Abstract
While many tumor associated antigens (TAAs) have been identified in human cancers, efforts to develop efficient TAA “cancer vaccines” using classical vaccine approaches have been largely ineffective. Recently, a process to specifically target proteins to exosomes has been established [1] which takes advantage of the ability of the Factor V like C1C2 domain of lactadherin to specifically address proteins to exosomes. Using this approach, we hypothesized that TAAs could be targeted to exosomes to potentially increase their immunogenicity, as exosomes have been demonstrated to traffic to antigen presenting cells (APC) [2]. To investigate this possibility, we created adenoviral vectors expressing the extracellular domain (ECD) of two non-mutated TAAs often found in tumors of cancer patients, carcinoembryonic antigen (CEA) and HER2, and coupled them to the C1C2 domain of lactadherin. We found that these C1C2 fusion proteins had enhanced expression in exosomes in vitro. We saw significant improvement in antigen specific immune responses to each of these antigens in naïve and tolerant transgenic animal models and could further demonstrate significantly enhanced therapeutic anti-tumor effects in a human HER2+ transgenic animal model. These findings demonstrate that the mode of secretion and trafficking can influence the immunogenicity of different human TAAs, and may explain the lack of immunogenicity of non-mutated TAAs found in cancer patients. They suggest that exosomal targeting could enhance future anti-tumor vaccination protocols. This targeting exosome process could also be adapted for the development of more potent vaccines in some viral and parasitic diseases where the classical vaccine approach has demonstrated limitations.
Keywords: Cancer Vaccines, exosomes, C1C2 domain, tumor antigens, adenovirus vectors, CEA, HER2
Introduction
In most infectious diseases, soluble or particle antigens are circulating in the blood and can easily be captured by the professional antigen presenting cells (APC). Vaccines delivering these antigens in a native or inactivated form associated with proper adjuvant typically elicit a very potent immune response. This classical vaccine approach has been used widely and successfully applied in human and animal populations for preventing deadly diseases.
Unfortunately, this classical approach shows very little efficacy in diseases where antigens remain mainly localized inside cells, such as in some viral and parasitic diseases. The identification of tumor associated antigens (TAA) in human cancers [3-4] triggered an enormous effort in the medical and scientific community to develop “cancer vaccines”. Except in the case where viral antigens could be identified [5], the delivery of TAA in various forms by various vectors in association with a variety of adjuvants has led, up to now, to rather disappointing results [6-7]. These studies have revealed several specific difficulties with this type of approach. A primary difficulty is that most TAA are cell-associated and probably not delivered efficiently to professional APCs. While TAA can include proteins [3-4] that are coded by the host and overexpressed in cancer cells (HER2, wild type p53), some are expressed in fetal development (CEA) and/or select tissues, but not widely expressed in adult life such as the cancer-testis antigen family. Others are expressed with somatic mutations (RAS, p53) or translational modifications (MUC1). However, in general, they are poorly antigenic or expressed in an immunosuppressive environment. “Cancer vaccines” are supposed to be used mainly as therapeutic vaccines when a state of TAA immune-tolerance is established, a situation quite different from that encountered in classical vaccines where xenogeneic antigens are delivered to naïve individuals. Although new immunomodulatory reagents that may reverse tolerance in advanced cancer patients are being developed for cancer immunotherapy, strategies to enhance the potency of cancer vaccines to break established tolerance are essential for vaccines that may be given to a wide range of cancer patients
The lactadherin C1C2 domain is a lipid binding domain, related to the C1C2 domain of Factor V. It is responsible for the specific addressing of lactadherin to exosomes as deletion in this domain abolishes exosome addressing [1, 8]. It has recently been shown that soluble proteins including intracellular proteins, when fused to the C1C2 domain of lactadherin are no longer found intracellularly but are released in extracellular compartment, almost exclusively associated to exosomes [1, 9]. As exosomes transfer intracellular antigens directly to antigen presenting cells (APCs) [2], it was proposed that targeting intracellular antigens to exosomes would increase their trafficking to APCs and therefore stimulate their immunogenicity [1]. These principles were tested in a recent study that compared the tumorigenicities of ovalbumin antigen expressing cells [9]. In the study, the malignant cells expressing the C1C2-lactadherin domain ovalbumin fusion protein released the ovalbumin protein bound to exosomes in contrast to cell expressing unmodified ovalbumin. The authors also found that cells expressing the C1C2-fusion albumin were strikingly less tumorigenic if tested in immune-competent mice but kept their tumorigenity in immune-suppressed mice. Furthermore, some animals treated with cells expressing C1C2-ovabulbin fusion could become immune against the cells expressing the unmodified ovalbumin. These results suggest that despite a tumor cell environment, the C1C2 fusion protein could induce an effective anti-tumor immune response most likely mediated by trafficking to exosomes [9].
In order to test the possibility of using this targeting strategy to improve the potency of vaccines, we generated recombinant adenoviral vectors expressing the extracellular domain (ECD) of carcinoembryonic antigen (CEA) or HER2 linked to the C1C2 domain of lactadherin in addition to native unlinked ECD versions of CEA and HER2. We tested the efficacy of these viruses using mice made transgenic for these antigens to mimic the state of immune-tolerance found in human patients. We found that adenoviral expression of a C1C2 modified CEA/ECD and HER2/ECD resulted in significantly higher protein expression in exosomal fractions compared to non-targeted CEA in both murine cell lines and antigen presenting cells. We also found that secreting the ECD of CEA or HER2 in vivo as a vesicle-associated form was superior in inducing antigen specific immune responses in naïve and tolerant animals and enhanced anti-tumor immune responses. Our results thus provide insight into the low immunogenicity of soluble TAAs in cancer patients and suggest new means to improve anti-tumor immune responses for vaccines targeting cancer or potentially other diseases.
MATERIALS AND METHODS
C1C2 cloning and Ad vector construction
Briefly, the extracellular domain (ECD) of either human CEA (nt 1-2025) or human HER2/neu (nt 1-1953) were inserted into the mouse Lactadherin expression plasmid p6mLC1C2 as described [1] to create exosomal cassettes containing the leader signal and C1C2 domains of mouse lactadherin fused in-frame to the respective constructs. Vectors were created using the pAdEasy system [10] and all stocks titered using AdEasy viral titer kit (Stratagene, Santa Clara, CA).
In vivo experiments
C57BL/6J and BALB/c mice were obtained from Jackson Labs (Bar Harbor, MA), human CEA-transgenic mice were a kind gift from Jeff Schlom (National Cancer Institute, Bethesda, MD), and HER2 transgenic mice were obtained from Dr. Wei-Zen Wei (Wayne State University, Detroit, MI). Adenoviral vectors were administered via the footpad at indicated times of 4-12 week old mice. All animal work was performed in accordance with Duke IACUC approved protocols.
ELISPOT and Antibody procedures
Mouse IFN-gamma ELISPOT assay (Mabtech Inc., Cincinnati, OH) was performed according to according to published methods [11]. Briefly, harvested splenocytes were stimulated with CEA peptide mix (2.6 μg/ml: BD Bioscience, San Jose, CA), HER2 overlapping peptide mixtures (1 μg/ml of 15mer peptides overlapping by 11 amino acids for HER2) or irrelevant HIV gag or CMV pp65 antigen controls (2.6 μg/ml: BD Bioscience, San Jose, CA). PMA (50 ng/ml) and Ionomycin (1 μg/ml) were used as positive controls for splenocyte responsiveness. Anti-HER2 IgG antibodies was detected by FACS analysis of BT474 and SKBR3 HER2+ cells using PE-conjugated anti-Mouse IgG (Dako, Cat # R0480) as a secondary detection antibody. CEA ELISA used recombinant CEA (TriChem Resources, Inc., West Chester, PA, 10ug/ml) as the capture antigen and an anti-mouse IgG secondary antibody (Jackson Immunoresearch, West Grove, PA) as the detection antibody.
In vitro Assays
The human BT474 and SKBR3 HER2+ breast cancer lines and the murine JAWSII DC lines were obtained, tested for contamination (cellular and mycoplasma), and maintained according to ATCC recommendations. The human-HER2 expressing 4T1 mouse mammary tumor line (4T1-HER2) was kindly provided by Dr. Michael Kershaw (Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia). Murine bone marrow derived dendritic cells (bmDCs) were prepared and cultured with standard methods using GM-CSF (10ng/ml) and IL-4 (10ng/ml) (PeproTech, Rocky Hill, NJ).
For assessment of exosome protein expression, cleared supernatants were harvested and exosomes concentrated using standard ExoQuick (SBI Biosciences, Mountain View, CA) procedures [12]. Briefly, cleared supernatants (centrifuged at 3000×g for 15 minutes) were filtered using a .45um PVDF filter and mixed with ExoQuick precipitation solution and incubated overnight at 4°C. After incubation, precipitated exosomes were centrifuged at 1500×g for 30 minutes at 4°C following ExoQuick protocols. Concentrated exosomal fractions were resuspended in then quantified used a BCA assay (Thermo Fisher, Rockford, IL), and subjected to western blot analysis using anti-CEA (Cell Signaling Technology, Danvers, MA) and tsg101 as an exosome marker loading control (Abcam, Cambridge, MA) antibodies.
For exosome-capture ELISA to measure CEA and HER2 protein levels, supernatants from transduced cells were used in an anti-CD81 cross-capture ELISA [13] with monoclonal anti-CD81 (BioLegend, San Diego, CA) as a capture antibody and a polyclonal anti-CEA rabbit Ab (AbCam, Cambridge, MA) or an anti HER2 (N-terminal) rabbit Ab (Santa Cruz Biotechnology, Santa Cruz, CA), and an anti-rabbit HRP linked antibody (AbCam, Cambridge, MA) as the detection antibody set. In all experiments, uninfected control wells were performed simultaneously to monitor and control for background absorbance.
For cellular proliferation assessments, HER2+ BT474 human mammary tumor cells were cultured with serum of Ad immunized mice (Ad-HER2/ECDC1C2 or control at 1:20 dilution with Trastuzumab (Herceptin) (1 μg/ml) as a positive control for 6 days (with a media change at day 3) and measured by MTT (absorbance 550nm).
To assess complement-dependent cytotoxicity (CDC), sera from mice immunized as above was diluted (1:100) and co-incubated with target cells (BT474) at 37°C for 1h and 1:100 diluted rabbit serum as the source of complement. After 2.5 h incubation, cytotoxicity was measured using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega) to measure LDH release in the culture media as evidence of cytotoxicity.
RESULTS
Construction of Adenoviral vectors targeting CEA and HER2 to exosome and exosome associated expression of CEA
To assess the effect of exosomal secretion on virally expressed tumor associated antigens (TAAs), we generated a series of recombinant adenoviral vectors expressing the extracellular domain (ECD) of carcinoembryonic antigen (CEA) and HER2 in addition to a control LacZ. Identical antigen constructs coupled to a C1C2 domain, previously demonstrated to target antigens to exosomal compartment, [1, 9] were then generated to assess the effect of antigen exosomal targeting (Fig. 1A).
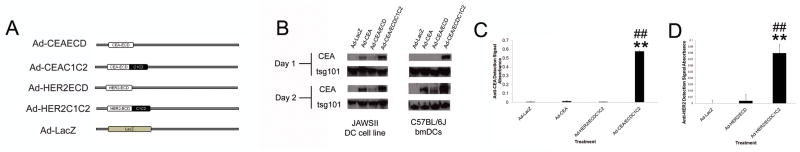
Figure 1. Exosomal Adenoviral Vectors.
A) Design of adenoviral vectors encoding CEA/ECD and HER2/ECD exosomal constructs and controls. B) Ad transduced (MOI=2000) JAWSII and bmDCs supernatant CEA expression at 24 and 48 hpi. C and D) Ad-transduced (MOI=2000) JAWSII cells 24hpi (C) and 48hpi (D) supernatants were assessed using an exosome-specific ELISA, which employed a CD81 capture antibody to isolate exosomes and CEA-specific detection antibody (C) and HER2-specific detection antibody (D) to assess exosomal CEA andHER2 expression. In all experiments, * and ** denotes conditions that showed p<0.05 and p<0.01 respectively, compared to control Ad-LacZ transduction and # and ## denote conditions that showed p<0.05 and p<0.01 respectively to Ad-CEA/ECD or Ad-HER2/ECD treatment. N=3 and error bars=SD.
Immortalized and bmDC-derived murine dendritic cells were then transduced with the various Ad-CEA vectors and exosome concentrated supernatants (using ExoQuick) assessed for CEA expression at 1 and 2 days post-transduction (dpi) by western blot analysis (Fig 1B). These results revealed a dramatic enhancement of CEA expression in extracellular exosome fractions compared to CEA and CEA/ECD controls (Fig. 1B). To further confirm CEA antigen presence in the exosomal fraction, we used an exosomal capture ELISA assay to isolate CD81+ exosome fractions and measure CEA protein levels (Fig. 1C). These assays confirmed our western blot analysis, demonstrating that only in exosomal fractions from JAWSII cells transduced with Ad-CEA/ECDC1C2 did we detect significant levels of CEA protein, thus demonstrating the C1C2 domain effectiveness in enhancing TAA exosome targeting. Similar experiments using Ad-HER2ECD and Ad-HER2/ECDC1C2 adenoviral vectors confirmed the secretion of HER2 in exosomal fractions (Fig. 1D), thus confirming that lactadherin C1C2 targeting of tumor antigen in adenoviral vector could effectively target these proteins to secreted exosomal vesicles.
Enhancement of T cell responses to CEA/ECD by targeting to exosomes
To determine the immunologic effects of the C1C2 modification, we vaccinated C57BL/6J mice with the various Ad-CEA or control adenoviral vectors and assessed CEA specific immune responses by CEA specific ELISPOT and ELISA at 2 weeks post-transduction (wpi). Both ELISPOT and ELISA analyses demonstrated that anti-CEA immunity in Ad-CEA/ECDC1C2 was significantly enhanced compared to Ad-CEA/ECD vectors (Fig. 2A). To determine if similar differences in anti-CEA responses could be seen at lower viral doses, Ad-CEA/ECD and Ad-CEA/ECDC1C2 were injected at two lower titers and T-cell and B-cell responses were again assessed by ELISPOT and ELISA assays. Remarkably, we found that Ad-CEA/ECDC1C2 elicited robust T-cell and antibody responses without significant abatement at lower titers, while the strength of Ad-CEA/ECD T-cell and B-cell responses were significantly reduced by the diminished viral titer (Fig. 2B).
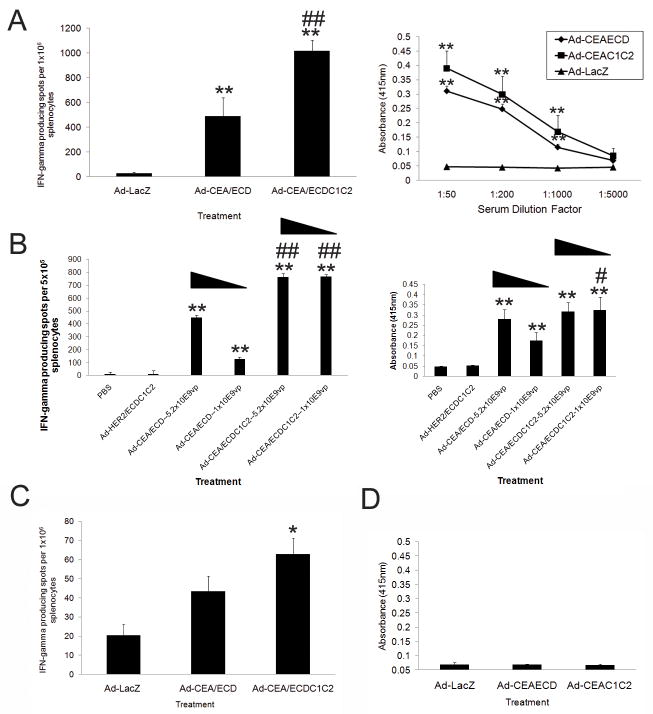
Figure 2. Vaccination with Ad-CEA C1C2 elicits superior anti-CEA adaptive immune responses.
A) -CEA T-cell responses at 14 days post-transduction (dpi) by IFN-γ ELISPOT (on left) and by anti-CEA IgG ELISA (on right) from C57BL/6J Ad vaccinated mice (2.6×1010 viral particles). B) Mice were vaccinated as in A) but using 5.2×109 or 1.05×109 vp of the indicated vectors with ELISPOT (on left) and ELISA (on right) performed (1:4000 Dilution) at 14 days-post-injection. Triangular bars indicate decreasing viral dose. C and D) ELISPOT And ELISA responses from C57BL/6J human CEA+ transgenic animals were treated and assessed as in A). In all experiments, * and ** denotes conditions that showed p<0.05 and p<0.01 respectively, compared to control Ad-LacZ transduction and # and ## denote conditions that showed p<0.05 and p<0.01 respectively to Ad-CEA/ECD treatment. N=5 and error bars=SD.
We next vaccinated CEA transgenic animals with the Ad-CEA/ECD, Ad-CEA/ECDC1C2 and control vectors to determine the effects of vaccination in a model of CEA tolerance. As expected, the tolerant human CEA transgenic mice were far less responsive to vaccination. While, Ad-CEA/ECDC1C2 vaccinated CEA transgenic mice did have a significant CEA specific T-cell response (Fig. 2C), vaccination with Ad-CEA/ECDC1C2 at this dose did not generate significant anti-CEA antibody responses in CEA transgenic mice (Fig 2D).
Enhancement of T and B cell responses to HER2/ECD by targeting to exosomes
As we demonstrated with CEA exosome targeted vaccines, we found that HER2/ECDC1C2 vaccination elicited significantly greater HER2 specific T-cell (Fig. 3A) and antibody responses compared to wild-type HER2/ECD in vivo (Fig. 3B).
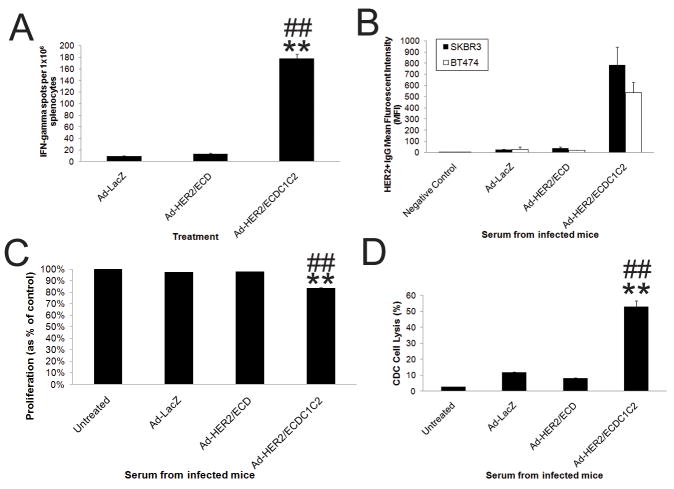
Figure 3. Vaccination with Ad-HER2-C1C2 elicits superior anti-HER2 adaptive responses.
A) Anti-HER2 T-cell responses assessed at 14 days post-transduction (dpi) by IFN-γ ELISPOT in BALB/c mice (2.6×1010 vp). Error bars represent standard deviation (n=4 mice per group at each time point). B) Anti-HER2 antibodies from serum of vaccinated mice (as in A) were measured using FACS to HER2+ cell lines (Average MFI from multiple experiments is shown). C) Anti-proliferative effects of vaccine induced antibodies were assessed by incubating Serum from C57BL/6J vaccinated mice (injected with 2.6×1010 vp, boosted 2.6×1010 vp at 14dpi, and harvested at 28dpi) with BT474 HER2+ cells in a MTT proliferation assay (N=4). D) Complement dependant cytotoxicity (CDC) mediated by vaccine induced antibodies (VIA) was assessed by incubating serum from mice treated as in C) with BT474 HER2+ cells in a CDC assay (N=4). In all experiments, * and ** denotes conditions that showed p<0.05 and p<0.01 respectively, compared to control Ad-LacZ transduction and # and ## denote conditions that showed p<0.05 and p<0.01 respectively to Ad-HER2/ECD treatment. Error bars denote SD.
As antibodies that target the extracellular domain of HER2 have proven therapeutic effects, we then assessed the HER2/ECD-specific antibodies in serum for anti-proliferative activity and complement-dependent cytotoxicity (CDC). Our results demonstrated a significantly greater inhibition of proliferation (Fig 3C) and CDC mediated lysis (Fig. 3D) of HER2+ BT474 cells with serum from mice post Ad-HER2/ECDC1C2 vaccination compared to the Ad-HER2/ECD vaccination (Fig. 3C).
To investigate if exosome targeting could enable therapeutic anti-tumor immunity in vivo, we implanted human HER2+ mouse mammary cancer cells (4T1-HER2) into human HER2 transgenic mice and vaccinated tumor bearing mice at the indicated intervals with Ad-LacZ, Ad-HER2/ECD, and Ad-HER2/ECDC1C2. Consistent with our previous CEA findings, we found that vaccination with Ad-HER2/ECDC1C2, but not Ad-HER2/ECD, significantly retarded HER2+ tumor growth in transgenic HER2+ mice (Fig 4A). When vaccinations were repeated against an Ad-CEA/ECDC1C2 control, we again found that Ad-HER2/ECDC1C2 significantly attenuated tumor growth, while Ad-CEA/ECDC1C2 did not, thus indicating the anti-tumor effect was specific to C1C2-tagged HER2 and not due to non-specific effects of C1C2 expression.
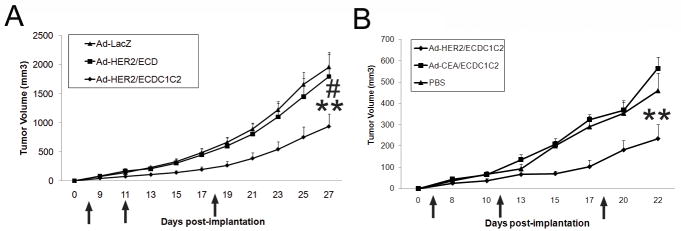
Figure 4. Vaccination with Ad-HER2-C1C2 elicits superior anti-tumor responses against HER2+ cells in human HER2 transgenic mice.
BALB/c HER2+ transgenic mice were implanted with 2×104 4T1-HER2+ cells at day zero and vaccinated with 2.6×1010 vp of the indicated Ad vectors (A and B) where indicated by arrows (4, 11, and 18 dpi). For all conditions, N=6-8 and error bars denote SE. In all samples, * and ** denotes conditions that showed p<0.05 and p<0.01 respectively, compared to Ad-LacZ (A) or PBS (B) controls. Additionally, # and ## denote conditions that showed p<0.05 and p<0.01 respectively to Ad-HER2/C1C2ECD injected animals (A).
Discussion
In agreement with previous proposal and observations [1], our study demonstrates that adenoviral transduction of cells in culture with constructs expressing the extracellular regions of different non-mutated TAAs fused to the C1C2 domain of lactadherin promotes high expression of exosome associated antigen in the extracellular medium. It is likely that the same phenomenon occurs in vivo. However, at this stage of the investigations, whether a fraction of the fusion proteins associates with other components in vivo cannot be ruled out.
The modality of lactadherin trafficking and binding to exosomes, which represent a very small fraction of the total membrane content of a cell is not yet clearly understood. Exosomes reside inside the multivesicular bodies (MVB) and are formed by inward budding of the MVB membrane [14]. The external surface of exosomes is never in direct contact with cytoplasmic medium. Recently, crystallographic structure of the C2 domain of bovine lactadherin was elucidated [15]. This study indicates that the C1C2 domain forms a high affinity reversible complex with membranes [16] through the intercalation of several aromatic and hydrophobic amino acids in the lipid layer. Since the external surface of exosomes seems to be never in contact with cytoplasmic constituents, Lactadherin targeting to exosomes would require its specific transport across the MVB membrane to allow its interaction with the exosomes inside the MVB compartment. Whether selective transport across MVB membrane and specific binding would account for the targeting of lactadherin and its C1C2-fusion analogues to exosomes in cells remains to be examined.
The injection of adenoviruses expressing the C1C2 domain fused to the ECD of either CEA or HER2 results in enhanced antigen-specific immune responses in both naïve and tolerant transgenic animals. This was in contrast to the in vivo expression of the soluble antigen not fused to C1C2 which, in naive and tolerant transgenic animals, triggered low or non-detectable immune responses.
Our work demonstrates enhancement of both T-cell and B-cell activation by this targeting process and extends these observations to common human TAAs overexpressed in the context of a viral vector. Furthermore, we demonstrated that TAA targeting to secreted membrane vesicles in vivo enhanced immune responses in both naïve and TAA tolerant settings. The utility of the approach to improve recombinant viral vaccines is significant, as these vectors can transduce many cell types and produce protein at much greater levels compare to naked DNA vaccines. Finally, we also found that the heightened immune responses mediated by exosomal targeting also translated into more effective therapeutic anti-tumor responses in a TAA tolerant animal model. The use of a tolerant animal model is critical for mimicking the situation found in human cancers.
The rationale to use exosome targeting is supported by the known physiological properties of exosomes. Indeed, one of the assumed exosome functions is to deliver antigens to antigen presenting cells and exosomes contain a large complex variety of proteins which could stimulate both adaptive [2, 17] and innate [18] immune responses.
It has been observed that exosomes released by some tumor cells could become immunosuppressive [19-21]. Tumors cells can modify the properties and the protein content of the exosomes they release. The ability of some tumor cells to turn exosomes into immunosuppressive vesicles could be one of the factors which allow malignant cells to escape host immunosurveillance, as previously discussed [22]. As viral vectors have been well demonstrated to activate immune responses in innate immune cells, as well as in many types of stromal cells, a viral mediated strategy may be of critical importance to generating exosomes with immunogenic content. As an initial demonstration of this strategy, the modified vaccinia system was shown to induce enhanced immune responses to exosome secreted PSA and PAP antigens in a prostate tumor model [23]. As adenoviral vectors have been demonstrated to be highly immunogenic in comparison to other viral platforms [24,25], we believe that their use could offer significant improvements in the use of exosomal targeting strategies. Thus, while the type of cell and the method of transduction are issues of critical importance for the type of exosome produced, we believe our adenoviral-mediated strategy is capable of significantly enhancing immune responses to exosomal-derived proteins. It must also be underlined that this targeting process allows many different types of protein to be delivered to exosomes, such as interleukins and GM-CSF [1]. In future work, the possibility of co-delivering antigens and adjuvant factors to exosomes to further increase vaccine potency will be investigated.
Finally, our study also suggests that involvement of antigen cross presentation [26] should be considered for vaccine development directed against intracellular antigens and that exosomes as [2] could be one element of this complex and not yet fully characterized process.
In conclusion, this is the first demonstration that viral vaccines can be enhanced by exosomal targeting, specifically demonstrating that both C1C2-fused CEA and HER2 ECD encoded by viral vectors are superior in generating antigen specific T and B cell response compared to soluble protein in wild type and transgenic animals. In line with this data, endogenous soluble CEA and HER2 ECD found in the circulation of cancer patients are poor immunogens that do not contribute to efficient anti-tumor responses. Modifying tumor antigens to exploit the exosome pathway for cross-presentation may represent a new way to generate effective immune-mediated anti-tumor activity. Significantly, while this work was performed with tumors antigens, this strategy could be also extended to other types of diseases, such as viral and parasitic transductions, to elicit highly effective and specifically targeted therapeutics.
Highlights.
Extracellular domians of CEA and HER2 as adenoviral exosome expressed proteins > infection of cells enables expression of CEA and HER2 in secreted exosomes > Exosome expressed CEA and HER2 stimulate enhanced immune responses > Vaccination with exosomal HER2 adenovirus enhances anti-tumor responses in vivo
Acknowledgments
This study was supported by grants from the National Cancer Institute (NCI P50 CA89496-01 (H.K.L.), NCI R01 CA95447 (T.M.C.)), Department of Defense Breast Cancer Research Program Clinical Translational Research Award (BC050221) (T.M.C.), and a Susan G. Komen Foundation Postdoctoral Fellowship Award (KG080627) (Z.C.H.).
Abbreviations
- TAA
tumor associated antigens
- APC
antigen presenting cells
- ECD
extracellular domain
- CEA
carcinoembryonic antigen
- HER2
human epidermal growth factor receptor 2
- MUC1
mucin 1
- CDC
complement-dependent cytotoxicity
- bmDC
bone marrow derived dendritic cells
- MVB
multivesicular bodies
Footnotes
The work was performed at: Duke University Medical Center, Durham, NC 27710.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Delcayre A, Estelles A, Sperinde J, Roulon T, Paz P, Aguilar B, et al. Exosome Display technology: applications to the development of new diagnostics and therapeutics. Blood Cells Mol Dis. 2005;35(2):158–68. doi: 10.1016/j.bcmd.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–79. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 3.Finn OJ. Cancer immunology. N Engl J Med. 2008;358(25):2704–15. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 4.Steer HJ, Lake RA, Nowak AK, Robinson BWS. Harnessing the immune response to treat cancer. Oncogene. 2010;29:6301–13. doi: 10.1038/onc.2010.437. [DOI] [PubMed] [Google Scholar]
- 5.Finn OJ, Edwards RP. Human Papillomavirus Vaccine for Cancer Prevention. N Engl J Med. 2009;361:1899–1901. doi: 10.1056/NEJMe0907480. [DOI] [PubMed] [Google Scholar]
- 6.Finn OJ. Cancer vaccines: Between the idea and the reality. Nat Rev Immunol. 2003;3:360–41. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 7.Pejawar-Gaddy S, Finn OJ. Cancer vaccines: accomplishments and challenges. Crit Rev Oncol Hematol. 2008;67(2):93–102. doi: 10.1016/j.critrevonc.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Oshima K, Aoki N, Kato T, Kitajima K, Matsuda T. Secretion of a peripheral membrane protein, MFG-E8, as a complex with membrane vesicles. Eur J Biochem. 2002;269(4):1209–18. doi: 10.1046/j.1432-1033.2002.02758.x. [DOI] [PubMed] [Google Scholar]
- 9.Zeelenberg IS, Ostrowski M, Krumeich S, Bobrie A, Jancic C, Boissonnas A, et al. Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res. 2008;68(4):1228–35. doi: 10.1158/0008-5472.CAN-07-3163. [DOI] [PubMed] [Google Scholar]
- 10.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95(5):2509–14. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartman ZC, Wei J, Osada T, Glass O, Lei G, Yang XY, et al. An adenoviral vaccine encoding full-length inactivated human Her2 exhibits potent immunogenicity and enhanced therapeutic efficacy without oncogenicity. Clin Cancer Res. 2010;16(5):1466–77. doi: 10.1158/1078-0432.CCR-09-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor DD, Zacharias W, Gercel-Taylor C. Exosome isolation for proteomic analyses and RNA profiling. Methods Mol Biol. 2011;728:235–46. doi: 10.1007/978-1-61779-068-3_15. [DOI] [PubMed] [Google Scholar]
- 13.Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods. 2002;270(2):211–26. doi: 10.1016/s0022-1759(02)00330-7. [DOI] [PubMed] [Google Scholar]
- 14.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113:3365–74. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 15.Lin L, Huai Q, Huang M, Furie B, Furie BC. Crystal Structure of the Bovine Lactadherin C2 Domain, a Membrane Binding Motif, Shows Similarity to the C2 Domains of Factor V and Factor VIII. J Mol Biol. 2007;371(3):717–24. doi: 10.1016/j.jmb.2007.05.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bardelle C, Furie B, Furie BC, Gilbert GE. Membrane binding kinetics of factor VIII indicate a complex binding process. J Biol Chem. 1993;268(12):8815–24. [PubMed] [Google Scholar]
- 17.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 18.Viaud S, Terme M, Flament C, Taieb J, André F, Novault S, Escudier B, Robert C, Caillat-Zucman S, Tursz T, Zitvogel L, Chaput N. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PLoS One. 2009;4(3):e4942. doi: 10.1371/journal.pone.0004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koga K, Matsumoto K, Akiyoshi T, Kubo M, Yamanaka N, Tasaki A, Nakashima H, Nakamura M, Kuroki S, Tanaka M, Katano M. Purification, characterization and biological significance of tumor-derived exosomes. Anticancer Res. 2005;25:3703–3708. [PubMed] [Google Scholar]
- 20.Clayton A, Tabi Z. Exosomes and the MICA-NKG2D system in cancer. Blood Cells Mol Dis. 2005;34(3):206–13. doi: 10.1016/j.bcmd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Zhang HG, Grizzle WE. Exosomes and cancer: a newly described pathway of immune suppression. Clin Cancer Res. 2011;17(5):959–64. doi: 10.1158/1078-0432.CCR-10-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delcayre A, Shu H, Le Pecq JB. Dendritic cell-derived exosomes in cancer immunotherapy: exploiting nature’s antigen delivery pathway. Expert Rev Anticancer Ther. 2005;5(3):537–47. doi: 10.1586/14737140.5.3.537. [DOI] [PubMed] [Google Scholar]
- 23.Rountree RB, Mandl SJ, Nachtwey JM, et al. Exosome targeting of tumor antigens expressed by cancer vaccines can improve antigen immunogenicity and therapeutic efficacy. Cancer Res. 2011 Aug 7; doi: 10.1158/0008-5472.CAN-10-4076. [DOI] [PubMed] [Google Scholar]
- 24.Cubillos-Zapata C, Guzman E, Turner A, et al. Differential effects of viral vectors on migratory afferent lymph dendritic cells in vitro predicts enhanced immunogenicity in vivo. J Virol. 2011;85(18):9385–94. doi: 10.1128/JVI.05127-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barefoot B, Thornburg NJ, Barouch DH, et al. Comparison of multiple vaccine vectors in a single heterologous prime-boost trial. Vaccine. 2008;26(48):6108–18. doi: 10.1016/j.vaccine.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rock KL, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol Rev. 2005;207:166–83. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]