Abstract
Aluminium (Al) toxicity and drought are two major factors limiting common bean (Phaseolus vulgaris) production in the tropics. Short-term effects of Al toxicity and drought stress on root growth in acid, Al-toxic soil were studied, with special emphasis on Al–drought interaction in the root apex. Root elongation was inhibited by both Al and drought. Combined stresses resulted in a more severe inhibition of root elongation than either stress alone. This result was different from the alleviation of Al toxicity by osmotic stress (–0.60 MPa polyethylene glycol) in hydroponics. However, drought reduced the impact of Al on the root tip, as indicated by the reduction of Al-induced callose formation and MATE expression. Combined Al and drought stress enhanced up-regulation of ACCO expression and synthesis of zeatin riboside, reduced drought-enhanced abscisic acid (ABA) concentration, and expression of NCED involved in ABA biosynthesis and the transcription factors bZIP and MYB, thus affecting the regulation of ABA-dependent genes (SUS, PvLEA18, KS-DHN, and LTP) in root tips. The results provide circumstantial evidence that in soil, drought alleviates Al injury, but Al renders the root apex more drought-sensitive, particularly by impacting the gene regulatory network involved in ABA signal transduction and cross-talk with other phytohormones necessary for maintaining root growth under drought.
Keywords: Abscisic acid, aluminum, callose, common bean, cytokinin, drought, gene expression, root growth
Introduction
Common bean (Phaseolus vlugaris L.) is the major food legume for human nutrition in the world, and a major source of calories and protein, particularly in many developing Latin American and African countries (Graham, 1978; Rao, 2001). Common bean production in the tropics is severely limited by two major abiotic stresses, drought and aluminium (Al) toxicity (Goldman et al., 1989; Ishitani et al., 2004). Generally, common bean has been regarded as an Al- and drought-sensitive crop (Rao, 2001; Beebe et al., 2008). In many regions of the developing world, drought and Al toxicity overlap (Wortmann et al., 1998; Thung and Rao, 1999; Beebe et al., 2011). Furthermore, on many acid soils, intermittent drought stress during the growing period could cause yield reduction of 30–60% (CIAT, 1992; Wortmann et al., 1998).
The root apex is the most Al-sensitive root zone (Horst et al., 1992; Delhaize and Ryan, 1995). In common bean, the transition zone (1–2 mm) and the elongation zone are targets of Al injury (Rangel et al., 2007). Excess Al will result in a rapid inhibition of root elongation and enhanced callose synthesis in the root tips; both are sensitive indicators of Al injury in roots (Delhaize and Ryan, 1995; Staß and Horst, 2009). Applying the pressure probe technique to 5 cm root tips of an Al-sensitive maize cultivar, Gunsé et al. (1997) found that Al treatment decreased both the cellular and whole root hydraulic conductivities and cell wall extensibility. However, by application of Al only to the 1 cm root apex, Sivaguru et al. (2006) did not find any impairment of xylem water flow.
Al resistance in common bean is related to lower Al accumulation in the root tips (Rangel et al., 2007). Lower Al accumulation and thus the detoxification of Al in the apoplast through root exudates, such as Al-activated exudation of citrate from root tips, play a key role in Al resistance in common bean (Miyasaka et al., 1991; Rangel et al., 2009, 2010; Horst et al., 2010). Eticha et al. (2010) showed that the Al-induced expression of a MATE (multidrug and toxin extrusion family protein) gene in root apices is a prerequisite for citrate exudation and Al resistance in common bean. In addition Al-induced inhibition of root elongation was positively correlated with the expression of an ACCO (1-aminocyclopropane-1-carboxylic acid oxidase) gene in the root apex (Eticha et al., 2010). The expression of MATE and ACCO has been used as a sensitive indicator of Al impact on the root apex in common bean (Yang et al., 2011).
Drought strongly affects the root apex, leading to inhibition of root elongation (Sharp et al., 2004). The maintenance of root growth during water deficit is a prerequisite for water uptake from the subsoil (Sponchiado et al., 1989; Serraj and Sinclair, 2002). In maize, three mechanisms involved in primary root growth maintenance under water deficit have been proposed: osmotic adjustment; modification of cell wall (CW) extension properties; and the role of abscisic acid (ABA) accumulation (Sharp et al., 2004; Yamaguchi et al., 2010).
To the authors’ knowledge the interaction of drought and Al at the level of the root apex has not yet been studied. In a first approach, polyethylene glycol (PEG) 6000 [osmotic stress (OS)] was used to simulate drought stress in hydroponics. It was found that OS alleviated Al toxicity by inhibiting Al accumulation in the root tip of the Mesoamerican common bean genotype VAX 1 (Yang et al., 2010). The positive PEG effect on root elongation in the presence of Al was confirmed by the expression of MATE and ACCO as sensitive indicators of the impact of Al on the root apex (Yang et al., 2011). The PEG-suppressed Al accumulation in the root tips was suggested to be due to the OS-induced reduction of CW porosity, involving the regulation by XTH (xyloglucan endotransglucosylase/hydrolase), BEG (glucan endo-1,3-β-glucosidase), and HRGP (hydroxyproline-rich glycoprotein).
In contrast to the PEG effect in hydroponics, it was expected and hypothesized that low soil moisture (drought) in an acid Al-toxic soil would aggravate Al toxicity, further impeding root growth, which may strongly restrict the aquisition of water from the subsoil and thus the ability of the plants to withstand drought stress (Goldman et al., 1989). Indeed, Butare et al. (2011) found that in an acid soil, inhibition of root development of Phaseolus acutifolius and the Mesoamerican common bean genotypes was strongly aggravated by combined Al and drought stress. However Al partially alleviated the negative effects of water stress in Al-resistant Phaseolus coccineus genotypes.
Since PEG 6000-induced water deficit in roots may differ greatly from the effect of low soil moisture, a technique was developed that allowed the study of the interaction of Al and low soil moisture in an acid, Al-toxic soil (designated drought stress). The main objective of the present study was to compare at the physiological and molecular level the short-term effects of combined Al toxicity and drought stress with a previous study in hydroponics using PEG as a substitute for drought (designated OS), with special emphasis on the root apex in the Al-sensitive common bean genotype VAX 1.
Materials and methods
Soil properties and preparation
The acid soil was obtained from Matazul farm (4''9'N, 72''39'W) in the Llanos region of Colombia. Soil chemical characteristics are shown in Table 1. The soil pH was measured in 0.01 M CaCl2 solution or distilled water with a 1:2 soil:extract ratio (w/v). The exchangeable acidity (H+, Al3+) was determined by NaOH titration using 1% phenolphthalein and 0.1% methyl orange after extracting with 1 M KCl. The effective cation exchange capacity (ECEC) was calculated as the sum of the exchangeable cations (Ca2+, Mg2+, Na+, K+, and Al3+); the exchangeable cations were extracted using the method of Sumner and Miller (1996) and determined by inductively coupled plasma mass spectroscopy (ICP-MS) (7500cx, Agilent Technology, Santa Clara, CA, USA). The Al saturation (%) of the soil was calculated as the ratio exchangeable Al3+/ECEC×100. The soil water retention was determined. The water retention curve and the soil water potential (SWP) at different soil moisture used for the drought treatment in this study are shown in Supplementary Fig. S1 available at JXB online.
Table 1.
Chemical characteristics of an Oxisol collected from Matazul farm in the Llanos region of Colombia
| Soil chemical characteristics | Oxisol |
| pH_CaCl2 | 4.05 |
| pH_H2O | 4.89 |
| Exchangeable acidity (cmolc kg−1 soil) | 1.57 |
| Exchangeable H+ (cmolc kg−1 soil) | 0.33 |
| Exchangeable Al (cmolc kg−1 soil) | 1.23 |
| Total Al content (mg kg−1 soil) | 111.0 |
| ECEC (cmolc kg−1 soil) | 1.49 |
| Al saturation (%) | 89.1 |
For the soil treatment, the limed [1.1 g Ca(OH)2 kg−1 soil] soil was incubated at 25 °C for 1 week. Then different levels of Al (AlCl3·6 H2O; 0, 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 g kg−1 soil) were added to the limed soils, mixed well, and incubated for2 weeks. Finally the soil pH_H2O was 6.5, 5.5, 5.0, 4.7, 4.3, 4.1, and 3.9, respectively, and the corresponding Al concentrations in the water extract were 0.4, 0.7, 1.0, 4.3, 40, 173, and 426 μM (see Supplementary Fig. S2 at JXB online). The treated soil was air-dried and stored for future use. Before transferring the seedling to the soil, deionized water was added to adjust soil moisture to the desired level and then the soil was incubated for 24 h. The soil solution from the incubated soil treated with 2 g of Al was extracted by centrifugation using porous cups at 4000 g for 20 min. No soil solution could be recovered at the lowest SWPs. The soil solution was collected in microfuge tubes and centrifuged again at 20 000 g for 10 min to remove soil debris, and then the concentration of Al, Ca, Mg, and K in the clear supernatant was measured using ICP-MS. The concentrations of all cations increased with decreasing soil moisture (Supplementary Fig. S3).
Plant materials and growing conditions
Seeds of common bean (P. vulgaris L.) genotype VAX 1 (Al sensitive) were germinated for 2 d or 3 d on filter paper sandwiched between sponges. For the soil experiments, uniform seedlings were transferred into the soil with different levels of Al application and/or soil moisture in falcon vials (one plant per vial), covered with Al foil, and kept in an upright position for 24 h. For the hydroponic experiments, uniform seedlings were transferred to a continuously aerated simplified nutrient solution containing 5 mM CaCl2, 1 mM KCl, and 8 μM H3BO3 (Rangel et al., 2007). The pH of the nutrient solution was gradually lowered to 4.5 within 2 d. Then the plants were transferred into the simplified nutrient solution (pH 4.5) containing AlCl3 (0, 25 μM Al) and PEG 6000 (0 or 150 g l−1) (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) for 24 h. The osmotic potential (OP) of 150 g l−1 PEG 6000 was –0.60 MPa, measured with a cryoscopic osmometer (Osmomat 030, Gonotec GmbH, Berlin, Germany). Plants were cultured in a growth chamber under controlled environmental conditions of a 16/8 h light/dark cycle, 27/25 °C day/night temperature, 70% relative air humidity, and a photon flux density of 230 μmol m−2 s−1 of photosynthetically active radiation at plant height. Root tips (1 cm) were harvested for Al analysis or immediately frozen in liquid nitrogen in Eppendorf vials for callose and phytohormone determination and RNA isolation.
Measurement of root elongation rate
Before transferring the plants into the soil or the nutrient solution, the tap roots were marked 1 cm (for soil) or 3 cm (for hydroponics) behind the primary root tip using a fine point permanent marker (Sharpie blue, Stanford Corporation, Oak Brook, IL, USA) which did not affect root growth during the experimental period. Root elongation was measured after treating the plants for 24 h using a millimetre scale.
RNA isolation and quantitative real-time PCR
After treating plants in soil with different Al supplies (0, 1.0, and 2.0 g kg−1 soil) and soil moisture (–0.05, –0.14, and –0.31 MPa SWP) for 24 h, primary root tips (1 cm long) from each plant were harvested and shock-frozen in liquid nitrogen. Nine root tips were bulked and ground to powder in liquid nitrogen. Total RNA was isolated using the NucleoSpin RNA plant kit (Macherey-Nagel GmbH and Co., KG, Düren, Germany) following the manufacturer’s protocol. After isolating the RNA from the root tips, first-strand cDNA was synthesized using a RevertAid H-Minus first-strand cDNA synthesis kit (Fermentas, www.fermentas.com) following the manufacturer’s protocol. Quantitative real-time PCR (qRT-PCR) was performed using the CFX96™ Real Time System plus the C1000™ Thermal Cycler (www.bio-rad.com) as described by Yang et al. (2011). Samples for qRT-PCR were run in three biological replicates and two technical replicates. Relative gene expression was calculated using the comparative ΔΔCT method according to Livak and Schmittgen (2001). For the normalization of gene expression, β-tubulin was used as an internal standard according to Eticha et al. (2010), and the control (–0.05 MPa SWP in the absence of Al application) plants of bean genotype VAX 1 were used as the reference sample.
Candidate gene selection and primer design for qRT-PCR
Candidate genes were selected either from the SuperSAGE library (Yang et al., 2011) or from a public database. The expressed sequence tags (ESTs) obtained from P. vulgaris were aligned; otherwise the EST sequences from other legumes were gathered for sequence alignment. The well-conserved regions were used for primer design. Primers were designed using Primer3 software (Rozen and Skaletsky, 2000). The specifications of the primers of the genes studied are given in Supplementary Table S1 at JXB online. The PCR efficiencies of the primer pairs were in the range of 90–110% as determined by dilution series of the cDNA template. Primer pairs with PCR efficiencies deviating from this range were discarded and new primers of the genes were designed to obtain more reliable quantification.
Determination of Al and other mineral elements
Root tips (1 cm) were digested in 500 μl of ultrapure HNO3 (65%, v/v) by overnight shaking on a rotary shaker. The digestion was completed by heating the samples in a water bath at 80 °C for 20 min. Al was measured with a Unicam 939 QZ graphite furnace atomic absorption spectrophotometer (GFAAS; Analytical Technologies Inc., Cambridge, UK) at a wavelength of 308.2 nm after appropriate dilution, and an injection volume of 20 μl. The concentrations of titanium (Ti) in root tips and Al, Ca, Mg, and K in the soil solution were measured using ICP-MS (7500cx, Agilent Technology) after appropriate dilution.
Determination of callose
Three primary root tips (1 cm long) for each sample were excised and instantly frozen in liquid N2. Samples were homogenized in 500 ml of 1 M NaOH with a mixer mill (MM 200; Retsch GmbH and Co. KG, Haan, Germany) at a speed of 20 cycles s−1 for 2 min. After homogenization, another 500 ml of 1 M NaOH was added, and callose was solubilized by heating in a water bath at 80 °C for 20 min. Callose was measured according to Kauss (1989), after addition of aniline blue reagent using a microplate fluorescence reader (FLx 800, Bio-Tek Instruments, Winooski, VT, USA) at excitation and emission wavelengths of 400 nm and 485 nm, respectively. Pachyman (1, 3-β-D-glucan) was used as a calibration standard, and, thus, root callose content was expressed as pachyman equivalents (PE) per cm root tip.
Analysis of phytohormones
Different forms of cytokinins (CKs), indole-3-acetic acid (IAA), ABA, jasmonic acid (JA), and salicylic acid (SA) were extracted and purified according to Albacete et al. (2008) with some modifications. Primary root tips (1 cm long) were excised from common bean genotype VAX 1 and immediately frozen in liquid N2. Root tips were ground to powder in liquid nitrogen. Afterwards, 1 ml of 80% (v/v) methanol was added to each sample and vortexed. Then 4 μl of internal standard mix (5 μg ml−1) composed of deuterium-labelled hormones ([2H5]Z (zeatin), [2H5]ZR (zeatin riboside), [2H5]ZOG (zeatin-O-glucoside), [2H5]ZROG (zeatin-O-glucoside riboside), [2H6]iP (riboside 5′-diphosphate), [2H5]DHZ (dehydro-zeatin), [2H5]DHZR (dehydro-zeatin riboside), [2H6]ABA, [2H3]IAA, and [2H5]JA, Olchemin Ltd, Olomouc, Czech Republic) was added, mixed well, and incubated for 30 min at 4 °C. Afterwards, the samples were centrifuged at 20 000 g and 4 °C for 15 min. The supernatant was passed through pre-equilibrated Chromafix C18 columns (Macherey-Nagel) with 80% (v/v) methanol. Samples were collected in 5 ml tubes on ice, and 1 ml of 80% (v/v) methanol was added and vortexed thoroughly. After centrifuging at 20 000 g and 4°C for 15 min, the filtration step was repeated. The collected samples were concentrated to dryness using a Thermo ISS110 centrifugal vacuum evaporator (Thermo Savant, Holbrook, NY, USA). The residue from each sample was re-dissolved in 500 μl of 20% (v/v) methanol, sonicated for 8 min, and filtrated through 0.22 μm syringe filters (Chromafil PES-20/25, Macherey-Nagel, Düren, Germany). The filtered samples were immediately frozen for phytohormone measurement.
Analyses were carried out on a UPCL-MS/MS system consisting of a Thermo ACCELA UPLC (Thermo Scientific, Waltham, MA, USA) coupled to a thermostated HTCPAL autosampler (CTC Analytics, Zwingen, Switzerland), and connected to a Thermo TSQ Quantum Acces Max Mass Spectrometer (Thermo Scientific) with a heated electrospray ionization interface. A 10 μl aliquot of each standard (known concentrations of each phytohormone) and the internal standards or sample were injected into a Thermo Hypersil Gold column (1.9 μm, 50×2.1 mm, Thermo Scientific) eluted at a flow rate of 250 μl min−1. Mobile phase A consisting of water/methanol/acetic acid (89.5/10/0.5, v/v/v) and mobile phase B consisting of methanol/acetic acid (99.5/0.5, v/v) were used for chromatographic separation. The elution consisted of 2 min of 95% A and a linear gradient from 5% to 100% of B in 8 min; 100% B was maintained for 6 min and afterwards the column was equilibrated with the starting composition (95% A) for 8 min before each analytical run. The mass spectrometer was operated in the positive mode for all the hormones analysed, except JA and SA that were measured in the negative mode. Capillary spray voltage was set to 4000 V, the nebulizer gas (He) pressure to 40 psi with a flow rate of 8.0 l s−1 at a temperature of 250 °C, and the scan cycle time was 0.5 s from 100 m/z to 600 m/z. The chromatogram of each hormone from both standards and samples was extracted, and the peak area quantified using the Thermo XCalibur software version 2.1.0.
Statistical analysis
A completely randomized design was used with 3–12 replicates in each experiment. Statistical analysis [analysis of variance (ANOVA)] was carried out using SAS 9.2 (SAS Institute, Cary, NC, USA). Means were compared using t-test or Tukey test depending on the number of treatments being compared. *, **, and *** denote significance at P < 0.05, 0.01, and 0.001, respectively.
Results
Application of AlCl3 (0–3.0 g Al kg−1 soil) to the acid soil limed to pH 6.5 reduced the soil pH (H2O) to 3.9 after incubation for 2 weeks, and the reduction of soil pH was correlated with an increase of Al concentration in the water extract (Supplementary Fig. S2 at JXB online). The root elongation rate of the common bean genotype VAX 1 was increasingly inhibited by the application of increasing Al rates (Fig. 1A). The supply of 1.0 and 2.0 g Al kg−1 soil reduced the root elongation rate by 29% and 52%, respectively, compared with the control (no Al). Decreasing the SWP from –0.05 to –0.87 MPa also drastically reduced root elongation. Medium to severe drought stess at –0.14 MPa and –0.31 MPa SWP inhibited the root elongation rate by 45% and 68%, respectively, compared with the well-watered control (–0.05 MPa SWP).
Fig. 1.
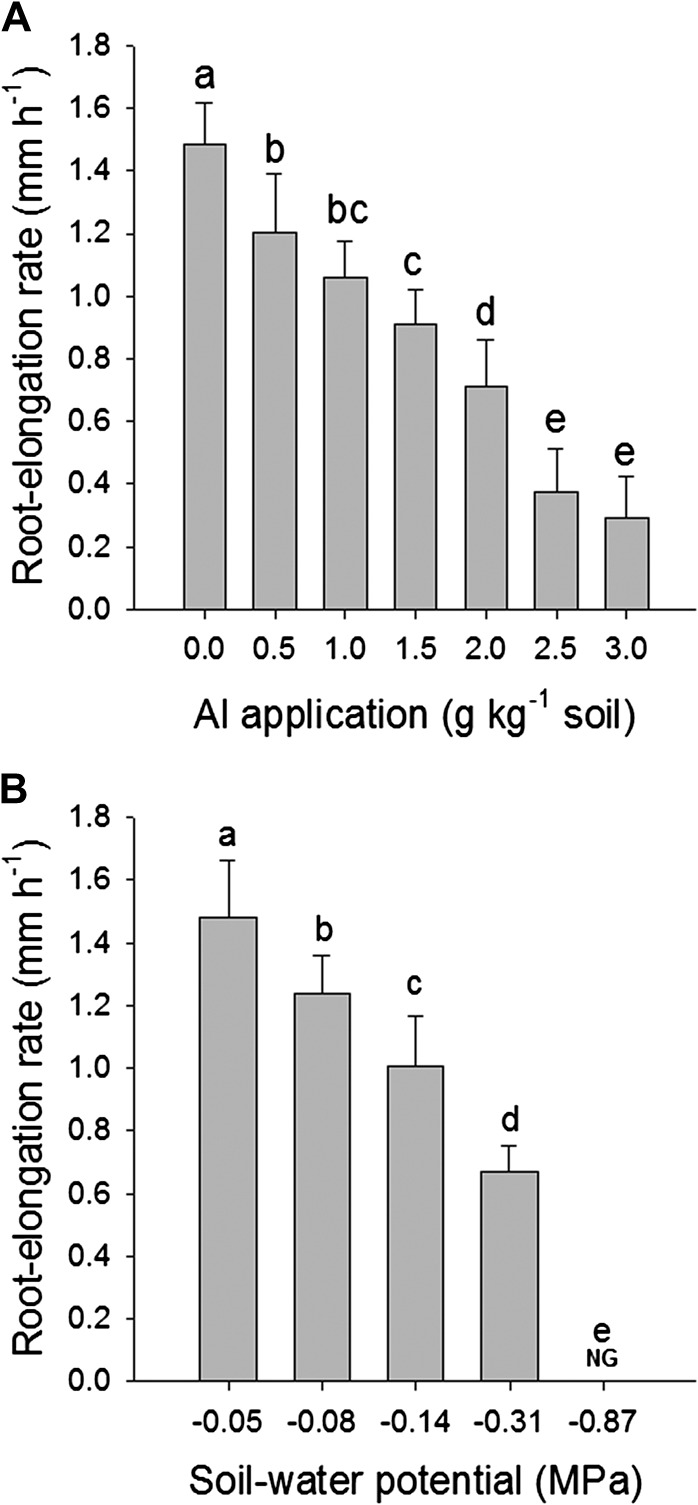
Root elongation rate at different levels of Al supply under well-watered conditions (A) and at different levels of soil water potentials in the absence of Al application (pH 6.5) (B). Two-day-old seedlings were grown in soil for 24 h. Bars represent means ±SD, n=12. Means with different letters are significantly different at P < 0.05 (Tukey test) for the comparison of treatments. NG, no growth.
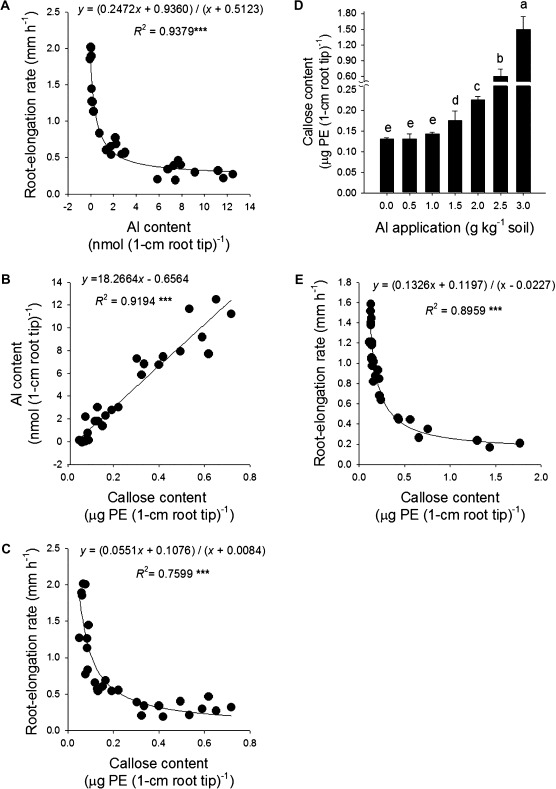
A major effort was made to analyse the Al contents of the apices of soil-grown root tips including desorption with high ionic strength solution, organic acids, and the use of Ti as an indicator of soil contamination of plant samples as suggested by Cook et al. (2009). However, none of the attempts was successful at removing Al contamination by soil particles, and the Ti-based quantification of the soil contamination failed since laser ablation ICP-MS analysis of root tips showed that Ti can also be absorbed into the root tissue (data not shown). Therefore, the suitability of the callose content of root tips as an indicator of the Al contents and Al injury in soil was evaluated. In hydroponics, a significant negative correlation (P < 0.001) between Al contents and root elongation was observed (Fig. 2A). Al induced callose formation in the root tips. The relationship between Al and callose contents could be described by a highly significant positive linear regression (Fig. 2C) very similar to the Al content–root elongation relationship (Fig. 2A). Thus the root tip callose and Al contents were highly significantly linearly related (Fig. 2C), suggesting that the callose content can be used as a sensitive indicator of Al contents and Al-induced inhibition of the root elongation rate in hydroponics. Similarly, in the soil culture experiment, addition of Al increased the root tip callose contents (Fig. 2D). The callose content proved to be a sensitive indicator of Al-induced inhibition of root elongation also in the soil culture experiment (Fig. 2E) and may thus be used as an indicator of the root tip Al content.
Fig. 2.
Correlations between root elongation rate, and Al and callose contents in the 1 cm root tips of common bean genotype VAX 1 in nutrient solution (A–C) or soil (E), and the effect of soil Al treatment on callose contents in the root tips (D). (A–C) Plants were pre-cultured in simplified nutrient solution containing 5 mM CaCl2, 1 mM KCl, and 8 μM H3BO3 for 48 h for acclimation and pH adaptation; then the plants were exposed to 25 μM Al in the simplified nutrient solution for 24 h, pH 4.5. (D and E) Two-day-old seedlings were grown in well-watered (–0.05 MPa SWP) soil with different levels of Al supply for 24 h. In D, bars represent means ±SD, n=4, and means with different letters are significantly different at P < 0.05 (Tukey test) for the comparison of treatments. For the regression analysis, *** denote significance at P < 0.001.
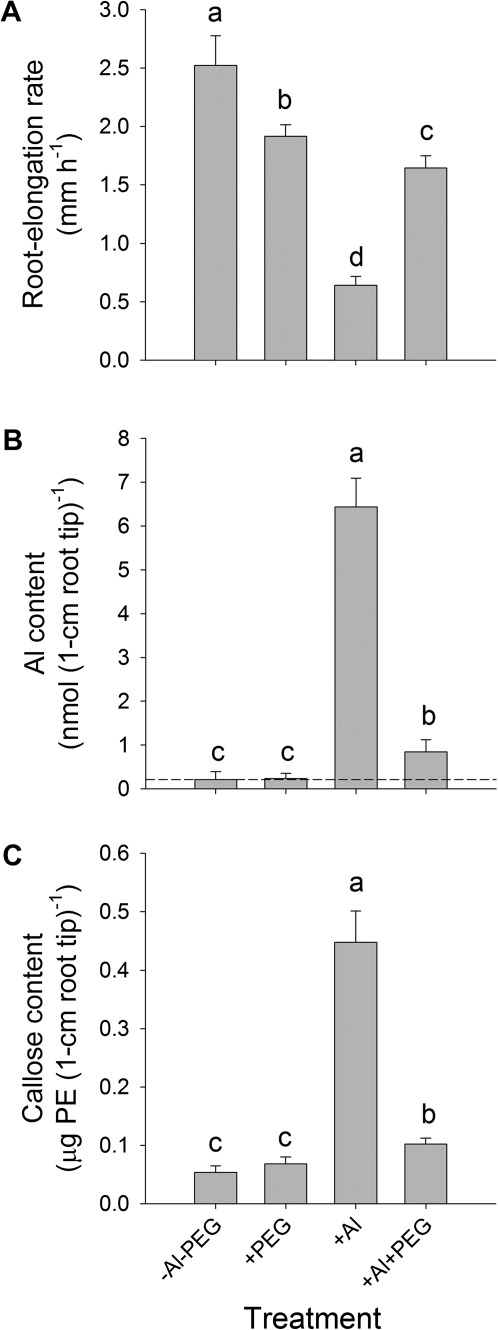
In order to verify whether Al-induced callose formation is also a reliable indicator of Al stress under combined Al and drought stress, a hydroponic experiment was first conducted using PEG 6000—which cannot penetrate into the root apoplast because of its high molecular weight (Carpita et al., 1979)—to simulate drought stress. PEG at –0.60 MPa OP significantly reduced root elongation (Fig. 3A) but did not stimulate callose formation (Fig. 3C). Al at 25 μM strongly inhibited root elongation (Fig. 3A) and increased root tip Al (Fig. 3B) and callose contents (Fig. 3C). Combined PEG/Al stress alleviated the Al-induced inhibition of root elongation (Fig. 3A) by reducing the Al accumulation in the root tips (Fig. 3B). The PEG-induced alleviation of the Al stress is also clearly shown by reduced callose formation (Fig. 3C), suggesting a high sensitivity and specificity of callose formation for root Al injury.
Fig. 3.
Root elongation rate (A), and Al (B) and callose (C) contents in 1 cm root tips of the common bean genotype VAX 1 under osmotic (0, –0.60 MPa OP) and Al stress (0, 25 μM Al). Plants were pre-cultured in a simplified nutrient solution containing 5 mM CaCl2, 1 mM KCl, and 8 μM H3BO3 for 48 h for acclimation and pH adaptation, then treated or not with 25 μM Al in the absence or presence of PEG (150 g l−1 PEG 6000) in the simplified nutrient solution for 24 h, pH 4.5. The background value (dashed line) in B presents the mean Al content of the root tips without Al treatment. Bars represent means ±SD, n=12 for A, and n=4 for B and C. Means with different letters are significantly different at P < 0.05 (Tukey test) for the comparison of treatments.
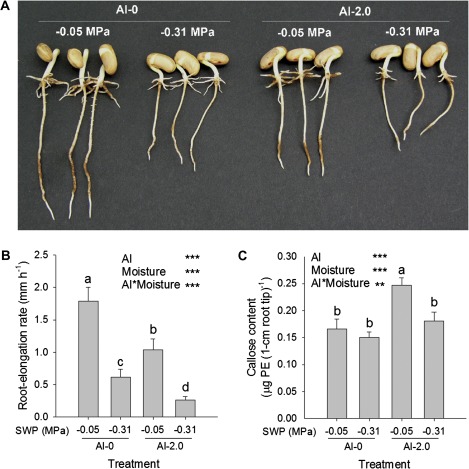
Unlike the hydroponic experiment with PEG (see Fig. 3), in the soil experiment, combined drought (–0.31 MPa SWP) and Al stresses enhanced the inhibition of root elongation beyond the effects of the individual stresses in an additive manner (Fig. 4A, B) in spite of reduced Al stress, as indicated by the significant reduction of callose formation in the root tips (Fig. 4C).
Fig. 4.
Seedling appearance (A), root elongation rate (B), and callose contents in the 1 cm root tips (C) of the common bean genotype VAX 1 as affected by soil moisture and Al supply (g kg−1 soil). Two-day-old seedlings were grown in soil for 24 h. Root browning is due to soil particles adhering particularly to the root hair zone. Bars represent means ±SD, n=12 for B, and n=4 for C. Means with different letters are significantly different at P < 0.05 (Tukey test) for the comparison of treatments. For the ANOVA, ** and *** denote significance at P < 0.01 and P < 0.001, respectively. SWP, soil water potential. (This figure is available in colour at JXB online.)
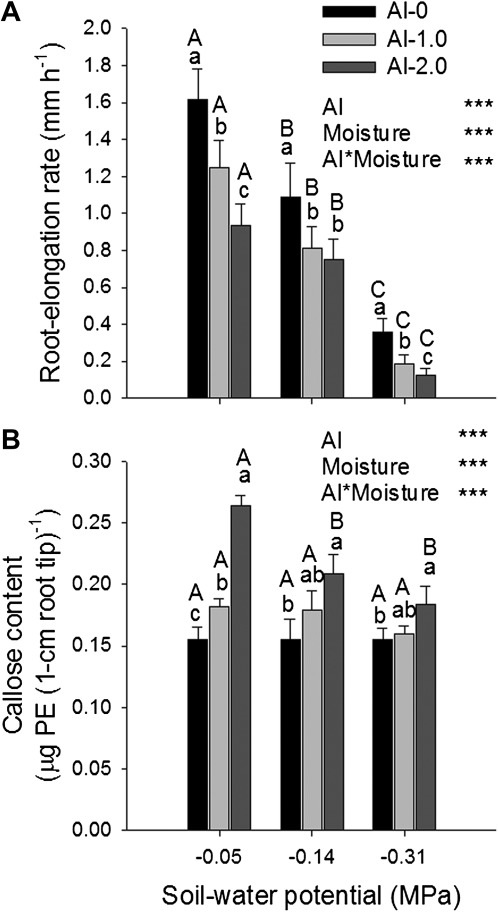
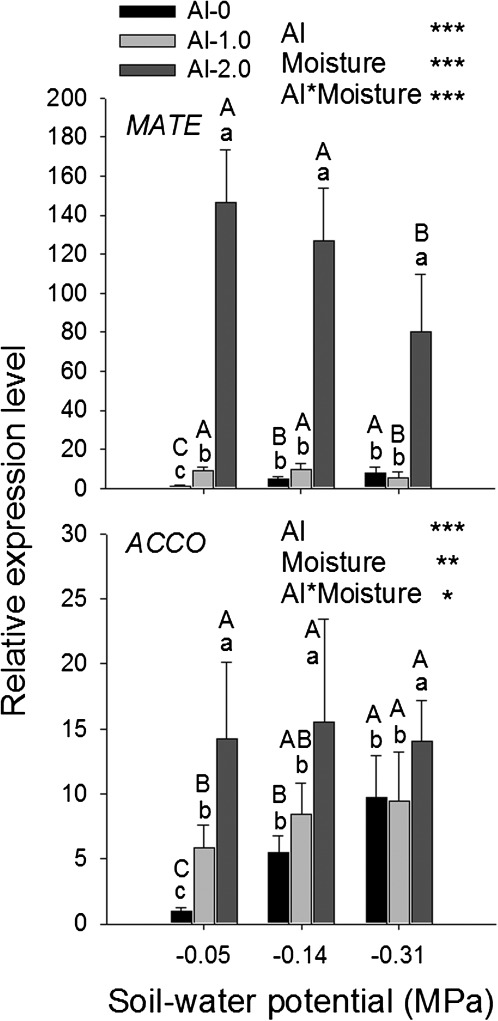
To better understand the interaction between Al toxicity and drought in soil, both Al supply and soil moisture were varied at three rates in a factorial combination (0, 1.0, and 2.0 g Al and –0.05, –0.14, and –0.31 MPa SWP). The results confirmed that Al supply enhanced drought-induced inhibition of root elongation at all stress levels (Fig. 5A). Similar to OS, increasing drought stress reduced Al-induced root tip callose formation, confirming the alleviation of Al toxicity by drought (Fig. 5B). This observation is supported by the expression of a citrate transporter MATE gene which sensitively responds to Al treatment. Particularly the high Al supply strongly enhanced the expression of MATE (Fig. 6A). Severe drought stress, which only slightly stimulated MATE expression, significantly suppressed the Al-induced gene expression, confirming reduced Al stress as indicated by reduced callose formation (see above, Figs 4, 5). ACCO sensitively responded to both Al and drought stresses (Fig. 6B). At all soil moisture levels, Al further enhanced the drought-induced up-regulated ACCO expression, which is in agreement with the enhanced inhibition of root elongation with combined Al and drought stress factors (see above, Figs 4, 5).
Fig. 5.
Root elongation rate (A) and callose contents in the 1 cm root tips (B) of the common bean genotype VAX 1 as affected by soil moisture and Al supply (g kg−1 soil). Two-day-old seedlings were grown in soil for 24 h. Bars represent means ±SD, n=12 for A, and n=4 for B. Means with different lower and upper case letters are significantly different at P < 0.05 (Tukey test) for the comparison of Al treatments within soil moisture and comparison of soil moisture treatments within Al treatments, respectively. For the ANOVA, *** denote significance at P < 0.001.
Fig. 6.
MATE and ACCO gene expression in the 1 cm root tips of the common bean genotype VAX 1 as affected by soil moisture and Al supply (g kg−1 soil). Two-day-old seedlings were grown in soil for 24 h. qRT-PCR was performed using the β-tubulin gene as internal standard. Bars represent means ±SD, n=3. Means with different lower and upper case letters are significantly different at P < 0.05 (Tukey test) for the comparison of Al treatments within soil moisture and comparison of soil moisture treatments within Al treatments, respectively. For the ANOVA, *, **, and *** denote significance at P < 0.05, P < 0.01, and P < 0.001, respectively.
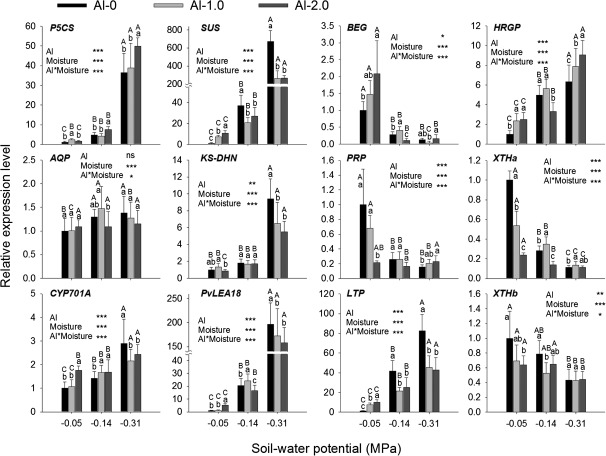
From an analysis of OS (PEG)-induced changes in gene transcription in common bean root tips using SuperSAGE (Yang et al., 2011), 12 genes with possible roles in the regulation of CW properties [BEG, HRGP, PRP (proline-rich protein), XTHa, XTHb, and LTP (lipid transfer protein)] and response to OS [P5CS (Δ1-pyrroline-5-carboxylate synthase), SUS (sucrose synthase), AQP (aquaporin), KS-DHN (KS-type dehydrin), PvLEA18 (late embryogenesis abundant protein), and CYP701A (cytochrome P450 monooxygenase 701A)] were selected. In agreement with these results, 11 of the selected genes were comparably affected by OS (Yang et al., 2011) and by drought stress in soil (Fig. 7): six genes (P5CS, SUS, HRGP, KS-DHN, PvLEA18, and LTP) were strongly up-regulated, one gene (AQP) was slightly up-regulated, while four genes (BEG, PRP, XTHa, and XTHb) were down-regulated. Only CYP701A was up-regulated by drought but down-regulated by OS.
Fig. 7.
Cell wall- and osmotic stress-associated gene expression in 1 cm root tips of common bean genotype VAX 1 as affected by soil moisture and Al supply (g kg−1 soil). Two-day-old seedlings were grown in soil for 24 h. qRT-PCR was performed using the β-tubulin gene as internal standard. Bars represent means ±SD, n=3. Means with different lower and upper case letters are significantly different at P < 0.05 (Tukey test) for the comparison of Al treatments within soil moisture and comparison of soil moisture treatments within Al treatments, respectively. *** denote significance at P < 0.001. ns, non-significant.
Al stress alone (optimum soil moisture, –0.05 MPa SWP) also significantly affected the expression of most genes in the same direction as drought stress (Fig. 7). However, in most cases, the effect was small compared with the drought effect. Exceptions were BEG, PRP, and XTHa, which were affected by Al to the same degree as by decreasing soil moisture. Only BEG expression was enhanced by Al but decreased by drought. The Al×soil moisture interaction was significant for all and highly significant for most genes. Al remarkably reduced the drought-enhanced expression of SUS, KS-DHN, PvLEA18, and LTP, but further increased the expression of P5CS and HRGP (only at the high Al supply).
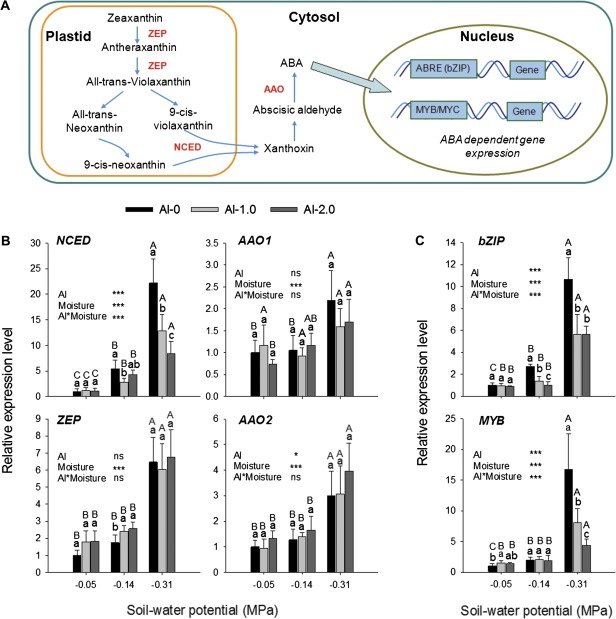
Drought stress significantly increased the expression of the genes NCED (9-cis-epoxycarotenoid dioxygenase), ZEP (zeaxanthin epoxidase), AAO1(abscisic aldehyde oxidase 1), and AAO2 (Fig. 8B) involved in ABA biosynthesis (Fig. 8A). Al markedly affected only NCED, which was down-regulated at low soil moisture (significant Al×soil moisture interaction), reversing the drought-enhanced expression of this gene (Fig. 8B). The expression of the two transcription factors bZIP (basic leucine zipper) and MYB (myeloblastosis) (Fig. 8C) that are involved in the ABA-dependent gene regulation under drought stress (Fig. 8A; Shinozaki and Yamaguchi-Shinozaki 1997) and selected from a previous analysis of PEG-induced gene expression in common bean root tips using SuperSAGE (Yang et al., 2011), was highly up-regulated by drought (Fig. 8B). In agreement with NCED expression, Al stress in addition to drought stress reversed the drought-enhanced gene expression of both transcription factors (highly significant Al×soil moisture interaction).
Fig. 8.
Schematic flow of ABA biosynthesis and ABA-dependent gene regulation pathways (A) and the expression of genes coding for enzymes involved in the pathways as shown in A (B, C) in 1 cm root tips of the common bean genotype VAX 1 as affected by soil moisture and Al supply (g kg−1 soil). Two-day-old seedlings were grown in soil for 24 h. qRT-PCR was performed using the β-tubulin gene as internal standard. Bars represent means ± SD, n=3. Means with different lower and upper case letters are significantly different at P < 0.05 (Tukey test) for the comparison of Al treatments within soil moisture and comparison of soil moisture treatments within Al treatments, respectively. For the ANOVA, * and *** denote significance at P < 0.05 and P < 0.001, respectively. ns, non-significant. ZEP, zeaxanthin epoxidase; NCED, 9-cis-epoxycarotenoid dioxygenase; AAO, abscisic aldehyde oxidase; ABRE, ABA-responsive element. (This figure is available in colour at JXB online.)
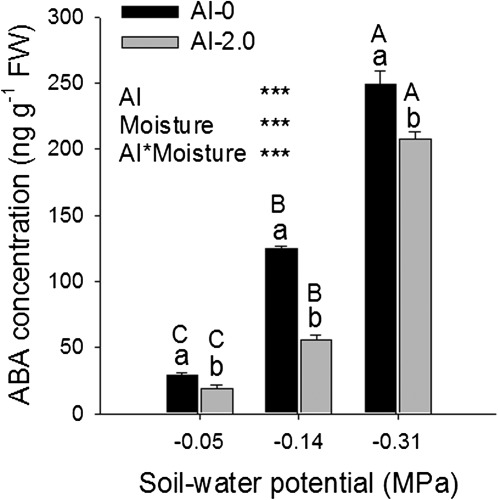
The observed changes in the expression of genes related to ABA biosynthesis and of transcription factors mediating ABA-dependent gene regulation were fully supported by the determination of ABA concentrations in the root tips (Fig. 9). Drought stress alone greatly increased the ABA concentration. Al supply alone only slightly decreased the ABA concentration in the root tips of well-watered plants. In combination with drought stress, Al markedly suppressed the drought-enhanced ABA accumulation in the root tips (highly significant Al×drought interaction).
Fig. 9.
Abscisic acid (ABA) concentration in the 1 cm root tips of the common bean genotype VAX 1 as affected by soil moisture and Al supply (g kg−1 soil). Two-day-old seedlings were grown in soil for 24 h. Bars represent means ±SD, n=3. Means with different lower and upper case letters are significantly different at P < 0.05 (Tukey test) for the comparison of Al treatments within soil moisture and comparison of soil moisture treatments within Al treatments, respectively. For the ANOVA, *** denote significance at P < 0.001.
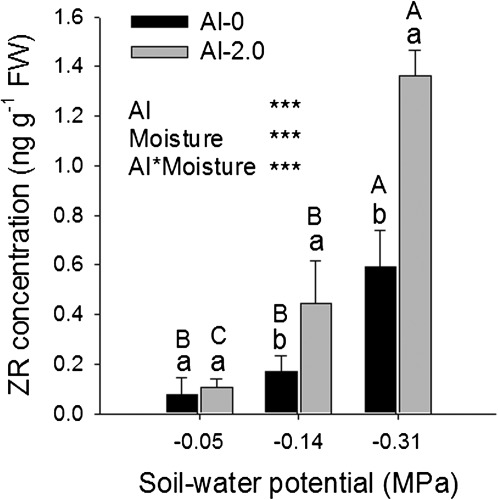
In addition to ABA, the concentrations of other phytohormones were analysed in the root tips. No significant effects of either drought or Al were found on SA and JA (Supplementary Fig. S4 at JXB online). Al stress alone significantly increased, but combined Al and drought stress reversed, the Al-enhanced IAA concentration. However, drought stress significantly increased not only the biologically active ZR (Fig. 10) but also the trans-zeatin (tZ) (Supplementary Fig. S4) concentration in the root tips. Among the CK storage forms, only the ZOG concentration was strongly increased at the lowest soil moisture (Supplementary Fig. S4). In well-watered soil, Al did not affect the CK concentrations. However, Al strongly enhanced the drought-increased ZR and ZOG concentrations in the root tips (Fig. 10; Supplementary Fig. S4).
Fig. 10.
Zeatin riboside (ZR) concentration in the 1 cm root tips of the common bean genotype VAX 1 as affected by soil moisture and Al supply (g kg−1 soil). Two-day-old seedlings were grown in soil for 24 h. Bars represent means ±SD, n=3. Means with different lower and upper case letters are significantly different at P < 0.05 (Tukey test) for the comparison of Al treatments within soil moisture and comparison of soil moisture treatments within Al treatments, respectively. For the ANOVA, *** denote significance at P < 0.001.
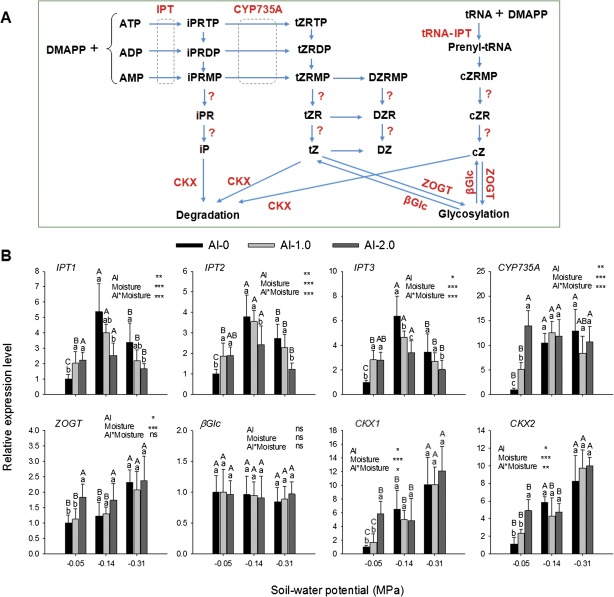
The clear changes in CK concentration prompted the study of the expression of the genes IPT (adenosine-phosphate isopentenyl-transferase) and CYP735A (cytochrome P450 monooxygenase 735A) involved in CK biosynthesis and ZOGT (zeatin-O-glucosyltransferase), βGlc (β-glucosidase), and CKX (cytokinin oxidase/dehydrogenase) involved in CK degradation/inactivation (Fig. 11A). Both drought and Al treatment individually significantly increased the gene expression of all genes except βGlc in the root tips (Fig. 11B). Under combined drought and Al stresses, Al treatment decreased the drought-enhanced expression of all IPT genes. The Al-enhanced expression of CYP735A, ZOGT, and CKX in well-watered plants disappeared under reduced soil moisture, maintaining the drought-stimulated expression level.
Fig. 11.
(A) Schematic flow of CK biosynthesis pathways. Unknown genes are marked with ‘?’. (B) Expression of genes coding for enzymes involved in CK biosynthesis as shown in A in the root tips of the common bean genotype VAX 1 as affected by soil moisture and Al supply (g kg−1 soil). Two-day-old seedlings were grown in soil for 24 h. qRT-PCR was performed using the β-tubulin gene as internal standard. Bars represent means ±SD, n=3. Means with different lower and upper case letters are significantly different at P < 0.05 (Tukey test) for the comparison of Al treatments within soil moisture and comparison of soil moisture treatments within Al treatments, respectively. For the ANOVA, *, **, and *** denote significance at P < 0.05, P < 0.01, and P < 0.001, respectively. ns, non-significant. DMAPP, dimethylallyl diphosphate; iP, N6-(Δ2-isopentenyl)-adenine; tZ, trans-zeatin; cZ, cis-zeatin; DZ, dihydrozeatin; iPR, N6-(Δ2-isopentenyl)-adenine riboside; tZR, trans-zeatin riboside, cZR, cis-zeatin riboside; DZR, dihydrozeatin riboside; iPRDP, iP riboside 5′-diphosphate; iPRTP, iP riboside 5'-triphosphate; iPRMP, iP riboside 5′-moophosphate; tZRDP, tZR 5-diphosphate; tZRTP, tZR 5′-triphosphate; tZRMP, tZR 5′-monophosphate; DZRMP, DZR 5′-monophosphate; cZRMP, cZR 5′-monophosphate; IPT, adenosine-phosphate isopentenyl-transferase, CYP735A, cytochrome P450 monooxygenase 735A; CKX, cytokinin oxidase/dehydrogenase; ZOGT, zeatin-O-glucosyltransferase; βGlc, β-glucosidase. (This figure is available in colour at JXB online.)
Discussion
In hydroponic culture, application of PEG 6000 to simulate drought stress greatly alleviated Al-induced inhibition of root elongation (Fig. 3A) without changing the mononuclear Al concentration in solution (Yang et al., 2010). This was due to reduced Al accumulation in the root tips in the presence of PEG nearly to the level of the control not treated with Al (Fig. 3B). Lower Al binding of PEG-treated root apices has been related to reduced accessibility of the Al-binding sites of the apoplast owing to dehydration and thus reduced porosity of the CW (Yang et al., 2010). An impact of PEG on CW structure has been supported by a transcriptomic analysis showing modification of the expression of genes encoding the CW-loosening enzymes XTH and BEG and the structural protein HRGP by PEG (Yang et al., 2011). The lack of Al impact on the root apex in the presence of PEG is confirmed by greatly reduced Al-induced callose formation (Fig. 3C). Induction of callose synthesis proved to be a sensitive indicator of Al injury in root apices (Fig. 2; Horst et al., 1997; Eticha et al. 2005; Staß and Horst, 2009). In addition, Al-induced expression of MATE and ACCO which have been successfully used as Al sensitivity indicators (Eticha et al., 2010) also confirms that PEG reduced Al accumulation and Al toxicity in the root tips of common bean (Yang et al., 2011).
In the discussion of the drought–Al interaction in the root apex in the soil-grown plants it might be necessary to consider that these plants were at the early seedling stage with still unfolded leaves lacking transpiration and photosynthesis (Fig. 4A). Thus, it cannot be excluded that in seedlings subjected to drought and Al stress at a later stage, cross-talk between the shoot and root may affect the studied physiological and molecular responses differently.
In agreement with the effect of PEG in hydroponics, low soil moisture also reduced the Al-induced enhancement of callose production (Figs 4C, 5B) and the expression of MATE (Fig. 6A) in the root tips of soil-grown common bean plants, particularly at the high Al level (2.0 g kg−1 soil), thus providing evidence that drought also reduced the impact of Al on the root apices of common bean. The expression of MATE was rather specific for Al (140-fold increase) compared with drought (10-fold increase). Compared with hydroponics (Eticha et al., 2010; Yang et al., 2011), the Al-induced MATE expression was less in soil, which can mainly be attributed to a higher basic level of gene expression in the well-watered, limed soil.
A lower impact of Al on root apices at decreasing soil moisture cannot be explained by lower Al concentrations of the soil solution (Supplementary Fig. S3 at JXB online). The similar induction of the expression of six CW-associated genes in root tips of common bean subjected to drought in soil (Fig. 7) compared with PEG in hydroponics (Yang et al., 2011) indicates that drought-induced reduction of the Al impact on the root apex in this study might also result from drought-induced alteration of CW structure. However, the possibility that the increased total ionic strength of other cations (Ca2+, Mg2+, and K+) in lower moisture soil solutions (Supplementary Fig. S3) may have reduced the ionic activity of Al3+ cannot be excluded. The consistency of the expression of the six CW- and six OS-associated genes (except CYP701A) by PEG and by drought in soil in root tips of common bean (Fig. 7; Yang et al., 2011) supports the use of PEG 6000 in hydroponics in determining short-term drought stress responses of root apices at the molecular level. The generally greater differences in expression levels of most genes in root apices exposed to dry soil compared with PEG in hydroponics might result from the possibility to acclimatize to OS (PEG 6000) in hydroponic culture, allowing sufficient water uptake to resume root elongation partly within 24 h, whereas in dried soil the adaptation to water deficit fails.
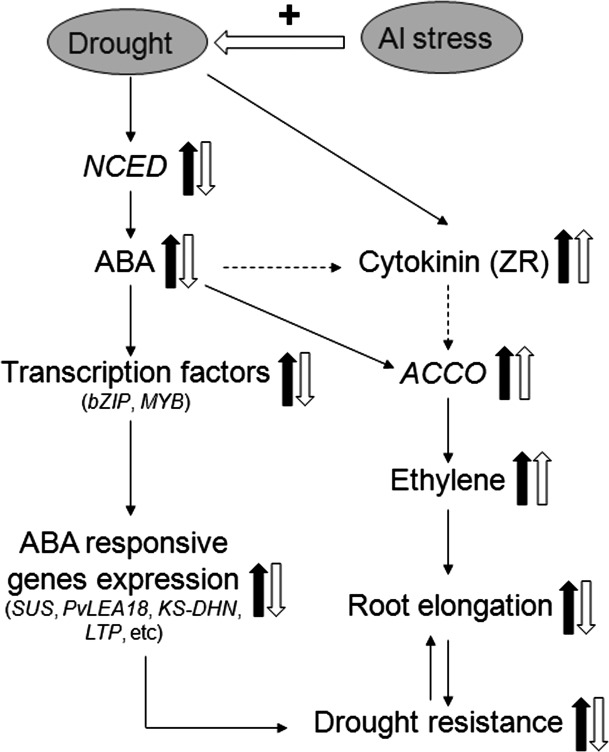
However, in spite of a lower impact of Al on the root apex and in contrast to the alleviation of Al toxicity in hydroponics by PEG, Al and drought additively inhibited root elongation (Figs 4A, B, 5A). It appears that in soil, the small amount Al in the root tips was sufficient to induce Al-specific responses, as reflected by slightly enhanced MATE expression (Fig. 6), and consequently could increase the susceptibility of roots to drought, as proposed by Goldman et al. (1989) in soybean, and thus aggravating the drought-induced inhibition of root elongation. This conclusion is supported by the following lines of evidence.
(i) Al suppressed drought-induced ABA accumulation (Fig. 9) and the expression of NCED (Fig. 8B) involved in ABA biosynthesis (Seo and Koshiba, 2002; Fig. 8A) in the root tips of common bean. The accumulation of ABA in roots mainly towards the root apex is necessary to maintain the primary root elongation at low water potentials (Saab et al., 1992; Sharp et al., 2004; Yamaguchi and Sharp, 2010). It has been reported that the overexpression of NCED in tobacco (Nicotiana plumbaginifolia) and Arabidopsis resulted in an increase in the endogenous ABA level and an improvement of drought tolerance (Iuchi et al., 2001; Qin and Zeevaart, 2002).
(ii) Consistent with the change in NCED expression and ABA accumulation in the root tips, Al reversed the drought-elevated expression of bZIP and MYB (Fig. 8C). The ABA-responsive element (ABRE)-binding bZIP transcription factor and the MYB transcription factor play key roles in ABA-regulated water deficit-induced gene expression, and thus drought tolerance (Shinozaki and Yamaguchi-Shinozaki, 1997, 2007). Therefore, Al might suppress drought-induced ABA-responsive gene expression via decreasing the regulation of ABA-dependent transcription factors such as bZIP and MYB, and thus reduce the drought tolerance of the root (Fig. 12).
(iii) Among the 12 OS- and CW-associated genes studied, Al further suppressed the drought-stimulated gene expression of SUS, PvLEA18, KS-DHN, and LTP in the root tips of common bean (Fig. 7). It has been reported that the expression of these genes plays crucial roles in plant cellular adaptation to drought (Colmenero-Flores et al., 1999; Wang et al., 2000; Bartels and Sunkar, 2005; Yang et al., 2011) and can be induced by ABA. For example, most genes encoding LEA proteins in Arabidopsis had ABREs in their promoters and were induced by ABA. Among these, two genes (At2g23110 and At2g23120) belong to the PvLEA18 group (Hundertmark and Hincha, 2008). Dehydrin (DHN) is the second biggest group of LEA proteins. The EST of KS-DHN in common bean has high sequence similarity to At1g54410 which codes for proteins belonging to the DHN group of LEA and also can be induced by ABA (Hundertmark and Hincha, 2008). In tomato (Solanum lycopersicum L.), overexpression of drought-induced SlAREB1 up-regulated the genes encoding LTP and LEA proteins (Orellana et al., 2010). Moreover, Saftner and Wyse (1984) observed that ABA increased sucrose uptake in the roots of sugar beet (Beta vulgaris), and the ABA-insensitive (abi8) mutant showed a strong reduction of the expression of SUS in Arabidopsis (Brocard-Gifford et al., 2004). Therefore, it appears that the suppression of ABA-dependent drought-induced gene regulation by Al may lead to an aggravated inhibition of root elongation under drought, as schematically depicted in Fig. 12.
(iv) Drought and Al (Al plus drought > drought > Al > control) clearly inhibited both primary and lateral root growth of common bean (Fig. 4A), and this observation is in agreement with the increasing concentration of ZR, the biologically active form of CKs in the root tips (Fig. 10). Massot et al. (1994) were the first to report that excess Al increased the endogenous levels of ZR and DHZR in roots of common bean. CKs strongly inhibit root growth (Werner et al., 2001). Overexpression of IPT involved in CK biosynthesis (Fig. 11A) enhanced the levels of biologically active CKs and inhibited primary root elongation in Arabidopsis (Kuderová et al., 2008). Overexpression of genes involved in CK degradation/inactivation, such as ZOGT in maize (Rodó et al., 2008) and CKX in Arabidopsis (Werner et al., 2010) and tobacco (Werner et al., 2001, 2010), resulted in enhanced root growth and branching. The root-specific reduction of CKs by overexpression of CKX strongly enhanced drought resistance in Arabidopsis and tobacco (Werner et al., 2010).
Fig. 12.
Schematic representation of the potential regulatory mechanisms involved in acclimation to drought of root apices of common bean and how Al stress interferes with this acclimation. The thick arrows indicate the up- and down-regulated changes. The thick filled arrows show the effect of the sole drought treatment, and the thick open arrows show the relative changes under combined drought and Al stress compared with the sole drought treatment. The thin dashed arrows indicate the potential connections. For further explanations, see the related discussion.
In the present study, both drought and Al treatment significantly affected the expression levels of IPT, CYP735A, ZOGT, and CKX in the root tips of common bean (Fig. 11B). However, the clearly highest levels of ZR and ZOG in the root tips exhibiting combined Al and drought stress (Fig. 10; Supplementary Fig. S4 at JXB online) are difficult to explain on the basis of the expression of the genes involved in CK biosynthesis and degradation/inactivation (Sakakibara, 2006; Kudo et al., 2010; Fig. 11A). Of course, gene expression does not allow unequivocal quantitative conclusions on protein activities which might be affected by post-translational regulation. Also, the specific genes and members of the gene families involved in ZR and ZOG synthesis and degradation are not yet known.
(v) Al enhanced drought-stimulated expression of ACCO involved in the biosynthesis of ethylene (Wang et al., 2002) in the root tips (Fig. 6). In interplay with auxin, ethylene strongly inhibits root growth (Stepanova et al., 2007; Swarup et al., 2007).
(vi) Al treatment triggers a cross-talk between phytohormones, leading to reduced drought resistance of the root apex and thus to enhanced inhibition of root elongation of common bean, as depicted in Fig. 12: Al may suppress drought-induced ABA accumulation in the root tips by promoting CK production which, subsequently, stimulate the synthesis of ethylene, leading to enhanced inhibition of root elongation. Several studies suggest interplay between ABA, CKs, and ethylene. ABA suppresses ethylene production, and the maintenance of root elongation under water deficit conditions requires increased ABA levels to prevent excess ethylene production (Sharp et al., 2000; Spollen et al., 2000; Sharp, 2002; LeNoble et al., 2004), which mediates the CK-induced inhibition of root elongation (Bertell and Eliasson, 1992; Cary et al., 1995; Massot et al., 2002; Růžička et al., 2009). CKs stimulate ethylene biosynthesis (Chae et al., 2003), and ABA mediates the activity of CKX and the expression of CYP735A1 and CYP735A2 involved in CK biosynthesis and thus CK concentrations (Takei et al., 2004; Vysotskaya et al., 2009). Recently, Guo and Gan (2011) demonstrated that the transcription factor atmyb2 mutant exhibited enhanced expression of IPT involved in CK biosynthesis and synthesis of CKs, particularly ZR (the biologically active form of CKs) and isopentenyladenosine in Arabidopsis.
The results provide circumstantial evidence that in common bean drought alleviates Al injury in the root tips based on less Al-induced callose formation and lower expression of a MATE gene. However, Al renders the root apex more drought sensitive, leading to enhanced inhibition of root elongation resulting from the disruption of the gene regulatory network involved in ABA signal transduction and ABA signal cross-talk with other phytohormones that are necessary for maintaining root growth under drought stress.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. The soil water retention curve of an oxisol from the Llanos region of Colombia.
Figure S2. Soil pH and Al concentrations of water extracts at different levels of Al application.
Figure S3. The concentrations of Al, Ca, Mg, and K in the soil solution under different levels of soil moisture.
Figure S4. Phytohormone concentrations in the root tips of the common bean genotype VAX 1 as affected by soil moisture and Al supply.
Table S1. List of genes and specific primer pairs used for quantitative gene expression analysis.
Acknowledgments
This research was supported by a restricted core project from the Bundesministerium für Wirtschaftliche Zusammenarbeit/Gesellschaft für Technische Zusammenarbeit (BMZ/GTZ) (no. 05.7860.9-001.00) granted to the International Center for Tropical Agriculture (CIAT). We thank Dr Steve Beebe, Leader of the Bean Program of CIAT, for the supply of seeds of the common bean genotype, J. Bachmann, M. Volkmann, and S.K. Woche, Institute of Soil Science, Leibniz Universität Hannover, for their help in establishing the pF curve of the experimental soil, and the China Scholarship Council for providing a scholarship to Z-BY.
References
- Albacete A, Ghanem ME, Martínez-Andújar C, Acosta M, Sánchez-Bravo J, Martínez V, Lutts S, Dodd IC, Pérez-Alfocea F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinised tomato (Solanum lycopersicum L.) plants. Journal of Experimental Botany. 2008;59:4119–4131. doi: 10.1093/jxb/ern251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels D, Sunkar R. Drought and salt tolerance in plants. Critical Reviews in Plant Sciences. 2005;24:23–58. [Google Scholar]
- Beebe S, Ramirez J, Jarvis A, Rao IM, Mosquera G, Bueno JM, Blair M. Genetic improvement of common beans and the challenges of climate change. In: Yadav SS, Redden RJ, Hatfield JL, Lotze-Campen H, Hall AE, editors. Crop adaptation to climate change. New York: John Wiley and Sons; 2011. pp. 356–369. [Google Scholar]
- Beebe S, Rao IM, Cajiao C, Grajales M. Selection for drought resistance in common bean also improves yield in phosphorus limited and favourable environments. Crop Science. 2008;48:582–592. [Google Scholar]
- Bertell G, Eliasson L. Cytokinin effects on root growth and possible interactions with ethylene and indole-3-acetic acid. Physiologia Plantarum. 1992;84:255–261. [Google Scholar]
- Brocard-Gifford I, Lynch TJ, Garcia ME, Malhotra B, Finkelstein RR. The Arabidopsis thaliana ABSCISIC ACID-INSENSITIVE8 encodes a novel protein mediating abscisic acid and sugar responses essential for growth. The Plant Cell. 2004;16:406–421. doi: 10.1105/tpc.018077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butare L, Rao I, Lepoivre P, Polania J, Cajiao C, Cuasquer J, Beebe S. New genetic sources of resistance in the genus Phaseolus to individual and combined stress factors of aluminium toxicity and progressive soil drying. Euphytica. 2011;185:385–404. [Google Scholar]
- Carpita N, Sabularse D, Montezinos D, Delmer DP. Determination of the pore size of cell walls of living plant cells. Science. 1979;205:1144–1147. doi: 10.1126/science.205.4411.1144. [DOI] [PubMed] [Google Scholar]
- Cary AJ, Liu W, Howell SH. Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings. Plant Physiology. 1995;107:1075–1082. doi: 10.1104/pp.107.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae HS, Faure F, Kieber JJ. The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. The Plant Cell. 2003;15:545–559. doi: 10.1105/tpc.006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIAT. Constraints to and opportunities for improving bean production. A planning document 1993–98 and an achieving document 1987–92. Cali, Colombia: CIAT; 1992. [Google Scholar]
- Colmenero-Flores JM, Moreno LP, Smith CE, Covarrubias AA. Pvlea-18, a member of a new late-embryogenesis-abundant protein family that accumulates during water stress and in the growing regions of well-irrigated bean seedlings. Plant Physiology. 1999;120:93–104. doi: 10.1104/pp.120.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook LL, McGonigle TP, Inouye RS. Titanium as an indicator of residual soil on arid-land plants. Journal of Environmental Quality. 2009;38:188–199. doi: 10.2134/jeq2007.0034. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR. Aluminum toxicity and tolerance in plants. Plant Physiology. 1995;107:315–321. doi: 10.1104/pp.107.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eticha D, Thé C, Welcker C, Narro L, Staß A, Horst WJ. Aluminium-induced callose formation in root apices: inheritance and selection trait for adaptation of tropical maize to acid soils. Field Crops Research. 2005;93:252–263. [Google Scholar]
- Eticha D, Zahn M, Bremer M, Yang Z, Rangel AF, Rao IM, Horst WJ. Transcriptomic analysis reveals differential gene expression in response to aluminium in common bean (Phaseolus vulgaris) genotypes. Annals of Botany. 2010;105:1119–1128. doi: 10.1093/aob/mcq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman IL, Carter TE, Jr, Patterson RP. A detrimental interaction of subsoil aluminum and drought stress on the leaf water status of soybean. Agronomy Journal. 1989;81:461–463. [Google Scholar]
- Graham PH. Some problems and potentials of field beans (Phaseolus vulgaris L.) in Latin America. Field Crops Research. 1978;1:295–317. [Google Scholar]
- Gunsé B, Poschenrieder C, Barceló J. Water transport properties of roots and root cortical cells in proton- and Al-stressed maize varieties. Plant Physiology. 1997;113:595–602. doi: 10.1104/pp.113.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Gan SS. AtMYB2 regulates whole plant senescence by inhibiting cytokinin-mediated branching at late stages of development in Arabidopsis. Plant Physiology. 2011;156:1612–1619. doi: 10.1104/pp.111.177022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst WJ, Asher CJ, Cakmak J, Szulkiewicz P, Wissemeier AH. Short-term responses of soybean roots to aluminium. Journal of Plant Physiology. 1992;140:174–178. [Google Scholar]
- Horst WJ, Püschel A-K, Schmohl N. Induction of callose formation is a sensitive marker for genotypic aluminium sensitivity in maize. Plant and Soil. 1997;192:23–30. [Google Scholar]
- Horst WJ, Wang Y, Eticha D. The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Annals of Botany. 2010;106:185–197. doi: 10.1093/aob/mcq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundertmark M, Hincha DK. LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genomics. 2008;9:118. doi: 10.1186/1471-2164-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani M, Rao I, Wenzl P, Beebe S, Tohme J. Integration of genomics approach with traditional breeding towards improving abiotic stress adaptation: drought and aluminum toxicity as case studies. Field Crops Research. 2004;90:35–45. [Google Scholar]
- Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K. Regulation of drought tolerance by gene manipulation of 9-cisepoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. The Plant Journal. 2001;27:325–333. doi: 10.1046/j.1365-313x.2001.01096.x. [DOI] [PubMed] [Google Scholar]
- Kauss H. Fluorometric measurement of callose and other 1,3-β-glucans. In: Linskens H-F, Jackson JF, editors. Modern methods of plant analysis. Berlin: Springer Verlag; 1989. pp. 127–137. [Google Scholar]
- Kuderová A, Urbánková I, Válková M, Malbeck J, Brzobohatý B, Némethová D, Hejátko J. Effects of conditional IPT-dependent cytokinin overproduction on root architecture of Arabidopsis seedlings. Plant and Cell Physiology. 2008;49:570–582. doi: 10.1093/pcp/pcn029. [DOI] [PubMed] [Google Scholar]
- Kudo T, Kiba T, Sakakibara H. Metabolism and long-distance translocation of cytokinins. Journal of Integrative Plant Biology. 2010;52:53–60. doi: 10.1111/j.1744-7909.2010.00898.x. [DOI] [PubMed] [Google Scholar]
- LeNoble ME, Spollen WG, Sharp RE. Maintenance of shoot growth by ABA: genetic assessment of the role of ethylene suppression. Journal of Experimental Botany. 2004;55:237–245. doi: 10.1093/jxb/erh031. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Massot N, Nicander B, Barceló J, Poschenrieder Ch, Tillberg E. A rapid increase in cytokinin levels and enhanced ethylene evolution precede Al3+-induced inhibition of root growth in bean seedlings (Phaseolus vulgaris L.) Plant Growth Regulation. 2002;37:105–112. [Google Scholar]
- Massot N, Poschenrieder Ch, Barceló J. Aluminium-induced increase of zeatin riboside and dihydrozeatin riboside in Phaseolus vulgaris L. cultivars. Journal of Plant Nutrition. 1994;17:225–265. [Google Scholar]
- Miyasaka SC, Buta JG, Howell RK, Foy CD. Mechanism of aluminum tolerance in snapbeans: root exudation of citric acid. Plant Physiology. 1991;96:737–743. doi: 10.1104/pp.96.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana S, Yañez M, Espinoza A, Verdugo I, González E, Ruiz-Lara S, Casaretto JA. The transcription factor SlAREB1 confers drought, salt stress tolerance and regulates biotic and abiotic stress-related genes in tomato. Plant, Cell and Environment. 2010;33:2191–2208. doi: 10.1111/j.1365-3040.2010.02220.x. [DOI] [PubMed] [Google Scholar]
- Qin X, Zeevaart JA. Overexpression of a 9-cis-epoxycarotenoid dioxygenase gene in Nicotiana plumbaginifolia increases abscisic acid and phaseic acid levels and enhances drought tolerance. Plant Physiology. 2002;128:544–551. doi: 10.1104/pp.010663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel AF, Rao IM, Braun H-P, Horst WJ. Aluminium resistance in common bean (Phaseolus vulgaris) involves induction and maintenance of citrate exudation from root apices. Physiologia Plantarum. 2010;138:176–190. doi: 10.1111/j.1399-3054.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- Rangel AF, Rao IM, Horst WJ. Spatial aluminium sensitivity of root apices of two common bean (Phaseolus vulgaris L.) genotypes with contrasting aluminium resistance. Journal of Experimental Botany. 2007;58:3895–3904. doi: 10.1093/jxb/erm241. [DOI] [PubMed] [Google Scholar]
- Rangel AF, Rao IM, Horst WJ. Intracellular distribution and binding state of aluminum in root apices of two common bean (Phaseolus vulgaris) genotypes in relation to Al toxicity. Physiologia Plantarum. 2009;135:162–173. doi: 10.1111/j.1399-3054.2008.01183.x. [DOI] [PubMed] [Google Scholar]
- Rao IM. Role of physiology in improving crop adaptation to abiotic stresses in the tropics: the case of common bean and tropical forages. In: Pessarakli M, editor. Handbook of plant and crop physiology. New York: Marcel Dekker; 2001. pp. 583–613. [Google Scholar]
- Rodó AP, Brugière N, Vankova R, Malbeck J, Olson JM, Haines SC, Martin RC, Habben JE, Mok DW, Mok MC. Over-expression of a zeatin O-glucosylation gene in maize leads to growth retardation and tasselseed formation. Journal of Experimental Botany. 2008;59:2673–2686. doi: 10.1093/jxb/ern137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics methods and protocols. Totowa, NJ: Humana Press; 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- Růžička K, Šimášková M, Duclercq J, Petrášek J, Zažímalová E, Simon S, Friml J, Van Montagu MC, Benková E. Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proceedings of the National Academy of Sciences, USA. 2009;106:4284–4289. doi: 10.1073/pnas.0900060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab IN, Sharp RE, Pritchard J. Effect of inhibition of abscisic acid accumulation on the spatial distribution of elongation in the primary root and mesocotyl of maize at low water potentials. Plant Physiology. 1992;99:26–33. doi: 10.1104/pp.99.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saftner RA, Wyse RE. Effect of plant hormones on sucrose uptake by sugar beet root tissue discs. Plant Physiology. 1984;74:951–955. doi: 10.1104/pp.74.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H. Cytokinins: activity, biosynthesis, and translocation. Annual Review of Plant Biology. 2006;57:431–449. doi: 10.1146/annurev.arplant.57.032905.105231. [DOI] [PubMed] [Google Scholar]
- Seo M, Koshiba T. Complex regulation of ABA biosynthesis in plants. Trends in Plant Science. 2002;7:41–48. doi: 10.1016/s1360-1385(01)02187-2. [DOI] [PubMed] [Google Scholar]
- Serraj R, Sinclair TR. Osmolyte accumulation: can it really help increase crop yield under drought conditions? Plant, Cell and Environment. 2002;25:333–341. doi: 10.1046/j.1365-3040.2002.00754.x. [DOI] [PubMed] [Google Scholar]
- Sharp RE. Interaction with ethylene: changing views on the role of ABA in root and shoot growth responses to water stress. Plant, Cell and Environment. 2002;25:211–222. doi: 10.1046/j.1365-3040.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Sharp RE, LeNoble ME, Else MA, Thorne ET, Gherardi F. Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: evidence for an interaction with ethylene. Journal of Experimental Botany. 2000;51:1575–1584. doi: 10.1093/jexbot/51.350.1575. [DOI] [PubMed] [Google Scholar]
- Sharp RE, Poroyko V, Hejlek LG, Spollen WG, Springer GK, Bohnert HJ, Nguyen HT. Root growth maintenance during water deficits: physiology to functional genomics. Journal of Experimental Botany. 2004;55:2343–2351. doi: 10.1093/jxb/erh276. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. Gene expression and signal transduction in water-stress response. Plant Physiology. 1997;115:327–334. doi: 10.1104/pp.115.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. Journal of Experimental Botany. 2007;58:221–227. doi: 10.1093/jxb/erl164. [DOI] [PubMed] [Google Scholar]
- Sivaguru M, Horst WJ, Eticha D, Matsumoto H. Aluminum inhibits apoplastic flow of high-molecular weight solutes in root apices of Zea mays L. Journal of Plant Nutrition and Soil Science. 2006;169:679–690. [Google Scholar]
- Spollen WG, LeNoble ME, Samuels TD, Bernstein N, Sharp RE. Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiology. 2000;122:967–976. doi: 10.1104/pp.122.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponchiado BN, White JW, Castillo JA, Jones PG. Root growth of four common bean cultivars in relation to drought tolerance in environments with contrasting soil types. Experimental Agriculture. 1989;25:249–257. [Google Scholar]
- Staß A, Horst WJ. Callose in abiotic stress. In: Bacic A, Fincher GB, Stone BA, editors. Chemistry, biochemistry, and biology of (1→3)-β-glucans and related polysaccharides. Burlington, MA: Academic Press; 2009. pp. 499–524. [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM. Multilevel interactions between ethylene and auxin in Arabidopsis roots. The Plant Cell. 2007;19:2169–2185. doi: 10.1105/tpc.107.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner ME, Miller WP. Cation exchange capacity and exchange coefficients. In: Sparks DL, editor. Methods of soil analysis. Part 3. Chemical methods. Madison, WI: Soil Science Society of America and American Society of Agronomy; 1996. pp. 1215–1217. [Google Scholar]
- Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GT, Sandberg G, Bhalerao R, Ljung K, Bennett MJ. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. The Plant Cell. 2007;19:2186–2196. doi: 10.1105/tpc.107.052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K, Yamaya T, Sakakibara H. Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-zeatin. Journal of Biological Chemistry. 2004;279:41866–41872. doi: 10.1074/jbc.M406337200. [DOI] [PubMed] [Google Scholar]
- Thung M, Rao IM. Integrated management of abiotic stresses. In: Singh SP, editor. Common bean improvement in the twenty-first century. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1999. pp. 331–370. [Google Scholar]
- Vysotskaya LB, Korobova AV, Veselov SY, Dodd IC, Kudoyarova GR. ABA mediation of shoot cytokinin oxidase activity: assessing its impacts on cytokinin status and biomass allocation of nutrient-deprived durum wheat. Functional Plant Biology. 2009;36:66–72. doi: 10.1071/FP08187. [DOI] [PubMed] [Google Scholar]
- Wang HL, Lee PD, Chen WL, Huang DJ, Su JC. Osmotic stress-induced changes of sucrose metabolism in cultured sweet potato cells. Journal of Experimental Botany. 2000;51:1991–1999. doi: 10.1093/jexbot/51.353.1991. [DOI] [PubMed] [Google Scholar]
- Wang KLC, Li H, Ecker JR. Ethylene biosynthesis and signaling networks. The Plant Cell. 2002;14:S131–S151. doi: 10.1105/tpc.001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Strnad M, Schmülling T. Regulation of plant growth by cytokinin. Proceedings of the National Academy of Sciences, USA. 2001;98:10487–10492. doi: 10.1073/pnas.171304098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Nehnevajova E, Köllmer I, Novák O, Strnad M, Krämer U, Schmülling T. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. The Plant Cell. 2010;22:3905–3920. doi: 10.1105/tpc.109.072694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortmann CS, Kirkby RA, Eledu CA, Allen DJ. Atlas of common bean (Phaseolus vulgaris L) production in Africa. Cali, Colombia: CIAT; 1998. [Google Scholar]
- Yamaguchi M, Sharp RE. Complexity and coordination of root growth at low water potentials: recent advances from transcriptomic and proteomic analyses. Plant, Cell and Environment. 2010;33:590–603. doi: 10.1111/j.1365-3040.2009.02064.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Valliyodan B, Zhang J, Lenoble ME, Yu O, Rogers EE, Nguyen HT, Sharp RE. Regulation of growth response to water stress in the soybean primary root. I. Proteomic analysis reveals region-specific regulation of phenylpropanoid metabolism and control of free iron in the elongation zone. Plant, Cell and Environment. 2010;33:223–243. doi: 10.1111/j.1365-3040.2009.02073.x. [DOI] [PubMed] [Google Scholar]
- Yang ZB, Eticha D, Rao IM, Horst WJ. Alteration of cell-wall porosity is involved in osmotic stress-induced enhancement of aluminium resistance in common bean (Phaseolus vulgaris L.) Journal of Experimental Botany. 2010;61:3245–3258. doi: 10.1093/jxb/erq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZB, Eticha D, Rotter B, Rao IM, Horst WJ. Physiological and molecular analysis of polyethylene glycol-induced reduction of aluminium accumulation in the root tips of common bean (Phaseolus vulgaris) New Phytologist. 2011;192:99–113. doi: 10.1111/j.1469-8137.2011.03784.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.