a major dust mite allergen Der p 2, and its biological implication. Journal of Molecular Biology. 2002;318:189–197. doi: 10.1016/S0022-2836(02)00027-X. [DOI] [PubMed] [Google Scholar]
- Frew AJ. Immunotherapy of allergic disease. Journal of Allergy and Clinical Immunology. 2003;111:S712–S719. doi: 10.1067/mai.2003.84. [DOI] [PubMed] [Google Scholar]
- Fujii S, Ono K, Takeuchi A, Aki T, Shigeta S, Suzuki O, Jyo T, Yamashita U. Identification of T-cell epitope sequences on an important mite antigen. Clinical and Experimental Allergy. 1997;27:1086–1094. doi: 10.1111/j.1365-2222.1997.tb01261.x. [DOI] [PubMed] [Google Scholar]
- Geli MI, Torrent M, Ludevid D. Two structural domains mediate two sequential events in γ-zein targeting: protein endoplasmic reticulum retention and protein body formation. The Plant Cell. 1994;6:1911–1922. doi: 10.1105/tpc.6.12.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto F, Yoshihara T, Shigemoto N, Toki S, Takaiwa F. Iron fortification of rice seed by the soybean ferritin gene. Nature Biotechnology. 1999;17:282–286. doi: 10.1038/7029. [DOI] [PubMed] [Google Scholar]
- Haida M, Okudaira H, Ogita T, Ito K, Miyamoto T, Nakajima T, Hongo O. Allergens of the house dust mite Dermatophagoides farinae – immunochemical studies of four allergenic fractions. Journal of Allergy and Clinical Immunology. 1985;75:686–692. doi: 10.1016/0091-6749(85)90094-6. [DOI] [PubMed] [Google Scholar]
- Hiroi T, Kaminuma O, Takaiwa F. Vaccination with transgenic rice seed expressing mite allergen: a new option for asthma sufferers? Expert Review of Vaccines. 2011;10:1249–1251. doi: 10.1586/erv.11.102. [DOI] [PubMed] [Google Scholar]
- Ichikawa S, Takai T, Yashiki T, Takahashi S, Okumura K, Ogawa H, Kohda D, Hatanaka H. Lipopolysaccharide binding of the mite allergen Der f 2. Genes to Cells. 2009;14:1055–1065. doi: 10.1111/j.1365-2443.2009.01334.x. [DOI] [PubMed] [Google Scholar]
- Inoue R, Matuoka T, Kondo N, Nishimura Y, Matsushita S. Identification of Dermatophagoides farinae-2-derived peptides and class II HLA molecules recognized by T cells from atopic individuals. International Archives of Allergy and Immunology. 1997;114:354–360. doi: 10.1159/000237694. [DOI] [PubMed] [Google Scholar]
- Johannessen BR, Skov LK, Kastrup JS, Kristensen O, Bolwig C, Larsen JN, Spangfort M, Lund K, Gajhede M. Structure of the house dust mite allergen Der f 2: implications for function and molecular basis of IgE cross-reactivity. FEBS Letters. 2005;579:1208–1212. doi: 10.1016/j.febslet.2004.11.115. [DOI] [PubMed] [Google Scholar]
- Kawakatsu T, Hirose S, Yasuda H, Takaiwa F. Reducing rice seed storage protein accumulation leads to changes in nutrient quality and storage organelle formation. Plant Physiology. 2010;154:1842–1854. doi: 10.1104/pp.110.164343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S, Aki T, Yamashita M, et al. Toward elucidating the full spectrum of mite allergens- state of the art. Journal of Bioscience and Bioengineering. 2002;94:285–298. doi: 10.1263/jbb.94.285. [DOI] [PubMed] [Google Scholar]
- Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nature Reviews Immunology. 2006;6:761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- Linhart B, Valenta R. Molecular design of allergy vaccines. Current Opinion in Immunology. 2005;17:645–655. doi: 10.1016/j.coi.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Llop-Tous I, Madurga S, Giralt E, Marzabal P, Torrent M, Ludevid MD. Relevant elements of a maize γ-zein domain involved in protein body biogenesis. Journal of Biological Chemistry. 2010;285:35633–35644. doi: 10.1074/jbc.M110.116285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainieri D, Rossi M, Archinti M, Bellucci M, De Marchis F, Vavassori S, Pompa A, Arcioni S, Vitale A. Zeolin. A new recombinant storage protein constructed using maize γ-zein and bean phaseolin. Plant Physiology. 2004;136:3447–3456. doi: 10.1104/pp.104.046409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama C, Fukuda M, Usui Y, Iwamoto N, Yuuki T, Okumura Y, Okudaira H. Analysis of the IgE-epitope of Der f 2, a major mite allergen, by in vitro mutagenesis. Molecular Immunology. 1995;32:1021–1029. doi: 10.1016/0161-5890(95)00091-7. [DOI] [PubMed] [Google Scholar]
- Nochi T, Takagi H, Yuki Y, et al. Rice-based mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination. Proceedings of the National Academy of Sciences, USA. 2007;104:10986–10991. doi: 10.1073/pnas.0703766104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RM, Thomas WR, Nicholson I, Lamb JR, Tait BD. An immunogenetic analysis of the T-cell recognition of the major house dust mite allergen Der p 2: identification of high- and low-responder HLA-DQ alleles and localization of T-cell epitopes. Immunology. 1995;86:176–182. [PMC free article] [PubMed] [Google Scholar]
- O’Hehir RE, Verhoef A, Panagiotopoulou E, Keswani S, Hayball JD, Thomas WR, Lamb JR. Analysis of human T cell responses to the group II allergen of Dermatophagoides species: localization of major antigenic sites. Journal of Allergy and Clinical Immunology. 1993;92:105–113. doi: 10.1016/0091-6749(93)90044-g. [DOI] [PubMed] [Google Scholar]
- Oono Y, Wakasa Y, Hirose S, Yang L, Sakuta C, Takaiwa F. Analysis of ER stress in developing rice endosperm accumulating β-amyloid peptide. Plant Biotechnology Journal. 2010;8:671–718. doi: 10.1111/j.1467-7652.2010.00502.x. [DOI] [PubMed] [Google Scholar]
- Park GM, Lee SM, Lee IY, Ree HI, Kim KS, Hong CS, Yong TS. Localization of a major allergen, Der p 2, in the gut and faecal pellets of Dermatophogoides pteronyssinus. Clinical and Experimental Allergy. 2000;30:1293–1297. doi: 10.1046/j.1365-2222.2000.00883.x. [DOI] [PubMed] [Google Scholar]
- Qu LQ, Takaiwa F. Evaluation of tissue specificity and expression strength of rice seed component gene promoters in transgenic rice. Plant Biotechnology Journal. 2004;2:113–125. doi: 10.1111/j.1467-7652.2004.00055.x. [DOI] [PubMed] [Google Scholar]
- Saito Y, Kishida K, Takata K, Takahashi H, Shimada T, Tanaka K, Morita S, Satoh S, Masumura T. A green fluorescence protein fused to rice prolamin forms protein body-like structures in transgenic rice. Journal of Experimental Botany. 2009;60:615–627. doi: 10.1093/jxb/ern311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten A, Roosien J, van Engelen FA, et al. The C-terminal KDEL sequence increases the expression level of a single-chain antibody designed to be targeted to both the cystosol and the secretory pathway in transgenic tobacco. Plant Molecular Biology. 1996;30:781–793. doi: 10.1007/BF00019011. [DOI] [PubMed] [Google Scholar]
- Seigneurin-Berny D, Rolland N, Garin L, Joyard J. Differential extraction of hydrophobic proteins from chloroplast envelope membranes: a subcellular-specific proteomic approach to identify rare intrinsic membrane proteins. The Plant Journal. 1999;19:217–228. doi: 10.1046/j.1365-313x.1999.00511.x. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Kaminuma O, Yang L, et al. Prevention of allergic asthma by vaccination with transgenic rice seed expressing mite allergen: induction of allergen-specific oral tolerance without bystander suppression. Plant Biotechnology Journal. 2011a;9:982–990. doi: 10.1111/j.1467-7652.2011.00613.x. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Yang L, Takaiwa F. Transgenic rice accumulating modified cedar pollen allergen Cry j 2 derivatives. Journal of Bioscience and Bioengineering. 2011b doi: 10.1016/j.jbiosc.2011.10.005. (in press) [DOI] [PubMed] [Google Scholar]
- Tada Y, Utsumi S, Takaiwa F. Foreign gene products can be enhanced by introduction into low storage protein mutants. Plant Biotechnology Journal. 2003;1:411–422. doi: 10.1046/j.1467-7652.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- Takagi H, Saito S, Yang L, Nagasaka S, Nishizawa N, Takaiwa F. Oral immunotherapy against a pollen allergy using a seed-based peptide vaccine. Plant Biotechnology Journal. 2005;3:521–533. doi: 10.1111/j.1467-7652.2005.00143.x. [DOI] [PubMed] [Google Scholar]
- Takagi H, Hiroi T, Hirose S, Yang L, Takaiwa F. Rice seed ER-derived protein body as an efficient delivery vehicle for oral tolerogenic peptides. Peptides. 2010;31:1421–1425. doi: 10.1016/j.peptides.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Saito Y, Kitagawa T, Morita S, Masumura T, Tanaka K. A novel vesicle derived directly from endoplasmic reticulum is involved in the transport of vacuolar storage proteins in rice endosperm. Plant Cell Physiology. 2005;46:245–249. doi: 10.1093/pcp/pci019. [DOI] [PubMed] [Google Scholar]
- Takai T, Ichikawa S, Hatanaka H, Inagaki F, Okumura Y. Effects of proline mutations in the major house dust mite allergen Der f 2 on IgE-binding and histamine-releasing activity. European Journal of Biochemistry. 2000a;267:6650–6656. doi: 10.1046/j.1432-1327.2000.01760.x. [DOI] [PubMed] [Google Scholar]
- Takai T, Ichikawa S, Yokota T, Hatanaka H, Inagaki F, Okumura Y. Unlocking the allergenic structure of the major house dust mite allergen Der f 2 by elimination of key intramolecular interactions. FEBS Letters. 2000b;484:102–107. doi: 10.1016/s0014-5793(00)02096-2. [DOI] [PubMed] [Google Scholar]
- Takai T, Mori A, Yuuki T, Okudaira H, Okumura Y. Non-anaphylactic combination of partially deleted fragments of the major house dust mite allergen Der f 2 for allergen-specific immunotherapy. Molecular Immunology. 1999;36:1055–1065. doi: 10.1016/s0161-5890(99)00098-x. [DOI] [PubMed] [Google Scholar]
- Takai T, Yokota T, Yasue M, Nishiyama C, Yuuki T, Mori A, Okudaira H, Okumura Y. Engineering of the major house dust mite allergen Der f 2 for allergen-specific immunotherapy. Nature Biotechnology. 1997;15:754–758. doi: 10.1038/nbt0897-754. [DOI] [PubMed] [Google Scholar]
- Takaiwa F. Transgenic rice seed as a nutriceutical delivery system. CAB reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 2, no. 2007:041. [Google Scholar]
- Takaiwa F. Seed-based oral vaccines as allergen-specific immunotherapies. Human Vaccines. 2011;7:357–366. doi: 10.4161/hv.7.3.14302. [DOI] [PubMed] [Google Scholar]
- Takaiwa F, Takagi H, Hirose S, Wakasa Y. Endosperm tissue is good production platform for artificial recombinant proteins in transgenic rice. Plant Biotechnology Journal. 2007;5:84–92. doi: 10.1111/j.1467-7652.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- Takaiwa F, Hirose S, Takagi H, Yang L, Wakasa Y. Deposition of a recombinant peptide in ER-derived protein bodies by retention with cysteine-rich prolamins in transgenic rice seed. Planta. 2009;229:1147–1158. doi: 10.1007/s00425-009-0905-7. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Sugimoto T, Ogawa M, Kasai Z. Isolation and characterization of two types of protein bodies in the rice endosperm. Agricultural and Biological Chemistry. 1980;44:1633–1639. [Google Scholar]
- Thomas WR, Chua KY. The major mite allergen Der p 2 – a secretion of the male mite reproductive tract. Clinical and Experimental Allergy. 1995;25:667–669. doi: 10.1111/j.1365-2222.1995.tb01117.x. [DOI] [PubMed] [Google Scholar]
- Thomas WR, Smith W. House-dust-mite allergens. Allergy. 1998;53:821–832. doi: 10.1111/j.1398-9995.1998.tb03987.x. [DOI] [PubMed] [Google Scholar]
- Thomas WR, Smith WA, Hales BJ, Mills KL, O’Brien RM. Characterization and immunobiology of house dust mite allergens. International Archives of Allergy and Immunology. 2002;129:1–18. doi: 10.1159/000065179. [DOI] [PubMed] [Google Scholar]
- Tian L, Sun SSM. A cost-effective ELP–intein coupling system for recombinant protein purification from plant production platform. Plos One. 2011;6:e24183. doi: 10.1371/journal.pone.0024183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrent M, Llompart B, Lasserre-Ramassamy S, Llop-Tous I, Bastida M, Marzabal P, Westerholm-Parvinen A, Saloheimo M, Heifetz PB, Ludevid MD. Eukaryotic protein production in designed storage organelles. BMC Biology. 2009;7:5. doi: 10.1186/1741-7007-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta R, Ferreira F, Focke-Tejkl M, Linhart B, Niederberger V, Swoboda I, Vrtala S. From allergen genes to allergy vaccines. Annual Reviews of Immunology. 2010;28:211–241. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]
- Valenta R, Niederberger V. Recombinant allergens for immunotherapy. Journal of Allergy and Clinical Immunology. 2007;119:826–830. doi: 10.1016/j.jaci.2007.01.025. [DOI] [PubMed] [Google Scholar]
- van Neerven RJJ, vant’Hof W, Ringrose JH, Jansen HM, Aalberse RC, Wierenga EA, Kapsenberg ML. T cell epitopes of house dust mite major allergen Der p II. Journal of Immunology. 1993;151:2326–2335. [PubMed] [Google Scholar]
- Wakasa Y, Yasuda H, Oono Y, Kawakatsu T, Hirose S, Takahashi H, Hayashi S, Yang L, Takaiwa F. Expression of ER quality control-related genes in response to changes in BiP1 levels in developing rice endosperm. The Plant Journal. 2011;65:675–689. doi: 10.1111/j.1365-313X.2010.04453.x. [DOI] [PubMed] [Google Scholar]
- Yang L, Kajiura H, Suzuki K, Hirose S, Fujiyama K, Takaiwa F. Generation of a transgenic rice seed-based edible vaccine against house dust mite allergy. Biochemical and Biophysical Research Communication. 2008;365:334–339. doi: 10.1016/j.bbrc.2007.10.186. [DOI] [PubMed] [Google Scholar]
- Yang L, Suzuki K, Hirose S, Wakasa Y, Takaiwa F. Development of transgenic rice seed accumulating a major Japanese cedar pollen allergen (Cry j 1) structurally disrupted for oral immunotherapy. Plant Biotechnology Journal. 2007;5:815–826. doi: 10.1111/j.1467-7652.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Hayashi Y, Jomori T, Takaiwa F. The correlation between expression and localization of foreign gene product in rice endosperm. Plant and Cell Physiology. 2006;47:756–763. doi: 10.1093/pcp/pcj049. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Hirose S, Kawakatsu T, Wakasa Y, Takaiwa F. Overexpression of BiP has inhibitory effects on the accumulation of seed storage proteins in endosperm cells of rice. Plant and Cell Physiology. 2009;50:1532–1543. doi: 10.1093/pcp/pcp098. [DOI] [PubMed] [Google Scholar]
- Yuuki T, Okumura Y, Ando T, Yamakawa H, Suko M, Haida M, Dohi M, Okudaira H. Cloning and expression of cDNA coding for the major house dust mite allergen Der f II in Escherichia coli. Agricultural and Biological Chemistry. 1991;55:1233–1238. [PubMed] [Google Scholar]
- Yuuki T, Okumura Y, Ando T, Yamakawa H, Suko M, Haida M, Okudaira H. Cloning and sequencing of cDNA corresponding to mite major allergen Der f II. Japanese Journal of Allergy. 1990;39:557–561. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.