Abstract
Hydroponic experiments were conducted to investigate the effect of radial oxygen loss (ROL) and external aeration on iron (Fe) plaque formation, and arsenic (As) accumulation and speciation in rice (Oryza sativa L.). The data showed that there were significant correlations between ROL and Fe concentrations in Fe plaque produced on different genotypes of rice. There were also significant differences in the amounts of Fe plaque formed between different genotypes in different positions of roots and under different aeration conditions (aerated, normal, and stagnant treatments). In aerated treatments, rice tended to have a higher Fe plaque formation than in a stagnant solution, with the greatest formation at the root tip decreasing with increasing distances away, in accordance with a trend of spatial ROL. Genotypes with higher rates of ROL induced higher degrees of Fe plaque formation. Plaques sequestered As on rice roots, with arsenate almost double that with arsenite, leading to decreased As accumulation in both roots and shoots. The major As species detected in roots and shoots was arsenite, ranging from 34 to 78% of the total As in the different treatments and genotypes. These results contribute to our understanding of genotypic differences in As uptake by rice and the mechanisms causing rice genotypes with higher ROL to show lower overall As accumulation.
Keywords: arsenic (As), arsenate, arsenite, iron plaque, radial oxygen loss, rice, spatial pattern of ROL
Introduction
Arsenic (As) contamination of groundwater has been reported worldwide, and there has been an increasing public health concern in many parts of the world such as Bangladesh and West Bengal, India (Williams et al., 2006; Stone, 2008; Zhu et al., 2008a). Groundwater in Bangladesh and West Bengal, India, has been used as a major source of drinking water (Williams et al., 2006). Long-term exposure to As can exert serious health problems among populations in the affected areas (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2004; Sun, 2004). Moreover, As-contaminated groundwater has also been used as a major source of irrigation water for crops, especially rice (Meharg, 2004; Geen et al., 2006; Williams et al., 2006). Rice is the staple food for 3 billion people (Stone, 2008). Unfortunately, rice grains grown in regions with As contamination of paddy soils also have high As concentrations, which contribute a significant potential As exposure pathway (Meharg, 2004; Stone, 2008; Zhu et al., 2008b). Furthermore, flooding of rice paddies greatly elevates As concentrations in rice (Xu et al., 2008), leading to higher As shoot assimilation compared with other crops, such as wheat and barley (Williams et al., 2007). Understanding the mechanism of As tolerance and uptake by rice is essential for estimating the risks posed by As contamination in rice soils and for reducing As accumulation in the grains.
A number of wetland plants, including rice, are known to form Fe plaque on their roots by oxidizing Fe2+ to Fe3+, resulting from the oxidizing activity of the plant roots and associated microorganisms (Crowder and St-Cyr, 1991). Over the oxidation period, substantial quantities of iron are transferred towards the root plane, producing a well-defined zone of ferric hydroxide accumulation. Previous studies have shown that Fe plaque can sequester metals on wetland plant roots (Hansel and Fendorf, 2001; Blute et al., 2004) and influence metal tolerance and uptake in aquatic plants (Ye et al., 1997; Batty et al., 2000; Chen et al., 2006). Fe plaque prevents translocation of As from roots to shoots (Liu et al., 2004a,b), and enhances arsenite uptake but decreases arsenate uptake in rice (Chen et al., 2005).
Aerenchyma is a spongy tissue with large air spaces, which provides a low-resistance internal pathway for the exchange of gases within plant tissues and between plants and environments (Armstrong, 1979; Deng et al., 2009; Pi et al., 2009; Deng et al., 2010). Oxygen transported within the root aerenchyma is consumed by cells in adjacent tissues, and diffuses towards the root apex or the rhizosphere (Justin and Armstrong, 1987; Colmer, 2003a,b). The transfer of oxygen from aerenchyma to the rhizoshphere is termed radial oxygen loss (ROL) (Colmer, 2003b). ROL can oxidize rhizosphere soil substances and cause precipitation of toxic metals on the rhizosphere soil and root surface (Otte et al., 1989; Smolders and Roelofs, 1996). Both abiotic and biotic factors control plaque formation (Macfie and Crowder, 1987; St-Cyr and Crowder, 1989; Batty et al., 2000), but the oxidizing capacity of the plant root is considered the most important biotic factor controlling plaque formation (Mendelssohn et al., 1995). ROL, as a part of this, may also play an important role in Fe plaque formation, As sequestration on roots, and As tolerance and uptake in rice plants (Wu et al., 2011).
It has been reported that ROL of roots and root anatomy is related to metal tolerance and uptake in wetland plants (Liu et al., 2009; Cheng et al., 2010; Pi et al., 2010), especially As tolerance and accumulation in rice (Mei et al., 2009; Deng et al., 2010). However, these previous studies focused only on root anatomy, ROL, and Fe plaque formation related to As tolerance and uptake in plants individually (Chen et al., 2005; Mei et al., 2009; Deng et al., 2010), and there is a lack of direct evidence on the effects of ROL on Fe plaque formation and As accumulation and speciation in rice. Our previous study (Wu et al., 2011) indicated that ROL is negatively correlated with As accumulation, which may be due to the effects of ROL on Fe plaque formation. Therefore, it is important to clarify the relationship between ROL and Fe plaque formation. Moreover, Wu et al. (2011) found that there were large variations between genotypes with regard to As uptake and tolerance, but the mechanisms involved were unclear. Xu et al. (2008) reported that As accumulation in rice shoots and grains was markedly increased under flooded conditions compared with aerated conditions, which demonstrated that growing rice aerobically would markedly decrease As accumulation. Moreover, ROL can vary significantly in stagnant and aerated conditions (Colmer et al., 1998). Based on these observations, it is important to investigate As accumulation and ROL in different aeration conditions.
The major aims of the present investigation were thus to study: (i) the quantitative relationships between ROL of entire roots, the spatial pattern of ROL, and Fe concentration on root surfaces; (ii) the effects of ROL and external oxygen contents on Fe plaque formation in rice plants; and (iii) the effects of ROL and Fe plaque on As accumulation and speciation in rice plants.
Materials and methods
ROL of entire roots and Fe plaque formation
Initial growth conditions:
Eight genotypes of rice (O. sativa) (Hejiang16, IAPAR9, Kinmaze, MANHAR, Nanyangzhan, TD71, TORO2, and Xiushui11) were selected in this investigation, including japonica and indica subspecies, as well as paddy and upland rice genotypes (Table 1). Seeds were obtained from the National Rice Research Institute, Hangzhou, and Guangdong Rice Research Institute, Guangzhou, PR China. All were sterilized in 30% H2O2 solution for 15 min, washed with deionized water, and then germinated in Petri dishes, each containing a piece of moist filter paper. After 1 week, uniform seedlings were transplanted to 2 l plastic vessels (four plants per vessel) with nutrient solution containing 40 mg l−1 N as NH4NO3, 10 mg l−1 P as NaH2PO4.H2O, 40 mg l−1 K as K2SO4, 40 mg l−1 Ca as CaCl2.2H2O, 40 mg l−1 Mg as MgSO4.7H2O, and traces of Mn, B, Zn, Cu, and Fe (Yoshida et al., 1976). The solution pH was maintained at around 5.8 with KOH. The nutrient solutions were renewed once every 5 d for a total growth period of 30 d. The solution was not aerated but was non-stagnant. The oxygen concentration in the solution, measured randomly over the 5 d period, ranged from 7.0 to 7.5 mg l−1. There were four replicates (four 2 l vessels) for each genotype and each analysis. All the vessels were arranged randomly in a controlled glasshouse at a temperature of 25/20 °C day/night, with natural light supplemented with sodium light and a day/night photoperiod of 12/12 h and a relative humidity of 70%.
Table 1.
Characteristics of root biomass and ROL in different genotypes of rice grown in Yoshida nutrient solution after a growth period of 30 d, and Fe concentrations in iron plaque after subjected to 30 mg l−1 Fe for 12 h
| Genotype | Type | Root biomass (mg per root DW) | ROL (μmol O2 g-1 DW h-1) | Fe in iron plaque (g kg-1) |
| Hejiang16 | Japonica, paddy | 35±10 c | 8.9±1.6 bcd | 19.4±5.7 abc |
| IAPAR9 | Japonica, upland | 61±20 b | 4.5±1.6 e | 12.5±5.6 de |
| Kinmaze | Japonica, paddy | 58±10 b | 13.4±1.3 a | 18.3±1.7 bcd |
| MANHAR | Indica, paddy | 86±20 a | 6.8±1.0 de | 15.6±2.8 bcde |
| Nanyangzhan | Japonica, paddy | 62±10 b | 7.2±1.2 cd | 11.2±2.0 e |
| TD71 | Indica, paddy | 54±10 bc | 11.1±2.1 ab | 25.1±5.5 a |
| TORO 2 | Japonica, paddy | 99±10 a | 10.1±1.7 bc | 20.5±2.5 ab |
| Xiushui11 | Indica, paddy | 50±10 bc | 9.7±3.0 bc | 13.6±1.9 cde |
Values followed by different letters on the same column are significantly different (P <0.05; least significant difference (LSD) test).DW, dry weight.
Fe plaque formation:
Seedlings were grown for 30 d after transplanting. Subsequently, half of the seedlings were used to induce Fe plaque formation, while the other half were used for ROL determination. Prior to Fe plaque induction, all seedlings were placed in deionized water for 12 h to minimize any interference from other elements with the Fe. They were then transferred into 2 l of solution with 30 mg l−1 ferrous ion for 12 h (Fe2+ as FeSO4.7H2O). The solution pH was adjusted to 5.5 using 0.1 M KOH or HCl. Solutions were maintained in a deoxygenated state by bubbling with N2 for the duration of the Fe plaque induction experiment period of 12 h (Taylor et al., 1984; Liu et al., 2004a,b). The solutions were prepared under the deoxygenated state and the pH was adjusted to 5.5 using 0.1 M KOH or HCl at the beginning of the experiment and every 3 h. After Fe plaque induction, Fe plaque on roots was extracted with dithionite-citrate-bicarbonate (DCB) for the determination of Fe concentration (Otte et al., 1989).
There were four replicates for each treatment and all treatments were conducted in the same glasshouse, as described above.
ROL measurement of entire rice roots:
The ROL rates of the entire root systems of seedlings were measured using a titanium(III) citrate buffer method (Kludze et al., 1994), as described in more detail in our previous study (Wu et al., 2011). The oxygen released was calculated using the following formula (Kludze et al., 1994):
where ROL is measured as μmol O2 plant−1 d−1, c is the initial volume of Ti3+ added to each test tube (in l), y is the concentration of Ti3+ in the solution of the control (without plant) (in μmol Ti3+ l−1), and z is the concentration of Ti3+ in solution after 6 h treatment with plants (in μmol Ti3+ in solution plant−1 l−1).
The rate of ROL (μmol O2 g−1 dry weight d−1) was calculated as:
where G is the root dry weight (in g).
DCB extraction of Fe plaque:
At harvest, after washing with deionized water, 0.5 g of root tissue was incubated for 60 min at room temperature (20–25 °C) in 40 ml of solution containing 0.03 M sodium citrate (Na3C6H5O7·2H2O) and 0.125 M sodium bicarbonate (NaHCO3), with the addition of 0.6 g of sodium dithionite (Na2S2O4). After incubation, the roots were rinsed three times with deionized water and added to the DCB extract. The resulting solution was made up to 100 ml with deionized water for analysis (Otte et al., 1989; Liu et al., 2004a,b).
Spatial pattern of ROL and spatial distribution of Fe plaque
Plant growth:
Four genotypes – TD71 and TORO2, with higher degrees of ROL rates and Fe plaque formation, and IAPAR9 and Nanyangzhan, with lower degrees of ROL rates and Fe plaque formation – selected from the Fe plaque induction experiment, were used in this investigation to include both japonica and indica subspecies, as well as paddy and upland rice genotypes. The seeds were germinated as described above. There were three treatments for each genotype. Plants were grown for 30 d in aerated, stagnant (deoxygenated nutrient solution containing 0.1%, w/v, agar, which more closely resembled the waterlogged soil than either semi-stagnant or N2-flushed solution because dilute agar prevents convective movements in the solution) and normal nutrient solution (neither stagnant nor aerated). The stagnant solution was bubbled with N2 gas for 24 h for deoxygenation before use. All solutions were renewed once every 5 d. The oxygen concentrations in the stagnant medium generally ranged from 0.4 to 1.0 mg l−1 (Visser et al., 1996).
After treatment, half of the plants were washed with distilled water and used to determine spatial patterns of ROL. The rest were transferred to 2 l of solution containing 30 mg l−1 Fe2+ (as FeSO4.7H2O) for 12 h. After treatment, plants were harvested and root samples cut into three sections: tip, middle, and base. Fe plaque on the fresh root surfaces of three root sections was extracted with DCB.
Measurement of ROL spatial patterns:
ROL spatial patterns from selected adventitious roots with lengths of 10 cm were measured using root-sleeving cylindrical platinum O2 electrodes (Armstrong, 1994; Colmer et al., 2006). After 30 d, the adventitious roots produced many lateral roots. As the spatial pattern of ROL is similar between lateral roots and adventitious roots in rice (Armstrong and Armstrong, 2001, 2005), the ROLs of lateral roots were not determined individually in the present study. The entire root systems of rice seedlings were immersed in a transparent chamber (30 cm high and 15 cm diameter) filled with O2-free 0.1% (w/v) agar solution containing 0.5 mM CaSO4 and 5 mM KCl, which was deoxygenated by autoclave and then sparged overnight with high-purity N2 gas. The root–shoot junction was held by wet cotton wool through a rubber bung sealed onto the top of the chamber to ensure that the shoots were upright.
After being transplanted into the chamber for 2 h, the selected intact lateral root was carefully passed through a cylindrical O2 electrode (internal diameter 2.25 mm, height 5.0 mm), fitted with a guide to keep the root near the centre of the electrode (Armstrong, 1971). Measurements were taken along the selected roots with the electrode centre located 5, 10, 20, 30, 40, and 50 mm from the root tip. After 30 d, the adventitious roots produced many lateral roots above 60 mm; measurements with the cylindrical electrode were not positioned above 60 mm. After measurements had been made, the root diameter at each position was determined using a Vernier microscope (150 mm Arc Headed Digital Caliper, Vernier). All measurements of ROL were carried out at 26±2 °C and with a photon flux density of approximately 120 μmol m−2 s−1.
As accumulation and speciation in rice plants
Plant growth:
The four genotypes used in the previous experiments were used in this investigation. Plant preparation and Fe plaque induction were as described above. After the induction of Fe plaque, all seedlings were grown in half-strength nutrient solution for 2 d, before exposure to As. Plants were then grown in a solution containing 4 mg l−1 arsenite [as sodium arsenite, NaAsO2, As(III)] or arsenate [as sodium arsenate, NaHAsO4, As(V)] for 24 h. The concentration chosen was the level of contaminated irrigation water (Tondel et al., 1999; Abedin et al., 2002). There were at least three replicates for each treatment, and all vessels were arranged randomly in the same glasshouse as used in the earlier studies. After exposure, the Fe plaque of roots was extracted with DCB. Rice plants were harvested after 1 d exposure to As for determination of As accumulation and speciation.
Plant analysis for As and Fe:
Sampling procedures followed those described by Abedin et al. (2002) and Liu et al. (2006). At maturity, plants were harvested, washed carefully and then oven dried at 50 °C. After recording the dry weight, all plant samples were ground to a fine powder for analysis. Dried plant samples were digested with 5 ml HNO3 until the digestion solution became clear. The certified reference material (CRM) 1568a from the National Institute of Standards and Technology (NIST; Gaithersburg, MD, USA) was used to verify the accuracy of elemental determinations. The acid digests of plant materials and DCB extracts were analysed for total As and Fe by inductively coupled plasma emission spectrometry (ICP; Elan 9000, PerkinElmer) (Allen, 1989).
Plant analysis for As speciation:
Plant samples of four genotypes of rice (IAPAR9, Nanyangzhan, TD71, and TORO2) collected in the As accumulation experiment were selected in this investigation. To speciate As in rice plants, a trifluoroacetic acid (TFA) extraction method was used (Heitkemper et al., 2001; Williams et al., 2005). Arsenic speciation was determined by HPLC-ICP-MS (HPLC 1100 series, Agilent Technologies). The speciation method has been described in more detail in Williams et al. (2005) and Wu et al. (2011).
Each analysis was performed within 24 h of sample extraction to minimize any changes in speciation during prolonged storage. The standard reference material NIST CRM 1568a rice flour was used to validate the method, and was also used to characterize its speciation (Williams et al., 2005; Liu et al., 2006). The mean total recovery (%), calculated as (sum of species recovered from TFA extraction/total As from acid digestion)×100, ranged from 83 to 111%, which was consistent with other studies (Heitkemper et al., 2001; Williams et al., 2005).
Statistical analyses
Analysis of variance (ANOVA) on plant biomass and concentrations of As and Fe was performed using the statistical package SPSS 13.0 for Windows (SPSS, College Station, TX, USA).
Results and discussion
Plant growth and Fe plaque formation
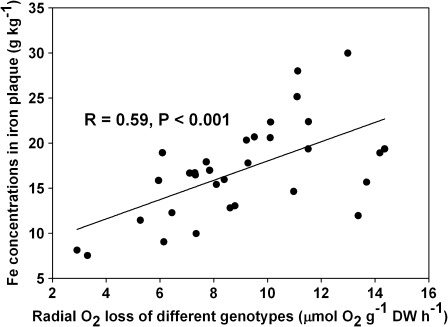
There were significant differences between genotypes (P <0.05) in root biomass, total ROL, and Fe concentrations in Fe plaque on roots. The rate of ROL in the genotypes was ranked in the following order: Kinmaze≈TD71>TORO2>Xiushui11>Heijiang16>Nanyangzhan>MANHAR>IAPAR9, with the highest rate being 13.4 and the lowest 4.5 μmol O2 g−1 dry weight h−1. The concentrations of Fe in Fe plaque were ranked in the order: TD71>TORO2>Hejiang16>Kinmaze>MANHAR>Xiushui11>IAPAR9>Nanyangzhan, with the highest value being 25.1 and the lowest 11.2 g kg−1 (Table 1). There was a significant positive correlation (R=0.59, P <0.001) between rates of ROL and concentrations of Fe plaque in the different genotypes of rice (Fig. 1). However, after 30 d, the adventitious roots produced many laterals, contributing to the total ROL and Fe plaque formation. The role of lateral roots in the total ROL and Fe plaque formation requires further investigation.
Fig. 1.
Relationships between ROL (μmol O2 g−1 dry weight h−1) and Fe concentrations in iron plaque (g kg−1) of rice plants grown in Yoshida nutrient solution for a growth period of 30 d and then subjected to 30 mg l−1 Fe for 12 h.
There were genotypic differences in ROL in the present study. The ROL in the non-aerated nutrient solution used may be different from wet soil and stagnant field conditions (Armstrong, 1979) but still provides important implications. Root aeration can be more complex in wet soil and is affected by soil structural characteristics, the distribution of water, the distribution and respiratory activities of microorganisms, and the distribution, internal diffusivity, diameter, and oxygen requirements of the roots themselves (Armstrong, 1979). In agreement with the present results in stagnant conditions, our previous study (Wu et al., 2011) found that different genotypes had different root anatomy demonstrated by entire root porosity (significantly different between genotypes). The latter was correlated with ROL (P <0.01). Liu et al. (2006) also indicated that there were significant variations in Fe plaque formation between genotypes. Fe plaque on the roots of wetland plants is an adaptation to a stressed environment, as in mangroves it can act as a filter for toxic metals, reacting with them and therefore immobilizing and preventing their uptake (Pi et al., 2010). The same has been shown in some mono- and dicotyledonous wetland species (Visser et al., 2000) and rice (Deng et al., 2010), and plaque can influence metal tolerance and uptake in wetland plants (Otte et al., 1989; Ye et al., 1997; Chen et al., 2005). It has also been reported that Fe plaque plays an important role in the behaviour of As on rice root surfaces, preventing translocation of As from roots to shoots (Liu et al., 2004a,b), enhancing arsenite and decreasing arsenate uptake in rice (Chen et al., 2005).
In the present study, genotypes with higher ROL released more O2 from the roots, which causes a higher degree of oxidation of Fe(II) to ferric oxide or hydroxide forming Fe plaque on the root surface. Fe plaque formation could decrease As toxicity and accumulation in rice, indicating that ROL may be significant in As tolerance and uptake in rice. The data suggest that, by using genotypes with high ROL and strong plaque-forming ability, it might be possible to reduce the transfer of As to above-ground parts of the rice plant. These results provide useful guidance for rice breeders. Furthermore, Fe plaque may also be responsible for the oxidation of arsenite to arsenate, and for reducing the toxicity of As contamination in soil–plant systems (Zhao et al., 2009). The dynamics of As in the rhizosphere of rice plants may play a primary role in the oxidation of arsenite to arsenate by the presence of Fe plaque or the release of O2/oxidants from rice roots. Hansel et al. (2002) showed that arsenate was the predominant species in Fe plaque with small amounts of arsenite, demonstrating that a plant-induced oxic–anoxic interface may exist at the root surface. Deng et al. (2010) found that, in stagnant conditions, rice could increase the root-specific surface area compared with aerated conditions and could form more Fe plaque. An increase in the active root surface area would thus increase the release of O2 into the rhizosphere (Kirk, 2004). The differences in root surface area between different genotypes may therefore contribute to the genotypic differences in Fe plaque formation. However, data derived from another experiment (D. Deng, C. Wu and M. Wong, unpublished data) under the same study conditions (conducted in the same laboratory and under the same growth conditions) showed that there was no significant relationship between root surface area and the degree of Fe plaque formation for four different genotypes. It is clear that the effect of root surface area on Fe plaque formation requires further investigation.
Spatial pattern of ROL and spatial distribution of Fe plaque
The four genotypes differed significantly (P <0.001, Table 2) in root length and shoot length at the end of the treatments (30 d), with IAPAR9 having the longest root and shoot lengths. Rice plants grown in aerated and normal nutrient solution had longer root lengths than plants grown in the stagnant solution (Table 2). A two-way ANOVA showed that there were significant differences in root length between genotypes (P <0.001), as well as among the three different treatments (P <0.001) (Table 2). There was also a significant difference in shoot length between genotypes (P <0.001) but not in shoot length among the three treatments (P >0.05).
Table 2.
Characteristics of root length and shoot height of four genotypes of rice grown in aerated, normal, and stagnant Yoshida nutrient solution for a growth period of 30 d
| Treatment | Root length (cm) |
Shoot height (cm) |
||||||
| IAPAR 9 | Nanyangzhan | TD71 | TORO 2 | IAPAR 9 | Nanyangzhan | TD71 | TORO 2 | |
| Aerated | 35±6.8ab | 31±11.4a | 26±3.1a | 28±12.7a | 64±9.1b | 56±3.2a | 54±14a | 39±1.7a |
| Normal | 37±7.4a | 24±1.0ab | 25±3.6a | 22±1.2a | 64±4.9b | 52±1.9a | 64±5.9a | 39±1.5a |
| Stagnant | 30±1.9b | 23±1.5b | 17±2.3b | 19±3.9b | 71±3.5a | 46±10.9a | 57±7.9a | 36±5.4a |
| Analysis of variance: | ||||||||
| G (genotype) | P <0.001 | P <0.001 | ||||||
| T (treatment) | P <0.001 | NS | ||||||
| G×T | NS | NS | ||||||
Values followed by different letters in the same column are significantly different (P <0.05; least significant difference (LSD) test).NS, not significant.
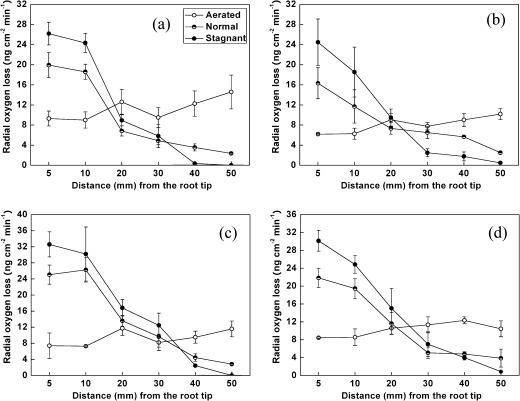
The rates of ROL along roots grown in aerated, normal, and stagnant nutrient solutions showed that the three treatments had a significant effect on the pattern of ROL from roots (P <0.01, Fig. 2). The rates of ROL in the tip were only lower than in the base in all genotypes for the aerated treatment, whilst in stagnant and normal treatments, the rates of ROL decreased with increasing distance from the tip. Moreover, there was an apparent barrier above 50 mm from the tip in stagnant treatment. The rates of ROL in the tip of rice roots in stagnant treatments were relatively higher than those in the aerated and normal treatments (Fig. 2). Genotypes TD71 and TORO2 showed higher rates of ROL compared with IAPAR9 and Nanyangzhan, in accordance with the total ROL rates (Table 1 and Fig. 2). For example, the rates of ROL in the tip were 1.3-fold higher in TD71 than in IAPAR9 in the stagnant treatment (Fig. 2). There were significant differences between different positions along the rice roots in all treatments (P <0.01). A significant interaction was also observed between the growth condition (i.e. aerated, normal, or stagnant) and the position of ROL measurement (P <0.05).
Fig. 2.
Spatial patterns of ROL along the roots of rice of four genotypes, IAPAR9 (a), Nanyangzhan (b), TD71 (c), and TORO2 (d) subjected to different aeration conditions (aerated, stagnant, and normal nutrient solutions) for a growth period of 30 d. Values are shown as means ±standard deviation (SD).
The use of deoxygenated stagnant nutrient solution to simulate semi-stagnant conditions used in the present experiment but commonly omitted in other hydroponic studies appeared to provide additional information. ROL and porosity can be enhanced when plants grow in a stagnant solution compared with growth in aerated conditions, with an induced barrier to ROL in the basal zones (Armstrong, 1979, 1987; Colmer et al., 1998). A subapical decline in root-wall permeability in wetland species (Armstrong, 1987) has been observed. Moreover, the porosities are different along rice roots, with the lowest in the root apex and increasing with distance from the apex, whereas ROL decreases with increasing distance (Armstrong, 1979, 1987; Kotula et al., 2009). Kotula et al. (2009) found that the levels of suberin and lignin increased along the roots towards the base, and concluded that ROL can be effectively restricted by the formation of a suberized exodermis and/or lignified sclerenchyma in the outer part of rice roots. These may all contribute to the formation of barriers in basal zones in stagnant conditions. Changes in spatial patterns of ROL in stagnant solutions compared with aerated solutions could enhance longitudinal diffusion in the aerenchyma to supply O2 to the internal tissues of rice, contributing to the physiological plasticity and enabling rice to succeed in diverse environments such as drained and flooded soils (Colmer et al., 1998, 2003a,b; Visser et al., 2000). These acclimations presumably contribute to waterlogging tolerance in rice (Armstrong, 1971; Colmer et al., 1998). The present results also revealed that improved aeration in a stagnant solution would enhance root growth (Table 2). Comis (1997) has shown that improved internal aeration may enhance root growth through compacted soil layers.
Fe plaque deposited on rice roots was observed as reddish coatings with different shades at different positions. The amount of Fe plaque formed on root surfaces was in ranked in the genotype order: TD71 (25.1 g kg−1)>TORO2 (20.5 g kg−1)>IAPAR9 (12.5 g kg−1)>Nanyanzhan (11.2 g kg−1) (Table 1). There was a significant difference in the amount of Fe plaque formation along the roots for the different genotypes (Table 3, P <0.01). There were also significant differences at different positions (Table 3, P <0.001) and under the three different aeration conditions (Table 3, P <0.001). Under aerated conditions, the amount of Fe plaque formed on the root base was higher than at the root tip (P >0.05). However, the amount of Fe plaque formed on the root base was lower (P <0.001) than at the root tip in the normal and stagnant treatments. Furthermore, the amount of Fe plaque formed at the root tip in the stagnant treatment was higher (P <0.001) than the other two treatments. The trends were in accordance with the spatial ROL pattern in different treatments.
Table 3.
Amounts of iron plaque on different root zones (base, middle, and tip) for the four genotypes with low and high rates of total ROL grown in three different aerated conditions (aerated, normal, and stagnant nutrient solutions) for 30 d and then subjected to 30 mg l−1 Fe for 12 h
| Fe concentration in plaque (g kg−1) |
||||
| Genotype | Condition | Root base | Root middle | Root tip |
| IAPAR 9 | Aerated | 8.0±1.4 | 7.1±0.38 | 7.7±1.5 |
| Normal | 3.6±0.7 | 5.6±2.1 | 9.6±1.7 | |
| Stagnant | 4.6±0.8 | 6.4±2.1 | 29.0±20 | |
| Nanyangzhan | Aerated | 7.5±1.5 | 6.5±1.8 | 7.1±0.5 |
| Normal | 3.8±0.8 | 4.8±1.0 | 7.61±1.3 | |
| Stagnant | 5.6±1.2 | 7.0±0.4 | 22.8±5.4 | |
| TD71 | Aerated | 9.1±0.9 | 7.5±0.3 | 6.3±0.5 |
| Normal | 6.4±1.5 | 8.6±2.8 | 17.0±4.5 | |
| Stagnant | 10.0±4.3 | 26.0±7.0 | 32.0±7.6 | |
| TORO 2 | Aerated | 8.4±0.7 | 6.9±1.2 | 5.8±0.5 |
| Normal | 5.2±1.2 | 7.2±0.6 | 13.9±4.6 | |
| Stagnant | 8.3±2.5 | 22.1±5.5 | 26.2±7.2 | |
| Analysis of variance: | ||||
| Genotype (G) | P <0.01 | |||
| Zone (Z) | P <0.001 | |||
| Treatment (T) | P <0.001 | |||
| G×Z | NS | |||
| G×T | NS | |||
| Z×T | P <0.001 | |||
| G×T×Z | NS | |||
Results were analysed by a three-way ANOVA. NS, not significant.
It has been observed that the distribution of Fe plaque on rice roots follows the order: tip>middle>base in a compartmented soil–glass bead culture system (Liu et al., 2006). In the present study, concentrations of Fe in Fe plaque were highest in the root tip in both stagnant and normal nutrient solutions (Table 3). This trend was again in accordance with the distribution of ROL. However, the present study was conducted using hydroponics. In the field, Fe plaque formation occurs at some radial distance from the root surfaces near the tip, and, as barrier formation increases towards the root base, the Fe plaque gradually approaches and finally reaches the root surface (Armstrong et al., 1992). This provides evidence of ROL being the controlling factor in plaque formation in the field.
As accumulation and speciation in rice plants
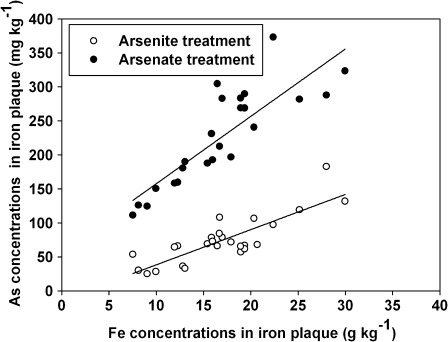
There were positive correlations between Fe concentrations and As concentrations in both arsenite (R=0.87, P <0.001) and arsenate (R=0.81, P <0.001) treatments (Fig. 3). However, the Fe plaque sequestered almost twice as much arsenate as arsenite (Fig. 3). Chen et al. (2005) indicated that arsenite concentrations in DCB extracts were generally lower than those of arsenate. This may be due to iron oxides having a higher affinity for arsenate than arsenite, with arsenite associated with Fe plaque much more easily desorbed than arsenate (Hansel et al., 2002; Meng et al., 2002). Thus, the Fe plaque may act as a ‘buffer’ or ‘reservoir’ for arsenate in the rhizosphere, leading to a lower influx into root cells.
Fig. 3.
Correlations between Fe concentrations (g kg−1) and As concentrations (mg kg−1) in Fe plaque of different genotypes of rice subjected to 30 mg l−1 Fe for 12 h and then 4 mg l−1 arsenite (open circles) or arsenate (filled circles) for 24 h.
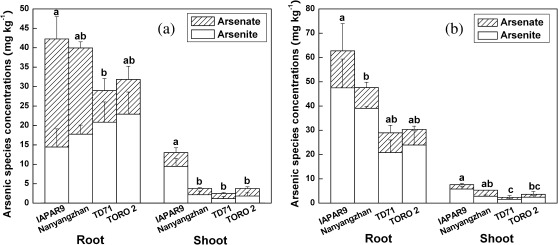
The present results also showed that genotypes had significant effects on As concentrations in Fe plaque (P <0.01, Fig. 3). There was a significant difference between genotypes (P <0.05) in total As concentrations in rice roots. Moreover, there was a significant difference (P <0.001) in As concentrations in shoots between genotypes (Fig. 4). Genotypes IAPAR9 and Nanyangzhan showed higher (P <0.05) As concentrations in the shoots and relatively higher (P <0.05) As translocation from the roots to the shoots compared with the other two genotypes (Fig. 4). Arsenic treatments (arsenite or arsenate treatment) showed significantly different effects (P <0.05) on As in rice roots and shoots in these genotypes. The ratios of As concentrations from shoots to roots varied significantly (F=9.351, P <0.05) with reference to genotype×treatment (arsenate or arsenite treatment) combinations according to a two-way ANOVA. The major As species detected in rice plants was arsenite, except for the roots of genotypes IAPAR9 and Nanyangzhan in both the arsenate and arsenite treatments (Fig. 4).
Fig. 4.
Arsenic species concentrations in rice plants of four genotypes subjected to 30 mg l−1 Fe for 12 h and then to 4 mg l−1 arsenate (a) or arsenite (b) for 24 h (values are means ±SD).
Previous studies have indicated that formation of Fe plaque can reduce As accumulation in above-ground parts of rice plants (Liu et al., 2004a,b). Our previous study showed that total ROL from entire roots was significantly different between genotypes, which was correlated with As tolerance (expressed as the percentage of the mean of control straw biomass, P <0.01) among 20 genotypes; total As concentrations (P <0.01) and inorganic As concentrations (P <0.05) in rice grains of different genotypes were negatively correlated with ROL (Wu et al., 2011). There were also significant genotypic variations in As accumulation and speciation in rice (Mei et al., 2009; Wu et al., 2011). Genotypes TD71 and TORO2 with a higher ROL induced higher amounts of Fe plaque formation, leading to a reduction in As accumulation in the shoots of rice (Fig. 4), which probably reduces the As accumulation in rice grains. It has thus been demonstrated that genotypic variations in ROL rates and Fe plaque formation could at least contribute to the observed genotypic variation in As accumulation in rice. Arsenate is the predominant As species found in rice straw (Abedin et al., 2002), and it can also be readily converted to arsenite and further metabolized to methylated species in plants (Meharg and Hartley-Whitaker, 2002). The major As species detected in this study was arsenite in both rice roots and shoots, even in the arsenate treatment. This may due to the rapid reduction of arsenate to arsenite in rice roots.
Conclusions
Our experiments have shown that Fe concentrations in Fe plaque were significantly correlated with ROL of different genotypes of the rice cultivars tested. The test plants tended to have higher ROL in stagnant solutions compared with aerated and normal nutrient solutions. There were significant differences in the amounts of Fe plaque formed in different genotypes, in different positions of the rice roots, and under different aerated conditions in accordance with the spatial patterns of ROL. Genotypes with higher ROL induced more Fe plaque formation on the roots, further decreasing As accumulation in rice roots and shoots. The greater proportion of speciated As detected was arsenite in the different treatments and for the different genotypes. The present study contributes to our understanding of the mechanisms that cause rice genotypes with higher ROL to have lower As accumulation, as well as any intrinsic genotypic variation.
Acknowledgments
We sincerely thank Professor Alan J.M. Baker (School of Botany, University of Melbourne, Australia) for improving this manuscript. Financial support from the Research Grants Council of Hong Kong (HKBU261407 and HKBU262009) and the National Natural Science Foundation of China (No. 30770417) is gratefully acknowledged.
Glossary
Abbreviations
- ANOVA
analysis of variance
- As
arsenic
- DCB
dithionite-citrate-bicarbonate
- Fe
iron
- ROL
radial oxygen loss
- SD
standard deviation
- TFA
trifluoroacetic acid
References
- Abedin MJ, Cresser MS, Meharg AA, Feldmann J, Cotter-Howells J. Arsenic accumulation and metabolism in rice (Oryza sativa L.) Environmental Science and Technology. 2002;36:962–968. doi: 10.1021/es0101678. [DOI] [PubMed] [Google Scholar]
- Allen SE. Chemical analysis of ecological materials. 2nd edn. Oxford, UK: Blackwell Science; 1989. [Google Scholar]
- Armstrong W. Radial oxygen losses from intact rice roots as affected by distance from the apex, respiration, and waterlogging. Physiologia Plantarum. 1971;25:192–197. [Google Scholar]
- Armstrong W. Aeration in higher plants. In: Woolhouse HW, editor. Advances in botanical research. Vol. 7. London: Academic Press; 1979. pp. 225–332. [Google Scholar]
- Armstrong W. Internal aeration and the development of stelar anoxia in submerged roots. A multishelled mathematical model combing axial diffusion of oxygen in the cortex with radial losses to the stele, the wall layers and the rhizosphere. New Phytologist. 1987;105:221–245. [Google Scholar]
- Armstrong J, Armstrong W. Rice and Phragmites: effects of organic acids on growth, root permeability, and radial oxygen loss to the rhizosphere. American Journal of Botany. 2001;88:1359–1370. [PubMed] [Google Scholar]
- Armstrong J, Armstrong W. Rice: sulfide-induced barriers to root radial oxygen loss, Fe2+ and water uptake, and lateral root emergence. Annals of Botany. 2005;96:625–638. doi: 10.1093/aob/mci215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J, Armstrong W, Beckett PM. Phragmites australis: Venturi- and humidity-induced pressure flows enhance rhizome aeration and rhizosphere oxidation. New Phytologist. 1992;120:197–207. [Google Scholar]
- Armstrong W. Polarographic oxygen electrodes and their use in plant aeration studies. Proceedings of the Royal Society of Edinburgh. 1994;102B:511–527. [Google Scholar]
- Batty LC, Baker AJM, Wheeler BD, Curtis CD. The effect of pH and plaque on the uptake of Cu and Mn in Phragmites australis (Cav.) Trin ex. Steudel. Annals of Botany. 2000;86:647–653. [Google Scholar]
- Blute NK, Brabander DJ, Hemond HR, Sutton SR, Newville MG, Rivers ML. Arsenic sequestration by ferric iron plaque on cattail roots. Environmental Science and Technology. 2004;38:6074–6077. doi: 10.1021/es049448g. [DOI] [PubMed] [Google Scholar]
- Chen RF, Shen RF, Gu P, Dong XY, Du CW, Ma JF. Response of rice (Oryza sativa) with root surface iron plaque under aluminium stress. Annals of Botany. 2006;98:389–395. doi: 10.1093/aob/mcl110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhu YG, Liu WJ, Meharg AA. Direct evidence showing the effect of root surface iron plaque on arsenite and arsenate uptake into rice (Oryza sativa) roots. New Phytologist. 2005;165:91–97. doi: 10.1111/j.1469-8137.2004.01241.x. [DOI] [PubMed] [Google Scholar]
- Cheng H, Liu Y, Tam NFY, Wang X, Li SY, Chen GZ, Ye ZH. The role of radial oxygen loss and root anatomy on zinc uptake and tolerance in mangrove seedlings. Environmental Pollution. 2010;158:1189–1196. doi: 10.1016/j.envpol.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Colmer TD. Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.) Annals of Botany. 2003a;91:301–309. doi: 10.1093/aob/mcf114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell and Environment. 2003b;26:17–36. [Google Scholar]
- Colmer TD, Cox MCH, Voesenek LACJ. Root aeration in rice (Oryza sativa): evaluation of oxygen, carbon dioxide, and ethylene as possible regulators of root acclimatizations. New Phytologist. 2006;170:767–777. doi: 10.1111/j.1469-8137.2006.01725.x. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Gibbered MR, Wiengweera A, Tinh TK. The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. Journal of Experimental Botany. 1998;49:1431–1436. [Google Scholar]
- Comis D. Aerenchyma. Lifelines for living underwater. US Department of Agriculture – Agricultural Research. 1997;45:4–8. [Google Scholar]
- Crowder AA, St-Cyr L. Iron oxide plaques on wetland roots. Trends in Soil Science. 1991;1:315–329. [Google Scholar]
- Deng D, Wu SC, Wu FY, Deng H, Wong MH. Effects of root anatomy and Fe plaque on arsenic uptake by rice seedlings grown in solution culture. Environmental Pollution. 2010;158:2589–2595. doi: 10.1016/j.envpol.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Deng H, Ye ZH, Wong MH. Lead, zinc and iron (Fe2+) tolerances in wetland plants and relation to root anatomy and spatial pattern of ROL. Environmental and Experimental Botany. 2009;65:353–362. [Google Scholar]
- Geen AV, Zheng Y, Cheng Z, He Y, Dhar RK, Garnier JM, Rose J, Seddique A, Hoque MA, Ahmed KM. Impact of irrigating rice paddies with groundwater containing arsenic in Bangladesh. Science of the Total Environment. 2006;367:769–777. doi: 10.1016/j.scitotenv.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Hansel CM, Fendorf S. Characterization of Fe plaque and associated metals on the roots of mine-waste impacted aquatic plants. Environmental Science and Technology. 2001;35:3863–3868. doi: 10.1021/es0105459. [DOI] [PubMed] [Google Scholar]
- Hansel CM, La Force MJ, Fendorf S, Sutton S. Spatial and temporal association of As and Fe species on aquatic plant roots. Environmental Science and Technology. 2002;36:1988–1994. doi: 10.1021/es015647d. [DOI] [PubMed] [Google Scholar]
- Heitkemper DT, Vela NP, Stewart KR, Westphal CS. Determination of total and speciated arsenic in rice by ion chromatography and inductively coupled plasma mass spectrometry. Journal of Analytical Atomic Spectrometry. 2001;16:299–306. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some drinking-water disinfectants and contaminants, including arsenic. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2004;84:1–477. [PMC free article] [PubMed] [Google Scholar]
- Justin SHFW, Armstrong W. The anatomical characteristics of roots and plant response to soil flooding. New Phytologist. 1987;106:465–495. [Google Scholar]
- Kirk G. The biogeochemistry of submerged soils. Chichester, UK: John Wiley & Sons; 2004. [Google Scholar]
- Kludze HK, DeLaune RD, Patrick WH. A colorimetric method for assaying dissolved oxygen loss from container-grown rice roots. Agronomy Journal. 1994;60:616–621. [Google Scholar]
- Kotula L, Ranathunge K, Schreiber L, Steudle E. Functional and chemical comparison of apoplastic barriers to radial oxygen loss in roots of rice (Oryza sativa L.) grown in aerated or deoxygenated solution. Journal of Experimental Botany. 2009;60:2155–2167. doi: 10.1093/jxb/erp089. [DOI] [PubMed] [Google Scholar]
- Macfie SM, Crowder AA. Soil factors influencing ferric hydroxide plaque formation on roots of Typha latifolia L. Plant and Soil. 1987;102:177–184. [Google Scholar]
- Meharg AA. Arsenic in rice – understanding a new disaster for South-East Asia. Trends in Plant Science. 2004;9:415–417. doi: 10.1016/j.tplants.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Meharg AA, Hartley-Whitaker J. Arsenic uptake and metabolism in arsenic resistant and non-resistant plant species. New Phytologist. 2002;154:29–43. [Google Scholar]
- Mei XQ, Ye ZH, Wong MH. The relationship of root porosity and radial oxygen loss on arsenic tolerance and uptake in rice grains and straw. Environmental Pollution. 2009;157:2550–2557. doi: 10.1016/j.envpol.2009.02.037. [DOI] [PubMed] [Google Scholar]
- Mendelssohn IA, Kleiss BA, Wakeley JS. Factors controlling the formation of oxidized root channels – a review. Wetlands. 1995;15:37–46. [Google Scholar]
- Meng XG, Korfiatis GP, Bang KW. Combined effects of anions on arsenic removal by iron hydroxides. Toxicology Letters. 2002;133:103–111. doi: 10.1016/s0378-4274(02)00080-2. [DOI] [PubMed] [Google Scholar]
- Liu WJ, Zhu YG, Hu Y, Williams PH, Gault AG, Meharg AA, Charnock JM, Smith FA. Arsenic sequestration in iron plaque, its accumulation and speciation in mature rice plants (Oryza sativa L.) Environmental Science and Technology. 2006;40:5730–5736. doi: 10.1021/es060800v. [DOI] [PubMed] [Google Scholar]
- Liu WJ, Zhu YG, Smith FA, Smith SE. Do iron plaque and genotypes affect arsenate uptake and translocation by rice seedlings (Oryza sativa L.) grown in solution culture? Journal of Experimental Botany. 2004a;55:1707–1713. doi: 10.1093/jxb/erh205. [DOI] [PubMed] [Google Scholar]
- Liu WJ, Zhu YG, Smith FA, Smith SE. Do phosphorus nutrition and iron plaque alter arsenate (As) uptake by rice seedlings in hydroponic culture? New Phytologist. 2004b;162:481–488. [Google Scholar]
- Liu Y, Tam NFY, Yang JX, Pi N, Wong MH, Ye ZH. Mixed heavy metals tolerance and radial oxygen loss in mangrove seedlings. Marine Pollution Bulletin. 2009;58:1843–1849. doi: 10.1016/j.marpolbul.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Otte ML, Rozema J, Koster L, Haarsma MS, Broekman RA. Iron plaque on roots of Aster tripolium L.: interaction with zinc uptake. New Phytologist. 1989;111:309–317. doi: 10.1111/j.1469-8137.1989.tb00694.x. [DOI] [PubMed] [Google Scholar]
- Pi N, Tam NFY, Wong MH. Effects of wastewater discharge on formation of Fe plaque on root surface and radial oxygen loss of mangrove roots. Environmental Pollution. 2010;158:381–387. doi: 10.1016/j.envpol.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Pi N, Tam NFY, Wu Y, Wong MH. Root anatomy and spatial pattern of radial oxygen loss of eight true mangrove species. Aquatic Botany. 2009;90:222–230. [Google Scholar]
- Smolders AJP, Roelofs JGM. The roles of internal iron hydroxide precipitation, sulphide toxicity and oxidizing ability in the survival of Stratiotes aloides roots at different iron concentrations in sediment pore water. New Phytologist. 1996;133:253–260. doi: 10.1111/j.1469-8137.1996.tb01892.x. [DOI] [PubMed] [Google Scholar]
- St-Cyr L, Crowder AA. Factors affecting iron plaque on the roots of Phragmites australis (Cav.) Trin. ex Steudel. Plant and Soil. 1989;116:85–93. [Google Scholar]
- Stone R. Arsenic and paddy rice: a neglected cancer risk. Nature. 2008;321:184–185. doi: 10.1126/science.321.5886.184. [DOI] [PubMed] [Google Scholar]
- Sun G. Arsenic contamination and arsenicosis in China. Toxicology and Applied Pharmacology. 2004;198:268–271. doi: 10.1016/j.taap.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Taylor GJ, Crowder AA, Rodden R. Formation and morphology of an iron plaque on the roots of Typha latifolia L. grown in solution culture. American Journal of Botany. 1984;71:666–675. [Google Scholar]
- Tondel M, Rahman M, Magnuson A, Chowdhury IA, Faruquee MH, Ahmad SA. The relationship of arsenic levels in drinking water and the prevalence rate of skin lesions in Bangladesh. Environmental Health Perspective. 1999;107:727–729. doi: 10.1289/ehp.99107727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser EJW, Cohen JD, Barendse GWM, Blom CWPM, Voesenek LACJ. An ethylene-mediated increase in sensitivity to auxin induces adventitious root formation in flooded Rumex palustris Sm. Plant Physiology. 1996;112:1687–1692. doi: 10.1104/pp.112.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser EJW, Colmer TD, Blom CWPM, Voesenek LACJ. Changes in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and dicotyledonous wetland species with contrasting types of aerenchyma. Plant, Cell and Environment. 2000;23:1237–1245. [Google Scholar]
- Williams PN, Islam MR, Adomako EE, Raab A, Hossain SA, Zhu YG, Feldmann J, Meharg AA. Increase in rice grain arsenic for regions of Bangladesh irrigating paddies with elevated arsenic in groundwaters. Environmental Science and Technology. 2006;40:4903–4908. doi: 10.1021/es060222i. [DOI] [PubMed] [Google Scholar]
- Williams PN, Price AH, Raab A, Hossain SA, Feldmann J, Meharg AA. Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environmental Science and Technology. 2005;39:5531–5540. doi: 10.1021/es0502324. [DOI] [PubMed] [Google Scholar]
- Williams PN, Villada A, Deacon C, Raab A, Figuerola J, Green AJ, Feldmann J, Meharg AA. Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environmental Science and Technology. 2007;41:6854–6859. doi: 10.1021/es070627i. [DOI] [PubMed] [Google Scholar]
- Wu C, Ye ZH, Shu WS, Zhu YG, Wong MH. Arsenic accumulation and speciation in rice are affected by root aeration and variation of genotypes. Journal of Experimental Botany. 2011;62:2889–2898. doi: 10.1093/jxb/erq462. [DOI] [PubMed] [Google Scholar]
- Xu XY, McGrath SP, Meharg AA, Zhao FJ. Growing rice aerobically markedly decrease arsenic accumulation. Environmental Science and Technology. 2008;42:5574–5579. doi: 10.1021/es800324u. [DOI] [PubMed] [Google Scholar]
- Ye ZH, Baker AJM, Wong MH, Willis AJ. Copper and nickel uptake, accumulation and tolerance in Typha latifolia with and without iron plaque on the root surface. New Phytologist. 1997;136:481–488. doi: 10.1046/j.1469-8137.1997.00758.x. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Forno DA, Cock J, Gomez KA. Laboratory manual for physiological studies of rice. 3rd edn. Los Banos, Laguna: IRRI; 1976. [Google Scholar]
- Zhao FJ, Ma JF, Meharg AA, McGrath SP. Arsenic uptake and metabolism in plants. New Phytologist. 2009;181:777–794. doi: 10.1111/j.1469-8137.2008.02716.x. [DOI] [PubMed] [Google Scholar]
- Zhu YG, Sun GX, Lei M, et al. High percentage inorganic arsenic content of mining impacted and nonimpacted Chinese rice. Environmental Science and Technology. 2008b;42:5008–5013. doi: 10.1021/es8001103. [DOI] [PubMed] [Google Scholar]
- Zhu YG, Williams PN, Meharg AA. Exposure to inorganic arsenic from rice: a global health issue? Environmental Pollution. 2008a;154:169–171. doi: 10.1016/j.envpol.2008.03.015. [DOI] [PubMed] [Google Scholar]